Abstract

Cycloaddition reactions are among the most practical strategies to assemble cyclic products; however, they usually require the presence of reactive functional groups in the reactants. Here, we report a palladium-catalyzed formal (4 + 2) cycloaddition that involves the activation of C(sp3)–H bonds and provides a direct, unconventional entry to tetrahydroquinoline skeletons. The reaction utilizes amidotolyl precursors and allenes as annulation partners, and is catalyzed by Pd(II) precursors in combination with specific N-acetylated amino acid ligands. The reactivity can be extended to ortho-methyl benzylamides, which provide for the assembly of appealing tetrahydro-2-benzazepines in a formal (5 + 2) annulation process.
Azaheterocycles form the scaffold of many drugs, agrochemicals, dyes, and fragrances and can be found in many natural products. Therefore, the assembly of these skeletons in a sustainable and atom economical fashion remains a primary goal in modern organic synthesis. In this context, one of the more appealing synthetic strategies to build these type of rings consists of the use of metal-catalyzed cycloadditions involving the direct activation of C–H bonds.1,2 This is exemplified by the synthesis of indoles from anilides through a formal (3 + 2) oxidative cycloaddition (Scheme 1A).3 The reaction involves an initial C(sp2)–H activation to form metallacycle A, followed by migratory insertion of the unsaturated partner and reductive elimination (Scheme 1A). One could envision a similar annulation to build tetrahydroquinolines (THQs) instead of indoles, which is a central scaffold in many bioactive alkaloids; however this would require the use of 3-carbon cycloaddition partners, which are not obvious to identify (Scheme 1B, left arrow).4 An alternative, more attractive disconnection for THQ skeletons could be based on a (4 + 2) instead a (3 + 3) disconnection, like that shown in Scheme 1B (right arrow), as this would entail the use of common 2-carbon unsaturated partners. Moreover, as 4-atom components, ortho-methylanilines are very appealing because of their availability.
Scheme 1. Metal-Catalyzed Annulations To Give Azaheterocycles.
However, synthetic reactions that fulfill this retrosynthetic analysis, enabling a formal (4 + 2) cycloaddition between ortho methylanilides and unsaturated partners, are unknown. Performing this transformation using transition metal catalysis is challenging, not only because of the well-known difficulties associated with the activation of sp3 C–H bonds5 but also because the subsequent steps (migratory insertion into the C(sp3)–metal bond and reductive elimination) are also more problematic than in the case of substrates with sp2 reacting carbons. Indeed, while a vast array of different types of annulations (especially formal cycloadditions) involving the activation of aromatic C(sp2)–H bonds have been described, mechanistically related processes based on the activation of sp3 C–H bonds are very scarce.6
Herein, we report the first examples of transition metal formal (4 + 2) annulations involving ortho-methylanilides, using allenes as two-carbon partners (Scheme 1C). Importantly, we also demonstrate that the reaction, which is catalyzed by Pd(II) species, can be extended to benzylamides, providing for the direct assembly of azepines in a formal (5 + 2) cycloaddition approach.
As previously established,7 the presence of strong electron-withdrawing groups at the nitrogen is key for successful C–H functionalization reactions in amino aromatic substrates. Therefore, we started our investigation by examining the reactivity of 1,1,1-trifluoro-N-(o-tolyl)methanesulfonamide (1a, Table 1). As partners we paid attention to allenes, owing to their successful performance in previous cycloadditions involving the activation of C(sp2)–H bonds.8
Table 1. Selected Optimization Resultsa.
