Abstract
A rapid method that provides information on the viability of organisms is needed to protect public health and ensure that remediation efforts following a release of a biological agent are effective. The rapid viability-polymerase chain reaction (RV-PCR) method combines broth culture and molecular methods to provide results on whether viable organisms are present in less than 15 h. In this study, a modified RV-PCR (mRV-PCR) method was compared to a membrane-filtration culture method for the detection of viable Bacillus spores in water matrices. Samples included small and large volumes of chlorine and non-chlorine treated tap water. Large volume water samples (up to 100 L), were processed by ultrafiltration using a semi-automated waterborne pathogen concentrator, followed by centrifugation as a secondary concentration technique. The concentrated samples were analyzed by mRV-PCR and culture methods. The overall agreement between the mRV-PCR and culture methods when seed concentrations were greater than 10 spores per sample volume analyzed was 96%. The total time from the start of sample processing to the final sample result for the mRV-PCR method was decreased by approximately 2 h, in comparison to the previously published RV-PCR method because of the incorporation of shorter, more efficient primary and secondary concentration steps and a shorter DNA extraction technique. Overall, this study confirmed that RV-PCR is a promising approach for identifying viable Bacillus spores in small- and large-volume water samples and for producing results in less time than traditional culture methods.
Keywords: Bacillus spores, Rapid, Viability, Quantitative PCR, Ultrafiltration, Water samples
1. Introduction
Quantitative polymerase chain reaction (qPCR) is a widely used molecular technique for the rapid detection and quantification of microorganisms; however, this technique alone does not provide information on whether the organisms are viable. Knowledge of viability is critical in particular situations; for example, public exposure to biological weapons such as Bacillus anthracis. Traditional culture methods to determine viability of bacteria generally take 24 h to obtain preliminary results, with confirmed results not available until days later. This time frame may be too long, particularly when there is a threat to public health. The rapid viability-PCR (RV-PCR) method combines molecular and broth culture methods and can determine the presence or absence of viable B. anthracis spores in under 15 h of total processing time (Kane et al., 2009; Létant et al., 2010; Létant et al., 2011; USEPA, 2011; USEPA, 2012).
Sampling after the release of a biological weapon can be divided into three phases: the initial assessment of contamination (Phase 1), the characterization of the extent of contamination (Phase 2), and then the clearance of the area following remediation (Phase 3) (Gillen, 2008). The qPCR method is appropriate for analyzing samples during phases 1 and 2 for the rapid identification and enumeration of the organism, followed by culture methods as a confirmation step. For Phase 3 samples, a method that can rapidly detect viable organisms is needed to ensure that risk of exposure to potentially infectious organisms is gone before clearance of the affected area. This allows for the protection of human health and confirmation that remediation efforts were effective.
Kane et al. (2013) evaluated the RV-PCR method during a study in which Bacillus atrophaeus, a commonly used surrogate of B. anthracis, was released into a facility and various decontamination techniques were assessed. Surface wipe samples were collected following decontamination and tested using the RV-PCR and culture methods. Results showed a 98% agreement between the results of the two methods. In another study, Létant et al. (2011) successfully used the RV-PCR method to detect viable B. anthracis spores from wipe, air filter, and water samples and described a shorter turnaround time and lower detection limit over the culture method.
Less testing has been done in different water matrices of varying volumes, particularly following the concentration of large volumes of water. The need for a method to detect biological weapons in water matrices does not come solely from the threat to a water supply or a distribution system, but there is also a need to detect whether viable organisms are present in collected wash water following decontamination efforts to determine whether mitigation steps were effective. Analysis of large volumes of water can be challenging due to the various concentration steps needed before the water sample can be analyzed. Along with the concentration of large volumes of water comes the concentration of potential inhibitors in the water that may negatively affect the qPCR reaction and cause false negative results.
In this study, we modified the previously published RV-PCR method by using centrifugation as the secondary concentration step, and by using a different DNA extraction technique in order to reduce the amount of time to obtain results. Additionally, for large volumes of water, a semi-automated ultrafiltration step was included for the primary concentration step. The results of the modified RV-PCR (mRV-PCR) were compared to results of the membrane-filtration culture method in small (less than 500 mL) and large (10 to 100 L) volumes of water that were seeded with varying concentrations of spores of B. atrophaeus. A subset of samples was treated with chlorine to represent a post-decontamination matrix and one large volume sample was amended with river sediment to evaluate method performance in the presence of particles and potential inhibitors. This paper describes the mRV-PCR method for the detection of viable Bacillus spores in water samples, with and without exposure to chlorine and potential PCR inhibitors. The criteria for success of the mRV-PCR method was based on whether there was agreement of results with the traditional culture method and whether the mRV-PCR method yielded results in a shorter amount of time than both the culture method and the previously published RV-PCR method.
2. Materials and methods
2.1. Experimental design
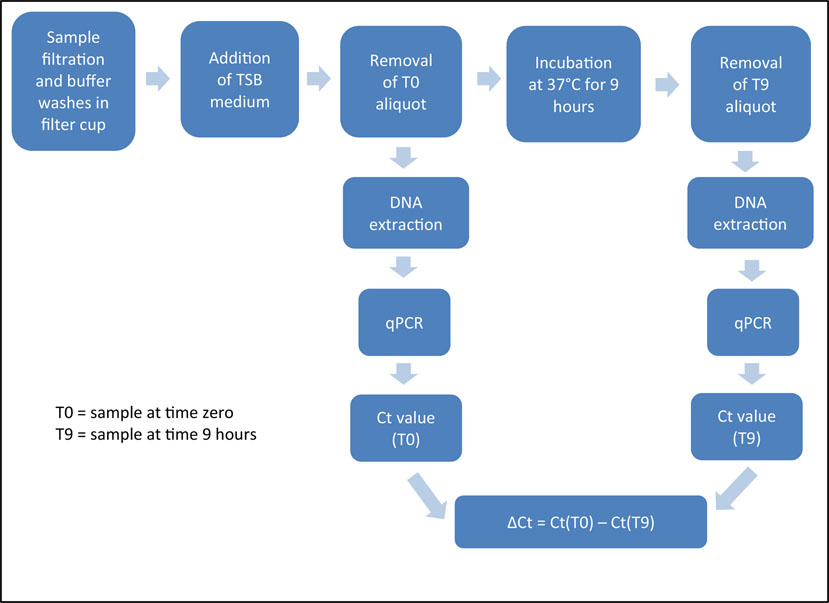
Fig. 1 shows the overall flow of the experimental design from original sample to analysis methods. Portions of the experimental design, including ultrafiltration, secondary concentration, and the DNA extraction step of the mRV-PCR method were modified from methods described in USEPA (2012), and are described below.
