ABSTRACT
Background
Infants born at very low birth weight (VLBW) are vulnerable to deficits in fatty acids (FAs) but little is known of factors that influence the intakes or composition of their human milk feeds.
Objectives
We aimed to identify sources of variability in the fat composition of human milk fed to VLBW infants and examine the impact of milk source (mother's own or donor) on fat and FA intakes.
Methods
Serial samples of mother's milk (n = 476) and donor milk (n = 53) fed to infants born weighing <1250 g (n = 114 infants from 100 mothers) were collected [Optimizing Mothers’ Milk for Preterm Infants (OptiMoM) randomized clinical trial]. Fat and FA were analyzed using a mid-infrared human milk analyzer and GC with flame ionization detection.
Results
At full enteral feeding, donor milk is estimated to provide 1.3 g · kg−1 · d−1 less total fat than mature mother's milk (recommended intake: 4.8 g · kg−1 · d−1), and 5–9 mg · kg−1 · d−1 less DHA (22:6n–3) and arachidonic acid (20:4n–6) (estimated average requirement: 55–60 and 35–45 mg · kg−1 · d−1, respectively) than colostrum or transitional milk. Similar deficits were observed in measured intakes of a subset of OptiMoM infants. In multivariable-adjusted models, maternal ethnicity had medium to large [≥0.5 SD score (SDS)] effects on DHA, SFAs, and MUFAs. Mothers with prepregnancy BMI in overweight and obese categories had higher milk total fat (β: 0.35; 95% CI: 0.10, 0.61 and β: 0.46; 95% CI: 0.16, 0.77 SDS, respectively). Those with BMI ≥30 in addition had higher proportions of SFAs (β: 0.61; 95% CI: 0.33, 0.89 SDS) and lower DHA (β: −0.54; 95% CI: −0.89, −0.20 SDS). Other factors, such as gestational age and income, were also associated with FA composition.
Conclusions
The fat and FA content of human milk fed to VLBW infants is variable. Care must be taken to ensure fat and FA intakes meet recommendations, particularly when feeding a high proportion of donor milk.
This trial was registered at clinicaltrials.gov as NCT02137473.
Keywords: human milk, breast milk, donor milk, very-low-birth-weight infants, preterm, fat, fatty acids, docosahexaenoic acid, arachidonic acid, intakes
Introduction
Feeding mother's own milk is associated with improved outcomes among infants born at a very low birth weight (VLBW, <1500 g), including better feeding tolerance; reduced risks of necrotizing enterocolitis, retinopathy of prematurity, and infection; and higher scores on neurodevelopmental tests (1). Nutrition is tightly managed for VLBW infants to optimize growth and development, often using standard reference values for macronutrients in human milk; however, concentrations of these nutrients are variable (2). Fat and fatty acids (FAs) are the most variable macronutrient component of human milk, and contribute 40%–50% of its energy (2, 3). Fat is also important for infant brain growth, accounting for >50% of the mass of the gray matter (4). DHA (22:6n–3) and arachidonic acid (ARA; 20:4n–6) are enriched in the brain and retina, where they play critical roles in membrane fluidity, signaling, neurogenesis, and modulating inflammation (5). These PUFAs must be obtained either from the diet/maternal circulation, or through limited elongation and desaturation from shorter-chain precursors (6). VLBW infants may be sensitive to deficits in fat and FA intake in early life because premature birth interrupts placental transfer during a period of rapid in utero growth and synaptogenesis in the third trimester (7). This is compounded by a lower efficiency of fat absorption in the first weeks of life, particularly for long-chain FAs (8). As a result, preterm infants are estimated to accumulate deficits in accretion of long-chain PUFAs (9).
Many factors contribute to the variation in the fat and FA content of human milk. These include diet and supplement use, as well as maternal factors (e.g., age or BMI), infant factors (such as gestational age and sex), sociodemographic factors (ethnicity, education, poverty), and environmental factors (smoking, season of delivery); however, these have been poorly studied among mothers of vulnerable VLBW infants (10–16). The composition of milk from mothers of VLBW infants may be influenced differently than term milk because both mammogenesis (e.g., mammary gland alveolar and ductal development) and lactogenesis (e.g., secretory initiation and activation) are interrupted owing to preterm birth. Intakes of fat and FAs may also be influenced by use of donor milk, as opposed to mother's milk, among VLBW infants. In the absence of sufficient volumes of mother's own milk, providing donor milk is standard of care in North America (17, 18). Despite its benefits, donor milk may not be equivalent to mother's milk with respect to fat and FA content because it is largely comprised of mature milk from mothers who gave birth at term, and undergoes additional processing (e.g., additional freeze-thaw cycles and container changes) that may remove fat or otherwise alter its FA composition (19–21). An improved understanding of the factors that influence human milk total fat and FA composition and intakes is important for planning nutrition for VLBW infants. Thus, our aim was to identify sources of variability in the fat composition of human milk fed to VLBW infants, and to examine the impact of milk source (mother's own or donor) on fat and FA intakes.
Methods
Serial samples from a 24-h supply of pooled human milk to be fed to hospitalized infants born weighing <1250 g were collected as part of the OptiMoM (Optimizing Mothers’ Milk for Preterm Infants) Fortifier Study (NCT02137473). Briefly, OptiMoM was a parallel-group randomized clinical trial that compared the effects of a bovine milk–based fortifier and a human milk–based fortifier on feeding tolerance during initial hospitalization. Participants were enrolled from neonatal intensive care units (NICUs) at Sinai Health or the Hospital for Sick Children in Toronto, Canada between August 2014 and November 2015. Full study methods, including feeding protocols, have been published previously (22). Infants were included if they were born weighing <1250 g and if their parents agreed to provide donor milk when sufficient volumes of mothers’ milk were unavailable. Infants were excluded if they had received formula or human milk fortifier before random assignment, if enteral feeding was not initiated by 14 d postbirth, if they had a known chromosomal or congenital anomaly affecting growth, if they were enrolled in another study that affected their feeding, or if they were likely to be transferred to a nonparticipating NICU. Both the feeding intervention as well as collection of milk samples continued after transfer to community NICUs (n = 16). The intervention continued until participants were 12 wk of age, were discharged from hospital, or consumed ≥2 oral feeds per day for ≥3 d, whichever occurred first. The protocol for OptiMoM was approved by the research ethics boards at all participating hospitals. Written informed consent was obtained for all participants. Infant baseline characteristics were obtained from medical records, whereas mothers reported characteristics such as their age, education, income, and ethnicity from fixed multiple-choice lists with the option to provide other answers. Gestational age was calculated from the date of the mother's last menstrual period. If this differed from the early ultrasound prediction by ≥2 wk, the estimate from the early ultrasound was used. Owing to small sample size for some responses, education level was binned to postsecondary or no postsecondary, and ethnicity was binned to European, East or Southeast Asian, South or West Asian, African or Caribbean, and Other (which included mothers who identified as Latin American or multiple ethnic origins) based on preliminary analysis indicating similar patterns of FA composition.
Mother's milk
As part of the standard of care at the participating NICUs, mother's milk was provided to infants as the preferred form of enteral nutrition whenever it was available. Until ∼34 wk postconception, this was almost exclusively expressed human milk administered by a feeding tube. Mothers of VLBW infants were encouraged to express human milk every 2–3 h using a double electric breast pump, allowing for a 5-h break overnight. They received instruction on how to ensure a full breast expression to stimulate supply. Breast pumps were available in the NICUs free of charge and a combination of free loaner and for payment programs existed for use of breast pumps at home as required. Mother's milk was stored at −20°C in hard plastic containers or polyethylene bags until thawed by hospital staff before the preparation of feeds the following day. At the time of the study, mother's milk was provided to infants in the order it was expressed and frozen. According to the original study protocol, once each week (targeted Monday), unfortified mother's milk remaining after the preparation of feeds was divided into aliquots and stored at −80°C until analysis. In the event >1 milk sample was collected during any given week, the first sample with sufficient volume was selected, except during the first week where all available samples were analyzed.
Donor milk
Pasteurized donor milk was provided primarily by the Rogers Hixon Ontario Human Milk Bank at Sinai Health System in Toronto, Canada, with additional milk provided from the NorthernStar Mothers Milk Bank in Calgary, Canada; both are members of the Human Milk Banking Association of North America (HMBANA) that use published HMBANA guidelines for screening donors and handling and processing human milk (23). Donors to the milk bank are instructed either to immediately freeze their milk in human milk freezer bags in their home freezer, or to refrigerate for ≤24 h before freezing. Frozen donated milk is collected by a courier a minimum of every 3 or 6 mo depending on home freezer type. Once at the milk bank, the milk is thawed in a refrigerator overnight, and the milk from ≥3 women is pooled into batches, divided into aliquots and pasteurized (Holder, 62.5°C for 30 min). It is then frozen at −20°C for a maximum of 6 mo before use. Samples of all donor milk batches (n = 311) fed to infants during the study were collected when available after the preparation of feeds as already described for mother's milk. A subset of 50 batches were randomly selected using a computer-generated table for FA analysis, with an additional 3 samples manually added to ensure the best coverage of study infants. All but 1 infant who received >5% donor milk during the intervention period had ≥1 of the batches of donor milk they were fed included in the analysis.
