Abstract
Objectives:
Carotid-femoral pulse wave velocity (PWV), an index of arterial stiffness and a proxy of arterial aging, has been reported to be an independent determinant of cardiovascular health. Whether the effects of antihypertensive treatment vary in the presence of accelerated arterial aging (stiffer artery, ie, PWV >10 m/s) has not been established. We tested this hypothesis in a longitudinal study in a large community-dwelling population.
Design:
Longitudinal population study with repeated measures.
Setting and Participants:
Study population consisted of a cohort of 6011 volunteers (2546 men and 3465 women, age range 14–101 years; 15,011 observations over a median follow-up of 6.8 years) participating in the SardiNIA Study.
Measures:
Repeated measures of PWV, blood pressure (BP), and metabolic risk factors and the antihypertensive medication trajectories of BP and PWV over time were assessed via mixed effects models.
Results:
Antihypertensive treatment significantly affected the trajectory of BP in both participants with (−0.47 ± 0.20 mmHg/y, P = .02) and participants without stiffer arteries (−0.47 ± 0.07 mmHg/y, P = .001). They also affected the trajectory of PWV in participants with stiffer artery, independent of the BP values.
Conclusions and Implications:
Antihypertensive treatment is effective in reducing both BP and PWV in older individuals with stiffer arteries.
Keywords: Antihypertensive treatment, arterial stiffness, longitudinal cohort study, pulse wave velocity
Blood pressure (BP) increases with advancing age and so does the prevalence of hypertension: from approximately 60% after age 60 years to approximately 75% among those aged ≥ 75 years.1 Hypertension represents the single major cause of cardiovascular morbidity and of the years lived with disability.2
The cultural paradigm considers older age a barrier to antihypertensive treatment because of assumed impaired perfusion to vital organs related to BP lowering; treating hypertension in older individuals still represents a challenge. Older patients are characterized by a high degree of heterogeneity in comorbidity,3 which exposes them to negative interaction4; polypharmacy including antihypertensive medications can cause serious adverse drug reactions.5 A particular comorbidity, primarily poorer cognitive function, may interfere with patients’ adherence to medical prescription and interfere with BP control and variability,6 which in turn may contribute to the progression of cognitive decline.7,8
Aging is associated not only with an increase in BP levels and prevalent hypertension but also with a progressive stiffening of large arteries,9 indexed by noninvasive measurement of carotid-femoral pulse-wave velocity (PWV).10 The positive association between BP and arterial stiffness is bidirectional: a stiffer artery predicts the development of systolic hypertension,11 and higher BP increases arterial stiffness.12
The stiffening of arteries with advancing age increases the complexity of management and treatment of hypertension at older ages. In fact, in the presence of stiffer arteries, the active treatment of hypertension has been reported as less effective13 and multiple organ damage (heart, vessel, and kidney) is more frequent.14 Additionally, with stiffer arteries, BP pulsatility is greater,15 which leads to progression of cognitive decline.6,16–18
The aim of the current study was to determine the impact of antihypertensive treatment on BP and PWV trajectories in people with or without stiffer arteries by measuring both PWV and BP trajectories during 6.8 years of follow-up in a large cohort of community-dwelling men and women 14 to 100 years of age at entry into the study.
Methods
Study Population
The SardiNIA Study was conceived as a study of a Sardinian founder population investigating the genetics of complex traits/phenotypes, including cardiovascular risk factors and arterial properties.19 The entire population aged ≥ 14 years was contacted via advertisements to participate in a free full screening over 3 years. Over this time, 6148 participants were enrolled, comprising 63% of those aged 14 to 102 years in a cluster of 4 towns. Each participant came to the clinic before breakfast, signed consent forms, and gave a sample of fasting blood so that all tests would be uninfluenced by meals at different times. Each participant underwent a detailed medical history and full medical examination, BP, and anthropometric measurements, a 12-lead resting electrocardiogram, and measurements of arterial structure and function.
Because we are investigating longitudinal changes in PWV and day-to-day variability in PWV measurements is about ±2 m/s,20 it seemed reasonable to exclude participants whose visit-to-visit changes in PWV were more than 5 m/s (ie, 2½ times the expected day-to-day variation).
The data used in the present analysis comprised 15,011 observations on 6011 volunteers (2546 men and 3465 women) who entered the study over a wide age range (14–101 years) and had a median follow-up of 6.8 years (mean 5.4 years and maximum 10.2 years).