Fig. 1.
Schematic of overall experimental workflow.
2.2. Strain and spore preparation
Spores of the B. atrophaeus (American Type Culture Collection, Cat. No. 9372) were used for this study. The spores were prepared using a procedure that was modified from a previous study (Francy et al., 2009). B. atrophaeus was streaked onto trypticase soy agar plus 5% sheep blood (TSA + SB) plates (Becton, Dickinson and Co., Sparks, Maryland) and incubated for 24 h at 35 °C. Eight isolated colonies were transferred to 5 mL of 1× phosphate buffered saline (PBS), pH 7.5 (Hardy Diagnostics, Santa Maria, California). The suspension was vortexed and 200 μL was spread plated onto five, 150-mm New Sporulation Medium (NSM) plates (Perdue et al., 2003) and incubated for 48 h at 35 °C. Five milliliters of cold (4 °C), sterile deionized (DI) water was pipetted onto each of the five NSM plates and the spores were scraped from the plate using a sterile, disposable spreader. The spore suspension was transferred to a centrifuge tube. Another 5 mL of DI water was pipetted onto each NSM plate, the surface was scraped, and the suspension was added to the centrifuge tube. The spore suspension was centrifuged at 12,300 xg for 10 min at 25 °C. The supernatant was discarded, and the spores were washed two more times with cold, sterile DI water, then resuspended in sterile DI water and stored at 4 °C for 24 h to allow for the lysis of any remaining vegetative cells. Purity of the spore stock was confirmed under a microscope and considered acceptable if spores visually made up greater than 90% of the total suspension. Spore stocks were stored in 1-mL aliquots at 4 °C for up to 3 months.
2.3. Chlorine inactivation of Bacillus spores
In order to evaluate the ability of the culture method and the RV-PCR or mRV-PCR method to detect low numbers of viable spores in the presence of chlorine-inactivated B. atrophaeus spores, a subset of samples was treated with chlorine. Various exposure times and chlorine concentrations were used to either inactivate all spores or a portion of the spores depending on the objective of the particular experiment.
Small volume disinfection experiments were performed using sterile chlorine demand-free (CDF) 0.05 M phosphate buffer. CDF buffer was prepared by adding 4.3 mL of a 1:20 dilution of 4–6% sodium hypochlorite solution to 5 L of 0.05 M Phosphate Buffer (34.0 g potassium phosphate monobasic into 5 L of Milli-Q water), pH adjusted to 7.0, allowed to stir at room temperature for at least 24 h, boiled for 10 min, and then exposed to ultraviolet light (254 nm) for at least 48 h. Once the chlorine concentration was less than 0.04 mg/L, the buffer was autoclaved and ready for use. CDF buffer was used to rinse glassware used in the disinfection experiments, prepare samples, dilute B. atrophaeus spores, and create quality-control blanks.
Water samples prior to spore addition were treated with chlorine by adding a 1:200 dilution of 4–6% sodium hypochlorite solution incrementally until the desired free chlorine concentration was achieved. Various concentrations of spores were added to the samples and at predetermined time points, subsamples were collected, and residual chlorine was neutralized with sodium thiosulfate at a concentration of 10 mg/L. Spores were then enumerated in the samples using the culture method and either the RV-PCR or the mRV-PCR method depending on the sample volume being analyzed.
2.4. Measurement of free chlorine concentrations
Free chlorine concentrations were measured in samples using a Pocket Colorimeter II instrument (Hach Company, Loveland, Colorado) and DPD (N, N,-diethylparaphenylenediamine) powder reagent packets. When mixed with DPD, free chlorine in the water forms a pink/red solution at a color intensity that is proportional to the chlorine concentration. Measurements were made using the instructions provided by the manufacturer (Hach Company, 2013). A blank and three secondary standards (Hach Company, Loveland, Colorado) were run prior to sample measurements. Standards were run and results were recorded each day that samples were analyzed.
2.5. Sample collection
Tap water samples ranging from 10 to 100 L were used for this study. Twenty-liter sterile cubitainers (collapsible containers) were used for the collection of water samples. Ten milliliters of sterile 10% sodium thiosulfate solution was added to each 20-L cubitainer the day of sampling to neutralize any residual chlorine in the water. Prior to collection of tap water samples, the inside and outside of the tap were swabbed with ethanol, flame-sterilized, then rinsed with sterile DI water. Sterile tubing was attached to the tap and water was allowed to run to waste for approximately 2 min before the sample was collected in the cubitainers. One 50-L tap water sample was amended with 13.4 g of river sediment mixed thoroughly to create a more turbid sample matrix. Samples were held in laboratory refrigerators at a temperature range of 1–4 °C for no longer than 24 h before processing.
2.6. Ultrafiltration
Water samples were concentrated by tangential flow ultrafiltration using a semi-automated waterborne pathogen concentration device (WPC) (Humrighouse et al., 2015). Single use, hollow-fiber Rexeed 25S hemodialyzers (Asahi Kasei, Tokyo, Japan) with a molecular cutoff of 30 kDa were used in this study.
Before filtering the sample, 1.5-L of a blocking solution was used to pretreat the filter and sample tubing by drawing it into the WPC and recirculating for 3 min prior to filtration of the water sample. The blocking solution consisted of 0.055% (v/v) Tween® 80 (Sigma-Aldrich, St. Louis, Missouri), 0.1% (w/v) sodium polyphosphate (Sigma-Aldrich), and 0.001% (v/v) Antifoam A (Sigma-Aldrich). The water sample was allowed to pass through the filter under conditions such that a proportion of the water sample is forced through the filter pores, while the remainder of the water and the suspended microorganisms are recirculated with the addition of new sample water. The recirculation helps to both reduce the fouling of the filtration media as well as keep microorganisms in suspension (Lindquist et al., 2007). After the water sample was filtered, 1 L of an elution solution, consisting of 0.001% Tween 80, was recirculated through the WPC to collect the organisms in a final retentate of approximately 400 mL. Sample retentates were stored at 4 °C until further processing.
