Abstract
Through our involvement in KEYNOTE-059, we unexpectedly observed durable responses in two patients with metastatic gastroesophageal adenocarcinoma (mGEA) who received ramucirumab (anti-VEGFR-2)/paclitaxel after immune checkpoint inhibition (ICI). To assess the reproducibility of this observation, we piloted an approach to administer ramucirumab/ paclitaxel after ICI in more patients, and explored changes in the immune microenvironment. Nineteen consecutive patients with mGEA received ICI followed by ramucirumab/paclitaxel. Most (95%) did not respond to ICI, yet after irRECIST-defined progression on ICI, all patients experienced tumor size reduction on ramucirumab/paclitaxel. The objective response rate (ORR) and progression-free survival (PFS) on ramucirumab/paclitaxel after ICI were higher than on the last chemotherapy before ICI in the same group of patients (ORR, 58.8% vs 11.8%; PFS 12.2 vs 3.0 months; respectively). Paired tumor biopsies examined by imaging mass cytometry showed a median 5.5-fold (range 4–121) lower frequency of immunosuppressive forkhead box P3+ regulatory T cells with relatively preserved CD8+ T cells, post-treatment versus pre-treatment (n = 5 pairs). We then compared the outcomes of these 19 patients with a separate group who received ramucirumab/paclitaxel without preceding ICI (n = 68). Median overall survival on ramucirumab/paclitaxel was longer with (vs without) immediately preceding ICI (14.8 vs 7.4 months) including after multivariate analysis, as was PFS. In our small clinical series, outcomes appeared improved on anti-VEGFR-2/paclitaxel treatment when preceded by ICI, in association with alterations in the immune microenvironment. However, further investigation is needed to determine the generalizability of these data. Prospective clinical trials to evaluate sequential treatment with ICI followed by anti-VEGF(R)/taxane are underway.
Keywords: anti-VEGFR2, gastric/gastroesophageal cancers, immune checkpoint inhibition, paclitaxel, tumor microenvironment
1 |. INTRODUCTION
Immune checkpoint inhibition (ICI) targeting programmed death (PD)-1 has shown activity in metastatic gastric/gastroesophageal junction (GEJ) adenocarcinoma (mGEA). Pembrolizumab (anti-PD-1) is approved for patients with PD-L1-expressing mGEA who progressed on ≥2 prior lines of therapy (KEYNOTE-059).1 However, responses after monotherapy occur in only 15%–16% of PD-L1-positive and 2%–6% of PD-L1-negative patients, underscoring the importance of identifying therapies that can benefit nonresponders.1,2 CD8+ T cells that mediate ICI activity are subject to resistance mechanisms relevant to the tumor microenvironment (TME) with regulatory T cells (Tregs) playing a role.3,4
Antivascular endothelial growth factor receptor (VEGFR)-2 treatment via ramucirumab, alone or combined with paclitaxel, is approved for mGEA after front-line therapy. However, these regimens, without prior ICI, induce responses in less than 30% of patients.5 Paclitaxel and ramucirumab without prior ICI have been reported to reduce Tregs.6,7
Through our participation in KEYNOTE-059 we previously reported durable responses to ramucirumab/paclitaxel after progression on pembrolizumab.8 We now report outcomes on ramucirumab/paclitaxel after ICI in more patients. We also explored alterations in the immune TME.
2 |. MATERIALS AND METHODS
2.1 |. Patients and treatment
Through our involvement in KEYNOTE-059, we unexpectedly observed durable responses in two (of two) subjects with mGEA who received post-study ramucirumab/paclitaxel after progression on pembrolizumab. After discussion as a group, interested physicians made a practice adjustment as a clinical pilot to treat patients with ramucirumab/paclitaxel after progression on PD-1 blockade. After approximately 1 year, we searched our databases to identify all patients with mGEA at Mayo Clinic who had received ramucirumab/paclitaxel (1 January 2014 to 1 April 2019) after anti-PD-1-containing therapy (data cutoff 20 September 2019). All results were confirmed by chart review. No patients were excluded with regard to PD-L1, HER2, or MMR status, prior response to PD-1 blockade, tumor volume, brain metastases, prior lines of therapy, ECOG performance status (PS), or other factors. Clinicopathologic, survival, and treatment-related data were collected using standardized intake forms via Research Electronic Data Capture.
