ABSTRACT
Background
RBC long-chain omega-3 (n–3) fatty acid (FA) percentages (of total fatty acids) are associated with lower risk for total mortality, but it is unknown if a suite of FAs could improve risk prediction.
Objectives
The objective of this study was to compare a combination of RBC FA levels with standard risk factors for cardiovascular disease (CVD) in predicting risk of all-cause mortality.
Methods
Framingham Offspring Cohort participants without prevalent CVD having RBC FA measurements and relevant baseline clinical covariates (n = 2240) were evaluated during 11 y of follow-up. A forward, stepwise approach was used to systematically evaluate the association of 8 standard risk factors (age, sex, total cholesterol, HDL cholesterol, hypertension treatment, systolic blood pressure, smoking status, and prevalent diabetes) and 28 FA metrics with all-cause mortality. A 10-fold cross-validation process was used to build and validate models adjusted for age and sex.
Results
Four of 28 FA metrics [14:0, 16:1n–7, 22:0, and omega-3 index (O3I; 20:5n–3 + 22:6n–3)] appeared in ≥5 of the discovery models as significant predictors of all-cause mortality. In age- and sex-adjusted models, a model with 4 FA metrics was at least as good at predicting all-cause mortality as a model including the remaining 6 standard risk factors (C-statistic: 0.778; 95% CI: 0.759, 0.797; compared with C-statistic: 0.777; 95% CI: 0.753, 0.802). A model with 4 FA metrics plus smoking and diabetes (FA + Sm + D) had a higher C-statistic (0.790; 95% CI: 0.770, 0.811) compared with the FA (P < 0.01) or Sm + D models alone (C-statistic: 0.766; 95% CI: 0.739, 0.794; P < 0.001). A variety of other highly correlated FAs could be substituted for 14:0, 16:1n–7, 22:0, or O3I with similar predicted outcomes.
Conclusions
In this community-based population in their mid-60s, RBC FA patterns were as predictive of risk for death during the next 11 y as standard risk factors. Replication is needed in other cohorts to validate this FA fingerprint as a predictor of all-cause mortality.
Keywords: lipids, omega-3 index, fatty acids, all-cause mortality, risk factors, myristic acid, palmitoleic acid, behenic acid
Introduction
The Framingham Heart Study provided unique insights into cardiovascular disease (CVD) risk factors (1) and led to the development of the Framingham Risk Score based on 8 baseline standard risk factors—that is, age, sex, smoking, hypertension treatment, diabetes status, systolic blood pressure, total cholesterol (TC), and HDL cholesterol (2). CVD is still the leading cause of death globally (3), and risk can be reduced by changing behavioral risk factors such as unhealthy diet, physical inactivity, and use of tobacco and alcohol. Therefore, biomarkers integrating lifestyle choices might help identify individuals at risk and be useful to assess treatment approaches, prevent morbidity, and delay death.
Among the diet-based biomarkers are fatty acids (FAs), whether measured in plasma or RBC membranes. The FAs most clearly associated with reduced risk for CVD and for total mortality (i.e., death from any cause) are the omega-3 FAs, EPA (20:5n–3) and DHA (22:6n–3) (4, 5). In a 2018 report (6) including 2500 participants in the Framingham Offspring Cohort followed for a median of 7.3 y (i.e., between ages ∼66 and 73 y), the baseline RBC EPA + DHA content [the omega-3 index (O3I)] was significantly and inversely associated with risk for death from all causes. Individuals in the highest quintile were 33% less likely to succumb during the follow-up years compared with those in the lowest quintile. Similar associations have been seen in the Women's Health Initiative Memory Study (7), the Heart and Soul Study (4), and the Ludwigshafen Risk and Cardiovascular Health Study (8). However, these prior investigations evaluated only 1 FA metric (i.e., the O3I) as an exposure variable. Other FA biomarker-based studies have focused only on linoleic acid (9, 10), FAs in the de novo lipogenesis pathway (11), trans FAs (12), dairy-derived FAs (13), or very-long-chain saturated FAs (VLCFAs) (14) in relation to select disease outcomes.
In 2009, Shearer et al. (15) attempted to define an “FA risk fingerprint” using a cross-sectional design with ∼1350 individuals, half of whom were confirmed acute coronary syndrome patients and half were outpatient controls. In that study, RBC FA profiles were quantified, and the association of each FA with acute coronary syndrome was systematically evaluated. A suite of 10 FAs was identified and compared with a suite of standard risk factors—that is, age, sex, TC, HDL cholesterol, smoking status, and self-reported history of hypertension and diabetes (16). The RBC FA profile discriminated cases from controls significantly better than did standard risk factors. However, that approach is not easily translated into clinical use, partly because it was cross-sectional and used a case–control design. In the current investigation, we posed a similar question in a prospective setting using the Framingham Offspring Cohort, which was followed for clinical events for 11 y after RBC FAs were measured. Here, we explore how a fingerprint or pattern of RBC FAs measured in older Americans compares with standard risk factors as predictors of risk of all-cause mortality.
Methods
This study was conducted in the framework of the Framingham Offspring Cohort (17). Participants (n = 3021) who attended their 8th examination cycle (2005–2008) were evaluated. They were excluded in hierarchical order if they were missing RBC FA measurements, were missing relevant clinical covariates, or had prevalent CVD (total exclusion n = 781), leaving 2240 eligible for the current investigation (Supplemental Figure 1). The study protocol was approved by the Institutional Review Board of the Boston University Medical Center. Written informed consent was provided by all participants.
Covariates and mortality outcomes
The primary endpoint was risk of all-cause mortality during 11 y of follow-up.