2.7. Secondary concentration
The secondary concentration step was done to reduce the 400-mL sample retentate down to 28 mL. Each sample was seeded with B. atrophaeus spores ranging from 56 to 1100 colony forming units (cfu)/sample, mixed thoroughly, and then split into equal volumes for each secondary concentration technique. In addition to the membrane filtration technique (USEPA, 2012), two other techniques were evaluated (centrifugation and secondary ultrafiltration) as potential modifications to the procedure described in USEPA (2012). These techniques were evaluated for their ability to recover B. atrophaeus spores, the amount of time to perform the technique, and the ease of the technique.
2.7.1. Membrane filtration
The 400-mL sample retentate was concentrated by membrane filtration through a 0.45-μm mixed cellulose ester filter (Advantec MFS, Inc., Dublin, California; Cat. No. A045H047W). The filter funnel was rinsed with 10 mL of 0.05% Tween 20. The filter was added to a 50-mL centrifuge tube with 28 mL of 0.05% Tween 20 and vortexed for 2 min to remove the spores from the filter. More turbid samples required splitting the 400-mL sample retentate among three filters. Each filter was added, one at a time, to the elution solution, vortexed for 2 min, and then discarded. The final eluate was collected and stored at 4 °C until further analysis within 24 h.
2.7.2. Centrifugation
The 400-mL sample retentate was split into two 250-mL centrifuge tubes and centrifuged at 3500 xg for 15 min. The supernatant in each tube was aspirated down to 5 mL, then the pellet was resuspended and transferred to a single sterile 50-mL centrifuge tube. The 250-mL centrifuge tubes were each rinsed with 5 mL of 0.005% Tween 20 and transferred to the single 50-mL centrifuge tube. The volume of the concentrated sample was brought up to 28 mL with 0.05% Tween 20. The concentrated sample was stored at 4 °C until further analysis within 24 h.
2.7.3. Secondary ultrafiltration
A tangential flow ultrafiltration system was used as a secondary concentration step to reduce the 400-mL retentate down to a 28 mL concentrate (Fig. 2). Single use, Minimate TFF Capsules (Pall Corporation, Port Washington, New York; Cat. No. 33213) with a molecular cutoff of 30 kDa were used. The storage solution in the Minimate TFF capsule was flushed out with 100 mL of 0.2-μm filtered DI water. The storage solution and water were drained to a waste container through the filtrate port, while the retentate port was closed. A filter blocking solution, 100 mL of 0.1% sodium polyphosphate (NaPP), was circulated through the system with the filtrate port closed for 1 h, then flushed with 100 mL of 0.2-μm filtered DI water. During filtration of the 400-mL retentate, the filtrate port was open and running to waste. The retentate port was open and spores were recirculated through the system as the sample volume was reduced. The sample continued to filter until only the hold-up volume in the capsule and tubing remained. The filtrate flow rate was approximately 30–40 mL/min. The sample concentrate was eluted using 25 mL of 0.001% Tween 80. The elution solution was recirculated with the filtrate port closed for 15 min. The retentate port was then closed, the filtrate port was opened, and a sterile tube was attached. Eluting solution was then flushed through the filter, via the peristaltic pump, and the final concentrate was collected in a sterile collection container. The final sample concentrate volume ranged from 28 to 30 mL and was stored at 4 °C until further analysis within 24 h.
Fig. 2.
Diagram of the minimate TFF capsule secondary ultrafiltration.
2.8. Culture methods
Culture methods for B. atrophaeus were used for two purposes: (1) enumeration of spore stocks to determine seed concentrations using a spread-plating technique, and (2) enumeration of spores in sample concentrates following secondary concentration, using a membrane filtration technique as described below.
2.8.1. Spread plating
One milliliter of the refrigerated stock spore suspension was vortexed at maximum speed for 60 s and then heat treated at 80 °C for 10 min. The suspension was serially diluted, spread plated on tryptic soy agar (TSA) plates, and incubated for 24 h at 35 °C. B. atrophaeus colonies on TSA plates are characterized by the orange/peach/salmon color, roughness in texture, and diameters of 1–3 mm.
2.8.2. Membrane filtration
Sample concentrates were heat-treated at 80 °C for 10 min to remove any vegetative cells in the sample. Following heat treatment, 1-mL and 10-mL aliquots of sample were filtered through a 0.45-μm mixed cellulose ester filter (Advantec MFS, Inc., Dublin, California; Cat. No. A045H047W). The filter funnel was rinsed with sterile PBS and the filters were placed on a TSA plate using sterile, disposable forceps. Plates were inverted and incubated at 36 °C for 24 h. Orange/peach/salmon color colonies were counted as B. atrophaeus.
2.8.3. Quality controls
Blank samples, consisting of sterile PBS, were analyzed by the spread plating and membrane filtration techniques each time these techniques were done to ensure the supplies were free from contamination.
2.9. Rapid Viability-Polymerase Chain Reaction (RV-PCR) method
Fig. 3 shows the processing steps of the RV-PCR method (Kane et al., 2009; Létant et al., 2010; Létant et al., 2011), which begins with the filtering of a sample, followed by buffer washes, and then resuspension of the material captured on the filter in a nutrient rich medium. An aliquot of sample is taken before (T0) and after (T9) the samples are incubated for 9 h. DNA is extracted from both aliquots and analyzed by qPCR. The difference ia results from the two aliquots will indicate whether viable spores are present in the sample.
Fig. 3.
Diagram showing the steps of the RV-PCR method. (Ct, cycle threshold)
2.9.1. Initial processing
Following secondary concentration, a 13-mL aliquot of sample concentrate was vacuum filtered through a filter cup (Whatman Autocup, VWR International, Radnor, Pennsylvania, Cat. No. 1602–0465) in a vacuum manifold. The cup was rinsed with 20 mL of cold (4 °C) 1× wash buffer (25 mM KH2PO4, pH 7.4). The manifold with filter cups was removed from the vacuum, and 3.5 mL of cold (4 °C) TSB medium was added to each filter cup and capped. The manifold was vortexed for 10 min on a platform vortexer, then 1 mL of sample from each cup was transferred to a sterile 2-mL Eppendorf tube and stored in a cold block (T0 samples). The 1-mL sample aliquots were centrifuged at 14,000 rpm for 10 min at 4 °C, 800 μL of supernatant was removed from each tube and discarded. The remaining 200 μL was stored at − 20 °C until DNA extraction. The remaining liquid (approximately 2.5 mL) in the filter cup was incubated for 9 h on an orbital platform shaker at 37 °C. After incubation, the filter cup manifold was vortexed for 10 min on the platform vortexer and a 1-mL aliquot was removed from each cup and transferred to a sterile 2-mL Eppendorf tube in a cold block (T9 samples). T9 sample aliquots were centrifuged at 14,000 rpm for 10 min at 4 °C, 800 μL of supernatant was removed from each tube and discarded. The remaining 200 μL was stored at − 20 °C until DNA extraction.