2.2 |. Tumor assessment
Tumor response and regression were determined based on REC-IST1.1. Disease progression was determined using RECIST1.1 while on ramucirumab/paclitaxel and immune-related RECIST (irRECIST) criteria when on immunotherapy.
2.3 |. Statistical methods
Endpoints were overall survival (OS), progression-free survival (PFS), duration of response (DOR), best objective response rate (ORR) per RECIST 1.1, and best tumor regression change from baseline. Survival analysis was performed from the time of initiation of ramucirumab/paclitaxel. OS was defined as death from any cause; PFS as disease progression or death; DOR as the time from the first documented response (complete response [CR] or partial response [PR]) to the date of progression or death (whichever occurred first). Time-to-event variables were estimated using Kaplan-Meier methods, chi-square for comparing independent groups, McNemar's for paired (intrapatient) response, and Wilcoxon Signed-Rank for tumor regression analysis. For continuous variables, Wilcoxon Rank-Sum test was used when treatment groups were considered independent. Univariate and multivariable Cox or logistic regression models, adjusted (adj) for potential confounders, where appropriate, with hazard ratios, 95% confidence intervals (CI), and likelihood ratio P-values were calculated. All P values are two-sided. Analysis was conducted using JMP 14.0 software (SAS Institute Inc., Cary, North Carolina).
2.4 |. Immune cell quantification
Formalin-fixed paraffin-embedded tissue sections were stained in parallel in the same batch and characterized using a 10-antibody immune panel (Fluidigm Hyperion imaging mass cytometry; Table S1) and, when noted, using direct immunofluorescence. Cells were quantified on a continuous scale.
Further methods can be found in the Supporting Information file.
3 |. RESULTS
We identified 19 consecutive patients with mGEA who received ICI followed by ramucirumab/paclitaxel between 1 January 2014 and 1 April 2019 (Figure S1; Figure 1A). Most patients were male, had an ECOG PS of 0–1, and received ≤2 lines of therapy prior to ramucirumab/paclitaxel (Table 1). Forty-two percent had ≥3 metastatic sites; ninety-five percent of tumors were mismatch-repair-proficient (pMMR), and thirty-five percent were PD-L1-negative. Median follow-up was 18.1 (1.8–53.3) months.
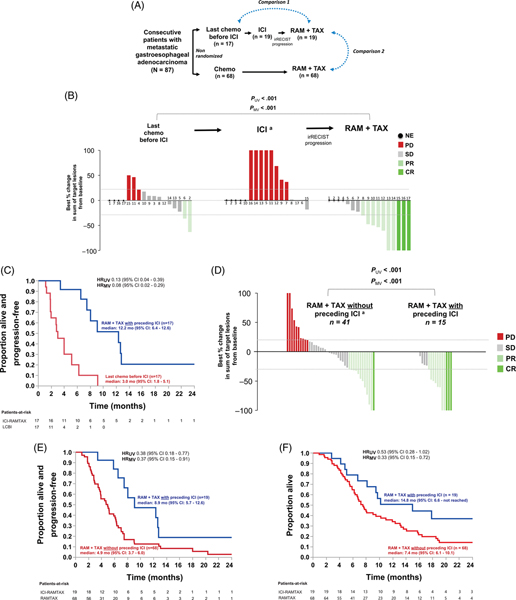
FIGURE 1.
Clinical activity of ramucirumab/paclitaxel in ICI-experienced patients as compared to last chemotherapy before ICI (LCBI) in the same group of patients (Comparison 1, B,C) or compared to ramucirumab/paclitaxel in ICI-naïve patients (Comparison 2, D-F). A, Analytic approach. Comparison 1 was restricted to all 17 patients who received chemotherapy before ICI, and multivariate models were adjusted for ECOG PS immediately prior to a given line of therapy. ICI always included an anti-PD-1 antibody. For Comparison 2, multivariate models were adjusted for the number of prior lines of therapy, age, ECOG PS, serum albumin, and number of metastatic sites—all collected immediately pre-ramucirumab/paclitaxel. B, Tumor regression rates for nonpaired analysis of Comparison 1. Each number (bar) denotes a unique patient. Dots denote patients that were unevaluable for tumor regression during that treatment segment. A separate paired (intrapatient) analysis restricted to 9 patients evaluable for tumor regression during both LCBI and ramucirumab/paclitaxel yielded consistent results (Table S5). C, Progression-free survival for Comparison 1. D, Tumor regression rates, (E) progression-free survival, and (F) overall survival for Comparison 2. aFor illustration only, the graphical upper limit for the increase in the sum of target lesions from baseline was set at +100% (five patients during the ICI segment had values of +780%, +468%, +270%, +150%, +104.8% [B] and one patient during ramucirumab/paclitaxel without preceding ICI had a value of +230% [D]). ICI, immune checkpoint inhibition; RAM, ramucirumab; TAX, paclitaxel; LCBI, last chemotherapy before ICI; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; irRECIST, Immune-related Response Evaluation Criteria In Solid Tumors; HR, hazard ratio; UV, univariate; MV, multivariate
TABLE 1.