| Entry | R | Solvent | Temp | Ligand L | Yieldb |
|---|---|---|---|---|---|
| 1 | Tf (1a) | Toluene | 105 °C | – | <5% |
| 2 | Tf | Toluene | 105 °C | Boc-Val-OH | 25% |
| 3 | Tf | Toluene | 105 °C | Ac-Gly-OH | 42% |
| 4 | Tf | Toluene | 105 °C | Ac-Ala-OH | 55% |
| 5 | Tf | Toluene | 105 °C | Ac-Leu-OH | 55% |
| 6 | Tf | Toluene | 105 °C | Formyl-Val-OH | 37% |
| 7 | Tf | Toluene | 105 °C | Pro-Val-OH | 52% |
| 8 | Tf | Toluene | 105 °C | Ac-Val-OH | 60% |
| 9 | Ms (1a′) | Toluene | 105 °C | Ac-Val-OH | 39% |
| 10 | Ns (1a″) | Toluene | 105 °C | Ac-Val-OH | 33% |
| 11 | Tf | p-Xylene | 105 °C | Ac-Val-OH | 49% |
| 12c | Tf | THF | 105 °C | Ac-Val-OH | 53% |
| 13 | Tf | 2-Me THF | 85 °C | Ac-Val-OH | 54% |
| 14d | Tf | 2-Me THF | 85 °C | Ac-Val-OH | 61% |
| 15d,e | Tf | 2-Me THF | 85 °C | Ac-Val-OH | 56% |
| 16d,f | Tf | 2-Me THF | 85 °C | Ac-Val-OH | 71% |
Conditions: 0.333 mmol of 1a, 0.167 mmol of allene 2a, 2 mL of solvent, under air, 2 equiv of Cu(OAc)2·H2O, 1.5 equiv of Cs2CO3, 16 h.
Yields calculated based on 2a. Calculated by using an internal standard (entries 1–11). Isolated yields (entries 12–16).
Reaction performed in sealed tube.
1 equiv of Cu(OAc)2·H2O and 1 equiv of Cs2CO3.
0.167 mmol of 1a, 0.167 mmol of allene 2a.
Slow addition over 1 h of 0.167 mmol of allene 2a in 1.5 mL of 2-Me THF to the reaction.
Using commercially available allene 5-vinylidenenonane (2a), we observed no reaction in the presence of 10 mol % of palladium acetate, and copper acetate as oxidant (in toluene at 105 °C). In line with previous reports on the role of monoprotected amino acids accelerating the rate of Pd-mediated C–H activations,9 we found that using 40 mol % of Boc-protected valine as ligand promotes the formation of the desired tetrahydroquinoline product 3aa in a promising 25% yield (based on allene), as a single regioisomer (entries 1 and 2). We tested other amino acid ligands, bearing different amino-protecting groups, discovering that N-acetyl-L-valine (Ac-Val-OH) produces the best results (3aa formed in 60% yield, entry 8). Other oxidants, including benzoquinone or silver carbonate, are clearly inferior to copper acetate (26% and 20% yield respectively). It is possible to use substrates with other electron-withdrawing groups at the nitrogen than triflyl, such as mesyl and nosyl, albeit the reactions are less efficient (entries 9–10). Interestingly, the reaction also works using the environmentally friendly solvent methyl-THF (54% yield), which even allowed it to proceed at lower temperature (85 °C). Decreasing the amount of Cu(OAc)2 and Cs2CO3 to 1 equiv resulted in the product being obtained in 61% yield (entry 14), while with a lesser amount of palladium salt, conversions were not complete. Finally, we found that a slow addition of allene over a 4 h period led to an increase in yield up to 71% (entry 16).
With the optimized conditions in hand, we investigated the scope of the reaction using different types of allene partners (Scheme 2). Similar to 2a, the 1,1-disubstituted allene vinylidenecyclohexane (2b) worked in good yield (61%). Symmetrical 1,3-disubstituted allenes such nona-4,5-diene (2c) also led to the quinoline product 3ac in 61% yield. Gratifyingly nonsymmetrical 1,3-allenes 2d and 2e led to the expected products, with excellent regio- and diastereoselectivities and an up to 76% yield. Furthermore, while ethyl 2,3-butadienoate did not work, probably because of the presence of an electron-withdrawing group, electron-rich monosubstituted allenes like cyclohexylallene (2f) or the aryl-substituted derivative 2g produced the cycloadducts 3af and 3ag as mixtures of E/Z isomers. Remarkably, trisubstituted allenes are also valid cycloaddition partners, and therefore products 3ah, 3ai, and 3aj were obtained (42–73% yields).10
Scheme 2. Scope of the Formal (4 + 2) Cycloaddition of ortho-Anilides and Allenes.

