Abstract
Global temperatures are increasing rapidly affecting species globally. Understanding if and how different species can adapt fast enough to keep up with increasing temperatures is of vital importance. One mechanism that can accelerate adaptation and promote evolutionary rescue is sexual selection. Two different mechanisms by which sexual selection can facilitate adaptation are pre- and postcopulatory sexual selection. However, the relative effects of these different forms of sexual selection in promoting adaptation are unknown. Here, we present the results from an experimental study in which we exposed fruit flies Drosophila melanogaster to either no mate choice or 1 of 2 different sexual selection regimes (pre- and postcopulatory sexual selection) for 6 generations, under different thermal regimes. Populations showed evidence of thermal adaptation under precopulatory sexual selection, but this effect was not detected in the postcopulatory sexual selection and the no choice mating regime. We further demonstrate that sexual dimorphism decreased when flies evolved under increasing temperatures, consistent with recent theory predicting more sexually concordant selection under environmental stress. Our results suggest an important role for precopulatory sexual selection in promoting thermal adaptation and evolutionary rescue.
Keywords: evolutionary rescue, local adaptation, climate change, Drosophila, sexual selection
Rapidly increasing local, regional, and global temperatures caused by anthropogenic activities can have devastating consequences for biodiversity, increasing population, and species extinction risks (Thomas et al. 2004; Bellard et al. 2012; Dirzo et al. 2014; Wiens 2016; Ceballos et al. 2017). However, predictions about elevated extinction risk typically do not consider the potential positive roles of natural or sexual selection to promote adaptation and facilitate evolutionary rescue (Lavergne et al. 2010; Bell 2017). Recent research has suggested that evolutionary rescue, the recovery of a population due to evolutionary processes (adaptation through sexual selection), is more likely in gradually changing environments (Bell 2017). However, even if evolutionary rescue would be operating, the critical question is: can species adapt fast enough to keep up with rapidly changing climatic conditions (Visser 2008; Hoffmann and Sgrò 2011; Radchuk et al. 2019)?
Sexual selection has been proposed to accelerate adaptation to novel or changing environmental conditions by selecting for beneficial mutations or against deleterious mutations (Whitlock and Agrawal 2009; Servedio and Boughman 2017; Cally et al. 2019). Although its role in promoting adaptation is a contentious issue (Holland 2002; Candolin and Heuschele 2008), recent research suggests that sexual selection can indeed increase population fitness and promote local adaptation (Servedio and Boughman 2017; Parrett and Knell 2018; Cally et al. 2019; Parrett et al. 2019; Gomez-Llano et al. 2020). A recent meta-analysis of experimental evolution studies revealed an overall positive effect of sexual selection on population fitness, and indicated that sexual selection might be especially important for adaptation to changing environments (Cally et al. 2019). Unfortunately, few studies have quantified the relative importance of precopulatory (mate competition and mate choice) versus postcopulatory (sperm competition and cryptic female choice) sexual selection (Cally et al. 2019), or rely on correlative proxies of postcopulatory sexual selection (i.e., testis size; Parrett et al. 2019), rather than direct manipulations of sexual selection.
Sexual selection can also increase nonsexual fitness through its link with overall condition, since most loci in the genome are thought to influence organismal condition (Rowe and Houle 1996; Long et al. 2009, 2010). Sexual selection can therefore potentially have an impact on most of the genome. For instance, males in poor overall condition may have greater difficulties in searching, competing for, and courting mates than males in good condition (Gomez-Llano et al. 2020). If environmental change reduces the condition of some individuals, it will expose genetic variance that sexual selection can act on. Condition-dependent sexual selection might therefore become more efficient in changing environments (Whitlock and Agrawal 2009). Condition-dependent sexual selection can facilitate adaptation by purging the genome of deleterious mutations, thereby accelerating adaptation and facilitating population persistence (Lorch et al. 2003; Whitlock and Agrawal 2009; Parrett et al. 2019; Baur and Berger 2020).
Sexual selection can also promote or prevent local adaptation indirectly, affecting traits correlated to fitness. In many species of insects and other ectotherms, body size is positively correlated with different fitness proxies: fecundity, mating success, and survival (Blanckenhorn 2000; Kingsolver and Huey 2008; Waller and Svensson 2017). Moreover, it is well-documented that higher temperatures can reduce adult body size (Angilletta and Dunham 2003; Angilletta 2009) as a response to the shorter developmental time caused by higher temperatures (Kingsolver and Huey 2008). Therefore, local adaptation can be hindered if high temperatures reduce body size in a way that decreases population fitness. Interestingly and somewhat unexpectedly, a recent meta-analysis revealed selection for large body size also at high temperatures (Siepielski et al. 2019). This could imply that reduced body size under increasing temperatures is maladaptive in some cases and that selection might instead oppose such thermal effect on body size.