Milk composition analysis
Total human milk fat was determined using the mid-infrared Miris Human Milk Analyzer according to manufacturer instructions. The correlation coefficient between fat measured by Miris and by the Mojonnier method (the AOAC method for measuring fat in milk) is 0.997 (24). Further, at the time of this study, our team participated in the international MAMAS (Milk Analysis using Milk Analyzers in a Standardized setting) validation study that included baseline calibration of this instrumentation and ongoing monitoring using high and low quality control samples (25). A homogeneous pooled milk control was measured on each experimental day, yielding an interassay variability <5%.
Milk FA analysis
Milk FAs were extracted using a modified Folch method, methylated in 0.3:1 hexane:boron trifluoride methanol, and quantified by GC with flame ionization detection using a Varian-430 gas chromatograph (Varian) as described previously (26). Chromatograms were analyzed in Compass CDS software (version 3.0.0.0.687, Scion Instruments), with FAs identified by comparison with authenticated FAME standards (NuChek Prep Inc.). CVs for the major FA classes (saturated, monounsaturated, n–6, and n–3) ranged from 4% to 11% between experimental days. FA data are expressed as mol% of FAs (i.e., mass adjusted for molecular weight) or as SD scores (SDSs). SDS, or z score, is calculated as the sample mean minus the group mean divided by the group SD. This allows for comparison of effect sizes between different variables.
Fat and FA intakes
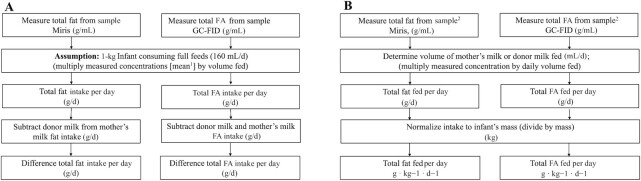
To quantify the impact of donor milk feeding on intakes of fat and FAs, we multiplied concentrations of fat and FAs for each milk type by 160 mL · kg−1 · d−1, which was the target volume for full enteral feeding for the VLBW infants in OptiMoM (22) (Figure 1A). We then subtracted the donor milk FA concentrations from those of mother's milk to provide an estimation of the deficit in intake with 100% donor milk, compared with 100% mother's milk feeding at different time points postpartum.
FIGURE 1.
Calculation of total fat and FA intakes among infants receiving mother's milk, donor milk, or a combination. (A) Calculation to model the estimated differences in fat or FA intakes for a 1-kg infant consuming full feeds (160 mL · kg−1 · d−1). (B) Calculation to determine actual differences in fat or FA intakes using infant feeding data. 1The mean of all samples in each group (donor milk, mother's milk) was used in the assumption made for this calculation. 2The closest available sample to each day of intake was selected for analysis. FA, fatty acid; GC-FID, GC with flame ionization detection.
To account for actual enteral intakes, which are often lower than prescribed target volumes, we then calculated the fat and FA intakes for a subset of OptiMoM participants at weeks 1 and 2 (days 1–14) and week 4 of life (days 22–28) (Figure 1B). This subset was restricted to infants not receiving parenteral nutrition, so that the fats from human milk were the primary source of dietary lipid. The analysis was further restricted to infants randomly assigned to the bovine milk–based fortifier group, because the human milk–based fortifier used in the other arm of the study displaced 20%–40% of other human milk (mother's own or donor) from the diet (22), resulting in lower intakes of both mother's own and donor milk in this group (P < 0.0001) and a lower proportion of donor milk usage (mean ± SE: 36.6% ± 5.9% compared with 21.0% ± 5.0%) over the intervention period (P < 0.0001). Weeks 1 and 2 were selected as a period when infants fed mother's milk would be receiving primarily colostrum or transitional milk (pooled owing to low sample size), whereas week 4 was selected as a period when infants fed mother's milk would be receiving early-mature milk. Daily intakes of mother's milk, donor milk, and fortifier were collected prospectively during the trial from infant medical records and recipes maintained in the 2 milk preparation rooms used for the study. Daily intakes of fat from mother's milk were calculated by multiplying the daily volume of mother's milk consumed normalized for daily infant weight (mL · kg−1 · d−1) by the measured concentrations of fat and FAs of the milk from the closest sample day available (mean ± SE day postpartum: 11.0 ± 0.46 for weeks 1 and 2; 25.2 ± 0.4 for week 4). Intakes of fat and FAs from donor milk were obtained by multiplying the daily infant weight–normalized donor milk intakes by the mean fat and FA composition of a panel of 53 donor milk batches fed to OptiMoM infants.
Statistical analysis
Analysis was carried out in SAS version 9.4 (SAS Institute). Baseline characteristics were compared between recruited participants with and without milk samples available for analysis using t tests or Wilcoxon's rank-sum tests and chi-square tests for continuous and categorical variables as appropriate (Table 1). Because of the high rate of follow-up and similarity between those with and without samples available, multiple imputation was not used for missing data.
TABLE 1.
Characteristics of participants1
| Characteristics | Fatty acid analysis | No milk sample available |
|---|---|---|
| Sex, female | 62/114 (54.4) | 9/11 (81.8) |
| Birth weight, g | 884.9 ± 19.1 | 914.5 ± 59.7 |
| Gestational age at birth,2 wk | 27.7 ± 0.2 | 27.7 ± 0.6 |
| Multiple birth status | 40/114 (35.1) | 4/11 (36.4) |
| Small for gestational age | 28/114 (24.6) | 1/11 (9.1) |
| Received antenatal steroids | 102/114 (89.5) | 8/11 (72.7) |
| SNAP-II score3 | 13.3 ± 1.0 | 17.6 ± 5.1 |
| Apgar score at 5 min | 7.3 ± 0.2 | 7.4 ± 0.5 |
| Any donor milk intake | 52/114 (45.6) | 11/11 (100.0)* |
| Randomization group HMBF | 59/114 (51.8) | 5/11 (45.5) |
| Morbidity composite4 | 50/114 (43.9) | 2/11 (18.2) |
| Brain injury5 | 16/114 (14.0) | 3/11 (27.3) |
| Cesarean delivery | 73/114 (64.0) | 4/11 (36.4) |
| Season of delivery | ||
| Winter | 35/114 (30.1) | 5/11 (45.5) |
| Spring | 31/114 (27.2) | 0/11 (0.0) |
| Summer | 24/114 (21.1) | 3/11 (27.3) |
| Fall | 24/114 (21.1) | 3/11 (27.3) |
| Mother's age, y | 33.1 ± 0.5 | 35.1 ± 2.3 |
| Mother's education | ||
| High school or less | 17/113 (15.0) | 2/10 (20.0) |
| College or vocational diploma | 39/113 (34.5) | 7/10 (70.0) |
| Baccalaureate | 40/113 (35.4) | 1/10 (10.0) |
| Postbaccalaureate | 17/113 (15.0) | 0/10 (0.0) |
| Mother's ethnicity | ||
| Eastern or Western European | 41/113 (36.3) | 1/10 (10.0) |
| East or Southeast Asian | 23/113 (20.4) | 4/10 (40.0) |
| South or West Asian | 21/113 (18.6) | 1/10 (10.0) |
| African or Caribbean | 24/113 (21.2) | 2/10 (20.0) |
| Other (including Latin American and mixed backgrounds) | 4/113 (3.5) | 2/10 (20.0) |
| Maternal parity >1 | 41/114 (36.0) | 5/11 (45.5) |
| Maternal BMI, kg/m2 | 25.5 ± 0.4 | 29.1 ± 1.0 |
| Maternal smoking | 7/114 (6.1) | 3/11 (27.3)* |
| Language spoken at home | ||
| English | 57/101 (56.4) | 4/8 (50.0) |
| Mixed English and other language | 31/101 (30.7) | 2/8 (25.0) |
| Language other than English | 13/101 (12.9) | 2/8 (25.0) |
| Not married | 6/101 (5.9) | 1/8 (12.5) |
| Family living below the poverty line6 | 23/112 (20.5) | 7/11 (63.6)* |
Values are mean ± SE or n (%). The trial initially enrolled 127 infants; however, 2 died before study day 1 and were not included in subsequent analyses. *Statistically significant difference, P < 0.05. Apgar, appearance pulse grimace activity respiration; HMBF, human milk–based fortifier group; SNAP-II, Score for Neonatal Acute Physiology II.
Gestational age determined using maternal estimates of last menstrual period. If the early ultrasound prediction differed by ≥2 wk, the gestational age estimate derived from the early ultrasound was used.
Scores may range from 0 to 100 with higher values indicating higher neonatal risk and newborn illness.
Includes late-onset sepsis, chronic lung disease, necrotizing enterocolitis, and retinopathy of prematurity requiring treatment.
Includes echodense intraparenchymal lesions, periventricular leukomalacia, porencephalic cysts, or ventriculomegaly with or without intraventricular hemorrhage.
Based on 2012 Statistics Canada family size–adjusted cutoff values.
For comparison of the FA percentage composition between human milk types, sample time points were selected to approximate colostrum (collected on postpartum days 1–4), transitional milk (days 8–14), early-mature milk (days 15–40), later-mature milk (>40 d), or donor milk. There was no association between fortifier group as randomly assigned and FA composition in repeated-measures ANOVA (data not shown), so the groups were pooled for all subsequent analyses.