Conditions: 0.333 mmol of 1, 0.167 mmol of allene 2, 2 mL of Me-THF, under air, 16 h. Regioisomeric ratios >20:1 and E/Z ratios >20:1, unless otherwise stated.
Yield after a gram-scale experiment.
The use of allenes as reaction partners is key for the success of the annulation. Alkynes, like diphenylacetylene, were essentially unreactive, while alkenes, such as ethyl acrylate, failed to give the cycloadducts, providing just traces of products resulting from addition/β-hydride elimination processes (olefination).11 This success with allenes is likely associated with several factors: (1) they are not as coordinating as alkynes, and thereby avoid the saturation of the metal coordination sphere to give nonactive complexes; (2) they favor the migratory insertion step owing to the formation of π-allyl intermediates; (3) they also facilitate the reductive elimination step because of the presence of an extra coordinating handle (double bond).12
We then explored the scope regarding the ortho-methyl anilides, by testing substrates 1b–1n, most of which were prepared by triflation of commercially available substrates. Precursors 1b and 1c with substituents ortho to the methyl group gave the corresponding products 3ba and 3ca in 69% and 50% yield, respectively. Substrates equipped with substituents meta to the methyl group such as phenyl or methoxy, or even with halogens (chloro, bromide), also led to moderate yields (3da–3ga), exhibiting better performance for the electron-rich substrates. The reaction is also compatible with substituents para to the methyl group (chloro, methyl ester, methoxy and phenyl), to give the expected products (3ha–3ka, 50–69% yield). Aryl-disubstituted substrates such 1l and 1m, as well as naphthyl anilide 1n, also led to effective reactions (3la–3na, 52–67% yield). Finally, as expected, the reaction is general for other allenes, as demonstrated with substrate 1f and product 3fh.
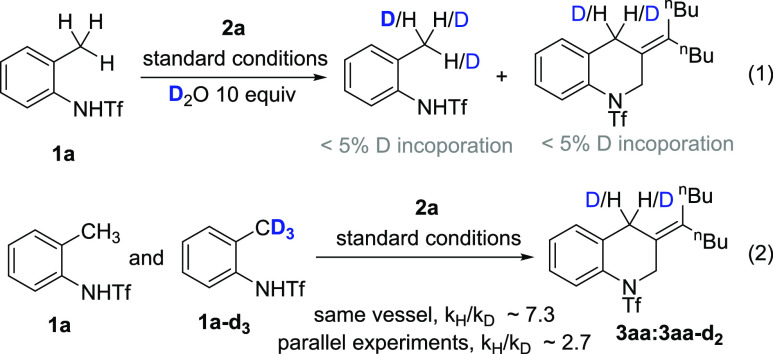
Running the reaction of 1a in the presence of D2O or Ac(d3)-OD under standard conditions revealed no deuterium incorporation in neither the starting material nor the product, which suggests the C–H activation step is irreversible (eq 1). We also measured the primary kinetic isotopic effect carrying out a competition between 1a and the deuterated analogue 1a-d3. When the competition experiments were carried out in the same vessel, we obtained a kH/kD ≈ 7.3. Using parallel experiments, the resulting value was 2.7. From both experiments we can conclude that the C–H bond cleavage is the turnover limiting step (eq 2).13
 |
1 |
At this stage, we wondered whether it would be possible to use ortho-methyl benzylamides instead of anilides as annulation precursors. In these substrates the amide directing group is further apart from the methyl substituent, and therefore the required C(sp3)–H bond activation was not warranted. The annulation is synthetically relevant, as it could allow the formation of seven-membered tetrahydro-2-benzazepines, through a novel type of formal (5 + 2) annulation.