Baseline host and tumor characteristics of patients immediately prior to administration of ramucirumab/paclitaxel (N = 87)
| Ramucirumab + paclitaxel with preceding ICI (n = 19) | Ramucirumab + paclitaxel without preceding ICI (n = 68) | P | ||
|---|---|---|---|---|
| Host | Age median (IQR), years | 58.2 (54.1, 70.3) | 62.2 (54.4, 67.6) | .64 |
| Male sex, n (%) | 15 (79) | 53 (78) | .92 | |
| ECOG performance status, n (%) | ||||
| 0 | 5 (26) | 31 (46) | .28 | |
| 1 | 11 (58) | 29 (43) | ||
| 2 | 3 (16) | 6 (9) | ||
| 3 | 0 (0) | 2 (3) | ||
| No. of metastatic sites, n (%) | ||||
| <3 | 11 (58) | 50 (74) | .20 | |
| ≥3 | 8 (42) | 18 (26) | ||
| Serum albumin median (IQR), g/dL | 3.9 (3.5, 4.1) | 3.8 (3.5, 4.0) | .98 | |
| Tumor | Primary tumor location, n (%) | |||
| Esophagus | 2 (11) | 16 (24) | .35 | |
| Gastroesophageal junction | 9 (47) | 23 (34) | ||
| Stomach | 8 (42) | 29 (43) | ||
| Grade, n (%) | ||||
| G1 | 8 (42) | 21 (32) | .57 | |
| G2 | 11 (58) | 44 (67) | ||
| G3 | 0 (0) | 1 (1) | ||
| Mismatch repair status, n (%) | ||||
| Proficient | 18 (95) | 24 (89) | -a | |
| Deficient | 1 (5) | 3 (11) | ||
| Unknown | 0 | 41 | ||
| PD-L1 status, n (%) | ||||
| Positive (combined positive score ≥1) | 11 (65) | 3 (21) | -a | |
| Negative (combined positive score <1) | 6 (35) | 11 (79) | ||
| Unknown | 2 | 54 | ||
| HER2 status, n (%) | ||||
| Positive | 4 (21) | 11 (16) | .36 | |
| Negative | 13 (68) | 55 (81) | ||
| Equivocal | 2 (11) | 2 (3) | ||
| Treatment | Prior lines of therapy, n (%) | |||
| 0–2 | 15 (79) | 60 (88) | <.001 | |
| ≥3 | 5 (21) | 8 (12) |
Abbreviations: ICI, immune checkpoint inhibition; PD-L1, programmed death-ligand 1.
P value not calculated since the majority of values were unknown for one group.
Most patients (95%) did not respond to ICI (Figure 1B). ICI always included anti-PD-1 (Table S2). All had irRECIST progression on ICI after a median of 3.0 months (95% CI, 1.8–4.2).
On ramucirumab/paclitaxel after ICI, tumor regression was observed in all patients (median size reduction −53.5% [range −7.5% to −100%]).