Associations of infant, maternal, sociodemographic, and environmental factors with human milk FAs were analyzed using PROC MIXED accounting for repeated measures using an autoregressive correlation structure. Percentage composition data were converted to SDS to allow for comparison of effect sizes. Infant factors included gestational age at birth (wk), multiple birth, and sex. Maternal factors included maternal prepregnancy BMI (in kg/m2) (underweight: <18.5; normal weight: 18.5–24.9; overweight: 25.0–29.9; or obese: ≥30), delivery method (cesarean, vaginal), and age. Sociodemographic variables included parity (>1 compared with 1), maternal education (postsecondary compared with no postsecondary), maternal self-reported ethnicity (European, East or Southeast Asian, South or West Asian, African or Caribbean, and Other), family income above or below the Statistics Canada 2012 Ontario family size–adjusted poverty line (yes/no) (27), and marital status. The environmental factors included maternal smoking and season of delivery. Associations were run unadjusted in Model 1 (Figure 2) and with adjustment in Model 2 (Figure 3) to determine whether the associations were independent and robust to adjustment. Adjustments in Model 2 included the strongest predictors of FAs from Model 1 and predictors of FA composition from the term-born literature (11). Before modeling, predictors were assessed for the presence of multicollinearity (tolerance statistic <0.4). Birth weight was significantly collinear with gestational age, and thus was not included in the adjusted model. Gestational age was kept in Model 2 rather than birth weight because it was considered a better marker of the interruption of normal mammogenesis by preterm birth. Marital status and smoking were not included in the adjusted model owing to small sample size.
FIGURE 2.
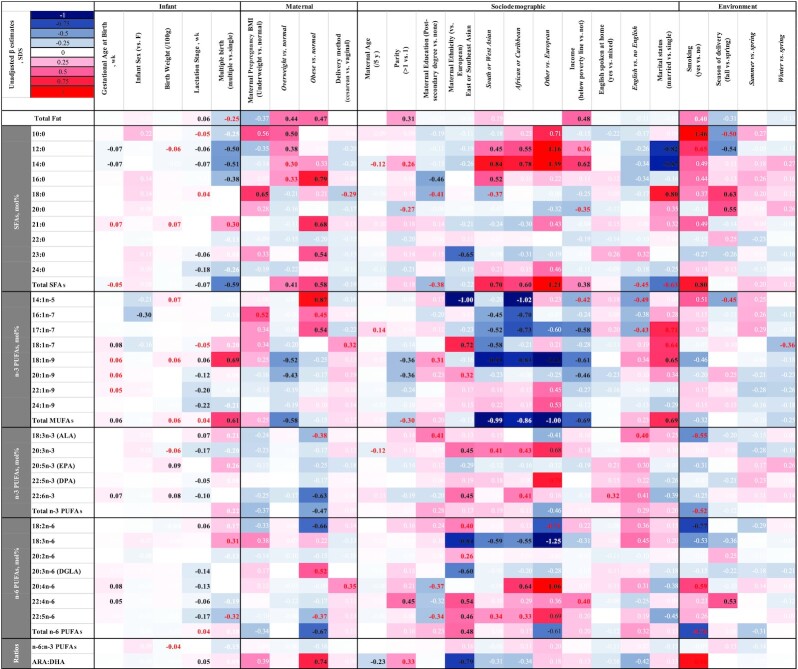
Determinants of fat and FA content of mother's milk: unadjusted β estimates (SDSs). Unadjusted associations (β estimates) of infant, maternal, sociodemographic, or environmental factors with mother's milk FA composition SDSs. A negative sign indicates a negative association for continuous variables (gestational age, lactation stage, and maternal age) or lower than the listed reference value for categorical variables (all others). Sample size is 100 mothers, 472 human milk samples. Associations were analyzed using PROC MIXED, followed by LSMEANS for pairwise comparisons where applicable, accounting for repeated measures using an autoregressive correlation structure. Estimates in black type represent associations that remained significant after adjusting for multiple testing (P < 0.007) whereas those in red are associated with P values < 0.05. Estimates in white type are P > 0.05. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; FA, fatty acid; SDS, SD score.
FIGURE 3.
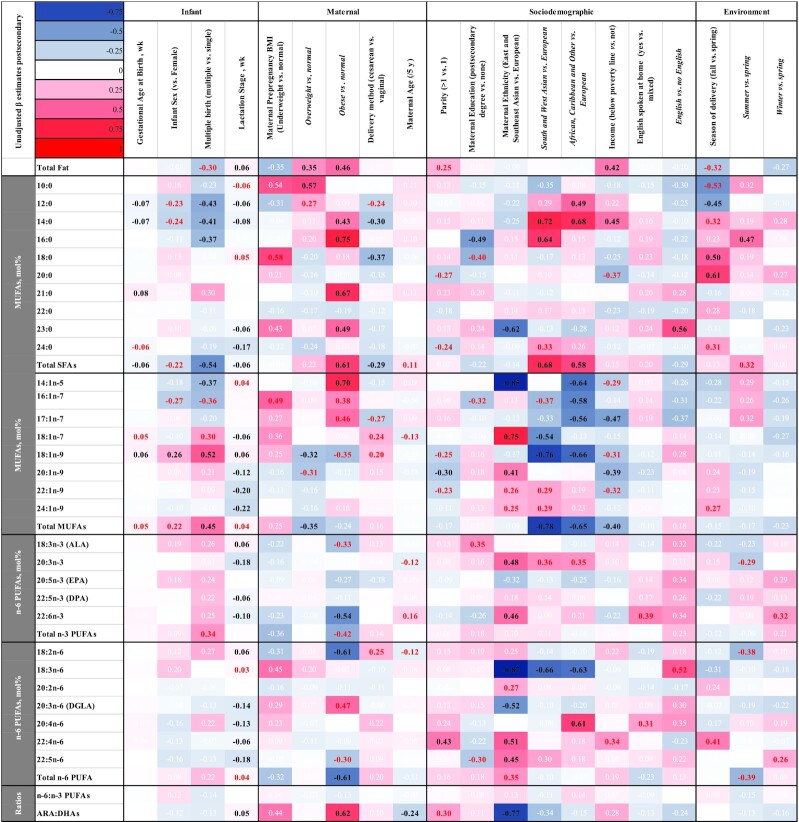
Determinants of fat and FA content of mother's milk: adjusted β estimates (SDSs). Adjusted associations (β estimates) of infant, maternal, sociodemographic, or environmental factors with mother's milk FA composition SDSs. Associations were analyzed using PROC MIXED, followed by LSMEANS for pairwise comparisons where applicable, accounting for repeated measures using an autoregressive correlation structure, adjusting for maternal prepregnancy BMI (in kg/m2) (underweight: <18.5; normal weight: 18.6–24.9; overweight: 25.0–29.9; or obese: ≥30), maternal education (postsecondary compared with none), maternal ethnicity (East or Southeast Asian, South or West Asian, European, African or Caribbean, or Other), family income above or below the family size–adjusted Ontario poverty line (y/n) and timing of sample collection (wk). A negative sign indicates a negative association for continuous variables (gestational age, lactation stage, and maternal age) or lower than the listed reference value for categorical variables (all others). Sample size is 99 mothers, 469 human milk samples (1 subject was not included from the unadjusted sample owing to missing covariate data). Estimates in black type represent associations that remained significant after adjusting for multiple testing (P < 0.007) whereas those in red are associated with P values < 0.05. Estimates in white type are P > 0.05. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; FA, fatty acid; SDS, SD score.
For comparison of fat and FA intakes, infants were grouped as predominately receiving donor human milk (>50% of enteral feeds) or 100% mother's milk during the weeks observed. For weeks 1 and 2, Model 1 was run unadjusted, and Model 2 adjusted only for maternal prepregnancy BMI owing to consideration of the sample size and because this variable had the strongest association with human milk fat and FA composition in preliminary bivariate analyses. For intakes at week 4 of life, Model 2 was adjusted for maternal prepregnancy BMI, self-reported ethnicity, and a composite social risk score, which included family structure (single- or dual-parent household), maternal education (less than university-educated or university and above), language spoken at home (no English or English), maternal age (<21 y or >21 y), and income (below or above the Statistics Canada 2012 Ontario family size–adjusted poverty line) (28).
All statistical tests of significance were 2-sided, with P < 0.05 considered statistically significant. Bonferroni correction was in addition used for Figures 2 and 3 based on 7 main outcomes of interest (total fat, saturated fat, monounsaturated fat, n–6 polyunsaturated fat, n–3 polyunsaturated fat, DHA, and ARA) to reduce the chance of type 1 error due to the large number of analyses. FA analysis was not one of the prespecified endpoints of the OptiMoM randomized clinical trial. This work was therefore exploratory, and aimed to identify factors that may influence human milk fat and FA composition and intakes to be pursued by future studies.