RBC fatty acid analysis
After a 10- to 12-h fast, blood was drawn into an EDTA tube, and RBCs were separated from plasma by centrifugation. The RBC fraction was frozen at –80°C immediately after collection. RBC FA composition was determined as described previously (18). Briefly, RBCs were incubated at 100°C with boron trifluoride–methanol and hexane to generate FA methyl esters that were then analyzed by GC with flame ionization detection. Twenty-seven FAs were quantified and expressed as a percentage of total RBC FAs, and the O3I was computed as the sum of EPA and DHA. For modeling purposes, we used the O3I (instead of its constituent FAs) and the remaining 25 FAs for a total of 26 FA metrics (Supplemental Table 1).
Statistical analysis
Sample characteristics were summarized using standard statistical metrics (e.g., means, SDs, and correlations). HRs were estimated on a per quintile basis using the survival package in R version 3.6.2 (R Foundation for Statistical Computing) (19). Primary analyses related quintiles of FAs to mortality established from reported date of death or date of censoring. Specifically, a forward, stepwise approach was used to systematically evaluate, in age- and sex-adjusted models, the association of additional risk factors (i.e., 6 standard risk factors and 26 FA metrics) with risk of death from all causes. We used 10-fold cross-validation to build and validate models. Specifically, the data set is divided into 10 randomly selected discovery data sets consisting of nonoverlapping sets of 90% of the data and 10 validation data sets corresponding to the other 10% of the data. This exercise builds 10 pairs of discovery–validation data sets, each composed of 90% (discovery) and 10% (validation).
We then applied the model-building process to each of the 10 discovery sets, each of which identified predictors (i.e., FAs and/or standard risk factors) to be potentially included in the final model. To be included in the final model, a predictor had to be statistically significant in ≥5 of the 10 discovery data sets. Models were constructed in a forward, stepwise approach using a predictor entry criterion of P < 0.05; adjusting for age and sex in all models, and then evaluating the 26 FA metrics and the 6 remaining standard risk factors (i.e., smoking status, hypertensive treatment, diabetes status, systolic blood pressure, TC, and HDL cholesterol). FA predictors used quintiles coded 1–5. Reported HRs and concordances (via Harrell's C-statistic) were averaged across the 10 validation data sets. C-statistics were compared between resulting models and the standard risk factors from Framingham using paired t tests across the 10 validation data sets. Kaplan–Meier survival curves were used to estimate survival proportions by age given different risk profiles. Sensitivity analyses explored 1) how concordance and HRs changed when using highly correlated FAs in place of model-selected FAs; 2) continuous and nonlinear, quintile-based analyses for model-selected FAs; and 3) how the addition of BMI, alcohol intake, and educational status into the final models affected outcomes.
Results
The average age at baseline was 65 y, with slightly more women (57%) than men (43%). The final data set consisted of 2240 individuals meeting the study inclusion/exclusion criteria (Table 1), and 384 participants died during the 11-y follow-up.
TABLE 1.
Sample characteristics at baseline (n = 2240)1
| Characteristic | |
|---|---|
| Male | 43% (972) |
| Age, y | 65.3 ± 8.7 |
| Systolic blood pressure, mm Hg | 128.1 ± 16.8 |
| Total cholesterol, mg/dL | 190.2 ± 35.7 |
| HDL cholesterol, mg/dL | 58.8 ± 18.4 |
| Current smoker | 9.3% (209) |
| Treatment for hypertension | 43.8% (981/2240) |
| Prevalent diabetes | 12.8% (286/2240) |
Values are percentage (n) or means ± SDs.
FA and standard risk factor models
The simplest model predicted all-cause mortality by only age and sex (A + S), with HRs of 1.12 per additional year and 0.66 for being female (Table 2). In an age- and sex-adjusted model including the remaining 6 standard FAs, only current smoking and prevalent diabetes were selected as significant predictors of total mortality, with average HRs of 1.89 and 1.65, respectively (model Sm + D). Using the same age- and sex-adjusted approach, only 4 of 26 FA metrics (i.e., 14:0, 16:1n–7, 22:0, and O3I) appeared in ≥5 of the discovery models as significant predictors of total mortality. Four metrics were selected into the model (FA), with HRs ranging between 0.85 and 1.25 (Table 2). Finally, in a model that simultaneously considered age, sex, and all 26 FA metrics and all 6 remaining standard risk factors, the forward selection procedure selected the same 4 FA metrics, again with similarly sized HRs (0.84–1.23), and the 2 standard risk factors—that is, current smoking (HR = 1.81) and prevalent diabetes (HR = 1.63). This latter model is hereafter called the FA + Sm + D model (Table 2).
TABLE 2.
FA and standard risk factors in predictive models for total mortality after forward selection (P < 0.05)1
| Risk factors in model | Model name | FA or standard risk factors (average HR; 95% CI) |
|---|---|---|
| Age and sex | A + S | Age (1.12; 1.11, 1.14)Female sex (0.66; 0.54, 0.81) |
| Age and sex + selected standard risk factors2,3 | Sm + D | Age (1.12; 1.11, 1.14)Female sex (0.69; 0.55, 0.85)Smoking status (1.89; 1.24, 2.88)Prevalent diabetes (1.65; 1.31, 2.07) |
| Age and sex + selected FAs2,4 | FA | Age (1.13; 1.11, 1.15)Female sex (0.61; 0.48, 0.77)14:0 (0.85; 0.74, 0.96)16:1n–7 (1.25; 1.11, 1.41)22:0 (0.93; 0.85, 1.01)O3I (0.85 0.79, 0.91) |
| Age and sex + selected FA + selected standard risk factors2,3,4 | FA + Sm + D | Age (1.13; 1.11, 1.15)Female sex (0.64; 0.49, 0.82)14:0 (0.84; 0.73, 0.96)16:1n–7 (1.23; 1.09, 1.39)22:0 (0.91; 0.83, 0.99)O3I (0.86; 0.81, 0.92)Current smoker (1.81; 1.14, 2.86)Prevalent diabetes (1.63; 1.24, 2.14) |
Average HRs across 10 validity models (n = 2240). A, age; D, diabetes; FA, fatty acid; O3I, omega-3 index; S, sex; Sm, smoking.