3.1 |. Comparison with the last chemotherapy before ICI
Time-to-progression on an experimental (vs earlier) regimen in the same patient is an emerging indicator of anticancer activity.9 Accordingly, we performed an intragroup analysis comparing outcomes on ramucirumab/paclitaxel after ICI with the last chemotherapy administered before ICI (LCBI). LCBI was 5-fluorouracil/oxaliplatin in most (82%) patients (Table S3). Tumor regression on ramucirumab/paclitaxel after ICI was greater than on LCBI (median size reduction −53.5% [−7.5% to −100%] vs +7.9% [−63.2% to +50%]) (Figure 1B; Figure S2). Responses were observed in PD-L1-positive and PD-L1-negative tumors (Figure S3). The ORR was higher on ramucirumab/paclitaxel after ICI than on LCBI (58.8% [95% CI 36.0%78.4%; 10/17] vs 11.8% [95% CI 3.3%–34.3%; 2/17]) (Table S4), as was PFS (median 12.2 [95% CI 6.4–12.6] vs 3.0 [95% CI 1.8–5.1] months; HRunadj 0.13 [95% CI 0.04–0.39]; HRadj 0.08 [95% CI 0.02–0.29]) (Figure 1C) including after adjustment for ECOG PS. We repeated the analysis in a paired (intrapatient) approach with each patient serving as his/her own “control,” and results were consistent for all endpoints (tumor regression and PFS in Table S5; ORR in Figure S4).
3.2 |. Comparison with patients who received ramucirumab/paclitaxel without preceding immunotherapy
We then compared the outcomes on ramucirumab/paclitaxel in these 19 patients with those who had not received immediately preceding ICI. A total of 68 consecutive patients received ramucirumab/paclitaxel without preceding ICI (Figure S1). Baseline characteristics between the groups were generally similar, except that ramucirumab/paclitaxel was mostly given third-line in ICI-experienced patients and second-line in ICI-naïve patients (Table 1; Figure S5).
Outcomes were improved on ramucirumab/paclitaxel in the ICI-experienced versus ICI-naïve patients for all endpoints: tumor regression (median size reduction −53.5% [−7.5% to −100%] vs −1.9% [−100% to +230%]), ORR (57.9% [95% CI 36.3%–76.9%; 11/19] vs 17.7% [95% CI 10.4%–28.4%; 12/68]), DOR (median 10.6 [95% CI 1.5-not reached] vs 4.3 [95% CI 1.6–5.2] months), PFS (median 8.9 [95% CI 5.7–12.6] vs 4.9 months [95% CI 3.7–6.0]; HRunadj 0.38[95% CI 0.18–0.77]; HRadj 0.37 [95% CI 0.15–0.91]), and OS (median 14.8 [95% CI 6.6-not reached] vs 7.4 [95% CI 6.1–10.1] months; HRunadj 0.53 [95% CI 0.28–1.02]; HRadj 0.33 [95% CI 0.15–0.72]), all respectively (Table S4; Figure 1D–F). Multivariate models were adjusted for age, number of prior lines of therapy, ECOG PS, serum albumin, and number of metastatic sites immediately prior to ramucirumab/ paclitaxel.
4 |. TUMOR MICROENVIRONMENT
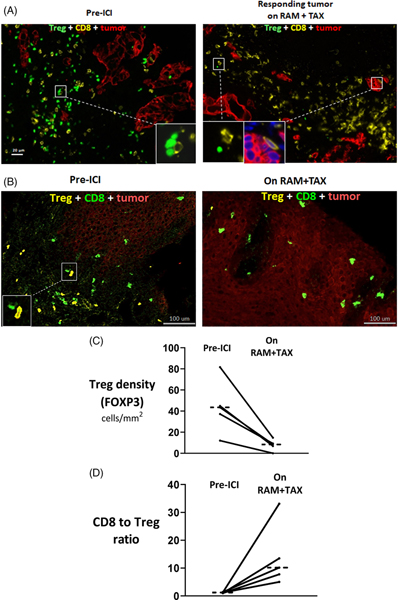
We explored immune mechanisms of response by first performing direct immunofluorescence on biopsies collected, pre-ICI and on subsequent ramucirumab/paclitaxel, in one patient responder. The frequency of immunosuppressive FOXP3+ Tregs was qualitatively lower, post-treatment versus pre-treatment, and that of antitumor CD8+ T cells was relatively preserved (Figure 2A).
FIGURE 2.