Results
Participants
Of the 127 infants enrolled in the original trial, 100 mothers of 114 infants had ≥1 milk sample available for analysis, for a total of 476 observations (Supplemental Figure 1). Samples were available from birth up to 12 wk postpartum, with most (83%) collected between postpartum days 5 and 48 (Supplemental Figure 2). Infants with samples available for analysis were born at a mean ± SE of 27.7 ± 0.2 weeks of gestation, weighing (mean ± SE) 884.9 ± 19.1 g (Table 1). Mothers were (mean ± SE) 33.1 ± 0.5 y of age and varied in ethnic background, with 36.3% self-identifying as Eastern or Western European, 20.4% as East or Southeast Asian, 18.6% as South or West Asian, 21.2% as Caribbean or African, and 3.5% as Other (Latin American or multiple ethnic backgrounds). Mean ± SE maternal prepregnancy BMI was 25.5 ± 0.4, with 28.1% of women meeting cutoffs for overweight, 15.8% for obesity, and 7.9% for underweight. Infants with no milk sample available for analysis were more likely to have received donor milk during hospitalization (100.0% compared with 45.6%, P < 0.001), reflecting a lower availability of mother's milk for both feeding and analysis, and were more likely to come from families living below the family size–adjusted poverty line (63.6% compared with 20.5%, P < 0.001).
Total fat and FA composition of human milk fed to infants born weighing <1250 g
The amount and type of fat in human milk fed to infants varied by lactation stage (colostrum, transitional, early-mature, or later-mature) and source of milk (mother's own or donor). Total fat content was lower in donor than in transitional, early-mature, and later-mature mother's milk (Table 2). Donor milk contained a lower proportion of SFAs and a higher proportion of MUFAs. Colostrum had a lower proportion of SFAs than transitional milk, whereas later-mature milk had a higher proportion of MUFAs than transitional or early-mature milk, but was not significantly different from colostrum. Donor milk also had lower proportions of DHA than colostrum or transitional milk, a lower proportion of ARA than colostrum, a higher proportion of ARA than later-mature milk, and a higher ratio of ARA:DHA than in colostrum, transitional, or early-mature mother's milk. Colostrum had a higher proportion of DHA than transitional, early-mature, and later-mature mother's milk and a higher proportion of ARA than early- and later-mature milk. Transitional milk had a higher proportion of DHA and ARA than both early- and later-mature milk. Later-mature milk had a lower proportion of ARA than early-mature milk and a higher ratio of ARA:DHA than colostrum and transitional mother's milk.
TABLE 2.
FA composition (mol% of total quantified FAs) of donor and mother's milk by lactation stage1
| Mother's milk2 | |||||
|---|---|---|---|---|---|
| Donor milk (n = 54) | Colostrum (n = 18) | Transitional (n = 80) | Early-mature (n = 92) | Later-mature (n = 45) | |
| Total fat, g/dL | 3.26 ± 0.13a | 3.21 ± 0.21a | 3.86 ± 0.11b | 3.78 ± 0.10b | 4.05 ± 0.14b |
| 10:0 | 0.31 ± 0.06a | 0.07 ± 0.08b | 0.38 ± 0.05a | 0.33 ± 0.05a | 0.32 ± 0.06a |
| 12:0 | 2.85 ± 0.34a | 3.08 ± 0.43a | 5.38 ± 0.26b | 4.85 ± 0.25c | 4.46 ± 0.32c |
| 14:0 | 4.58 ± 0.34a | 6.46 ± 0.42b | 8.30 ± 0.26d | 7.62 ± 0.25c | 7.16 ± 0.32c |
| 16:0 | 18.15 ± 0.31a | 22.35 ± 0.46b | 21.14 ± 0.25c | 21.14 ± 0.23c | 20.88 ± 0.32c |
| 18:0 | 5.14 ± 0.14a | 5.58 ± 0.20abc | 5.47 ± 0.11ab | 5.79 ± 0.10c | 5.60 ± 0.14bc |
| 20:0 | 0.13 ± 0.00a | 0.17 ± 0.01b | 0.16 ± 0.00bc | 0.16 ± 0.00bc | 0.16 ± 0.01bc |
| 21:0 | 0.13 ± 0.01a | 0.08 ± 0.00b | 0.08 ± 0.01b | 0.09 ± 0.01b | 0.08 ± 0.01b |
| 22:0 | 0.05 ± 0.00a | 0.08 ± 0.01b | 0.07 ± 0.00bc | 0.07 ± 0.00bc | 0.06 ± 0.00c |
| 23:0 | 0.10 ± 0.01a | 0.12 ± 0.01ab | 0.13 ± 0.01b | 0.13 ± 0.01bc | 0.11 ± 0.01ac |
| 24:0 | 0.04 ± 0.00a | 0.12 ± 0.01b | 0.09 ± 0.00c | 0.07 ± 0.00d | 0.06 ± 0.00e |
| Total SFAs | 31.86 ± 0.74a | 39.22 ± 0.97b | 41.68 ± 0.57c | 40.83 ± 0.55bc | 39.39 ± 0.71bc |
| 14:1n–5 | 0.21 ± 0.01a | 0.14 ± 0.02b | 0.18 ± 0.01b | 0.19 ± 0.01ac | 0.18 ± 0.01bc |
| 16:1n–7 | 2.64 ± 0.08a | 2.17 ± 0.10bc | 2.15 ± 0.06c | 2.28 ± 0.06b | 2.32 ± 0.08b |
| 17:1n–7 | 0.19 ± 0.01a | 0.17 ± 0.01bc | 0.16 ± 0.00c | 0.17 ± 0.00b | 0.17 ± 0.01bc |
| 18:1n–7 | 1.89 ± 0.04ac | 2.15 ± 0.06b | 1.99 ± 0.03c | 1.88 ± 0.03a | 1.91 ± 0.04ac |
| 18:1n–9 | 39.78 ± 0.55a | 34.95 ± 0.74bc | 33.86 ± 0.43b | 34.17 ± 0.41b | 35.20 ± 0.54c |
| 20:1n–9 | 0.47 ± 0.01a | 0.67 ± 0.02b | 0.59 ± 0.01c | 0.51 ± 0.01d | 0.49 ± 0.02ad |
| 22:1n–9 | 0.06 ± 0.00a | 0.15 ± 0.01b | 0.11 ± 0.00c | 0.08 ± 0.00d | 0.07 ± 0.00e |
| 24:1n–9 | 0.02 ± 0.00a | 0.18 ± 0.01b | 0.12 ± 0.00c | 0.07 ± 0.00d | 0.05 ± 0.00e |
| Total MUFAs | 46.02 ± 0.58a | 41.16 ± 0.78bc | 40.03 ± 0.45b | 40.23 ± 0.43b | 41.33 ± 0.57c |
| 18:3n–3 (ALA) | 2.20 ± 0.07a | 1.71 ± 0.10bcd | 1.65 ± 0.06d | 1.77 ± 0.05b | 1.94 ± 0.07c |
| 20:3n–3 | 0.05 ± 0.00a | 0.14 ± 0.01b | 0.11 ± 0.00c | 0.09 ± 0.00d | 0.08 ± 0.00e |
| 20:5n–3 (EPA) | 0.14 ± 0.01a | 0.08 ± 0.01b | 0.09 ± 0.01b | 0.09 ± 0.01b | 0.08 ± 0.01b |
| 22:5n–3 (DPA) | 0.21 ± 0.01a | 0.26 ± 0.01b | 0.20 ± 0.01c | 0.19 ± 0.01c | 0.18 ± 0.01c |
| 22:6n–3 | 0.30 ± 0.22ad | 0.45 ± 0.03b | 0.40 ± 0.02c | 0.34 ± 0.16a | 0.28 ± 0.20d |
| Total n–3 PUFAs | 2.87 ± 0.09a | 2.61 ± 0.13ab | 2.41 ± 0.71b | 2.43 ± 0.07b | 2.59 ± 0.09b |
| 18:2n–6 | 18.30 ± 0.42a | 15.30 ± 0.57b | 14.50 ± 0.33bc | 15.47 ± 0.31ad | 16.05 ± 0.41ad |
| 18:3n–6 | 0.15 ± 0.01a | 0.07 ± 0.01b | 0.08 ± 0.01bc | 0.11 ± 0.01d | 0.11 ± 0.01d |
| 20:2n–6 | 0.27 ± 0.01a | 0.67 ± 0.02b | 0.53 ± 0.01c | 0.44 ± 0.01d | 0.38 ± 0.02e |
| 20:3n–6 (DGLA) | 0.40 ± 0.02a | 0.69 ± 0.04b | 0.58 ± 0.02c | 0.51 ± 0.02d | 0.43 ± 0.03ae |
| 20:4n–6 | 0.61 ± 0.02ac | 0.71 ± 0.03b | 0.66 ± 0.02ab | 0.57 ± 0.02c | 0.51 ± 0.02d |
| 22:4n–6 | 0.23 ± 0.02a | 0.33 ± 0.03b | 0.22 ± 0.02a | 0.19 ± 0.02a | 0.16 ± 0.02c |
| 22:5n–6 | 0.03 ± 0.00a | 0.09 ± 0.01b | 0.07 ± 0.00c | 0.04 ± 0.03d | 0.04 ± 0.00ae |
| Total n–6 PUFAs | 20.09 ± 0.43a | 17.98 ± 0.60b | 16.82 ± 0.34c | 17.43 ± 0.32cd | 17.79 ± 0.43d |
| n–6:n–3 PUFA | 7.76 ± 0.53 | 7.07 ± 0.87 | 7.34 ± 0.42 | 7.88 ± 0.40 | 7.19 ± 0.57 |
| ARA:DHA | 2.26 ± 0.09a | 1.76 ± 0.09b | 1.77 ± 0.68b | 1.87 ± 0.07bc | 2.05 ± 0.08ac |
Values are means ± SEs. With the exception of total fat (g/dL), all values are percentage composition (mol%). Associations were analyzed using PROC MIXED followed by LSMEANS for pairwise comparisons, accounting for repeated measures using an autoregressive correlation structure. a–eStatistically significant differences, P < 0.05. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; FA, fatty acid.