The present study complies with the Declaration of Helsinki. The locally appointed ethics committee has approved the research protocol and informed consent has been obtained from the participants (or their legally authorized representative).
Variables Measured
Blood pressure
Morning BP was measured in both arms with a mercury sphygmomanometer using an appropriately sized cuff. Values for systolic BP (SBP) and diastolic BP (DBP) were defined by Korotkoff phase I and V, respectively. The average of the second and third measurements on both the right and left arm were used in the analysis. Pulse pressure (PP) was computed as PP = (SBP − DBP); mean BP (MBP) was computed as MBP = DBP + (PP/3).
Anthropometry
Height, weight, and waist circumference were determined for all participants. Body mass index was calculated as body weight (kg)/[height (m)]2.
Fasting plasma lipids
Blood samples were drawn from the antecubital vein between 7 and 8 am after an overnight fast. Participants were not allowed to smoke, engage in significant physical activity, or take medications before collection of samples. Plasma triglycerides and total cholesterol were determined by an enzymatic method (ABA-200 ATC Biochromatic Analyzer; Abbott Laboratories, Irving, TX). High-density lipoprotein cholesterol was determined by a dextran sulfate magnesium precipitation. Low-density lipoprotein cholesterol concentrations were estimated using the Friedewald formula. Fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments Inc, Fullerton, CA).
Arterial stiffness: PWV
Aortic stiffness was assessed noninvasively based on the carotid-femoral PWV,21 using nondirectional transcutaneous Doppler probes (9- to 10-MHz) (Model 810A; Parks Medical Electronics, Inc, Aloha, OR) and adopting the subtraction method for estimating aorta length from the distance between the carotid and femoral measurement sites. Accordingly, exaggerated stiff artery was defined as a PWV >1000 cm/s (or 10 m/s).22
Group Definitions
Volunteers were assigned to 4 different groups on the basis of 2 categorical variables assessed at any visit: high BP, that is, office BP > 140 mmHg for SBP and >90 mmHg for DBP (yes = 1; no = 0); use of antihypertensive medications (yes = 1; no = 0).
Accordingly, groups were identified as follows:
Normotensive: no high BP in the absence of antihypertensive treatment
Hypertensive, controlled: no high BP in the presence of antihypertensive treatment
Hypertensive, treated but not controlled: high BP despite antihypertensive treatment
Hypertensive, not treated and not controlled: high BP in the absence of antihypertensive treatment
Statistical Analyses
Because participants enter the SardiNIA study at different ages and were followed thus far for 2 or 3 visits, both cross-sectional and longitudinal perspectives can be gleaned from the measured variables. All analyses were performed using R software.23 The analyses included 6011 participants for 15,011 observations. Baseline characteristics are presented as mean ± standard deviation unless otherwise specified. Linear mixed effects models, the best available analytical tools for unbalanced, unequally spaced observations, such as those of the SardiNIA and PROGENIA studies, were used to assess average longitudinal trajectories of indexed parameters.24 The fit of the mixed effects models to the data are addressed by plots of the residuals and plots of observed vs predicted values.
Each model fit represents a specific hypothesis being tested, and each model contains a number of model effects. The mixed effects model included as covariates the following: entering age, time, body mass index, high BP, and antihypertensive treatment and the interaction terms of the last 2 variables plus entering age with time.
Results
Distribution of PWV and BP measurements according to BP groups is described in Table 1. To evaluate the impact of stiffer arteries on the response to antihypertensive treatment, the study population was divided into 2 subgroups: participants with PWV ≤10 m/s or >10 m/s (Table 2).
Table 1.
Distribution of PWV and BP Measurements According to BP Groups (Number of Participants)
| 1 Visit | 2 Visits | 3–4 Visits | Total Observations | |
|---|---|---|---|---|
| NT | 766 | 1752 | 2132 | 10,709 |
| HT, treated and controlled | 58 | 79 | 91 | 509 |
| HT, treated and not controlled | 266 | 429 | 577 | 2931 |
| HT, untreated and not controlled | 107 | 100 | 170 | 862 |
HT, hypertensive; NT, normotensive.
Table 2.