Model adjusted for age and sex.
Of the 6 standard risk factors (smoking status, hypertensive treatment, diabetes status, systolic blood pressure, total cholesterol, and HDL cholesterol), only smoking and prevalent diabetes from Table 1 were selected into the model.
Of the 26 FA metrics tested, only the 4 listed FA metrics [14:0, 16:1n–7, 22:0, and O3I (20:5n–3 + 22:6n–3)] remained as significant predictors. HR is per FA quintile or per presence/absence of Sm and D.
Concordances for 5 predictive models, 4 models from Table 2 and a fifth model [all SRFs that forced in all standard risk factors], are presented in Figure 1. The addition of a combination of 4 FA metrics improved concordance compared with the A + S model alone (0.778; 95% CI: 0.759, 0.797 compared with 0.748; 95% CI: 0.721, 0.775; paired t test, P = 0.001), as did the addition of smoking status and prevalent diabetes (Sm + D) compared with A + S alone (0.766; 95% CI: 0.739, 0.794 compared with 0.748; 95% CI: 0.721, 0.775; paired t test, P = 0.01). The age- and sex-adjusted FA and Sm + D models had similar concordances (0.778; 95% CI: 0.759, 0.797 compared with 0.766; 95% CI: 0.739, 0.794; paired t test, P = 0.22), whereas the combined age- and sex-adjusted model (FA + Sm + D) had significantly better concordance than either model alone (0.790; 95% CI: 0.770, 0.811 compared with 0.778; 95% CI: 0.759, 0.797; paired t test, P = 0.04; and 0.790; 95% CI: 0.770, 0.811 compared with 0.766; 95% CI: 0.739, 0.794; paired t test, P = 0.001, respectively). The FA + Sm + D model also had significantly better concordance than the age- and sex-adjusted all SRFs model (0.790; 95% CI: 0.770, 0.811 compared with 0.777; 95% CI: 0.753, 0.802; paired t test, P = 0.01) and an age- and sex-adjusted model with Sm + D + O3I only (0.790; 95% CI: 0.770, 0.811 compared with 0.772; 95% CI: 0.743, 0.801; paired t test, P = 0.007) (the latter comparison is not presented in Figure 1). When BMI, alcohol intake, or educational status were added to the FA + Sm + D model, they were not significant predictors of all-cause mortality, and all other predictors (A, S, FA, Sm, and D) remained statistically significant.
FIGURE 1.
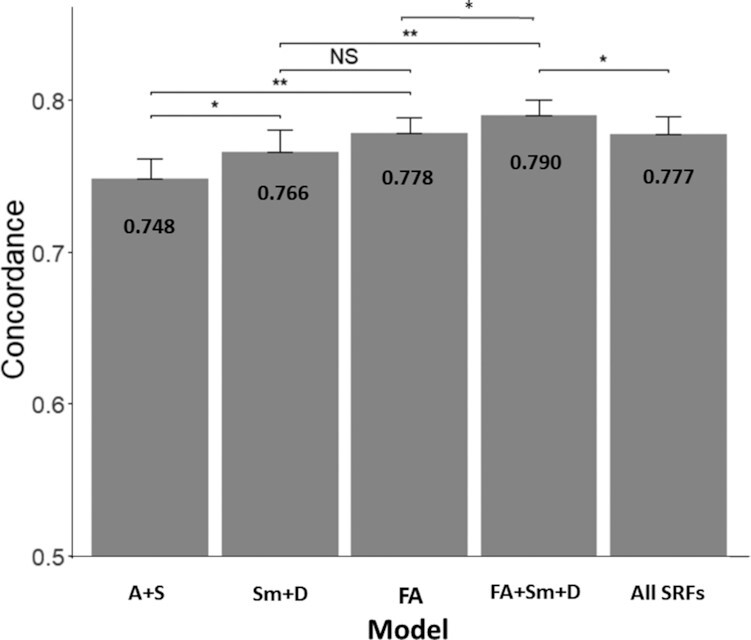
Concordance and SEs for 5 different predictive models for all-cause mortality (n = 2,240). A + S model includes only age and sex. Sm + D model includes age, sex, current smoking status, and prevalent diabetes. The FA model includes age, sex, and the 4 FA metrics [14:0, 16:1n–7, 22:0, and O3I (20:5n–3 + 22:6n–3)]. The FA + Sm + D model includes age, sex, 4 FA metrics, smoking status, and prevalent diabetes. The all SRFs model includes all of the standard risk factors (age, sex, current smoking status, prevalent diabetes, hypertensive treatment, systolic blood pressure, total cholesterol, and HDL cholesterol). *P < 0.01, **P < 0.001. A, age; D, diabetes; FA, fatty acid; O3I, omega-3 index; S, sex; Sm, smoking; SRF, standard risk factor.
Predicted effects of changing model-selected FAs or Standard Risk Factors on risk of death
Theoretical effects on risk of death during the 11 y of follow-up per a 1-quintile change in percentage of the 4 selected FA metrics were compared with the estimated effects of smoking (compared with nonsmoking) and prevalent (or not prevalent) diabetes (Table 3). A 1-quintile increase in 14:0, 22:0, or O3I was associated with an estimated increase in life span of 1.41, 0.79, or 1.18 y, respectively. The reverse held for RBC 16:1n–7, where a 1-quintile reduction was associated with 1.66 additional years of life. Regarding the standard risk factors, being female (compared with male) was associated with an additional 3.42 y, prevalent diabetes (compared with not prevalent) was associated with living 3.9 fewer y, and smoking (compared with not smoking) was associated with living 4.73 fewer years. To estimate effects on risk of death during follow-up associated with changes in 2 of the most modifiable factors in this study—the O3I and smoking—we compared risk for death in the highest compared with the lowest quintile O3I, with and without smoking (Figure 2). In this analysis, 11-y survival was predicted to range from ∼85% for nonsmokers with a high O3I to ∼71% for either alone and ∼47% for current smokers with a low O3I.