Colostrum was collected on postpartum days 1–4, transitional milk on days 8–14, early-mature milk on days 15–40, and later-mature milk at >40 d. The earliest sample available for each participant during each stage was selected for analysis.
Determinants of FA composition of mother's milk fed to infants born weighing <1250 g
Maternal, infant, sociodemographic, and environmental factors were associated with the FA composition of mother's milk provided to VLBW infants in both unadjusted and adjusted models (summary data in Figures 2 and 3, detailed results in Supplemental Tables 1 and 2). Statistically significant results for the fully adjusted models [presented as SDSs (also known as z score) and 95% CIs] are briefly summarized here. Data for the unadjusted models are also provided in an unstandardized format (units: g/dL or mol%) in Supplemental Table 3. SDS is used to allow for comparison of effect sizes between variables. Gestational age at birth was negatively associated with the proportion of total SFAs in human milk (β: −0.06; 95% CI: −0.10, −0.02 SDS/wk). Milk from mothers who gave birth to multiples (twins or triplets) had a lower proportion of total SFAs (β: −0.54; 95% CI: −0.76, −0.31 SDS) and a higher proportion of MUFAs (β: 0.45; 95% CI: 0.23, 0.67 SDS). Increasing weeks of lactation was associated with a reduction of total SFAs (β: −0.06; 95% CI: −0.09, −0.03 SDS/wk), DHA (β: −0.10; 95% CI: −0.14, −0.07 SDS/wk), and ARA (β: −0.13; 95% CI: −0.17, −0.10 SDS/wk) and an increase in total fat (β: 0.06; 95% CI: 0.02, 0.09 SDS/wk) and the ratio of ARA:DHA (β: 0.05; 95% CI: 0.03, 0.08 SDS/wk). Mothers with prepregnancy BMIs in the overweight or obese ranges had higher amounts of total fat in their human milk than those in the normal range (β: 0.35; 95% CI: 0.10, 0.61 and β: 0.46; 95% CI: 0.16, 0.77 SDS, respectively). Milk from mothers with BMIs in the overweight category also contained lower proportions of MUFAs (β: −0.35; 95% CI: −0.57, −0.13 SDS). Mothers with prepregnancy BMIs in the obese category had higher proportions of total SFAs (β: 0.61; 95% CI: 0.33, 0.89 SDS) and lower proportions of DHA (β: −0.54; 95% CI: −0.89, −0.20 SDS) and total n–6 PUFAs (β: −0.61; 95% CI: −0.94, −0.28 SDS), including linoleic acid (18:2n–6) (β: −0.61; 95% CI: −0.93, −0.29 SDS), than mothers in the normal-weight category prepregnancy. Mothers who experienced a cesarean delivery had lower proportions of human milk total SFAs (β: −0.29; 95% CI: −0.48, −0.10 SDS) than those who delivered vaginally. Increasing maternal age was associated with a lower ratio of ARA:DHA (β: −0.24; 95% CI: −0.39, −0.08 SDS per 5 y increased age). East or Southeast Asian ethnicity was associated with higher human milk DHA (β: 0.46; 95% CI: 0.14, 0.78 SDS) and a lower ratio of ARA to DHA (β: −0.77; 95% CI: −1.17, −0.37 SDS) than European ethnicity. Milk from mothers of South or West Asian or Caribbean or African descent was higher in SFAs (β: 0.68; 95% CI: 0.38, 0.99 and β: 0.58; 95% CI: 0.32, 0.85 SDS, respectively) and lower in MUFAs (β: −0.78; 95% CI: −1.07, −0.48 and β: −0.65; 95% CI: −0.91, −0.39 SDS, respectively) than milk from European mothers. Mothers of African or Caribbean descent in addition had higher human milk ARA than those of European ethnicity (β: 0.61; 95% CI: 0.28, 0.94 SDS). Milk from mothers living below the family size–adjusted Ontario poverty line had higher concentrations of total fat (β: 0.42; 95% CI: 0.14, 0.71 SDS), with lower proportions of MUFAs (β: −0.40; 95% CI: −0.65, −0.14 SDS), than milk from mothers above the poverty line.
Other factors that had statistically significant associations with individual FAs after adjustment, but not with total fat or categories of FAs, included maternal education [lower palmitic acid (16:0) in mothers with postsecondary education than in those with none], parity [>1 associated with lower proportions of gondoic acid (20:1n–9) and higher proportions of adrenic acid (22:4n–6)], language spoken at home [higher proportion of tricosylic acid (23:0) in non-English speakers], season of delivery [lower lauric acid (12:0) and higher stearic acid (18:0) and arachidic acid (20:0) in fall than in spring, higher palmitic acid in summer than in spring], and infant sex [higher oleic acid (18:1n–9) in milk from mothers of male infants].
Donor milk use and FA intakes
At full enteral feeding volumes (160 mL · kg−1 · d−1), a simulated diet of 100% donor milk would provide 1.3 g · kg−1 · d−1 (21%) less total fat than mature mother's milk, driven primarily by lower amounts of SFAs (deficit of 0.89 g · kg−1 · d−1) with donor milk feeding (Supplemental Table 4). Compared with feeding 100% colostrum or transitional milk, feeding 100% donor milk at full enteral feeding volumes would provide 8 and 9 mg · kg−1 · d−1 less DHA (50%–56%) and 5 and 9 mg · kg−1 · d−1 less ARA (16%–28%), respectively.
To validate these simulated estimates, daily intakes of fat and FAs for weeks of life 1 and 2 and week 4 (adjusted: Table 3; unadjusted: Supplemental Table 5) were compared between a subset of participants consuming >50% donor milk or 100% mother's milk (n = 13 and 31 for weeks 1 and 2 or 4, respectively). During weeks 1 and 2, infants consuming >50% donor milk (n = 6) consumed less total fat than those receiving 100% mother's milk (n = 7) even after adjustment for maternal prepregnancy BMI (Model 2, β: −2.60; 95% CI: −3.87, −1.44 g · kg−1 · d−1). This was driven primarily by lower daily intakes of SFAs, including capric acid (10:0), lauric acid, myristic acid (14:0), palmitic acid, stearic acid, arachidic acid, tricosylic acid, and lignoceric acid (24:0), and MUFAs, including palmitoleic acid (16:1n–7), heptadecenoic acid (17:1n–7), vaccenic acid (18:1n–7), oleic acid, gondoic acid, erucic acid (22:1n–9), and nervonic acid (24:1n–9). Infants receiving >50% donor milk also consumed lower amounts of DHA (β: −11.7; 95% CI: −21.5, −1.8 mg · kg−1 · d−1) and ARA (β: −11.1; 95% CI: −17.8, −4.3 mg · kg−1 · d−1) in the adjusted model during weeks 1 and 2 of life.
TABLE 3.