Characteristics of Participants With or Without Exaggerated Aortic Stiffness (Defined as PWV >10 m/s) at Their Study Entry
| PWV ≤10 m/s at Baseline (n = 5514) | PWV > 10 m/s at Baseline (n = 497) | |
|---|---|---|
| Age, y, mean ± SD | 41.3 ± 16.3 | 67.2 ± 11.9 |
| Age group, n (%) | ||
| <35 y | 2117 (38.4) | 10 (2.0) |
| 35–49 y | 1688 (30.6) | 29 (5.9) |
| 50–64 y | 1152 (20.9) | 146 (29.4) |
| ≥65 y | 557 (10.1) | 312 (62.7) |
| Women | 3219 (57.8) | 279 (55.7) |
| BMI | 25.0 ± 4.5 | 29.0 ± 4.9 |
| Waist circumference, cm | 83.6 ± 12.5 | 96.1 ± 12.3 |
| Fasting glucose, mg/dL | 88.4 ± 21 | 105.9 ± 35.4 |
| Total cholesterol, mg/dL | 206.7 ± 41.7 | 227.2 ± 43.8 |
| HDL cholesterol, mg/dL | 64 ± 14.9 | 65.5 ± 14.7 |
| LDL cholesterol, mg/dL | 125.6 ± 35.0 | 139.0 ± 37.8 |
| Triglycerides, mg/dL | 85.7 ± 66.4 | 113.2 ± 79.3 |
| Serum creatinine, mg/mL | 0.80 ± 0.19 | 0.85 ± 0.36 |
| SBP, mmHg | 123.6 ± 16.9 | 147.6 ± 21.0 |
| DBP, mmHg | 76.3 ± 10.5 | 84.7 ± 11.5 |
| MBP, mmHg | 91.9 ±11.7 | 105.5 ± 13.2 |
| PP, mmHg | 47.3 ± 11.7 | 62.9 ± 16.4 |
| NT | 75.7 | 22.0 |
| HT, treated and controlled | 3.1 | 8.2 |
| HT, treated and not controlled | 16.9 | 47.4 |
| HT, untreated and not controlled | 4.3 | 22.4 |
BMI, body mass index; HDL, high-density lipoprotein; HT, hypertensive; LDL, low-density lipoprotein; NT, normotensive; PP, pulse pressure.
Unless otherwise noted, values are mean ± SD.
Table 3 lists the estimates for the fitted models for SBP, MBP, and PWV in participants without or with stiffer artery. The graphs in Figures 1 and 2 are generated from the equations provided by the mixed effects models in Table 2.
Table 3.
Mixed Effects Models Results for BPs According to the Presence of Exaggerated Aortic Stiffness
| PWV at Baseline ≤10 m/s | PWV at Baseline >10 m/s | |||||
|---|---|---|---|---|---|---|
| Value | SE | P Value | Value | SE | P Value | |
| SBP | ||||||