Immune microenvironment in paired serial tumors collected pre-ICI and on-treatment with immediately subsequent ramucirumab/ paclitaxel in five patients with metastatic gastroesophageal adenocarcinoma. A, Direct immunofluorescence on paired tumor biopsies collected pre-ICI and during response on subsequent ramucirumab/paclitaxel from a single patient with metastatic gastroesophageal adenocarcinoma. In the pretreatment biopsy, Tregs are abundant and in direct contact with CD8+ T cells (inset). Post-treatment, Tregs are sparse and CD8+ T cells remain abundant (left inset shows an uncommon FOXP3+ Treg, right inset shows CD8+ T cell with confirmed DAPI-staining nuclei in dark blue in close proximity to tumor cells). B, Representative mass cytometry images from a single patient with paired biopsies collected endoscopically from the primary tumor. In the pretreatment tumor, Tregs were in close proximity with CD8+ T cells (inset). C, Cell-count densities of FOXP3+ Tregs and (D) ratio of CD8+ T cells to FOXP3+ Tregs on imaging mass cytometry with median values shown as horizontal dotted lines. Mean fold-change on-ramucirumab/paclitaxel vs pre-ICI is 28.4 (95% CI, −35.7 to 92.5) for FOXP3+ Treg frequency and 11.9 (95% CI 1.0 to 22.9) for the CD8/Treg ratio. Tregs, regulatory T cells; ICI, Immune checkpoint inhibition; RAM, ramucirumab; TAX, paclitaxel
We then assessed the reproducibility of this finding using an independent platform (imaging mass cytometry) in all five patients with available paired tumor biopsies collected pre-ICI and during response on subsequent ramucirumab/paclitaxel (Figure 2B; time between biopsy and ICI initiation: median 3.8 [range 1.9–5.2] months; time between initiation of ramucirumab/paclitaxel and biopsy on-ramucirumab/paclitaxel: median 4.1 [range 2.6–17.6] months; anatomic biopsy location was primary [n = 8], liver [n = 1], and retroperitoneum [n = 1]). The frequency of Tregs was lower in all patients on ramucirumab/paclitaxel versus pre-ICI (median −5.5-fold [range −4.0 to −121.0]; baseline median 43.4 [range 12.1–81.7] cells/mm2; Figure 2C). Most Tregs (>99%) were CD45RO+ (antigen-experienced or memory), pre and post. The CD8/Treg ratio was higher after treatment (median +8.0-fold [+4.2 to +26.5]; baseline median 1.2 [range 1.0–1.4]; Figure 2D). The proportion of pre-ICI CD8+ T cells exhibiting Granzyme B or Ki67 expression was 12.1% and 7.2%, respectively, and did not change noticeably while on ramucirumab/paclitaxel.
5 |. DISCUSSION
After our initial case reports,8 we now report data on 19 patients with mGEA who received ICI followed, after progression, by ramucirumab/paclitaxel. In this slightly larger series, outcomes (tumor regression, ORR, and PFS) on ramucirumab/paclitaxel after ICI were better as compared to during LCBI in the same group of patients. Results were consistent in paired analysis with each patient serving as his/her own “control.” Patients who received ramucirumab/paclitaxel after ICI also had improved outcomes (ORR, DOR, PFS, and OS) compared to a separate group who received ramucirumab/paclitaxel without preceding ICI. Together, these data suggest sequential therapy involving ICI and anti-VEGF(R)/cytotoxic agents may have cooperativity and represent a novel investigative strategy to enhance outcomes in mGEA. However, given limitations intrinsic to single-institution retrospective data, the generalizability of our findings requires confirmation in a prospective controlled trial.
With this understanding, it is notable that the patient group that received sequential ICI followed by ramucirumab/paclitaxel had aggressive disease characteristics commonly observed in chemorefractory mGEA. This included poor outcomes during LCBI (eg, median PFS, 3 months; ORR, 11.8%) and ICI (95% of patients had irRECIST progression as their best response). Baseline clinicopathologic characteristics in the ICI-experienced group were generally similar to that of the ICI-naïve group at the time of ramucirumab/paclitaxel initiation, including ECOG PS and serum albumin, although the ICI-experienced (vs ICI-naïve) group was younger and had involvement of more metastatic sites. Our ICI-naïve group had a generally lower ORR and median OS, but longer median PFS, than in similarly treated cohorts (Table S6). Outcomes on ramucirumab/paclitaxel after ICI were improved compared to those on ramucirumab/paclitaxel without prior ICI reported previously in other cohorts (Table S6). Acknowledging the possibility that other variables could contribute to the difference in outcomes, we repeated the analysis adjusting for age, number of prior lines of therapy, ECOG PS, serum albumin, and number of metastatic sites immediately prior to ramucirumab/ paclitaxel, and the results were generally strengthened or maintained.