The route requires use of ortho disubstituted benzylamide precursors, to avoid the activation of the C(sp2)–H of the aromatic ring (see the Supporting Information). The reaction works well (Scheme 3) and even leads to better yields than that of the homologous anilides. The annulations were better performed using N-acetyl-L-valine as an amino acid ligand and toluene as solvent, at 105 °C. It is also beneficial to use 2 equiv of allene and of copper acetate. Several interesting azepine products (5aa–5da) were obtained from readily available starting materials in good to excellent yields (61–90% yield). Substitution in the α-position to the amino group (5ea, 87%) are also tolerated. The reaction can also be performed with allenes other than 2a, illustrated with the formation of 5ch (61%).14
Scheme 3. Scope of the (5 + 2) Formal Cycloaddition of ortho-Methylbenzylamides and Allenes.
Conditions: 0.167 mmol of 1a, 0.333 mmol of allene 2a, 2 equiv of Cu(OAc)2·H2O, 2 mL of toluene, 15 equiv of DMSO, 1.5 equiv of Cs2CO3, under air, 16 h.
Racemic Ac-Val-OH was used.
We have also made a preliminary exploration of a kinetic resolution with substrates 4e and 4f. After a brief screening of ligands, we found out that with Boc-l-Leu-NHOMe, using standard reaction conditions at 60 °C, the cycloadduct 5fa was produced with a promising 90:10 enantiomeric ratio (Scheme 4).15,16 This result indicates that we can generate optically active tetrahydrobenzazepine skeletons in only three steps from commercially available starting materials and warrants further studies to optimize the process.
Scheme 4. Preliminary Results on a (5 + 2) Enantioselective Annulation.
2 equiv of allene.
In conclusion, we have developed a palladium-catalyzed annulation between ortho-methyl anilides or benzylamides and allenes involving the activation of benzylic methyl groups. The technology represents a substantial addition to the yet very scarce arsenal of metal cycloaddition tools lying on the activation of C(sp3)–H bonds. The approach allows a straightforward assembly of highly substituted tetrahydroquinoline or benzazepine skeletons from inexpensive and readily available starting materials.
Acknowledgments
This work has received financial support from Spanish grants (SAF2016-76689-R, PID2019-108624RB-I00, CTQ2016-77047-P, PID2019-110385GB-I00, and FPU fellowship to X.V.), the Consellería de Cultura, Educación e Ordenación Universitaria (ED431C 2017/19, 2015-CP082 and Centro Singular de Investigación de Galicia accreditation 2019-2022, ED431G 2019/03), the European Regional Development Fund (ERDF), and the European Research Council (Advanced Grant No. 340055). The orfeo-cinqa network CTQ2016-81797-REDC is also kindly acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c01594.
Experimental procedures and spectroscopic data for new compounds; X-ray data for 3fa and for 6 (PDF)
Accession Codes
CCDC 2025994 and 2026002 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- For selected recent reviews, see:; a Gensch T.; Hopkinson M. N.; Glorius F. Wencel-Delord, Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. 10.1039/C6CS00075D. [DOI] [PubMed] [Google Scholar]; b Engle K. M.; Mei T.-S.; Wasa M.; Yu J.-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 2012, 45, 788–802. 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang Q.; Shi B.-F. Site-selective functionalization of remote aliphatic C–H bonds via C–H metallation. Chem. Sci. 2021, 12, 841–852. 10.1039/D0SC05944G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review on the field of formal cycloadditions involving the direct activation of C–H bonds, see:Gulías M.; Mascareñas J. L. Metal-catalyzed annulations through activation and cleavage of C–H bonds. Angew. Chem., Int. Ed. 2016, 55, 11000–11019. 10.1002/anie.201511567. [DOI] [PubMed] [Google Scholar]
- For pioneering work, see:; a Stuart D. R.; Bertrand-Laperle M.; Burgess K. M. N.; Fagnou K. Indole Synthesis via Rhodium Catalyzed Oxidative Coupling of Acetanilides and Internal Alkynes. J. Am. Chem. Soc. 2008, 130, 16474–16475. 10.1021/ja806955s. [DOI] [PubMed] [Google Scholar]; b Stuart D. R.; Alsabeh P.; Kuhn M.; Fagnou K. Rhodium(III)-Catalyzed Arene and Alkene C–H Bond Functionalization Leading to Indoles and Pyrroles. J. Am. Chem. Soc. 2010, 132, 18326–18339. 10.1021/ja1082624. [DOI] [PubMed] [Google Scholar]
- Sridharan V.; Suryavanshi P. A.; Menéndez J. C. Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]; See the Supporting Information for more references.