TABLE 3.
Theoretical implications on risk of death during follow-up in the FA + Sm + D model1
| Risk factor | β (HR) | β ÷ 0.1252 | Interpretation |
|---|---|---|---|
| Age (per year) | 0.125 (1.13)3 | 1.00 | |
| Sex (female) | –0.453 (0.64) | –3.62 | Being female changes risk of death equivalent to adding 3.62 years of life expectancy vs being male |
| 14:0 | –0.176 (0.84) | –1.41 | Having a 14:0 level 1 quintile higher changes risk of death equivalent to adding 1.41 years (or 5.63 years for four quintiles higher) |
| 16:1n–7 | 0.207 (1.23) | 1.66 | Having a 16:1n-7 level 1 quintile lower changes risk of death equivalent to adding 1.66 years of life expectancy (or 6.62 years for four quintiles lower) |
| 22:0 | –0.099 (0.91) | –0.79 | Having a 22:0 level 1 quintile higher changes risk of death equivalent to adding 0.79 years (or 3.17 years for four quintiles higher) |
| O3I | –0.148 (0.86) | –1.18 | Having a O3I level 1 quintile higher changes risk of death equivalent to adding 1.18 years younger (or 4.74 y for four quintiles higher) |
| Current smoker | 0.591 (1.81) | 4.73 | Being a nonsmoker changes risk of death equivalent to adding 4.73 y |
| Prevalent diabetes | 0.487 (1.63) | 3.90 | Not having diabetes changes risk of death equivalent to adding 3.90 y |
The FA + Sm + D model consists of age, sex, 4 FA metrics [14:0, 16:1n–7, 22:0, and O3I (20:5n–3 + 22:6n–3)], current smoking status, and prevalent diabetes. The model (Figure 1) has a concordance = 0.790 and n = 2240. A, age; D, diabetes; FA, fatty acid; O3I, omega-3 index; S, sex; Sm, smoking.
Dividing β by 0.125 yields a value indicating how the relative risk of death changes for a risk factor compared to the change in risk from being 1 y older (i.e., smoking status or diabetes prevalence) relative to the change in risk for death from being 1 y younger. These estimates are from a model (Figure 1) that uses age, sex, 4 FA metrics, smoking status, and prevalent diabetes.
The HR per year for age is 1.13, or 13% more likely to die for each additional year of age.
FIGURE 2.
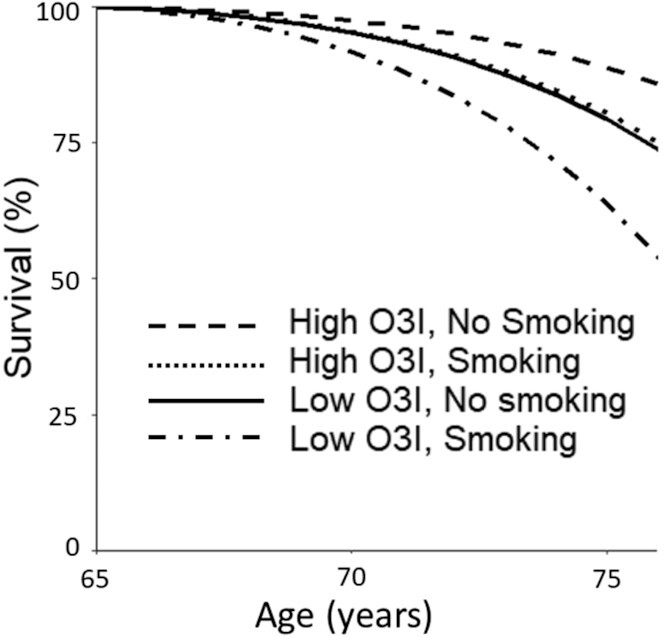
Estimated Kaplan–Meier survival curves by age using estimated HRs per year according to the highest/lowest O3I quintile and smoking status for individuals reaching 65 y (average baseline age). O3I, omega-3 index.
Sensitivity analysis
Because some FA metrics in this study were highly correlated with others (Supplemental Table 2), the sensitivity of the FA + Sm + D model was evaluated by exchanging 16:1n–7, 22:0, and O3I with other highly correlated FAs (r > 0.50) (Table 4). In almost all cases, concordances decreased (as expected), but not by a statistically significant amount (P > 0.05), and never by more than a concordance change of >0.0001. None of the FA metrics in the final model (FA + Sm + D) was highly correlated (r > 0.50) with any of the standard risk factors. Finally, sensitivity analyses forcing BMI and alcohol intake [which have both been associated with risk for death (20, 21)] did not materially change the original estimates associated with the FA or FA + Sm + D models (Supplemental Table 3). For each of the 4 FA metrics, we tested whether there was evidence of nonlinearity using quintiles (separate estimates per quintile or quadratic fit across quintiles) or continuous versions of FAs (using a cubic spline); there was no statistically significant evidence of improved fit for any model for any of the 4 FA metrics (P > 0.20 in all cases). Linear models for the continuous versions of the 4 metrics yielded similar results to the quintile analysis presented previously.
TABLE 4.