| (Intercept) | 108.148 | 0.7331 | <.001 | 120.1778 | 3.1942 | <.001 |
| Gender (male) | 4.4568 | 0.2403 | <.001 | 1.4701 | 0.9392 | .12 |
| Age c | 0.2095 | 0.0101 | <.001 | 0.1376 | 0.049 | .005 |
| BMI | 0.356 | 0.0292 | <.001 | 0.0538 | 0.0985 | .59 |
| High BP | 22.5232 | 0.342 | <.001 | 31.0445 | 1.188 | <.001 |
| antiHT | 2.4489 | 0.5173 | <.001 | −0.1607 | 1.2062 | .89 |
| Time | 0.1032 | 0.0419 | .014 | −0.1055 | 0.3661 | .77 |
| Time × high BP | −0.0762 | 0.0862 | .38 | −0.9055 | 0.3057 | .003 |
| Time × antiHT | −0.5348 | 0.0931 | <.001 | −0.3186 | 0.3113 | .31 |
| Age c × time | 0.0089 | 0.0025 | <.001 | 0.0144 | 0.0144 | .32 |
| MBP | ||||||
| (Intercept) | 78.2005 | 0.5026 | <.001 | 87.4365 | 2.1765 | <.001 |
| Gender (male) | 2.8203 | 0.1638 | <.001 | 0.2944 | 0.6506 | .65 |
| Age c | 0.1549 | 0.007 | <.001 | −0.0308 | 0.0329 | .35 |
| BMI | 0.3779 | 0.02 | <.001 | 0.1799 | 0.0674 | .008 |
| High BP | 16.166 | 0.2402 | <.001 | 19.1756 | 0.7845 | <.001 |
| antiHT | 0.848 | 0.3604 | .019 | 0.1914 | 0.8024 | .81 |
| Time | 0.165 | 0.0299 | <.001 | 0.1128 | 0.2353 | .63 |
| Time × high BP | −0.0203 | 0.0611 | .74 | −0.5746 | 0.1982 | .004 |
| Time × antiHT | −0.4681 | 0.066 | <.001 | −0.4747 | 0.2014 | .019 |
| Age c × time | 0.0015 | 0.0018 | .41 | 0.007 | 0.0093 | .45 |
| PWV | ||||||
| (Intercept) | 469.442 | 11.036 | <.001 | 1079.324 | 65.298 | <.001 |
| Gender (male) | −3.430 | 3.535 | .33 | 21.743 | 18.985 | .25 |
| Age c | 5.044 | 0.160 | <.001 | 1.825 | 1.013 | .07 |
| BMI | 6.488 | 0.439 | <.001 | 2.966 | 2.005 | .14 |
| High BP | 50.743 | 5.715 | <.001 | −7.758 | 24.767 | .75 |
| antiHT | 7.835 | 8.355 | .35 | −18.893 | 25.039 | .45 |
| Time | 10.843 | 0.745 | <.001 | −75.097 | 7.761 | <.001 |
| Time × high BP | 7.168 | 1.494 | <.001 | 12.967 | 6.443 | .045 |
| Time × antiHT | 0.159 | 1.622 | .92 | 17.135 | 6.568 | .009 |
| Age c × time | 0.167 | 0.044 | <.001 | 1.503 | 0.303 | <.001 |