2.10. DNA extraction
Two DNA extraction techniques were used in this study. The Promega MagneSil® Blood Genomic kit uses paramagnetic particles for binding nucleic acids in solution and then separating and purifying nucleic acids from the rest of the sample using a magnetic system. The Qiagen DNA Mini kit is a column-based system that utilizes a silica membrane for binding DNA in the presence of a high salt concentration and allows for the elution of DNA in a small volume of low-salt buffer.
2.10.1. Promega magnesil blood genomic kit
DNA was extracted from the T0 and T9 sample aliquots using the DNA extraction protocol from the Promega Magnesil Blood Genomic kit (Promega Corporation, Madison, Wisconsin; Cat. No. MD1360), as described in USEPA (2012). DNA from 200 μL of sample was extracted using the Promega kit, with the final extracted DNA eluted in a volume of 300 μL of elution buffer.
2.10.2. Qiagen DNA mini kit
DNA was extracted from the sample using a Qiagen DNA Mini Kit (Qiagen, Valencia, California; Cat. No. 51306). Twenty microliters of proteinase K was added to the 200 μL T0 and T9 sample aliquots and vortexed for 5 s. Two hundred microliters of Buffer AL was added to the sample and vortexed for 15 s. The sample was incubated at 56 °C for 10 min and then 200 μL of ethanol (96–100%) was added to the samples and vortexed for 15 s. The sample was transferred to a spin column and centrifuged for 1 min at 6000 xg. The spin column was transferred to a clean collection tube, 500 μL of Buffer AW1 was added, and the column was centrifuged for 1 min at 6000 xg. The spin column was transferred to a clean collection tube, 500 μL of Buffer AW2 was added, and the column was centrifuged for 3 min at 20,000 x g. The spin column was transferred to a clean collection tube and centrifuged for 1 min at 20,000 x g. The spin column was transferred to a sterile 1.5-mL microcentrifuge tube, 50 μL of Buffer AE was added to the center of the white filter membrane, and allowed to incubate at room temperature for 5 min, followed by centrifugation for 1 min at 6000 xg to collect the DNA extract. Another 50 μL of Buffer AE was added to the center of the white filter membrane and allowed to incubate at room temperature for 1 min, followed by centrifugation for 1 min at 6000 xg. The spin filter was discarded, and the DNA extract (100 μL final volume) was stored at 4 °C if qPCR was performed within 24 h or stored at − 20 °C if the time before qPCR analysis was greater than 24 h.
2.10.3. Quantitative PCR
Quantitative PCR reactions were carried out in optical 96-well reaction plates in an Applied Biosystems StepOnePlus System (Applied Biosystems, Foster City, California). The PCR targeted the recF gene and an amplicon length of 63 base pairs by use of the forward primer CGCGCCCGAGGACTTAA, reverse primer ATGTCAAGAAACCGCCGTC, and probe FAM-TCTCGTAAAGGGCAGCCCGCAAG-BHQ1. Each 25-μL reaction mixture contained 12.5 μL of TaqMan Fast Advanced 2× PCR MasterMix, 5 μL of DNA extract, 1.25 μL of 10 μM forward primer (Bg42F), 1.25 μL of 10 μM reverse primer (Bg104R), 2.5 μL of 1 μM probe (Bg60F/BHQ1), and 2.5 μL of molecular-grade water. Thermal cycling conditions were as follows: 2 min at 50 °C, 20 s at 95 °C, and 45 cycles of 5 s at 95 °C and 20 s at 60 °C.
2.10.4. Data interpretation
Samples were analyzed by qPCR in duplicate and average cycle threshold (Ct) values were used for comparison of the T0 and T9 sample aliquots. Non-detections by qPCR were assigned a Ct value of 45 in order to calculate the delta Ct, as 45 was the maximum number of cycles in the PCR. In samples where one of the duplicate Ct values was a non-detect and the other was a detection, the Ct value of the detection was used for the calculation of the delta Ct. The delta Ct was calculated by subtracting the Ct value for the T9 sample aliquot from the Ct value for the corresponding T0 sample aliquot (Fig. 3). A delta Ct value greater than or equal to 9, representing a three-log difference in DNA concentrations, indicated a positive detection for viable B. atrophaeus, as described in Kane et al. (2009); Létant et al. (2010); Létant et al. (2011); and USEPA (2011, 2012).
2.10.5. Quality controls
Each qPCR run included a high and low concentration positive control of previously extracted B. atrophaeus spores. Because the results of the RV-PCR method are either presence or absence, standard curves to calculate concentrations of spores were not needed. Extraction blanks (aliquots of DNA-free molecular-grade water) are processed through the entire extraction step then analyzed by qPCR with each batch of samples. Non-detections in the extraction blanks indicate that the extraction step is free from contamination for that particular run. A no template control (NTC) is a qPCR negative control that consists of DNA-free molecular-grade water analyzed with each run of qPCR. A non-detection in an NTC indicates that the qPCR step is free from contamination for that particular run. All quality-control samples were analyzed by qPCR in duplicate.
2.11. Statistical analysis
Statistical analysis to test for significant differences in recovery of B. atrophaeus using three different secondary concentration techniques was performed using the Kruskal-Wallis test (Kruskal and Wallis, 1952). The Kruskal-Wallis test is a nonparametric test that is used to determine whether there is a statistically significant difference between the medians of three or more independent groups. The Shapiro-Wilk goodness-of-fit test (Shapiro and Wilk, 1965) was used to test for normally distributed data. Spearman’s rank correlation coefficient was used to determine whether the relations between delta Ct values and membrane-filtration concentrations versus chlorine concentrations in samples were statistically significant. Spearman’s rho is a nonparametric measure of rank correlation between two variables. An alpha value of 0.05 was used for tests of significance.