- For recent reviews on metal C(sp3)–H activations, see:; a Zhang M.; Wang Q.; Peng Y.; Chen Z.; Wan C.; Chen J.; Zhao Y.; Zhang R.; Zhang A. Q. Transition Metal-Catalyzed sp3 C–H Activation and Intramolecular C–N Coupling to Construct Nitrogen Heterocyclic Scaffolds. Chem. Commun. 2019, 55, 13048–13065. 10.1039/C9CC06609H. [DOI] [PubMed] [Google Scholar]; b Gandeepan P.; Müller T.; Zell D.; Cera G.; Warratz S.; Ackermann L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]; c He J.; Wasa M.; Chan K. S. L.; Shao Q.; Yu J.-Q. Palladium-Catalyzed Transformations of Alkyl C–H Bonds. Chem. Rev. 2017, 117 (13), 8754–8786. 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xu Y.; Dong G. sp3 C–H Activation via exo-Type Directing Groups. Chem. Sci. 2018, 9, 1424–1432. 10.1039/C7SC04768A. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chu J. C. K.; Rovis T. Complementary Strategies for Directed C(sp3)–H Functionalization: A Comparison of Transition-Metal-Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem., Int. Ed. 2018, 57, 62–101. 10.1002/anie.201703743. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Chen Z.; Rong M.-Y.; Nie J.; Zhu X.-F.; Shi B.-F.; Ma J.-A. Catalytic alkylation of unactivated C(sp3)–H bonds for C(sp3)–C(sp3) bond formation. Chem. Soc. Rev. 2019, 48, 4921–4942. 10.1039/C9CS00086K. [DOI] [PubMed] [Google Scholar]; g Zhang Q.; Shi B.-F. Site-selective functionalization of remote aliphatic C–H bonds via C–H metallation. Chem. Sci. 2021, 12, 841–852. 10.1039/D0SC05944G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A few examples of [n + 2] annulations involving the activation of C(sp3)–H bonds have been reported:; a Rakshit S.; Patureau F. W.; Glorius F. Pyrrole Synthesis via Allylic sp3 C–H Activation of Enamines Followed by Intermolecular Coupling with Unactivated Alkynes. J. Am. Chem. Soc. 2010, 132, 9585–9587. 10.1021/ja104305s. [DOI] [PubMed] [Google Scholar]; b Romanov-Michailidis F.; Ravetz B. D.; Paley D. W.; Rovis T. Ir(III)-Catalyzed Carbocarbation of Alkynes through Undirected Double C–H Bond Activation of Anisoles. J. Am. Chem. Soc. 2018, 140, 5370–5374. 10.1021/jacs.8b02716. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nakao Y.; Morita E.; Idei H.; Hiyama T. Dehydrogenative [4 + 2] Cycloaddition of Formamides with Alkynes through Double C–H Activation. J. Am. Chem. Soc. 2011, 133, 3264–3267. 10.1021/ja1102037. [DOI] [PubMed] [Google Scholar]; d Chan C.-M.; Zhou Z.; Yu W.-Y. Rhodium-Catalyzed Oxidative Cycloaddition of N-tert-Butoxycarbonylhydrazones with Alkynes for the Synthesis of Functionalized Pyrroles via C(sp3)–H Bond Functionalization. Adv. Synth. Catal. 2016, 358, 4067. 10.1002/adsc.201600900. [DOI] [Google Scholar]; e Archambeau A.; Rovis T. Rhodium(III)-Catalyzed Allylic C(sp3)–H Activation of Alkenyl Sulfonamides: Unexpected Formation of Azabicycles. Angew. Chem., Int. Ed. 2015, 54, 13337–13340. 10.1002/anie.201504150. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Cendón B.; Font M.; Mascareñas J. L.; Gulías M. Palladium-Catalyzed Formal (4 + 2) Cycloaddition between Alkyl Amides and Dienes Initiated by the Activation of C(sp3)–H Bonds. ACS Catal. 2020, 10, 3425–3430. 10.1021/acscatal.0c00664. [DOI] [Google Scholar]; g Park H.; Yu J.-Q. Palladium-Catalyzed [3 + 2] Cycloaddition via Twofold 1,3-C(sp3)-H Activation. J. Am. Chem. Soc. 2020, 142, 16552–16556. 10.1021/jacs.0c08290. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Zhang Z.-Z.; Han Y.-Q.; Zhan B.-B.; Wang S.; Shi B.-F. Synthesis of Bicyclo[n.1.0]alkanes by a Cobalt-Catalyzed Multiple C(sp3)–H Activation Strategy. Angew. Chem., Int. Ed. 2017, 56, 13145–13149. 10.1002/anie.201707638. [DOI] [PubMed] [Google Scholar]
- The presence of the triflate increases the acidity of the NH moiety and facilitates the formation of the Pd–N bond while the palladium remains electrophilic enough to promote C–H activation.; a Engle K. M.; Mei T.-S.; Wasa M.; Yu J.-Q. Weak Coordination as Powerful Means for Developing Broadly Useful C–H Functionalization Reactions. Acc. Chem. Res. 2012, 45, 788. 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples from our group showcasing the influence of electron-withdrawing substituents in directing groups, in C–H functionalization reactions, see:; b Seoane A.; Comanescu C.; Casanova N.; García-Fandiño R.; Diz X.; Mascareñas J. L.; Gulías M. Rhodium-catalyzed annulation of ortho-alkenylanilides with alkynes: Formation of unexpected naphthalene adducts. Angew. Chem., Int. Ed. 2019, 58, 1700–1704. 10.1002/anie.201811747. [DOI] [PubMed] [Google Scholar]; c Font M.; Cendón B.; Seoane A.; Mascareñas J. L.; Gulías M. Rhodium(III)-Catalyzed Annulation of 2-Alkenylanilides with Alkynes via C-H Activation: a Direct Access to 2-substituted Indolines. Angew. Chem., Int. Ed. 2018, 57, 8255–8259. 10.1002/anie.201802830. [DOI] [PubMed] [Google Scholar]
- For selected examples of allenes in metal-catalyzed C–H activation and formal cycloaddition reactions, see:; a Gandeepan P.; Rajamalli P.; Cheng C.-H. Rhodium(III)-Catalyzed [4 + 1] Annulation of Aromatic and Vinylic Carboxylic Acids with Allenes: An Efficient Method Towards Vinyl-Substituted Phthalides and 2-Furanones. Chem. - Eur. J. 2015, 21, 9198–9203. 10.1002/chem.201501106. [DOI] [PubMed] [Google Scholar]; b Tran D. N.; Cramer N. syn-Selective Rhodium(I)-Catalyzed Allylations of Ketimines Proceeding through a Directed C―H Activation/Allene Addition Sequence. Angew. Chem., Int. Ed. 2010, 49, 8181–8184. 10.1002/anie.201004179. [DOI] [PubMed] [Google Scholar]; c Thrimurtulu N.; Dey A.; Maiti D.; Volla C. M. R. Cobalt-catalyzed sp2-C–H activation: intermolecular heterocyclization with allenes at room temperature. Angew. Chem., Int. Ed. 2016, 55, 12361–12365. 10.1002/anie.201604956. [DOI] [PubMed] [Google Scholar]; d Casanova N.; Seoane A.; Mascareñas J. L.; Gulías M. Rhodium-Catalyzed (5 + 1) Annulations Between 2-Alkenylphenols and Allenes: A Practical Entry to 2,2-Disubstituted 2H-Chromenes. Angew. Chem., Int. Ed. 2015, 54, 2374–2377. 