Age c, age at entry; antiHT, antihypertensive medication; BMI, body mass index; SE, standard error.
The models are age-centered and included 15,011 observations from 6001 participants.
Fig. 1.
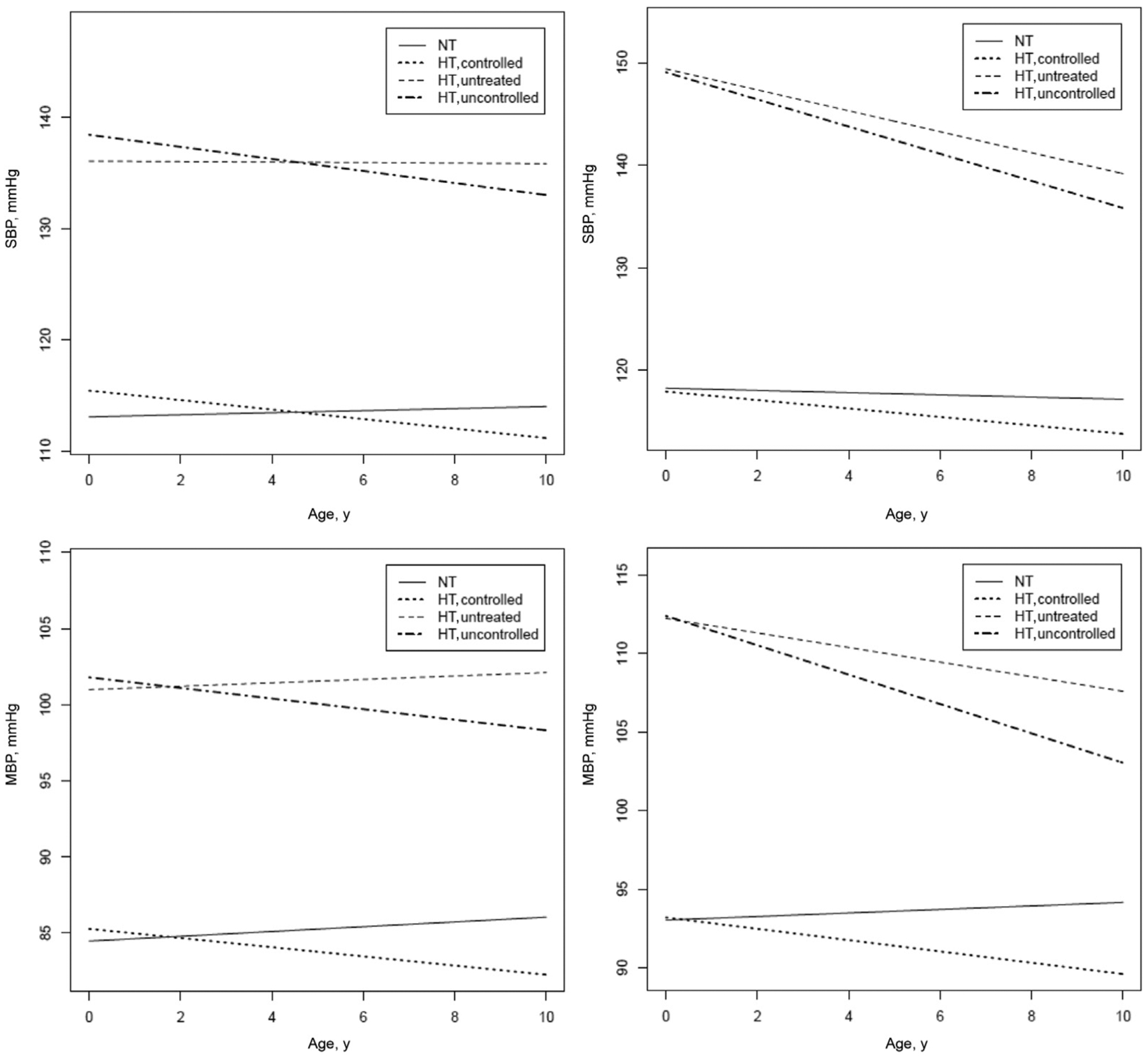
Longitudinal trajectories in BPs from mixed effects models according to BP levels and antihypertensive treatment at each visit. The longitudinal plots of the modeled data are constructed centering for population mean age (43.5 ± 17.5 years) and mean body mass index (25.3 ± 4.7). The equations used for these predictions are described in Table 2.
Fig. 2.
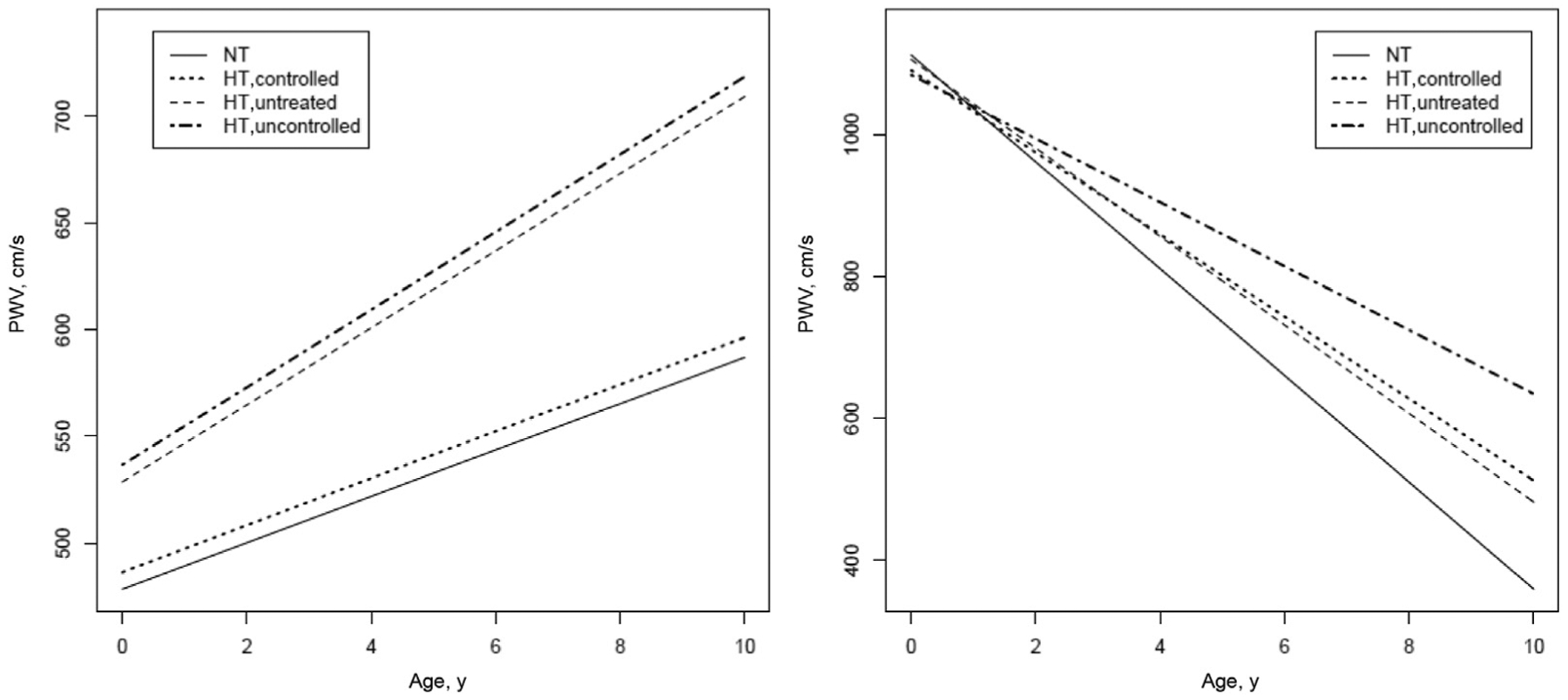
Longitudinal trajectories in PWV and PWV normalized for MBP from mixed effects models according to BP levels and antihypertensive treatment at each visit. The longitudinal plots of the modeled data are constructed centering for population mean age (43.5 ± 17.5 years) and mean body mass index (25.3 ± 4.7). The equations used for these predictions are described in Table 2.
In participants with PWV at baseline ≤10 m/s, participants receiving antihypertensive medication showed a significantly greater decline in SBP over time, whether they attained BP control or not (Figure 1, top left panel). A similar trend was observable for MBP (Figure 1, bottom left panel) and DBP (data not shown). In participants with PWV >10 m/s at study entry, SBP started significantly higher but decreased with a steeper trajectory in participants with high BP; antihypertensive treatment was accompanied by a significantly greater decrease in SBP over time (Figure 1, top right panel).
A similar trend was observable for MBP (Figure 1, bottom right panel) and DBP (data not shown). Concerning trajectory of PWV, in participants with PWV at baseline ≤10 m/s, the increase over time was slightly greater in those participants with higher BP and was not significantly influenced by antihypertensive medications (Figure 2, left panel). In participants with stiffer arteries, PWV significantly decreased over time; in hypertensive participants, treatment was associated with a greater decrease in PWV over time as compared to hypertensive participants not achieving BP control (Figure 2, right panel).