Sensitivity analysis of the FA + Sm + D model1 using highly correlated FAs
| FA2 (HR from FA + Sm + D model) | Highly correlated3 FA (correlation) | Average HR ± SE with highly correlated FA4 | Average concordance ± SE using highly correlated FA4 |
|---|---|---|---|
| 16:1n–7 (1.25) | 16:0 (0.62) | 1.12 ± 0.06 | 0.786 ± 0.01 |
| 22:0 (0.93) | 24:0 (0.80) | 0.91 ± 0.03 | 0.787 ± 0.01 |
| 24:1n–9 (0.73) | 0.90 ± 0.03 | 0.789 ± 0.01 | |
| O3I (0.85) | 22:4n–6 (–0.59) | 1.09 ± 0.06 | 0.789 ± 0.01 |
| 22:5n–6 (–0.52) | 1.12 ± 0.04 | 0.791 ± 0.01 |
The FA + Sm + D model consists of age, sex, 4 FA metrics [14:0, 16:1n–7, 22:0, and O3I (20:5n–3 + 22:6n–3)], current smoking status, and prevalent diabetes. The model (Figure 1) has a concordance ± SE = 0.790 ± 0.01 and n = 2240. A, age; D, diabetes; FA, fatty acid; O3I, omega-3 index; S, sex; Sm, smoking.
14:0 had no highly correlated FAs except 16:1n–7, which was already in the model.
Correlation of ≥0.5 from Supplemental Table 2 (except for 20:5n–3 and 22:6n–3 because these 2 FAs constitute the O3I).
Across the 10 validation models.
Discussion
Identifying individuals without known pre-existing conditions who may be at increased risk of dying is a serious public health challenge. The current study examined RBC FAs as a potential biomarker to improve upon standard risk factor–based predictions of risk of death during 11-y follow-up for individuals in their mid-60s. We found that a suite of 4 RBC FA metrics had the same predictive power as the standard risk factors originally identified in the Framingham study (albeit for CVD events) and that adding select RBC FA percentages produced a small but statistically significantly improvement in total mortality prediction. The finding that any FA-based metric would have predictive power similar to that of these well-established standard risk factors was unexpected, and it suggests that RBC FA—via unknown mechanisms—somehow reflects an in vivo milieu that consolidates into 1 measure the impact on the body of all these standard risk factors.
As noted in a previous cross-sectional, case–control study in patients with acute coronary syndrome (15), a suite of 10 RBC FAs discriminated cases from controls better than did a model based on standard risk factors (15), but only 16:1n–7 and DHA (the primary constituent of the O3I) were included in that suite; neither 14:0 nor 22:0 was examined, and the FA most strongly associated with case status [i.e., linoleic acid (18:2n–6)] was not selected in the current analysis. Hence, although Shearer et al. (15) identified a different suite of RBC FAs (and for a different outcome), our findings support their general conclusion that RBC FA patterns appear to carry heretofore underappreciated prognostic information.
Three of the 4 FA metrics identified in this study (14:0, 16:1n–7, and 22:0) each comprises <0.4% of RBC FAs, and the mean O3I is only ∼5%. It is remarkable that FAs present in such low percentages could carry such predictive power; however, it is possible that other highly correlated FAs might serve nearly as well (Supplemental Table 2, Table 3). RBC membrane FA percentages can be influenced by metabolic as well as dietary factors. Next, we consider each of the model-selected RBC FA metrics in that light.
The mean RBC percentage of 14:0 (myristic acid) in this cohort was 0.37%, and it was inversely related to risk for all-cause mortality. The average intake of this FA is estimated to be ∼2 g/d (22), and sources include dairy products, coconut milk, and baked goods (23). Feeding 14:0 to rats leads to a dose-dependent increase in tissue and plasma concentrations of 14:0 and 16:1n–7 (24) (consistent with the high correlation between these 2 FAs observed in this study) (Supplemental Table 1). Studies in healthy adults found a higher 14:0 intake to be associated with higher plasma triglycerides and apoCIII concentrations (25), and sometimes HDL cholesterol, LDL cholesterol, 20:5n–3, and 20:3n–6 concentrations (26, 27). Twenty-five-year mortality data from the Seven Countries Study (initiated between 1958 and 1964) associated 14:0 intake with increased TC concentrations (28), but evidence linking circulating 14:0 percentages to clinical coronary outcomes is heterogeneous (29). Although 14:0 constitutes 11% of the FA in cow milk (30), it is not known if increased dairy fat intake raises RBC 14:0 percentages [although 15:0 percentages do increase in this setting (31)]. We speculate that the association of 14:0 with dairy intake and the observation that dairy intake is associated with lower risk for type 2 diabetes mellitus (13) and possibly CVD (32) may explain its beneficial association with total mortality observed in this study.
The mean RBC percentage of 16:1n–7 (palmitoleic acid) was similar to that of 14:0 (i.e., 0.39%), but unlike 14:0, 16:1n–7 was directly linked with total mortality risk. The average intake of this FA is estimated to be ∼2 g/d (33), and the single richest source is macadamia oil. Most 16:1n–7 is believed to arise from de novo lipogenesis in the liver and adipose tissue (34, 35), although this is somewhat controversial (36). Palmitoleic acid percentages do respond directly to carbohydrate intake (37), but the extent to which an effect on mortality risk could be attributed to the FA or to other metabolic responses to such a dietary change is unknown.