Model 2—adjusted fatty acid intakes of very-low-birth-weight infants fed >50% DM compared with 100% MoM1
| Weeks 1 and 2 | Week 4 | |||||
|---|---|---|---|---|---|---|
| Intakes, g · kg−1 · d−1 | Adjusted means ± SEs | Model 22 | Adjusted means ± SEs | Model 23 | ||
| >50% DM | 100% MoM | β ± SE | >50% DM | 100% MoM | β ± SE | |
| Total fat | 3.980 ± 0.332 | 6.585 ± 0.373 | −2.605 ± 0.506* | 4.390 ± 0.410 | 0.327 ± 5.243 | −0.854 ± 0.507 |
| 10:0 | 0.004 ± 0.008 | 0.047 ± 0.009 | −0.043 ± 0.012* | 0.014 ± 0.005 | 0.004 ± 0.018 | −0.005 ± 0.006 |
| 12:0 | 0.074 ± 0.071 | 0.420 ± 0.082 | −0.346 ± 0.107* | 0.136 ± 0.023 | 0.018 ± 0.251 | −0.115 ± 0.028* |
| 14:0 | 0.160 ± 0.048 | 0.477 ± 0.056 | −0.317 ± 0.073* | 0.205 ± 0.034 | 0.027 ± 0.367 | −0.162 ± 0.042* |
| 16:0 | 0.689 ± 0.075 | 1.449 ± 0.084 | −0.759 ± 0.114* | 0.795 ± 0.106 | 0.085 ± 1.108 | −0.313 ± 0.131* |
| 18:0 | 0.192 ± 0.036 | 0.371 ± 0.041 | −0.179 ± 0.054* | 0.216 ± 0.037 | 0.030 ± 0.313 | −0.096 ± 0.046* |
| 20:0 | 0.005 ± 0.001 | 0.011 ± 0.001 | −0.005 ± 0.001* | 0.005 ± 0.001 | 0.001 ± 0.010 | −0.005 ± 0.001* |
| 21:0 | 0.005 ± 0.001 | 0.006 ± 0.001 | −0.001 ± 0.001 | 0.006 ± 0.001 | 0.001 ± 0.003 | 0.004 ± 0.001* |
| 22:0 | 0.002 ± 0.001 | 0.005 ± 0.001 | −0.003 ± 0.001* | 0.002 ± 0.001 | 0.001 ± 0.005 | −0.003 ± 0.001* |
| 23:0 | 0.004 ± 0.001 | 0.008 ± 0.001 | −0.003 ± 0.001* | 0.005 ± 0.001 | 0.001 ± 0.005 | 0.000 ± 0.001 |
| 24:0 | 0.002 ± 0.001 | 0.005 ± 0.001 | −0.003 ± 0.001* | 0.001 ± 0.001 | 0.001 ± 0.005 | −0.003 ± 0.001* |
| Total SFAs | 1.129 ± 0.162 | 2.907 ± 0.182 | −1.778 ± 0.246* | 1.405 ± 0.180 | 0.144 ± 2.118 | −0.713 ± 0.223* |
| 14:1n–5 | 0.008 ± 0.001 | 0.011 ± 0.001 | −0.002 ± 0.001 | 0.011 ± 0.001 | 0.001 ± 0.008 | 0.003 ± 0.001 |
| 16:1n–7 | 0.102 ± 0.010 | 0.151 ± 0.011 | −0.050 ± 0.015* | 0.121 ± 0.012 | 0.010 ± 0.104 | 0.017 ± 0.015 |
| 17:1n–7 | 0.007 ± 0.001 | 0.013 ± 0.001 | −0.006 ± 0.002* | 0.009 ± 0.001 | 0.001 ± 0.008 | 0.000 ± 0.001 |
| 18:1n–7 | 0.074 ± 0.008 | 0.124 ± 0.009 | −0.050 ± 0.012* | 0.083 ± 0.009 | 0.007 ± 0.094 | −0.011 ± 0.011 |
| 18:1n–9 | 1.621 ± 0.151 | 2.161 ± 0.171 | −0.540 ± 0.229* | 1.741 ± 0.157 | 0.125 ± 1.859 | −0.118 ± 0.195 |
| 20:1n–9 | 0.019 ± 0.003 | 0.033 ± 0.003 | −0.014 ± 0.004* | 0.019 ± 0.003 | 0.002 ± 0.031 | −0.012 ± 0.003* |
| 22:1n–9 | 0.002 ± 0.000 | 0.006 ± 0.001 | −0.003 ± 0.001* | 0.002 ± 0.000 | 0.000 ± 0.005 | −0.002 ± 0.001* |
| 24:1n–9 | 0.001 ± 0.001 | 0.006 ± 0.001 | −0.004 ± 0.001* | 0.001 ± 0.000 | 0.000 ± 0.004 | −0.003 ± 0.000* |
| Total MUFAs | 1.864 ± 0.172 | 2.589 ± 0.195 | −0.725 ± 0.461* | 2.023 ± 0.181 | 0.144 ± 2.167 | −0.144 ± 0.224 |
| 18:3n–3 (ALA) | 0.090 ± 0.008 | 0.093 ± 0.009 | −0.004 ± 0.013 | 0.103 ± 0.006 | 0.005 ± 0.086 | 0.017 ± 0.007* |
| 20:3n–3 | 0.002 ± 0.000 | 0.005 ± 0.000 | −0.003 ± 0.001* | 0.002 ± 0.000 | 0.000 ± 0.004 | −0.002 ± 0.000* |
| 20:5n–3 (EPA) | 0.006 ± 0.001 | 0.004 ± 0.001 | 0.001 ± 0.001 | 0.007 ± 0.000 | 0.000 ± 0.004 | 0.003 ± 0.000* |
| 22:5n–3 (DPA) | 0.009 ± 0.001 | 0.010 ± 0.001 | −0.001 ± 0.001 | 0.010 ± 0.001 | 0.001 ± 0.007 | 0.003 ± 0.001* |
| 22:6n–3 | 0.012 ± 0.003 | 0.023 ± 0.003 | −0.012 ± 0.004* | 0.014 ± 0.001 | 0.001 ± 0.014 | −0.001 ± 0.002 |
| Total n–3 PUFAs | 0.119 ± 0.010 | 0.135 ± 0.011 | −0.017 ± 0.015 | 0.134 ± 0.009 | 0.007 ± 0.113 | 0.021 ± 0.011 |
| 18:2n–6 | 0.750 ± 0.072 | 0.934 ± 0.082 | −0.184 ± 0.110 | 0.788 ± 0.065 | 0.052 ± 0.817 | −0.029 ± 0.800 |
| 18:3n–6 | 0.006 ± 0.001 | 0.007 ± 0.001 | −0.001 ± 0.001 | 0.007 ± 0.001 | 0.001 ± 0.005 | 0.002 ± 0.001* |
| 20:2n–6 | 0.010 ± 0.003 | 0.031 ± 0.004 | −0.020 ± 0.005* | 0.010 ± 0.002 | 0.002 ± 0.024 | −0.014 ± 0.002* |
| 20:3n–6 (DGLA) | 0.016 ± 0.003 | 0.032 ± 0.003 | −0.016 ± 0.004* | 0.018 ± 0.003 | 0.002 ± 0.022 | −0.004 ± 0.003 |
| 20:4n–6 | 0.024 ± 0.002 | 0.035 ± 0.002 | −0.011 ± 0.003* | 0.027 ± 0.002 | 0.002 ± 0.024 | 0.003 ± 0.002 |
| 22:4n–6 | 0.009 ± 0.001 | 0.009 ± 0.001 | 0.001 ± 0.001 | 0.010 ± 0.001 | 0.000 ± 0.005 | 0.005 ± 0.001* |
| 22:5n–6 | 0.001 ± 0.000 | 0.003 ± 0.000 | −0.002 ± 0.000* | 0.001 ± 0.000 | 0.000 ± 0.002 | −0.001 ± 0.000* |
| Total n–6 PUFAs | 0.822 ± 0.080 | 1.061 ± 0.091 | −0.238 ± 0.121 | 0.867 ± 0.071 | 0.057 ± 0.905 | −0.039 ± 0.088 |
| n–6:n–3 PUFA | 7.025 ± 0.447 | 8.056 ± 0.526 | −1.031 ± 0.676 | 5.752 ± 1.632 | 1.298 ± 9.008 | −3.256 ± 2.019 |
| ARA:DHA | 1.993 ± 0.147 | 1.566 ± 0.175 | 0.427 ± 0.222 | 1.940 ± 0.112 | 0.089 ± 1.743 | 0.197 ± 0.138 |
Difference in intakes calculated for a subset of infants in the OptiMoM (Optimizing Mothers’ Milk for Preterm Infants) study at weeks 1 and 2 of life (n = 13; n = 6, >50% DM; n = 7, 100% MoM; days 1–14) and week 4 (n = 31; n = 13, >50% DM; n = 18, 100% MoM; days 22–28) not receiving parenteral nutrition, and in the bovine milk–based fortifier group between those receiving >50% DM and 100% MoM. Associations were analyzed using PROC MIXED followed by LSMEANS for pairwise comparisons, accounting for repeated measures using an autoregressive correlation structure. *Statistically significant difference in β estimates, P < 0.05. ALA, α-linolenic acid; ARA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DM, donor milk; DPA, docosapentaenoic acid; MoM, mother's own milk.
Weeks 1 and 2: adjusted for repeated measures within subjects, and maternal prepregnancy BMI.
Week 4: adjusted for repeated measures within subjects, maternal ethnicity, maternal BMI, and a composite social risk score that includes maternal age, maternal education, language spoken at home, and marital status.
During week 4, infants fed >50% donor milk consumed less total SFAs than infants receiving 100% mother's milk, even after adjustment for maternal ethnicity, maternal prepregnancy BMI, and a composite social risk score (Model 2, β = −0.71; 95% CI: −1.18, −0.25 g · kg−1 · d−1). This was driven by lower intakes of most SFAs measured, including lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, heneicosylic acid (21:0), behenic acid (22:0), and lignoceric acid. After adjustment (Model 2) no statistically significant differences in intakes between infants fed >50% donor milk and 100% mother's milk were found for total fat (β: −0.85; 95% CI: −1.91, 0.20 g · kg−1 · d−1), DHA (β: −0.53; 95% CI: −4.3, 3.2 mg · kg−1 · d−1), or ARA (β: 3.2; 95% CI: −1.7, 8.0 mg · kg−1 · d−1).
Discussion
To our knowledge, this is the largest study to date of the determinants of the FA composition of human milk fed to VLBW infants. Milk from mothers of VLBW infants may differ because preterm birth disrupts normal development of mammary gland structure and milk secretion. Our sample size and availability of serially collected samples enabled evaluation of many factors that may influence human milk total fat and FA content over a VLBW infant's initial hospitalization. These data, with daily measured human milk intakes, allowed estimation of the magnitude of the deficit in total fat and nutritionally important FAs from mother's milk, donor milk, or both. Understanding the sources of variation in intakes or content of fat and FAs from human milk and estimated intakes may aid in optimizing nutrition to promote growth and development of VLBW infants.