The observed responses on ramucirumab/paclitaxel after progression on ICI are unlikely to represent response after ICI-related pseudoprogression. Pseudoprogression is infrequent in most solid tumor types, particularly mGEA.1 Not a single case of pseudoprogression was observed in KEYNOTE-059, the largest mGEA cohort to examine pseudoprogression; we used the same definition of irRECIST progression as in KEYNOTE-059.1 However, PD-1-blocking antibodies have been shown to persist in patients greater than 20 weeks after the last infusion, bound to its receptor.10 It is therefore possible that anti-VEGF(R2)/paclitaxel administered after progression on ICI could be concurrent with ICI, mediating recovery from prior primary ICI resistance and enhancing overall response.10
Our exploratory findings suggesting ICI may enhance outcomes on subsequent therapy are consistent with emerging data. Recent pan-cancer data, which included gastrointestinal tumors, suggest chemotherapy after ICI has better PFS as compared to LCBI, but this benefit was limited to ICI responders.11 To our knowledge, our study is the first to show improved PFS after ICI as compared to LCBI in a primarily ICI-refractory group. A recent report from Japan12 in 233 patients with advanced gastric cancer indicated a higher ORR on ramucirumab/paclitaxel in ICI-experienced vs ICI-naïve patients (61% vs 20%). As in our study, most tumors were pMMR, and responses occurred regardless of PD-L1 status, suggesting these findings could have relevance in patients who are considered less likely to benefit from immunotherapy. Novelties of our study include non-Asia patients, intragroup comparisons with LCBI, and intergroup data on DOR and OS. Recent exploratory analysis of the global KEYNOTE-061 trial of patients with PD-L1-positive mGEA found that median OS was longer in patients randomized to second-line pembrolizumab who received post-study treatment, including ramucirumab/paclitaxel, versus patients randomized to second-line paclitaxel who received post-study treatment.13 This enhanced response when chemotherapy is preceded by ICI has also been observed in lung cancer.14,15 These clinical observations are consistent with our preclinical data demonstrating that initiation of anti-PD-1 prior to (vs concurrently with) paclitaxel/carboplatin led to greater suppression of tumor growth in a mouse me model.16 Earlier initiation of anti-PD-1 resulted in expansion of tumor-specific effector CD8+ T cells that could survive the toxic effects of chemotherapy through drug efflux and induce immune-mediated tumor cell destruction.16
Mechanisms supporting the efficacy of chemotherapy after ICI treatment have been postulated to include the elimination of Tregs and myeloid-derived suppressor cells, induced expression of costimulatory molecules, enhanced antigen presentation/processing, and induction of immunogenic tumor cell death.17 In our study, exploratory analysis of paired biopsies collected pre-ICI and on subsequent ramucirumab/paclitaxel revealed a lower frequency of immunosuppressive FOXP3+ Tregs and higher CD8+/Treg ratio, post-treatment versus pre-treatment. This raises the possibility that serial treatment with ICI followed by anti-VEGF(R2)/taxane may lead to a more immunocompetent microenvironment in which tumor rejection by cytotoxic CD8+ T cells could be enhanced as suppression by Tregs is reduced. Other mechanisms implicated in interval increases of CD8/Tregs include changes in the gut microbiome, exercise, and sepsis-like states.18 While much investigation remains, emerging data suggest anti-PD-1, anti-VEGF(R), and taxane therapy have the potential to cooperate to impair Treg survival and/or proliferation in mGEA tissue and to modulate the tumor endothelium to reshape the myeloid/adaptive immune microenvironment. Both paclitaxel and ramucirumab as monotherapy or in combination—without prior ICI—have been reported to reduce Tregs, albeit only modestly.6,7 Anti-PD-1 monotherapy has not been associated with altered Treg counts,19 but PD-1 has recently been identified as a critical homeostatic regulator for Tregs.20 In this regard PD-1 expression prolonged survival of circulating Tregs, and PD-1 blockade has been shown to promote Treg apoptosis,20 as well as expand and/or activate effector CD8+ T cells.3,16 In mGEA patients, recent data showed that intratumoral Treg proliferation generally decreased after anti-PD-1 monotherapy, except in hyperprogressors.4 The precise impact of PD-1 blockade on Tregs needs further investigation, as PD-1 blockade has also been shown to promote recovery of immunosuppressive properties of PD-1+ Tregs.3 Furthermore, anti-VEGF(R) treatment modulates the tumor endothelium, which can act as a selective barrier that allows certain T cell subsets, notably Tregs, to traffic more effectively.21 Activation of the VEGF-VEGFR2 axis in gastric tumors has been reported to promote VEGFR2+ Treg proliferation, and correlates with an immunosuppressive TME which can be reversed with ramucirumab.7 Inhibiting the VEGF axis to increase tumor infiltration by CD8+ T cells relative to Tregs has been found to enhance immunotherapy.22,23 Further examination of the TME including myeloid and other markers is the focus of future research.