The mean RBC percentage of 22:0 (behenic acid) in this cohort was 0.23%. Very little 22:0 is consumed directly from the few foods that contain it (e.g., canola oil, peanuts, and macadamia nuts). The primary source of 22:0 is via metabolism of 18:0 by elongases 1 and 3 (38). Our results are in agreement with reports in which higher percentages of VLCFAs in RBC were associated with lower risk of coronary heart disease (39, 40) or sudden cardiac arrest (41). VLCFAs are important constituent of sphingolipids, such as ceramides and sphingomyelins. The high correlation between 22:0 and 24:0 and 24:1 observed here confirms that this FA is a surrogate of a suite of these complex lipid molecules. In a previous study in Framingham Offspring (examination 8, the same as in the current study), Walker et al. (42) found an inverse relation between the 22:0/16:0 ratio in plasma ceramides with both CV and total mortality. Zhao et al. (43) reported an inverse relation between plasma VLCFA, including 22:0, and risk of metabolic syndrome. Although the relation between VLCFAs in RBC and plasma has not been thoroughly explored, a higher RBC 22:0 could reasonably reflect a lower risk milieu.
The mean O3I in this cohort was 5.8%, similar to 5.2% measured in US family physicians, where 78% reported using an ω-3 supplement ≤1/wk and 57% reported consuming <2 servings/wk of fish (44). Among US adults aged ≥19 y, the average daily amount of EPA and DHA consumed from food and dietary supplements is 113 mg (45). The O3I is primarily determined by the direct intake of these 2 FAs (46, 47). In this Framingham cohort, dietary factors and “heritability” have been reported to explain 40% and 24% of the variation in percentages (48), respectively, but heritability could be as high as 70% (49). On the other hand, in genome-wide association studies, very few single-nucleotide polymorphisms were significantly associated with 20:5n–3 or 22:6n–3 percentages (50). The O3I (and RBC 20:5n–3 and 22:6n–3 percentages individually) has been inversely associated with total mortality in this cohort (6) and the Women's Health Initiative Memory Study (7). Multiple mechanisms of action have been documented to support evidence for beneficial relations of the long-chain n–3 PUFAs on human health (51–54). These FAs have hypolipidemic, antihypertensive, and antiplatelet effects and can improve endothelial function and autonomic balance. Together, these are mediated by effects on membrane physiochemistry, gene expression, and the production of a myriad of bioactive oxylipins. Hence, finding that the O3I was selected into the final model was not unexpected, but whether other RBC FA associations would have modified that relation was not known and motivated this investigation. Although we had no a priori hypothesis regarding which other FAs, if any, would add to the predictive power of the O3I, it is fair to say that none of the 3 FAs we identified would have been among our obvious choices.
In the final combined model (FA + Sm + D), smoking and the O3I seem to be the most easily modified risk factors. Being a current smoker (at age 65 y) is predicted to subtract ∼4.7 y of life (compared with not smoking), a life shortening equivalent to being in the lowest compared with highest O3I quintile. In the current cohort, the O3I cutoff for quintile 1 was <4.2%, and it was >6.8% for quintile 5 (6). It is interesting to note that in Japan, where the mean O3I is >8% (55), the expected life span is ∼5 y longer than it is in the United States (56), where the mean O3I is ∼5%. Hence, in practice, dietary choices that change the O3I may prolong life (4, 57).
The most obvious limitation of this study is applying standard risk factors originally identified in Framingham for CVD risk by following younger individuals (baseline age 49 y) to estimate risk of all-cause mortality in an older population (baseline age 65 y). Other risk markers, such as plasma C-reactive protein (58, 59) and ankle brachial index (60), might have been considered in efforts to improve prediction of all-cause mortality. Another limitation is the definition of “diabetes” used in this analysis, which includes type 1 diabetes—a disease that cannot be reverted. We acknowledge that diabetes is a modifiable risk factor with weight loss typically required to achieve normoglycemia. We are unaware of any other set of surrogate endpoints or biomarkers that have been shown to be superior to these standard risk factors in a cohort similar to that studied here. The FA + Sm + D model predictions for risk of death should be limited to individuals between the ages of ∼65 and 76 y (Figure 2); extrapolating to younger people or beyond the years of observation included here is unwarranted. Model predictions in the upper age range should be done with care because data are available from fewer individuals. Finally, comparing the potential effects of a change in a continuous variable (O3I) with a dichotomous variable (e.g., smoking) is challenging, but continuous data for smoking were not available for comparison.
In conclusion, in this cohort followed for 11 y, the information carried in the concentrations of 4 RBC FA metrics was as useful as that carried in lipid levels, blood pressure, smoking, and diabetic status with regard to predicting total mortality. The best predictions were made with the FA metrics and smoking/diabetes status. In the future, when larger data sets are available, additional model-building approaches may be worth exploring (e.g., random forest, partial least-squares–discriminant analysis, and principal component analysis), along with replication in other cohorts; however, the cross-validation approach is robust and suggests a strong association between this RBC FA fingerprint and risk for all-cause mortality.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—WSH: conceived the study; NLT and WSH: developed the analytical plan; NLT: performed the statistical analyses; MIM: drafted the manuscript; and all authors: contributed to the writing of the manuscript and read and approved the final manuscript. MIM serves on the Board of Directors of the American Society for Nutrition and consults with Council for Responsible Nutrition; Church & Dwight; DSM Nutritional Products; International Life Sciences Institute, North America; McCormick; OmegaQuant Analytics; PepsiCo; and VitaMe Technologies. AS-V has received institutional research funding and support to attend professional meetings from the California Walnut Commission. WSH holds an interest in OmegaQuant Analytics, a lab that offers ω-3 blood testing, and is a member of the RB Schiff Science and Innovation Advisory Board. All other authors report no conflicts interest.
Notes
This work was supported in part by the Institute for the Advancement of Food and Nutrition Sciences (IAFNS) through an International Life Sciences Institute North America Lipid Committee grant. IAFNS is a nonprofit science organization that pools funding from industry collaborators and advances science through the in-kind and financial contributions from public and private sector participants. The Framingham Heart Study (FHS) acknowledges the support of contracts N01-HC-25195, HHSN26820150000II, and 75N92019D00031 from the National Heart, Lung, and Blood Institute.