Maternal BMI, ethnicity, family income, and infant gestational age at birth all significantly influenced milk composition in the present study (Figures 2, 3). Maternal ethnicity had a strong (>0.5 SD) influence on concentrations of SFAs and MUFAs, γ-linolenic acid (18:3n–6), DHA, and ARA. BMIs in the overweight or obese ranges associated with higher concentrations of total fat. Further, milk collected from women who had a BMI indicative of obesity contained higher concentrations of SFAs and lower proportions of monounsaturates, DHA, and total n–6 PUFAs. To our knowledge, no study has examined the association between maternal BMI and FA composition of milk from mothers who delivered a VLBW infant; however, associations observed in the present study are similar to those of term-born infants (11, 12, 29–31). Interestingly, although negative associations existed between maternal BMI and DHA concentration, these differences disappeared in calculation of total intakes owing to the higher total fat content of milk from women in the upper range of the BMI continuum. Family income below the poverty line was associated with increased human milk fat content in the present study; in studies of term infants or mixed samples, no such relation has been reported (11, 32). It is possible that dietary differences between women above and below the poverty line may explain our observations; however, maternal diet was not assessed and hence will need to be confirmed in future studies. We found no associations in adjusted models between other maternal (e.g., education), infant (e.g., sex), environmental (e.g., season), or other factors reported to affect composition of term milk in some studies, and total fat or the main classes of FA in milk from mothers of VLBW infants (11, 13, 32–36). The sample size for several of these variables was small, so we may have lacked power to detect these associations if they existed.
Fat and FA composition was also influenced by the source and maturity of the human milk (Table 2). Donor milk contained similar concentrations of DHA and ARA (0.30% and 0.61%, respectively) to mature VLBW infant mother's milk, but less fat (−0.5 to −0.8 g/dL) than transitional, early-, or later-mature mother's milk. Although in line with the global average DHA in human milk reported in a meta-analysis of 65 studies (0.32%), the percentage in the present study is higher than has been previously reported in Canada (0.12%–0.30%) (37). This may be because our study drew from a multiethnic population in a large urban center, and because women who volunteer to donate milk may have diets higher in DHA; however, this was not measured. A recent systematic review of compositional changes to donor milk due to collection and processing suggested minimal changes in total fat or FA composition with refrigeration, freezing, or pasteurization per se but noted that fat globules may adhere to freezing containers thereafter (38). Donor milk undergoes ≥1 more freeze-thaw cycle and container change than mother's milk which may contribute to its lower fat content (39).
Donor milk use was a key source of variation in the fat and FA intakes of VLBW infants in this study. By comparison, although maternal prepregnancy BMI had a substantial impact on the FA composition of mother's milk, it was not associated with intakes of fat or any major classes of FAs during weeks 1 and 2 or 4 (data not shown). Donor milk accounted for ≥50% of the human milk intake of 19% (24 of 125) of OptiMoM infants in the first week of life, which remained consistent throughout initial hospitalization (22 of 125, 18%). A simulated diet of 100% donor milk at full enteral feeding volume (160 mL · kg−1 · d−1) would provide 1.3 g · kg−1 · d−1 less fat (∼27% of the recommended 4.8 g · kg−1 · d−1) during initial hospitalization than later-mature mother's milk (40). This is consistent with the deficits observed in OptiMoM infants when actual enteral intakes were considered. In a previous study conducted by our group in infants born weighing <1500 g, 34% did not reach recommended intakes of total lipid in the first week of life (41). Infants who met macronutrient recommendations in early life exhibited greater gains in weight, length and head circumference (42). Deficits in intake of total fat due to high donor milk feeding may therefore be clinically meaningful in VLBW infants.
The simulated 100% donor milk diet provided 5–9 mg · kg−1 · d−1 less DHA and ARA than colostrum or transitional mother's milk at full enteral feeding volume. This is similar to the daily deficits in intake observed in the sample of OptiMoM infants fed either >50% donor milk or 100% mother's milk of DHA (β: −11.7; 95% CI: −21.5, −1.8 mg · kg−1 · d−1) and ARA (−11.1; 95% CI:−17.8, −4.3 mg · kg−1 · d−1) during weeks 1 and 2 of life. Deficits in both the simulated and actual intake data accounted for 8%–16% of recommended intakes for DHA and 11%–26% for ARA (55–60 and 35–45 mg · kg−1 · d−1, respectively) (2); although neither group of infants reached recommended intakes from human milk during this period. Differences in ARA and DHA intakes were no longer present at week 4 of life, which reflects the increasing maturity of the mother's milk.
Strengths of this study include our large sample of mothers of VLBW infants, and repeated sampling within individuals. This allowed for an evaluation of many factors that could affect the fat and FA content of human milk from mothers of VLBW infants. A limitation is the lack of data on other characteristics that may influence FA composition of term human milk, such as fatty acid desaturase (FADS) 1 and 2 genotype, timing and frequency of human milk expression, or maternal health status (11, 43–45). Another strength of our study is the quantification of total fat and the availability of data on human milk intake. This enabled an examination of the effect of donor or mother's milk feeding on intakes of fat and FAs in the context of recommendations, which enhances the clinical relevance of this work. This was limited, however, by the small sample of infants available for the intake analysis, although those included were not significantly different from the rest of the OptiMoM cohort for any baseline characteristics (Supplemental Tables 6 and 7). Because of this, the magnitude of intake differences between infants receiving >50% donor milk or 100% mother's milk should be interpreted with caution, especially for weeks 1 and 2 of life. Although we attempted to address this gap by providing simulated intakes of fat and FA at full enteral feeding volumes, which demonstrated a similar pattern of results to the observed intakes in our sample, future confirmatory studies are needed to quantify the effect of donor milk feeding on fat and FA intakes.
In conclusion, the fat and FA composition of human milk fed to VLBW infants is influenced by lactation stage, milk type (mother's own or donor), and demographic factors such as maternal prepregnancy BMI, ethnicity, and sociodemographic status. These sources of variation may introduce error into intake calculations relying on reference values for the fat content of human milk, as opposed to individualized measures. A high proportion of donor milk feeding may produce clinically significant deficits in intakes of total fat, saturated fat, and, in the first weeks of life, ARA and DHA. Although the use of donor milk in the absence of sufficient volumes of mother's milk is an important strategy for reducing the risk of necrotizing enterocolitis among infants born at a VLBW, this work suggests that it is not equivalent to mother's own milk with respect to fat and FAs, and that extra care should be taken to ensure that infants receiving a high proportion of donor milk meet nutrient intake recommendations.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the OptiMoM Feeding group at the Hospital for Sick Children, Sinai Health System, Trillium Health Partners, St. Michael's Hospital, Humber River Hospital, Lakeridge Health, Markham Stouffville Hospital, North York General Hospital, Scarborough and Rouge Hospital, Mackenzie Health, Michael Garron Hospital, St. Joseph's Health Centre, Southlake Regional Health Centre, and the William Osler Health System for their assistance with study design, oversight, and data collection, as well as the Rogers Hixon Ontario Human Milk Bank for providing the donor milk for the study.
The authors’ responsibilities were as follows—DLO, SLU, and KEH: designed the research; KEH: analyzed the data and drafted the manuscript with input from all coauthors; DLO: had primary responsibility for the final content; and all authors: participated in conducting the research, contributed to the revision and production of the final version, and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Canadian Institutes of Health Research (CIHR) Programmatic Grants in Food and Health: OptiMoM, FHG 129919 (to DLO and SLU) and CIHR Foundation Scheme—Live Pilot Grant New: MaxiMoM, FDN 143233 (to DLO and SLU). KEH was supported by a CIHR Fellowship. The sources of support had no role in the design or conduct of the research study, statistical analysis, data interpretation, or writing of the manuscript.
Supplemental Figures 1 and 2 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ARA, arachidonic acid; FA, fatty acid; HMBANA, Human Milk Banking Association of North America; NICU, neonatal intensive care unit; OptiMoM, Optimizing Mothers’ Milk for Preterm Infants; SDS, SD score; VLBW, very low birth weight.
Contributor Information
Kathryn E Hopperton, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Michael A Pitino, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
Raphaël Chouinard-Watkins, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
Sara Shama, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Natasha Sammut, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
Nicole Bando, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Brock A Williams, Department of Food, Nutrition and Health, University of British Columbia, Vancouver, British Columbia, Canada.
Kathryn Walton, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada.
Alex Kiss, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Evaluative and Clinical Sciences, Sunnybrook Research Institute and the Institute of Health Policy, Toronto, Ontario, Canada.
Sharon L Unger, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada; Division of Neonatology, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Paediatrics, University of Toronto, Toronto, Ontario, Canada; Department of Paediatrics, Sinai Health System, Toronto, Ontario, Canada.
Richard P Bazinet, Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada.
Deborah L O'Connor, Translational Medicine Program, The Hospital for Sick Children, Toronto, Ontario, Canada; Department of Nutritional Sciences, University of Toronto, Toronto, Ontario, Canada; Department of Paediatrics, Sinai Health System, Toronto, Ontario, Canada.
Data Availability
Data described in the article, code book, and analytic code will not be made available in order to protect the privacy and confidentiality of our participants; we do not have consent from participant families to share their anonymized data, nor do we have permission from the research ethics boards of our participating hospitals.