3. Results and discussion
A series of experiments were done to modify steps in the RV-PCR method for the detection of viable B. atrophaeus spores in water samples and compare the results of the mRV-PCR method to the membrane-filtration culture method. Results presented first in this paper cover the secondary concentration and DNA extraction modifications. Modifications were evaluated for their ability to recover B. atrophaeus spores, along with the analytical time to perform the technique and the ease of the technique. These steps were evaluated early in the study so that modifications could be incorporated into later experiments.
3.1. Secondary concentration technique comparison
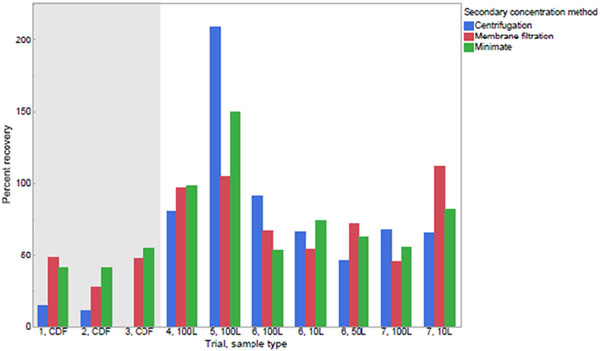
Three secondary concentration techniques were tested in this study using the culture method in order to compare the number of spores recovered, the analytical time of the technique, and ease of use. The samples and volumes used for this comparison were either 400 mL of CDF buffer (trials 1–3) or 400 mL of concentrated tap water samples (trials 4–7) with starting volumes ranging from 10 to 100 L. Each sample was seeded with B. atrophaeus spores ranging from 230 to 3100 cfu/sample, mixed thoroughly, and then split three ways for each secondary concentration technique. The percent recovery of spores per secondary concentration technique in each of the seeded samples is shown in Fig. 4. All buffers used during the comparison were membrane filtered and plated on TSA to check for contamination. Results for all buffer blank quality-control samples showed no contamination.
Fig. 4.
Comparison of percent recoveries for three secondary concentration methods in 7 trials. The chlorine-demand-free (CDF) buffer in trials 1 through 3 was seeded with a concentration of B. atrophaeus spores as follows: 900, 1000, and 540 cfu/sample, respectively. Concentrated tap water samples in trials 4 through 7 were seeded with concentrations of B. atrophaeus spores as follows (from left to right): 690, 230, 2100, 2200, 2100, 2600, and 3100 cfu/sample, respectively.
Based on a Kruskal-Wallis test, the percent recovery for the three secondary concentration techniques were not significantly different at α = 0.05; therefore, other factors were considered for determining which technique to use in the rest of the study. The centrifugation technique was the easiest technique to perform, and the amount of time to perform the technique did not change regardless of the sample matrix. Recoveries of spores in CDF buffer were low using the centrifugation technique; however, recoveries increased in concentrated tap water likely because the particulates in the sample assisted with capturing spores in the pellet. The phenomenon of higher recoveries in more turbid samples was also seen in similar studies (Rhodes et al., 2016; Gallardo et al., 2019).
For the membrane filtration technique, only 1 filter was needed for the CDF buffer samples; however, 3–5 filters were needed in order to filter concentrated tap water samples. The process of eluting spores from each filter and then combining the elution solutions was cumbersome. Filtration was slow for the more turbid samples (approximately 60 min per sample) and overall was slower than the centrifugation technique but faster than the Minimate technique.
The Minimate technique took the longest amount of time at approximately 45 min for CDF buffer and 90 min for concentrated tap water samples. This technique was easy to perform once it was set up; however, it was the lengthiest and the most expensive of the three techniques. Given the above challenges with the membrane filtration and Minimate techniques, the centrifugation technique was used for the rest of the study. Centrifugation as the secondary concentration step is one of the modifications to the RV-PCR method.
3.2. DNA extraction comparison
The DNA extraction step is the part of the RV-PCR method in which the DNA is separated from other material in the sample and purified before qPCR analysis. Two DNA extraction techniques were evaluated in the beginning of the study. In preliminary experiments (data not shown), 8 CDF buffer samples were seeded with B. atrophaeus spores at concentrations ranging from 1 to 8500 spores per sample. The samples were processed according to the initial processing steps (Section 2.9.1) of the RV-PCR method. The T0 and T9 sample aliquots were split equally and processed by the Promega and Qiagen extraction kits. Because the DNA extraction techniques resulted in different final DNA elution volumes, a direct comparison of Ct values was not done. Instead, the delta Ct for each split sample was calculated and compared. Other factors such as blank sample results and timing of the techniques were considered. In all 8 samples, the delta Ct results from the two DNA extraction techniques were in agreement for the presence or absence of viable B. atrophaeus spores. Along with the sample analyses, a total of 10 extraction blanks were analyzed using the Qiagen kit and 16 extraction blanks were analyzed using the Promega kit. The extraction blanks for Qiagen extractions resulted in Ct values that were non-detections. The extraction blanks for the Promega extractions resulted in detections in 13 of the blanks analyzed, with an average Ct value of 37.14 ± 0.83. The results of the six NTCs run alongside the extraction blanks were also non-detections.
The Promega extraction protocol included a total of 44 steps, including 2 salt washes, 4 alcohol washes, and a step in which the tubes were opened and heated in a heat block until the paramagnetic particles dried (approximately 20 min). The open tubes and the number of steps in the Promega extraction could have been the reason for the detections in the extraction blanks. The Qiagen extraction protocol included 16 steps and was able to be completed in a substantially less amount of time. Additionally, the tubes in the Qiagen extraction were only opened one at a time. Because the delta Ct results from the Qiagen kit agreed with the Promega kit in preliminary experiments, and because the Qiagen took less time and was easier to perform, it was used for the rest of the study. Using the Qiagen kit for the DNA extraction step is another modification to the RV-PCR method.