10.1002/anie.201410350. [DOI] [PubMed] [Google Scholar]; e Wang H.; Glorius F. Mild Rhodium(III)-Catalyzed C-H Activation and Intermolecular Annulation with Allenes. Angew. Chem., Int. Ed. 2012, 51, 7318–7322. 10.1002/anie.201201273. [DOI] [PubMed] [Google Scholar]; f Cendón B.; Casanova N.; Comanescu C.; García-Fandiño R.; Seoane A.; Gulías M.; Mascareñas J. L. Palladium-Catalyzed Formal (5 + 2) Annulation between ortho-Alkenylanilides and Allenes. Org. Lett. 2017, 19, 1674–1677. 10.1021/acs.orglett.7b00467. [DOI] [PubMed] [Google Scholar]; g Vidal X.; Mascareñas J. L.; Gulías M. Palladium-catalyzed, enantioselective formal cycloaddition between benzyltriflamides and allenes: Straightforward access to enantioenriched isoquinolines. J. Am. Chem. Soc. 2019, 141, 1862–1866. 10.1021/jacs.8b12636. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a review on metal-catalyzed C–H functionalization with allenes, see:; h Santhoshkumar R.; Cheng C.-H. Fickle Reactivity of Allenes in Transition-Metal-Catalyzed C–H Functionalizations. Asian J. Org. Chem. 2018, 7, 1151–1163. 10.1002/ajoc.201800133. [DOI] [Google Scholar]
- For examples on the use of amino acids as ligands enabling C–H functionalization reactions, see:; a Engle K. E.; Wang D.-H.; Yu J.-Q. Ligand-Accelerated C–H Activation Reactions: Evidence for a Switch of Mechanism. J. Am. Chem. Soc. 2010, 132, 14137–14151. 10.1021/ja105044s. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Novák P.; Correa A.; Gallardo-Donaire J.; Martin R. Synergistic Palladium-Catalyzed C(sp3)—H Activation/C(sp3)—O Bond Formation: A Direct, Step-Economical Route to Benzolactones. Angew. Chem., Int. Ed. 2011, 50, 12236–12239. 10.1002/anie.201105894. [DOI] [PubMed] [Google Scholar]; c Chan K. S. L. Ligand-enabled cross-coupling of C(sp3)–H bonds with arylboron reagents via Pd(II)/Pd(0) catalysis. Nat. Chem. 2014, 6, 146–150. 10.1038/nchem.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhuang Z.; Herron A. N.; Fan Z.; Yu J.-Q. Ligand-Enabled Monoselective β-C(sp3)–H Acyloxylation of Free Carboxylic Acids Using a Practical Oxidant. J. Am. Chem. Soc. 2020, 142, 6769–6776. 10.1021/jacs.0c01214. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Shao Q.; He J.; Wu Q.-F.; Yu J.-Q. Ligand-Enabled γ-C(sp3)–H Cross-Coupling of Nosyl-Protected Amines with Aryl- and Alkylboron Reagents. ACS Catal. 2017, 7, 7777–7782. 10.1021/acscatal.7b02721. [DOI] [Google Scholar]; f Liu L.; Liu Y.-H.; Shi B.-F. Synthesis of amino acids and peptides with bulky side chains via ligand-enabled carboxylate-directed γ-C(sp3)–H arylation. Chem. Sci. 2020, 11, 290–294. 10.1039/C9SC04482E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The E/Z selectivity and the regioselectivity observed in the annulations most probably stems from steric factors during the reductive elimination step (mostly related to minimizing the clash between allene substituents). On the other hand, for aryl-substituted substrates regioselectivity favors the formation of products in which the alkene is conjugated to the aryl substituent.