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: A, age; CVD, cardiovascular disease; D, diabetes; FA, fatty acid; O3I, omega-3 index; RF, risk factor; S, sex; Sm, smoking; SRF, standard risk factor; TC, total cholesterol; VLCFA, very-long-chain saturated fatty acid.
Contributor Information
Michael I McBurney, The Fatty Acid Research Institute, Sioux Falls, SD, USA; Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Canada; Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Nathan L Tintle, The Fatty Acid Research Institute, Sioux Falls, SD, USA; Department of Statistics, Dordt University, Sioux Center, IA, USA.
Ramachandran S Vasan, Schools of Medicine and Epidemiology, Boston University, Boston, MA, USA.
Aleix Sala-Vila, The Fatty Acid Research Institute, Sioux Falls, SD, USA; Hospital del Mar Medical Research Institute, Barcelona, Spain.
William S Harris, The Fatty Acid Research Institute, Sioux Falls, SD, USA; Sanford School of Medicine, University of South Dakota, Sioux Falls, SD, USA.
Data Availability
Data described in the article and code book can be requested through formal application to the Framingham Heart Study Executive Committee. Analytic code will be made available upon request to the corresponding author.
References
- 1.Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44(6):1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119(24):3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richie H, Roser M. Causes of death. Our World in Data. 2018. [Internet] Available from: https://ourworldindata.org/causes-of-death. [Google Scholar]
- 4.Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: the Heart and Soul Study. Circ Cardiovasc Qual Outcomes. 2010;3:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, Siscovick DS. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158(7):515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris WS, Tintle NL, Etherton MR, Vasan RS. Erythrocyte long-chain ω-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: the Framingham Heart Study. J Clin Lipidol. 2018;12:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris WS, Luo J, Pottala JV, Espeland MA, Margolis KL, Manson JE, Wang L, Brasky TM, Robinson JG. Red blood cell polyunsaturated fatty acids and mortality in the Women's Health Initiative Memory Study. J Clin Lipidol. 2017;11(1):250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleber ME, Delgado GE, Lorkowski S, Marz W, von Schacky C. ω-3 Fatty acids and mortality in patients referred for coronary angiography: the Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis. 2016;252:175–81. [DOI] [PubMed] [Google Scholar]
- 9.Marklund M, Wu JHY, Imamura F, Del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan Set al. Biomarkers of dietary ω-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139(21):2422–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris WS, Tintle NL, Ramachandran VS. Erythrocyte n–6 fatty acids and risk for cardiovascular outcomes and total mortality in the Framingham Heart Study. Nutrients. 2018;10(12):2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura F, Fretts AM, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Virtanen JKet al. Fatty acids in the de novo lipogenesis pathway and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2020;17(6):e1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sala-Vila A, Jackson KH, Harris WS. Parallel declines in erythrocyte trans fatty acids and US fatal ischemic heart disease rates. Nutr Res. 2019;71:111–4. [DOI] [PubMed] [Google Scholar]
- 13.Imamura F, Fretts A, Marklund M, Ardisson Korat AV, Yang WS, Lankinen M, Qureshi W, Helmer C, Chen TA, Wong Ket al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: a pooled analysis of prospective cohort studies. PLoS Med. 2018;15(10):e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fretts AM, Imamura F, Marklund M, Micha R, Wu JHY, Murphy RA, Chien KL, McKnight B, Tintle N, Forouhi NGet al. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2019;109(4):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shearer GC, Pottala JV, Spertus JA, Harris WS. Red blood cell fatty acid patterns and acute coronary syndrome. PLoS One. 2009;4:e5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:3, 281–90. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr. 2012;142(7):1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The R Project for Statistical Computing . R version 3. [Internet]. Available from: www.r-project.org.
- 20.Global B, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson Ch Let al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166(22):2437–45. [DOI] [PubMed] [Google Scholar]
- 22.Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, Hu FB, Sun Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ. 2016;355:i5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdock GA, Carabin IG. Safety assessment of myristic acid as a food ingredient. Food Chem Toxicol. 2007;45(4):517–29. [DOI] [PubMed] [Google Scholar]
- 24.Rioux V, Catheline D, Bouriel M, Legrand P. Dietary myristic acid at physiologically relevant levels increases the tissue content of C20:5n–3 and C20:3n–6 in the rat. Reprod Nutr Dev. 2005;45(5):599–612. [DOI] [PubMed] [Google Scholar]
- 25.Olivieri O, Speziali G, Castagna A, Pattini P, Udali S, Pizzolo F, Liesinger L, Gindlhuber J, Tomin T, Schittmayer Met al. The positive association between plasma myristic acid and ApoCIII concentrations in cardiovascular disease patients is supported by the effects of myristic acid in HepG2 cells. J Nutr. 2020;150(10):2707–15. [DOI] [PubMed] [Google Scholar]
- 26.Temme EH, Mensink RP, Hornstra G. Effects of medium chain fatty acids (MCFA), myristic acid, and oleic acid on serum lipoproteins in healthy subjects. J Lipid Res. 1997;38:1746–54. [PubMed] [Google Scholar]
- 27.Dabadie H, Motta C, Peuchant E, LeRuyet P, Mendy F. Variations in daily intakes of myristic and α-linolenic acids in sn-2 position modify lipid profile and red blood cell membrane fluidity. Br J Nutr. 2006;96(2):283–9. [DOI] [PubMed] [Google Scholar]