3.3. Performance of the RV-PCR and culture methods in detecting viable Bacillus spores in the presence of chlorine-inactivated Bacillus spores
A total of 15 samples, consisting of 100-mL CDF buffer seeded with 1.2 × 106 spores, were subjected to varying concentrations of chlorine for 90 min to inactivate a percentage of seeded spores. The chlorine was then neutralized with sodium thiosulfate before analyzing by RV-PCR and culture methods. The agreement of RV-PCR and culture method results are shown in Table 1. Because the concentration of viable spores decreased over the 90 min, a range of volumes was analyzed by the culture method and results are presented as cfu/mL. In 10 out of the 15 samples analyzed, the culture method and RV-PCR method results were in agreement. Of the five samples with results that did not agree, four of the samples had low concentrations of spores (0.05 to 1 cfu/mL as determined by the culture method). Such discrepancies are expected at spore concentrations that low because of spore clumping and difficulties splitting samples equally. Additionally, previous studies reported the detection limit of the RV-PCR method as 10 spores/sample (Kane et al., 2009; USEPA, 2011; Kane et al., 2013; Létant et al., 2011). At concentrations greater than 10 (cfu/mL) spores as measured by the culture method, the results for all but one sample were in agreement. The sample with a free chlorine concentration of 1.16 mg/L did not meet the delta Ct criteria for a positive result by the RV-PCR method but showed a positive qPCR response in the T9 sample with an average Ct value of 28.1. As this experiment was run at the beginning of the study, the Promega DNA extraction kit was used. The average Ct value of the DNA extraction blanks associated with the T0 sample aliquots was 37.66, and the average Ct value of the extraction blanks associated with the T9 sample aliquots was 36.54. The NTCs resulted in non-detections implying that contamination occurred in the DNA extraction step using the Promega kit, which may have affected the interpretation of RV-PCR results and therefore, the agreement of results between the two methods.
Table 1.
RV-PCR and culture method results for CDF-buffer samples seeded with B. atrophaeus spores and exposed to various concentrations of chlorine.
| Free chlorine concentration (mg/L) | RV-PCR method |
Culture method |
Results agree? | |
|---|---|---|---|---|
| Delta Ct | Present/Absent | Membrane filtration (cfu/mL) | ||
| 4.60 | 4.36 | Absent | 1 | No |
| 3.80 | 10.41 | Present | <0.05 | No |
| 3.76 | −0.36 | Absent | <0.05 | Yes |
| 2.33 | −0.50 | Absent | <0.05 | Yes |
| 1.79 | 10.82 | Present | 0.05 | Yes |
| 1.73 | 6.47 | Absent | 1 | No |
| 1.72 | 3.57 | Absent | 1 | No |
| 1.54 | 11.87 | Present | 7 | Yes |
| 1.16 | 7.11 | Absent | 85 | No |
| 1.14 | 23.01 | Present | 840 | Yes |
| 0.75 | 17.06 | Present | 7300 | Yes |
| 0.59 | 17.34 | Present | 4600 | Yes |
| 0.04 | 13.58 | Present | 4800 | Yes |
| 0.00 | 11.94 | Present | 8500 | Yes |
| 0.00 | 15.41 | Present | 12,000 | Yes |
Acronyms: mg/L, milligrams per liter; Ct, cycle threshold; cfu/mL, colony forming units per milliliter; <, less than.
Fig. 5 shows RV-PCR delta Ct values (A) or membrane filtration results (B) versus chlorine concentrations. Because the data for membrane filtration versus chlorine concentrations were not normally distributed according to the Shapiro-Wilk goodness-of-fit test (Shapiro and Wilk, 1965), nonparametric Spearman’s correlations were run. The relation between the delta Ct values and chlorine concentrations represented in Fig. 5A was statistically significant (rho = − 0.74; p = 0.0015). The relation between log membrane filtration concentrations and chlorine concentrations represented in Fig. 5B was also statistically significant (rho = − 0.93; p < 0.0001).
Fig. 5.
RV-PCR delta Ct values (A) and membrane filtration results (B) versus chlorine concentrations in CDF-buffer samples seeded with B. atrophaeus spores. The line in plot B indicates the detection limit of the membrane filtration method (0.05 spore per milliliter).
3.4. Performance of the mRV-PCR and culture methods in detecting viable Bacillus spores in the presence of chlorine-inactivated Bacillus spores in a large volume sample
An experiment was done to compare the results of the culture and mRV-PCR methods in a sample seeded with B. atrophaeus spores and exposed to chlorine for varying amounts of time. The intention of the experiment was not to evaluate the efficacy of chlorine as a disinfectant, but rather it was to evaluate spores as varying levels of exposure. Table 2 lists the results from a 100-L tap water sample that was concentrated by ultrafiltration and then further concentrated by centrifugation. Chlorine was added to the sample concentrate at a concentration of 3.92 mg/L, and then the 200-mL sample was seeded with 370 B. atrophaeus spores. At specified time intervals, 10-mL subsamples were removed, neutralized with sodium thiosulfate, then 5 mL were analyzed by mRV-PCR and 5 mL were analyzed by membrane filtration. By the 30-min time interval, there were no viable spores detected by either method. In all 8 subsamples, there was 100% agreement between the results of the mRV-PCR and culture methods: three subsamples had viable spores and five subsamples had no viable spores.
Table 2.
mRV-PCR and culture method results for a concentrated tap water sample seeded with B. atrophaeus spores and exposed to chlorine for varying contact times.
| Chlorine contact time (min) | mRV-PCR method |
Culture method |
Results agree? | |
|---|---|---|---|---|
| Delta Ct | Present/Absent | Membrane filtration (cfu/5 mL) | ||
| 0 | 19.92 | Present | 39 | Yes |
| 2 | 22.54 | Present | 23 | Yes |
| 15 | 21.03 | Present | 8 | Yes |
| 30 | 0.00 | Absent | <1 | Yes |
| 45 | 0.00 | Absent | <1 | Yes |
| 60 | 0.00 | Absent | <1 | Yes |
| 75 | 0.00 | Absent | <1 | Yes |
| 90 | 7.18 | Absent | <1 | Yes |
Acronyms: min, minutes; Ct, cycle threshold; cfu/5 mL, colony forming units per 5 mL; <, less than.