Discussion
This is the first extended longitudinal study in the general population of men and women of a broad age spectrum and extensive clinical information available, including and modeling repeated measurements of both BP and PWV over time. The present study showed that antihypertensive treatment reduced the trajectory of SBP and MBP over time both in participants with and without stiffer arteries. The impact of antihypertensive treatment on trajectory of PWV over time was greater in participants with stiffer arteries.
In participants without arterial stiffness, the trajectory of BP and PWV over time can be interpreted in light of the known vicious circle between BP and PWV, indicating the importance of BP control in limiting the progress of this detrimental vicious cycle.25,26 Thus, the lower the achieved BP, the slower the increase in PWV over time, that is, the rate of arterial aging.
The observed trajectory in BP and PWV in participants with stiffer arteries is more complex to interpret. If we cannot rule out that part of the observed effects in participants with stiffer artery may be attributable to the regression to the mean phenomenon rather than to antihypertensive treatment, however, the selective impact of antihypertensive treatment on BP levels and arterial stiffness suggests that the reduction is not attributable exclusively to the regression to the mean phenomenon.
It is tempting to speculate that a smaller aortic root with higher impedance to flow27 and large artery remodeling28 underlying the stiffer artery may affect the dissociation between the magnitude in trajectory of BP and PWV reduction over time in response to antihypertensive treatment.