- For examples of Pd(II)-catalyzed C(sp3)–H activation and olefination with acrylates and related molecules, see:; a Wasa M.; Engle K. M.; Yu J.-Q. Pd(II)-Catalyzed Olefination of sp3 C–H Bonds. J. Am. Chem. Soc. 2010, 132, 3680–3681. 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Stowers K. J.; Fortner K. C.; Sanford M. S. Aerobic Pd-Catalyzed sp3 C–H Olefination: A Route to Both N-Heterocyclic Scaffolds and Alkenes. J. Am. Chem. Soc. 2011, 133, 6541–6544. 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jiang H.; He J.; Liu T.; Yu J.-Q. Ligand-Enabled γ-C(sp3)–H Olefination of Amines: En Route to Pyrrolidines. J. Am. Chem. Soc. 2016, 138, 2055–2059. 10.1021/jacs.5b13462. [DOI] [PMC free article] [PubMed] [Google Scholar]; d He C.; Gaunt M. J. Ligand-Assisted Palladium-Catalyzed C–H Alkenylation of Aliphatic Amines for the Synthesis of Functionalized Pyrrolidines. Chem. Sci. 2017, 8, 3586–3592. 10.1039/C7SC00468K. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Zhuang Z.; Yu C.-B.; Chen G.; Wu Q.-F.; Hsiao Y.; Joe C. L.; Qiao J. X.; Poss M. A.; Yu J.-Q. Ligand-Enabled β-C(sp3)–H Olefination of Free Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 10363–10367. 10.1021/jacs.8b06527. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Zhan B.-B.; Li Y.; Xu J.-W.; Nie X.-L.; Fan J.; Jin L.; Shi B.-F. Site-Selective δ-C(sp3)–H Alkylation of Amino Acids and Peptides with Maleimides via a Six-Membered Palladacycle. Site-Selective Alkenylation of δ-C(sp3)–H Bonds with Alkynes via a Six-Membered Palladacycle. Angew. Chem., Int. Ed. 2018, 57, 5858–5862. 10.1002/anie.201801445. [DOI] [PubMed] [Google Scholar]; For a rare C(sp3)–H functionalization with alkynes, see:; g Xu J.-W.; Zhang Z.-Z.; Rao W.-H.; Shi B.-F. Site-Selective Alkenylation of δ-C(sp3)–H Bonds with Alkynes via a Six-Membered Palladacycle. J. Am. Chem. Soc. 2016, 138, 10750–10753. 10.1021/jacs.6b05978. [DOI] [PubMed] [Google Scholar]; h Ding Y.; Han Y.-Q.; Wu L.-S.; Zhou T.; Yao Q.-J.; Feng Y.-L.; Li Y.; Kong K.-X.; Shi B.-F. Pd(II)-Catalyzed Tandem Enantioselective Methylene C(sp3)–H Alkenylation–Aza-Wacker Cyclization to Access β-Stereogenic γ-Lactams. Angew. Chem., Int. Ed. 2020, 59, 14060–14064. 10.1002/anie.202004504. [DOI] [PubMed] [Google Scholar]
- For a discussion on the role of allenes:; a Casanova N.; Del Rio K. P.; García-Fandiño R.; Mascareñas J. L.; Gulías M. Palladium(II)-Catalyzed Annulation Between ortho-Alkenylphenols and Allenes. Key Role of the Metal Geometry in Determining the Reaction Outcome. ACS Catal. 2016, 6, 3349–3353. 10.1021/acscatal.6b00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See the Supporting Information for a mechanistic proposal for this reaction.
- For a derivatization of 5ch see the Supporting Information.
- For a review of the use of chiral monoprotected amino acids in asymmetric C–H functionalization reactions, see:Shao Q.; Wu K.; Zhuang Z.; Qian S.; Yu J.-Q. From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C–H Functionalization Reactions. Acc. Chem. Res. 2020, 53 (4), 833–851. 10.1021/acs.accounts.9b00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The absolute configuration of 5ea and 5fa was not determined.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