We caution that our findings are hypothesis-generating. Limitations of our study include its retrospective design, modest nonprespecified sample size, and lack of paired tumor samples from ICI-naïve patients and interim samples collected post-ICI/pre-ramucirumab/paclitaxel from ICI-experienced patients. Given the median 4-month interval between ramucirumab/paclitaxel initiation and the on-ramucirumab/paclitaxel biopsies, we cannot rule out that ramucirumab/paclitaxel alone might have contributed toward the interval CD8/Treg increase, although the greater magnitude of increase in our study suggests preceding ICI might play a role.6,7 Although the observed activity was seen in conjunction with subsequent ramucirumab/paclitaxel, the efficacy may not be limited to ramucirumab/ paclitaxel.11 While it is possible that ICI followed by other chemotherapeutic agents may also be beneficial, our small sample size precluded this evaluation. This sequential approach will be prospectively assessed in a recently opened randomized phase 2 trial (NCT04069273) and other planned trials in mGEA. Several ongoing trials are investigating treatments after true progression on immunotherapy.17 Moving forward, understanding the impact of prior ICI may have more relevance given that more patients will receive upfront ICI in this disease.24,25
Supplementary Material
What’s New?
For many cancers, the response to immune-checkpoint inhibition (ICI) has been disappointing. Could a modified strategy be useful? In this pilot study of patients with metastatic gastroesophageal adenocarcinoma (mGEA), the authors found that following ICI with anti-VEGFR/paclitaxel yielded considerably better response rates, overall survival (OS), and progression-free survival (PFS) than either modality alone. They also found alterations in the tumor microenvironment. These results support further studies on this sequential approach to therapy, and prospective clinical trials are currently underway.
ACKNOWLEDGMENTS
This work was supported by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding information
National Center for Advancing Translational Science (NCATS), Grant/Award Number: UL1 TR002377
CONFLICT OF INTEREST
JSS reports grant, research, and/or clinical trial support from Rafael Pharmaceuticals, Macrogenics, Molecular Templates, Daiichi Sankyo, Vedanta Biosciences, Bristol-Myers Squibb, Xencor, Aprea, Merus, Cardiff Oncology, Incyte, and consulting support from Pfizer, Natera, Tesera, and Ipsen. PMK reports grant funding from Boston Scientific, AstraZeneca; and honoraria (consultancy/advisory boards) from Taiho Oncology, Natera, Foundation Medicine, Merck Sharp & Dohme (MSD). HHY reports grant, research, and/or clinical trial support from Merck & Co., Inc, Macrogenics, Bristol Myer Squibb; and honoraria (advisory boards and/or steering committees) from Merck, Macrogenics, BeiGene, ALX Oncology, Bristol Myer Squibb, and AstraZeneca. The other authors have no conflict of interest to disclose.