References
- 1.Panczuk J, Unger S, O'Connor D, Lee SK. Human donor milk for the vulnerable infant: a Canadian perspective. Int Breastfeed J. 2014;9(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koletzko B, Poindexter B, Uauy Reditors. Nutritional care of preterm infants: scientific basis and practical guidelines. Basel (Switzerland): Karger; 2014. [Google Scholar]
- 3.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford MA, Casperd NM, Sinclair AJ. The long chain metabolites of linoleic and linolenic acids in liver and brain in herbivores and carnivores. Comp Biochem Physiol B Comp Biochem. 1976;54(3):395–401. [DOI] [PubMed] [Google Scholar]
- 5.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15(12):771–85. [DOI] [PubMed] [Google Scholar]
- 6.Lacombe RJS, Chouinard-Watkins R, Bazinet RP. Brain docosahexaenoic acid uptake and metabolism. Mol Aspects Med. 2018;64:109–34. [DOI] [PubMed] [Google Scholar]
- 7.Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr. 2014;99(3):734S–41S. [DOI] [PubMed] [Google Scholar]
- 8.Rings EHHM, Minich DM, Vonk RJ, Stellaard F, Fetter WPF, Verkade HJ. Functional development of fat absorption in term and preterm neonates strongly correlates with ability to absorb long-chain fatty acids from intestinal lumen. Pediatr Res. 2002;51(1):57–63. [DOI] [PubMed] [Google Scholar]
- 9.Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):143–50. [DOI] [PubMed] [Google Scholar]
- 10.Bokor S, Koletzko B, Decsi T. Systematic review of fatty acid composition of human milk from mothers of preterm compared to full-term infants. Ann Nutr Metab. 2007;51(6):550–6. [DOI] [PubMed] [Google Scholar]
- 11.Miliku K, Duan QL, Moraes TJ, Becker AB, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, Subbarao P, Field CJet al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am J Clin Nutr. 2019;110(6):1370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panagos PG, Vishwanathan R, Penfield-Cyr A, Matthan NR, Shivappa N, Wirth MD, Hebert JR, Sen S. Breastmilk from obese mothers has pro-inflammatory properties and decreased neuroprotective factors. J Perinatol. 2016;36(4):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachour P, Yafawi R, Jaber F, Choueiri E, Abdel-Razzak Z. Effects of smoking, mother's age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin A concentrations of human milk. Breastfeed Med. 2012;7(3):179–88. [DOI] [PubMed] [Google Scholar]
- 14.Nommsen LA, Lovelady CA, Heinig MJ, Lönnerdal B, Dewey KG. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: the DARLING Study. Am J Clin Nutr. 1991;53(2):457–65. [DOI] [PubMed] [Google Scholar]
- 15.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55(1–3):97–122. [DOI] [PubMed] [Google Scholar]
- 16.Gibson RA, Neumann MA, Makrides M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur J Clin Nutr. 1997;51(9):578–84. [DOI] [PubMed] [Google Scholar]
- 17.Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics. 2017;139(1):e20163440. [DOI] [PubMed] [Google Scholar]
- 18.Pound C, Unger S, Blair B. Pasteurized and unpasteurized donor human milk. Paediatr Child Health. 2020;25:549–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrin MT, Belfort MB, Hagadorn JI, McGrath JM, Taylor SN, Tosi LM, Brownell EA. The nutritional composition and energy content of donor human milk: a systematic review. Adv Nutr. 2020;11(4):960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentine CJ, Morrow G, Fernandez S, Gulati P, Bartholomew D, Long D, Welty SE, Morrow AL, Rogers LK. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J Pediatr. 2010;157(6):906–10. [DOI] [PubMed] [Google Scholar]
- 21.Valentine CJ, Morrow G, Reisinger A, Dingess KA, Morrow AL, Rogers LK. Lactational stage of pasteurized human donor milk contributes to nutrient limitations for infants. Nutrients. 2017;9(3):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, Daneman A, Francis J, Kotsopoulos K, Shah PSet al. Nutrient enrichment of human milk with human and bovine milk–based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108(1):108–16. [DOI] [PubMed] [Google Scholar]
- 23.Human Milk Banking Association of North America (HMBANA). Guidelines for the establishment and operation of a donor human milk bank. 10th ed. Fort Worth (TX): HMBANA; 2018. [Google Scholar]
- 24.Parat S, Groh-Wargo S, Merlino S, Wijers C, Super DM. Validation of mid-infrared spectroscopy for macronutrient analysis of human milk. J Perinatol. 2017;37(7):822–6. [DOI] [PubMed] [Google Scholar]
- 25.Kwan C, Fusch G, Rochow N, Fusch C; MAMAS Study collaborators. Milk analysis using milk analyzers in a standardized setting (MAMAS) study: a multicentre quality initiative. Clin Nutr. 2020;39(7):2121–8. [DOI] [PubMed] [Google Scholar]
- 26.Pitino MA, Alashmali SM, Hopperton KE, Unger S, Pouliot Y, Doyen A, O'Connor DL, Bazinet RP. Oxylipin concentration, but not fatty acid composition, is altered in human donor milk pasteurised using both thermal and non-thermal techniques. Br J Nutr. 2019;122(1):47–55. [DOI] [PubMed] [Google Scholar]
- 27.Statistics Canada. Income Research Paper Series: low income lines, 2011-2012. Catalogue no. 75F0002M, no. 002. Ottawa (Ontario): Income Statistics Division, Statistics Canada; 2013. [Google Scholar]
- 28.Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44(5):276–80. [DOI] [PubMed] [Google Scholar]
- 29.Leghi GE, Netting MJ, Middleton PF, Wlodek ME, Geddes DT, Muhlhausler BS. The impact of maternal obesity on human milk macronutrient composition: a systematic review and meta-analysis. Nutrients. 2020;12(4):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel AI, Shama S, Ismail S, Bourdon C, Kiss A, Mwangome M, Bandsma RHJ, O'Connor DL. Maternal BMI is positively associated with human milk fat: a systematic review and meta-regression analysis. Am J Clin Nutr. 2021;113(4):1009–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de la Garza Puentes A, Martí Alemany A, Chisaguano AM, Montes Goyanes R, Castellote AI, Torres-Espínola FJ, García-Valdés L, Escudero-Marín M, Segura MT, Campoy Cet al. The effect of maternal obesity on breast milk fatty acids and its association with infant growth and cognition—the PREOBE Follow-Up. Nutrients. 2019;11(9):2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brasil AL, Vitolo MR, Lopez FA, De Nóbrega FJ. Fat and protein composition of mature milk in adolescents. J Adolesc Health. 1991;12(5):365–71. [DOI] [PubMed] [Google Scholar]
- 33.Hopkinson JM, Schanler RJ, Fraley JK, Garza C. Milk production by mothers of premature infants: influence of cigarette smoking. Pediatrics. 1992;90(6):934–8. [PubMed] [Google Scholar]
- 34.Agostoni C, Marangoni F, Grandi F, Lammardo AM, Giovannini M, Riva E, Galli C. Earlier smoking habits are associated with higher serum lipids and lower milk fat and polyunsaturated fatty acid content in the first 6 months of lactation. Eur J Clin Nutr. 2003;57(11):1466–72. [DOI] [PubMed] [Google Scholar]
- 35.Fujita M, Roth E, Lo Y-J, Hurst C, Vollner J, Kendell A. In poor families, mothers’ milk is richer for daughters than sons: a test of Trivers–Willard hypothesis in agropastoral settlements in Northern Kenya. Am J Phys Anthropol. 2012;149(1):52–9. [DOI] [PubMed] [Google Scholar]
- 36.Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22(1):50–4. [DOI] [PubMed] [Google Scholar]
- 37.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–64. [DOI] [PubMed] [Google Scholar]
- 38.Gao C, Miller J, Middleton PF, Huang Y-C, McPhee AJ, Gibson RA. Changes to breast milk fatty acid composition during storage, handling and processing: a systematic review. Prostaglandins Leukot Essent Fatty Acids. 2019;146:1–10. [DOI] [PubMed] [Google Scholar]
- 39.Unger S, Gibbins S, Zupancic J, O'Connor DL. DoMINO: donor milk for improved neurodevelopmental outcomes. BMC Pediatr. 2014;14(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koletzko B, Poindexter B, Uauy R. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. World Rev Nutr Diet. 2014;110:297–9. [DOI] [PubMed] [Google Scholar]
- 41.Ng DVY, Brennan-Donnan J, Unger S, Bando N, Gibbins S, Nash A, Kiss A, O'Connor DL. How close are we to achieving energy and nutrient goals for very low birth weight infants in the first week?. JPEN J Parenter Enteral Nutr. 2017;41(3):500–6. [DOI] [PubMed] [Google Scholar]
- 42.Asbury MR, Unger S, Kiss A, Ng DVY, Luk Y, Bando N, Bishara R, Tomlinson C, O'Connor DL. Optimizing the growth of very-low-birth-weight infants requires targeting both nutritional and nonnutritional modifiable factors specific to stage of hospitalization. Am J Clin Nutr. 2019;110:1384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Kang S, Jung B-M, Yi H, Jung JA, Chang N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br J Nutr. 2017;117(4):556–61. [DOI] [PubMed] [Google Scholar]
- 44.Koletzko B. Human milk lipids. Ann Nutr Metab. 2016;69(Suppl. 2):28–40. [DOI] [PubMed] [Google Scholar]
- 45.Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available in order to protect the privacy and confidentiality of our participants; we do not have consent from participant families to share their anonymized data, nor do we have permission from the research ethics boards of our participating hospitals.