- 28.Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med. 1995;24:3, 308–15. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SGet al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 30.Månsson HL. Fatty acids in bovine milk fat. Food Nutr Res. 2008;52::1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slim M, Ha C, Vanstone CA, Morin SN, Rahme E, Weiler HA. Evaluation of plasma and erythrocyte fatty acids C15:0, t-C16:1n–7 and C17:0 as biomarkers of dairy fat consumption in adolescents. Prostaglandins Leukot Essent Fatty Acids. 2019;149:24–9. [DOI] [PubMed] [Google Scholar]
- 32.Drouin-Chartier JP, Brassard D, Tessier-Grenier M, Côté JA, Labonté M, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodge AM, Simpson JA, Gibson RA, Sinclair AJ, Makrides M, O'Dea K, English DR, Giles GG. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr Metab Cardiovasc Dis. 2007;17(6):415–26. [DOI] [PubMed] [Google Scholar]
- 34.Frigolet ME, Gutiérrez-Aguilar R. The role of the novel lipokine palmitoleic acid in health and disease. Adv Nutr. 2017;8(1):173s–81s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JJ, Lambert JE, Hovhannisyan Y, Ramos-Roman MA, Trombold JR, Wagner DA, Parks EJ. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am J Clin Nutr. 2015;101(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosqvist F, McNeil CA, Pramfalk C, Parry SA, Low WS, Cornfield T, Fielding BA, Hodson L. Fasting hepatic de novo lipogenesis is not reliably assessed using circulating fatty acid markers. Am J Clin Nutr. 2019;109(2):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, Artistizabal JC, Fernandez ML, Bruno RS, Maresh CM, Kraemer WJet al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One. 2014;9(11):e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem (Tokyo). 2012;152(5):387–95. [DOI] [PubMed] [Google Scholar]
- 39.Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB, Sun Q. Circulating very-long-chain saturated fatty acids and incident coronary heart disease in us men and women. Circulation. 2015;132(4):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papandreou C, Sala-Vila A, Galié S, Muralidharan J, Estruch R, Fitó M, Razquin C, Corella D, Ros E, Timiraos Jet al. Association between fatty acids of blood cell membranes and incidence of coronary heart disease. Arterioscler Thromb Vasc Biol. 2019;39(4):819–25. [DOI] [PubMed] [Google Scholar]
- 41.Lemaitre RN, King IB, Rice K, McKnight B, Sotoodehnia N, Rea TD, CO J, Raghunathan TE, Cobb LA, Mozaffarian Det al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91(4):149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker ME, Xanthakis V, Peterson LR, Duncan MS, Lee J, Ma J, Bigornia S, Moore LL, Quatromoni PA, Vasan RSet al. Dietary patterns, ceramide ratios, and risk of all-cause and cause-specific mortality: the Framingham Offspring Study. J Nutr. 2020;150(11):2994–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Li X, Li X, Chu Q, Zhou Y, Li Z, Zhang H, Brenna TJ, Song Y, Gao Y. Associations of plasma very-long-chain SFA and the metabolic syndrome in adults. Br J Nutr. 2018;120(8):855–62. [DOI] [PubMed] [Google Scholar]
- 44.Matusheski NV, Marshall K, Hartunian-Sowa S, McBurney MI. US family physicians overestimate personal ω-3 fatty acid biomarker status: associations with fatty fish and ω-3 supplement intake. Curr Dev Nutr. 2018;2(2):nzx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papanikolaou Y, Brooks J, Reider C, Fulgoni VL. U.S. adults are not meeting recommended levels for fish and ω-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J. 2014;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poppitt SD, Kilmartin P, Butler P, Keogh GF. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipid Health Dis. 2005;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block RC, Harris WS, Pottala JV. Determinants of blood cell ω-3 fatty acid content. Open Biomark J. 2008;1:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012;225:425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lemaitre RN, Siscovick DS, Berry EM, Kark JD, Friedlander Y. Familial aggregation of red blood cell membrane fatty acid composition: the Kibbutzim Family Study. Metabolism. 2008;57(5):662–8. [DOI] [PubMed] [Google Scholar]
- 50.Kalsbeek A, Veenstra J, Westra J, Disselkoen C, Koch K, McKenzie KA, O'Bott J, Vander Woude J, Fischer K, Shearer GCet al. A genome-wide association study of red-blood cell fatty acids and ratios incorporating dietary covariates: Framingham Heart Study Offspring Cohort. PLoS One. 2018;13(4):e0194882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darwesh AM, Sosnowski DK, Lee TY, Keshavarz-Bahaghighat H, Seubert JM. Insights into the cardioprotective properties of n–3 PUFAs against ischemic heart disease via modulation of the innate immune system. Chem Biol Interact. 2019;308:20–44. [DOI] [PubMed] [Google Scholar]
- 52.Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 2019;16(10):581–601. [DOI] [PubMed] [Google Scholar]
- 53.Endo J, Arita M. Cardioprotective mechanism of ω-3 polyunsaturated fatty acids. J Cardiol. 2016;67(1):22–7. [DOI] [PubMed] [Google Scholar]
- 54.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophy Acta. 2015;1851(4):397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanagisawa N, Shimada K, Miyazaki T, Kume A, Kitamura Y, Ichikawa R, Ohmura H, Kiyanagi T, Hiki M, Fukao Ket al. Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J Atheroscler Thromb. 2010;17:285–94. [DOI] [PubMed] [Google Scholar]
- 56.Infoplease . Life expectancy for countries. [Internet]. 2019.; Available from https://www.infoplease.com/world/health-and-social-statistics/life-expectancy-countries. [Google Scholar]
- 57.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine ω-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the Cardiovascular Health Study. Circulation. 2005;112(1):25–31. [DOI] [PubMed] [Google Scholar]
- 59.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8):C19–-31. [DOI] [PubMed] [Google Scholar]
- 60.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker Get al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article and code book can be requested through formal application to the Framingham Heart Study Executive Committee. Analytic code will be made available upon request to the corresponding author.