3.5. Comparison of mRV-PCR and culture methods in non-chlorine treated samples
A total of 26 large-volume tap water samples were concentrated first by ultrafiltration and then by centrifugation. In some of these experiments, the 50-L and 100-L samples were seeded with B. atrophaeus spores (referred to as “sample” in Fig. 1 and Table 3). For other experiments, the seeding of spores occurred after concentration by ultrafiltration (referred to as “retentate”), and others, seeding of spores occurred after concentration by ultrafiltration and centrifugation (referred to as “concentrate”). Samples were seeded at different steps in the overall process to determine whether specific steps might have an effect on the agreement of the methods at lower seed concentrations. All samples were then analyzed by mRV-PCR and culture methods. Two of the 26 samples consisted of 100-L of sample volume that were not seeded with B. atrophaeus spores and served as method blanks. The results for these two samples were negative for both the mRV-PCR and culture methods. Six samples had expected spore concentrations of less than 10 per volume analyzed. Of these six samples, three of them did not show agreement between the two methods (two samples were positive by mRV-PCR and negative by the culture method, and one sample was negative by mRV-PCR and positive by the culture method). A total of 18 large volume samples were seeded with spore concentrations of 10 or greater. All 18 samples showed positive results by both methods. One of the 18 samples was amended with river sediment to represent a turbid sample with potential inhibitors. Although this sample showed positive agreement between the methods, the culture method was difficult to read because of the buildup of sediment on the membrane filter. A lawn of the typical orange/peach/salmon color was present underneath the sediment and was therefore considered positive with a result of >1 cfu/13 mL. Ultimately, the RV-PCR method is to be applied to the detection of B. anthracis, of which the colonies do not have a typical or distinct color. Because of this, the culture method would not be able to confirm the presence of the organism unless a PCR was done, further delaying the reporting of results.
Table 3.
mRV-PCR and culture method results for tap water samples seeded with B. atrophaeus spores.
| Sample volume (L) | Step where spores were seeded1 | Number of spores expected in each analysis (13 mL)2 | mRV-PCR method |
Culture method |
Results agree? | |
|---|---|---|---|---|---|---|
| Delta Ct | Present/Absent | Membrane filtration (cfu/13 mL) | ||||
| 100 | Concentrate | 55 | 14.77 | Present | 46 | Yes |
| 100 | Concentrate | 29 | 16.19 | Present | 23 | Yes |
| 100 | Concentrate | 14 | 17.31 | Present | 6 | Yes |
| 100 | Concentrate | 6 | 15.97 | Present | 3 | Yes |
| 100 | Concentrate | 3 | 14.63 | Present | <3 | No |
| 100 | Concentrate | NS | 0.00 | Absent | <3 | Yes |
| 50 + sediment3 | Concentrate | 25 | 15.22 | Present | >1 | Yes |
| 10 | Retentate | 3700 | 22.55 | Present | 2600 | Yes |
| 10 | Retentate | 380 | 17.36 | Present | 350 | Yes |
| 10 | Retentate | 25 | 25.47 | Present | 28 | Yes |
| 10 | Retentate | 37 | 22.87 | Present | 29 | Yes |
| 10 | Retentate | 6 | 24.82 | Present | 2 | Yes |
| 10 | Retentate | <6 | 18.25 | Present | <1 | No |
| 100 | Retentate | 3800 | 28.77 | Present | 3100 | Yes |
| 100 | Retentate | 470 | 16.36 | Present | 340 | Yes |
| 100 | Retentate | 18 | 14.44 | Present | 51 | Yes |
| 100 | Retentate | 31 | 22.29 | Present | 50 | Yes |
| 100 | Retentate | 18 | 15.81 | Present | 5 | Yes |
| 100 | Retentate | <6 | 0.00 | Absent | 1 | No |
| 100 | Sample | 600 | 27.60 | Present | 200 | Yes |
| 100 | Sample | 650 | 28.91 | Present | 390 | Yes |
| 100 | Sample | 350 | 24.98 | Present | 230 | Yes |
| 100 | Sample | NS | 0.00 | Absent | <1 | Yes |
| 100 | Sample | 8 | 10.83 | Present | 19 | Yes |
| 100 | Sample | 120 | 13.81 | Present | 33 | Yes |
| 100 | Sample | 1200 | 12.37 | Present | 270 | Yes |
Acronyms: mg/L, millligrams per liter; Ct, cycle threshold; cfu/mL, colony forming units per milliliter; <, less than; NS, not seeded.
Concentrate, spores were seeded after concentration by ultrafiltration and centrifugation; Retentate, spores were seeded after concentration by ultrafiltration and before centrifugation; Sample, spores were seeded before concentration by ultrafiltration and centrifugation.
The seeded, concentrated sample was split equally between the mRV-PCR and culture method. This value represents the expected number of spores to be present for each analysis, based on the concentration of spores seeded into the sample.
A 50-L tap water sample amended with 13.4 g of river sediment.
Fig. 6 shows the results of the average Ct values for the T0 and T9 samples versus the number of spores by the culture method. The Ct values for the T0 samples are higher because they have not been incubated and the spores have not had a chance to replicate. Many of the T0 sample results are non-detections and the Ct values are set at 45; therefore, the relation between T0 samples or delta Ct values and membrane filtration results was not statistically significant. After the 9-h incubation, the Ct values decrease if viable spores are present, as seen in the T9 samples. The relation between the Ct value of the T9 samples and membrane filtration results with equal number of spores was statistically significant (rho = − 0.7927; p < 0.0001).
Fig. 6.
Ct values for T0 and T9 samples versus membrane filtration results in samples with equal numbers of B. atrophaeus spores. Non-detections by qPCR were assigned Ct values of 45. Non-detections by membrane filtration were assigned concentrations of 0.5.
4. Summary and conclusions
In this study, preliminary experiments were done to evaluate two modifications (secondary concentration and DNA extraction) to the process of analyzing large volume water samples for viable Bacillus spores by the mRV-PCR method. For the secondary concentration comparison, results from the centrifugation technique were not statistically different from either the membrane filtration or secondary ultrafiltration (Minimate) techniques; however, centrifugation was much easier to perform and took less time, as the centrifugation step was completed in 15 min regardless of the sample matrix, making it the preferred technique for this study. For the DNA extraction step, the Qiagen extraction technique was selected for use in this study because it took considerably less time and was easier to perform than the Promega extraction technique. Extraction blanks processed with the Qiagen kit resulted in non-detects by qPCR, whereas, extraction blanks processed with the Promega kit resulted in low-level detections by qPCR in 13 out of 16 blanks. Previous studies reported that the overall time to obtain results using the RV-PCR method was approximately 15 h (Létant et al., 2011; USEPA, 2012); however, the incorporation of these two modifications and the use of the semi-automated ultrafiltration water pathogen concentrator in this study resulted in a reduction of the overall time by approximately 2 h in comparison to the previously published RV-PCR method.