Another possibility relates to the prescribed antihypertensive drug classes in participants with stiffer artery and their ability to reduce arterial characteristic impedance and fibrosis, dilate muscular artery, decrease vascular tone, and modulate vascular remodeling. In fact, selective antihypertensive drugs, namely, those blocking the angiotensin II cascade, have been shown able to reduce PWV independently and beyond their effect on BP levels.29–31 Superiority of selected antihypertensive drug classes in reducing arterial stiffness has not been proven in human studies. This reflects the complexity of approaching this issue. First, most hypertensive participants need 2 or more drugs to achieve an adequate BP control.6 Meta-analysis including participants in monotherapy and adopting complex statistical modeling faced the limitation of the relatively short duration of treatment, likely not sufficient to produce those structural changes in the vessel wall able to modify arterial stiffness.32 Last, but not least, the contribution of poor adherence to antihypertensive therapy may be relevant. With advancing age, the issue of adherence to antihypertensive therapy is further complicated by the greater occurrence of cognitive impairment.33
The major strength of this study is the long follow-up time and the availability of repeated measures at regular intervals, allowing assessment of structural rather than functional arterial changes that likely underline changes in PWV. A further strength is the classification of groups according to treatment and BP levels at each visit. This characterization and description of trajectory in arterial aging, thus, becomes more accurate by partly overcoming the limitation of the usual intention-to-treat analysis adopted in clinical trials.
Although the present study included a large number of participants observed over time with repeated measurement of both BP and PWV, a relevant limitation is represented by the considerable interval between assessments (on average 3 years), typical in population studies. More BP measures at shorter interval might provide a more rigorously defined trajectory of BP over time and the impact of therapy. An additional limitation is represented by the population size, which is too small to provide the power for an accurate analysis of the arterial effects of specific antihypertensive drug classes. Thus, we cannot distinguish whether the impact of antihypertensive therapy on arterial aging may result only from BP lowering or a direct effect on aortic remodeling, or it is affected in part from adverse drug reaction and discontinuation of therapy attributable to specific drug classes.
The results of the present study cannot be generalized and need to be confirmed. However, they contribute to an innovative conceptual approach to aging population. In fact, the outcome adopted for therapeutic intervention was an integrated marker of aging (aortic stiffness measured as PWV) rather than disease, as conventionally is done.
Conclusions and Implications
In summary, antihypertensive therapy seems able to reduce arterial stiffness even in participants who have exaggerated aortic stiffness, a proxy of accelerated arterial aging.
The present findings need to be confirmed in larger population consortia. If confirmed, a better characterization of the potential specific pathways underlying selective changes in PWV, independently of BP reduction, may lead to the discovery of new therapeutic targets to slow arterial aging. Additionally, they may contribute to establish whether stiffer arteries identify older participants with an exaggerated and/or less predictable BP response to treatment, making them frailer and at greater risk of functional loss.34
Acknowledgments
This research was supported in part by the Intramural Research Program of the US National Institutes of Health, National Institute on Aging (No. HHSN271201600005C).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 2.Olsen MH, Angell SY, Asma S, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on Hypertension. Lancet 2016;388: 2665–2712. [DOI] [PubMed] [Google Scholar]
- 3.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Pérez-Zepeda MU, Marzetti E. Frailty and multimorbidity: Different ways of thinking about geriatrics. J Am Med Dir Assoc 2017;18:361–366. [DOI] [PubMed] [Google Scholar]
- 5.Onder G, Bonassi S, Abbatecola AM, et al. on behalf of the Geriatrics Working Group of the Italian Medicines Agency (AIFA). High prevalence of poor quality drug prescribing in older individuals: A nationwide report from the Italian Medicines Agency (AIFA). J Gerontol A Biol Sci Med Sci 2014;69:430–437. [DOI] [PubMed] [Google Scholar]
- 6.Di Daniele N, Fegatelli DA, Rovella V, et al. Circadian blood pressure patterns and blood pressure control in patients with chronic kidney disease. Atherosclerosis 2017;267:139–145. [DOI] [PubMed] [Google Scholar]