Abbreviations:
- ACE
advanced cohort explorer
- BSA
bovine serum albumin
- CD
cluster of differentiation
- CI
confidence interval
- CPS
combined positive score
- CPT
current procedural terminology
- CR
complete response
- Cyanine
Cy
- CyTOF
time-of-flight mass cytometer
- DAPI
4’,6-diamidino-2-phenylindole
- DNA
deoxyribonucleic acid
- DOR
duration of response
- ECOG
Eastern Cooperative Oncology Group
- FDA
Food and Drug Administration
- FFPE
formalin-fixed paraffin-embedded
- FLO
5-FU + leucovorin + oxaliplatin
- FOLFOX
folinic acid (leucovorin) “FOL” + fluorouracil (5-FU) “F” + oxaliplatin “Ox”
- FOXP3
Forkhead box P3
- GEJ
gastroesophageal junction
- H&E
hematoxylin and eosin
- HER2
human epidermal growth factor receptor 2
- HICDA
Hospital International Classification of Diseases Adapted
- ICD
International Classification of Disease
- ICI
immune checkpoint inhibition
- IHC
immunohistochemistry
- IMC
imaging mass cytometry
- irRECIST
immune-related response evaluation criteria in solid tumors
- LCBI
last chemotherapy administered before ICI
- mGEA
metastatic gastroesophageal adenocarcinoma
- MLH1
MutL homolog 1
- MMR
DNA mismatch repair
- MSH2
MutS homolog 2
- MSH6
MutS homolog 6
- MV
multivariable
- ORR
objective response rate
- OS
overall survival
- PBS
phosphate-buffered saline
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- PFS
progression-free survival
- PMS2
MS1 homolog 2
- PR
partial response
- PS
performance status
- RAM
ramucirumab
- RECIST
response evaluation criteria in solid tumors
- REDCap
research electronic data capture
- TAX
paclitaxel
- TME
tumor microenvironment
- Tregs
regulatory T cells
- VEGFR
vascular endothelial growth factor receptor
Footnotes
ETHICS STATEMENT
The study was approved by the Institutional Review Board of Mayo Clinic, and all participating patients providing biospecimens signed informed consent prior to inclusion in the study.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as Supporting Information. Raw data remain available upon reasonable request.
REFERENCES
- 1.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. [DOI] [PubMed] [Google Scholar]
- 3.Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;31:1–3. [DOI] [PubMed] [Google Scholar]
- 4.Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci. 2019;116(20):9999–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 6.Vicari AP, Luu R, Zhang N, et al. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58(4):615–628. 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada Y, Togashi Y, Kotani D, et al. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8+ T cells in the tumor microenvironment. J Immunother Cancer. 2018;6(1):106. 10.1186/s40425-018-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti S, Dong H, Paripati HR, Ross HJ, Yoon HH. First report of dramatic tumor responses with ramucirumab and paclitaxel after progression on pembrolizumab in two cases of metastatic gastroesophageal adenocarcinoma. Oncologist. 2018;23(7):840–843. 10.1634/theoncologist.2017-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirkel GA, Weeber F, Bins S, et al. The time to progression ratio: a new individualized volumetric parameter for the early detection of clinical benefit of targeted therapies. Ann Oncol. 2016;27(8):1638–1643. 10.1093/annonc/mdw223. [DOI] [PubMed] [Google Scholar]
- 10.Osa A, Uenami T, Koyama S, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3:59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aspeslagh S, Matias M, Palomar V, et al. In the immuno-oncology era, is anti-PD-L1 or anti-PD-L1 immunotherapy modifying the sensitivity to conventional cancer therapies? Eur J Cancer. 2017;87:65–74. 10.1016/j.ejca.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki A, Kawazoe A, Eto T, et al. Improved efficacy of taxanes and ramucirumab combination chemotherapy after exposure to anti-PD-1 therapy in advanced gastric cancer. ESMO Open. 2020; 4(suppl 2):e000775. 10.1136/esmoopen-2020-000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon HH, Fuchs CS, Özgüroğlu M, et al. O-12 KEYNOTE-061: response to subsequent therapy following second-line pembrolizumab or paclitaxel in patients with advanced gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2020; 31(suppl 3):236.31959340 [Google Scholar]
- 14.Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients with advanced NSCLC and PD-L1 TPS =50% enrolled in KEYNOTE-024. J Clin Oncol. 2017;35(15):9000. [Google Scholar]
- 15.Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018; 13(1):106–111. 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Cao S, Liu X, et al. CX3CR1 identifies PD-1 therapy-responsive CD8+ T cells that withstand chemotherapy during cancer chemoimmunotherapy. JCI Insight. 2018;3(8):e97828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol 2020;21(10):e463–e476. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M, Khong H, Fa'ak F, et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat Commun 2020;11:661. 10.1038/s41467-020-14471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asano T, Meguri Y, Yoshioka T, et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129:2186–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty S, Weston CJ, Oo YH, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J Immunol. 2011;186:4147–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. [Google Scholar]
- 25.Kato K, Sun JM, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: the phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192–S1193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as Supporting Information. Raw data remain available upon reasonable request.