This study demonstrated that the mRV-PCR method can be used to detect viable Bacillus spores in large volume water samples, including those with turbid matrices and those that have been treated with chlorine. Overall, the results of the mRV-PCR method agreed with the membrane-filtration culture method in 96% of samples when the seed concentration was greater than 10 spores per sample volume analyzed. Results for sample comparisons with seed concentrations lower than 10 spores were less consistent likely because of spore clumping, pipetting variations, and heterogeneities in splitting samples. In order to evaluate the correlation between the two methods, the average Ct value of the post-incubation mRV-PCR samples (T9) was compared to the culture method concentrations and showed a statistically significant correlation. These results are consistent with previous studies that also showed a high percentage of agreement between the methods, and a detection limit of 10 spores/sample for the RV-PCR method (Kane et al., 2009; Létant et al., 2011; USEPA, 2011; Kane et al., 2013). With a total processing time of approximately 13 h to detect the presence or absence of viable Bacillus spores in large volumes of water, the mRV-PCR method allows for more timely results than the RV-PCR method and the traditional culture method which is critical during the cleanup phase of the response to a biological weapon release. Further work is needed to evaluate the mRV-PCR method in large volume water samples that include the quantity and types of inhibitors that might be encountered during the decontamination and remediation of affected areas and buildings.
Acknowledgements
The U.S. Environmental Protection Agency (USEPA) through its Office of Research and Development managed and funded the research described herein under an Interagency Agreement with the U.S. Geological Survey (DW-14-95807410-0). It has been subjected to the Agency’s review and has been approved for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. This article has been peer reviewed and approved for publication consistent with U.S. Geological Survey Fundamental Science Practices (https://pubs.usgs.gov/circ/1367/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply an endorsement by the U.S. Government or USEPA. The USEPA does not endorse any commercial products, services, or enterprises.
Special thanks to Staci Kane and Gloria Murphy from the Lawrence Livermore National Laboratory in Livermore, California for providing hand-on training to the U.S. Geological Survey on the RV-PCR method. Thanks are also extended to Sanjiv Shah and Eugene Rice, U.S. Environmental Protection Agency, for guidance throughout the project.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data collected for this study are available at https://doi.org/10.23719/1519576.
References
- Francy DS, Bushon RN, Brady AMG, Bertke EE, Kephart CM, et al. , 2009. Comparison of traditional and molecular analytical methods for detecting biological agents in raw and drinking water following ultrafiltration. J. Appl. Microbiol. 107, 1479–1491. [DOI] [PubMed] [Google Scholar]
- Gallardo VJ, Morris BJ, Rhodes ER, 2019. The use of hollow fiber dialysis filters operated in axial flow mode for recovery of microorganisms in large volume water samples with high loadings of particulate matter. J. Microbiol. Methods 160, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen M, 2008. The role of sampling in the phases of a biological event: fact and fiction in an airport scenario. In: Emanuel P, Roos JW, Niyogi K (Eds.), Sampling for Biological Agents in the Environment. ASM Press, Washington DC, pp. 73–94. [Google Scholar]
- Hach Company, 2013. Pocket colorimeter II analysis systems instruction manual chlorine (Cl2). Hach Company No. 59570–88. USA. [Google Scholar]
- Humrighouse B, Pemberton A, Gallardo V, Lindquist HAD, 2015. A method detection limit for Bacillus anthracis spores in water using an automated waterborne pathogen concentrator. J. AOAC Int. 98, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Kane SR, Létant SE, Murphy GA, Alfaro TM, Krauter PW, et al. , 2009. Rapid, high-throughput, culture-based PCR methods to analyze samples for viable spores of Bacillus anthracis and its surrogates. J. Microbiol. Methods 76, 278–284. [DOI] [PubMed] [Google Scholar]
- Kane S, Shah S, Létant S, Murphy G, Alfaro T, et al. , 2013. Operational evaluation of the rapid viability PCR method for post-decontamination clearance sampling. J. Bioterr. Biodef S3, 016. [Google Scholar]
- Kruskal WH, Wallis WA, 1952. Use of ranks in one criterion variance analysis. J. Am. Stat. Assoc. 47, 583–621. [Google Scholar]
- Létant SE, Kane SR, Murphy GA, Alfaro TM, Hodges LR, et al. , 2010. Most-probable-number rapid viability PCR method to detect viable spores of Bacillus anthracis in swab samples. J. Microbiol. Methods 81, 200–202. [DOI] [PubMed] [Google Scholar]
- Létant SE, Murphy GA, Alfaro TM, Avila JR, Kane SR, et al. , 2011. Rapid-viability PCR method for detection of live, virulent Bacillus anthracis in environmental samples. Appl. Environ. Microbiol. 77, 6570–6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist HAD, Harris S, Lucas S, Hartzel M, Riner D, et al. , 2007. Using ultrafiltration to concentrate and detect Bacillus anthracis, Bacillus atrophaeus subspecies globigii, and Cryptosporidium parvum in 100-liter water samples. J. Microbiol. Methods 70, 484–492. [DOI] [PubMed] [Google Scholar]
- Perdue ML, Karns J, Higgins J, Van Kessel JA, 2003. Detection and fate of Bacillus anthracis (Sterne) vegetative cells and spores added to bulk tank milk. J. Food Prot. 66, 2349–2354. [DOI] [PubMed] [Google Scholar]
- Rhodes ER, Huff EM, Hamilton DW, Jones JL, 2016. The evaluation of hollowfiber ultrafiltration and celite concentration of enteroviruses, adenoviruses and bacteriophage from different water matrices. J. Microbiol. Methods 228, 31–38. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB, 1965. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611. [Google Scholar]
- USEPA, 2011. Development and Verification of Rapid Viability Polymerase Chain Reaction (RV-PCR) Protocols for Bacillus anthracis - For Application to Air Filters, Water and Surface Samples. U.S. Environmental Protection Agency, Washington DC, USA. EPA/600/R-10/156. [Google Scholar]
- USEPA, 2012. Protocol for detection of Bacillus anthracis in environmental samples during the remediation phase of an anthrax event. U.S. Environmental Protection Agency, Washington DC, USA. EPA/600/R-12/577. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collected for this study are available at https://doi.org/10.23719/1519576.