- 7.Lv YB, Zhu PF, Yin ZX, et al. A U-shaped association between blood pressure and cognitive impairment in Chinese elderly. J Am Med Dir Assoc 2017;18:193. e7–193.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng J, Lu F, Wang Z, et al. Excessive lowering of blood pressure is not beneficial for progression of brain white matter hyperintensive and cognitive impairment in elderly hypertensive patients: 4-year follow-up study. J Am Med Dir Assoc 2014;15:904–910. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson PN, Laurent S, Cunha PG, et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: The global Metabolic syndrome and Artery REsearch (MARE) Consortium. J Hypertens 2018;36: 2340–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 11.Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2008;51:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scuteri A, Morrell CH, Orrù M, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014;64:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton-Tyrrell K, Wildman R, Newman A, Kuller LH. Extent of cardiovascular risk reduction associated with treatment of isolated systolic hypertension. Arch Intern Med 2003;163:2728–2731. [DOI] [PubMed] [Google Scholar]
- 14.Scuteri A, Rovella V, Alunni Fegatelli D, et al. An operational definition of SHATS (Systemic Hemodynamic Atherosclerotic Syndrome): Role of arterial stiffness and blood pressure variability in elderly hypertensive subjects. Int J Cardiol 2018;263:132–137. [DOI] [PubMed] [Google Scholar]
- 15.Scuteri A, Wang H. Pulse wave velocity as a marker of cognitive impairment in the elderly. J Alzheimers Dis 2014;42:S401eS410. [DOI] [PubMed] [Google Scholar]
- 16.van Middelaar T, Richard E, Moll van Charante EP, et al. Visit-to-visit blood pressure variability and progression of white matter hyperintensities among older people with hypertension. J Am Med Dir Assoc 2019;20:1175–1177.e1. [DOI] [PubMed] [Google Scholar]
- 17.Scuteri A, Tesauro M, Guglini L, et al. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol 2013;169:371–377. [DOI] [PubMed] [Google Scholar]
- 18.Scuteri A, Coluccia R, Castello L, et al. Left ventricular mass increase is associated with cognitive decline and dementia in the elderly independently of blood pressure. Eur Heart J 2009;30:1525–1529. [DOI] [PubMed] [Google Scholar]
- 19.Scuteri A, Najjar SS, Orru’ M, et al. Age- and gender-specific awareness, treatment, and control of cardiovascular risk factors and subclinical vascular lesions in a founder population: The SardiNIA Study. NutrMetab Cardiovasc Dis 2009;19:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huck CJ, Bronas UG, Williamson EB, et al. Noninvasive measurements of arterial stiffness: Repeatability and interrelationships with endothelial function and arterial morphology measures. Vasc Health Risk Manag 2007;3:343–349. [PMC free article] [PubMed] [Google Scholar]
- 21.Scuteri A, Orru M, Morrell C, et al. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study. Atherosclerosis 2011;215:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values”. Eur Heart J 2010;31: 2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org; 2015. AccessedMarch 6, 2019. [Google Scholar]
- 24.Morrell CH, Brant LJ, Ferrucci L. Model choice can obscure results in longitudinal studies. J Gerontol A Biol Sci Med Sci 2009;64:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–1207. [DOI] [PubMed] [Google Scholar]
- 26.Vallée A, Yannoutsos A, Temmar M, et al. Determinants of the aortic pulse wave velocity index in hypertensive and diabetic patients: Predictive and therapeutic implications. J Hypertens 2018;36:2324–2332. [DOI] [PubMed] [Google Scholar]
- 27.Farasat SM, Morrell CH, Scuteri A, et al. Pulse pressure is inversely related to aortic root diameter implications for the pathogenesis of systolic hypertension. Hypertension 2008;51:196–202. [DOI] [PubMed] [Google Scholar]
- 28.Scuteri A, Chen CH, Yin FCP, et al. Functional correlates of central arterial geometric phenotypes. Hypertension 2001;38:1471–1475. [DOI] [PubMed] [Google Scholar]
- 29.Laurent S, Boutouyrie P. Vascular Mechanism Collaboration. Dose-dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension 2014;64:709–716. [DOI] [PubMed] [Google Scholar]
- 30.Ahimastos AA, Natoli AK, Lawler A, et al. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension 2005;45:1194–1199. [DOI] [PubMed] [Google Scholar]
- 31.Lacourcière Y, Béliveau R, Conter HS, et al. Canadian Hypertension Society. Effects of perindopril on elastic and structural properties of large arteries in essential hypertension. Can J Cardiol 2004;20:795–799. [PubMed] [Google Scholar]
- 32.Ye L, Yang X, Hu J, et al. Impact of antihypertensive agents on arterial stiffness in hypertensive patients. Int J Cardiol 2018;273:207–212. [DOI] [PubMed] [Google Scholar]
- 33.Kalaitzidis RG, Panagiotopoulou T, Stagikas D, et al. Arterial stiffness, cognitive dysfunction, and adherence to antihypertensive agents. Is there a link to hypertensive patients? Curr Vasc Pharmacol 2019April15. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Costanzo L, Cesari M, Ferrucci L, et al. Predictive capacity of frailty phenotype toward patterns of disability identified using latent class analysis. J Am Med Dir Assoc 2019;20:1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]