Despite the potential importance of sexual selection in promoting adaptation and population persistence, there is limited research on the impact of sexual selection on adaptation to gradually increasing temperatures. A recent study using Indian meal moths Plodia interpunctella, found that populations experiencing strong sexual selection had higher fecundity and higher offspring survival under increasing temperatures, compared with populations exposed to weak sexual selection, and such adaptation occurred rapidly, after only 8 generations of selection (Parrett and Knell 2018). However, in this and previous studies, sexual selection is typically not partitioned into pre- and postcopulatory components. Investigating how pre- and postcopulatory sexual selection might promote adaptation to increasing temperatures will help us understand the potential for evolutionary rescue in response to climate change, and how this might differ between species with different reproductive strategies or mating systems.
Here, we exposed replicate Drosophila melanogaster populations to different mating regimes simultaneously with different thermal regimes (stable, gradual change, and sudden change) in an experimental evolution study. Our aim was to simulate a rapid climate change scenario and investigate how pre- and postcopulatory sexual selection influenced female fitness. Specifically, we ask 1) if sexual selection facilitated adaptation to increasing temperatures, 2) what is the contribution of pre- and postcopulatory sexual selection to such adaptation, and 3) if the effect of temperature on body size might indirectly prevent adaptation and evolutionary rescue by decreasing female fecundity. Overall, we predicted that populations evolving under sexual selection would adapt to increasing temperatures, but we have no a priori prediction for which form of sexual selection would be most important in that effect, and whether both might play a role.
Materials and Methods
Stock population
We used laboratory-adapted wild type (LHM) D. melanogaster kept in Jessica Abbott’s laboratory in Lund University since 2012. This population of flies originated from 400 flies collected in central California by L. Harshman in 1991 (Rice et al. 2005). These flies have been maintained by L. Harshman (1991–1995), W. Rice (1995–2004), E. Morrow (2004–2012), and J. Abbott (2012–present). The stock flies have been kept since 2013 on standard cornmeal food at 25°C and 50% humidity and 12:12 h light:dark cycle at a density of 100–150 individuals per vial.
Selection experiments
We exposed 18 experimental populations of D. melanogaster to 2 different temperature treatments (stable and gradual change) and 3 different mating regimes: single mating with no choice (no choice), single mating with mate choice (precopulatory sexual selection), and multiple mating (postcopulatory sexual selection). Thus, we had 3 replicate lines per experimental combination. Each replicate line (hereafter experimental lines) contained 10 vials with a single female per vial. The small population size is of particular interest for future scenarios of environmental change, as populations exposed to novel or changing environments are expected to decline before adaptation can rescue the populations from extinction (Bell 2017). The stable temperature treatment is identical to the conditions at which the stock population has been maintained (25°C, 50% humidity, 12:12 h light:dark cycle). In the gradual change temperature treatment, we increased temperature 1°C per generation until reaching 29°C, after which temperature was maintained constant at 29°C for 2 generations until we performed our fitness assays (see below). Temperature in the gradual change treatment was increased after oviposition by the females, and therefore each new generation developed from eggs at the new temperature and experienced the full life cycle (hatching, development, reproduction, and oviposition) in that novel temperature regime. We kept humidity and light/dark cycle constant during the entire multigeneration experiment and equal for all the replicate populations. Although the rate of temperature change in our experiment is faster than natural change, evidence of adaptation to this fast rate of change would indicate that adaptation to a slower rate would also be possible.
We collected virgin individuals, females and males, 4–6 h after eclosion in all the different mating regimes and temperature treatments. Males and females were kept in separate vials for 24 h to allow sexual maturation but prevent mating, after which they were paired in new vials. We controlled the number and time females were exposed to males. In the no choice regime, we matched each female with 1 randomly chosen male in new vials, thereby experimentally excluding sexual selection. After 2 h, the male was removed to avoid remating and further male mating harassment on the female. In the precopulatory sexual selection regime, we exposed each female to 3 males in new vials for 2 h, after which all males were removed to avoid remating and further mating harassment. Finally, in the postcopulatory sexual selection regime, we paired each female with 3 random virgin males in new vials for 24 h to allow for both mating, remating, and harassment to occur. Individual males and females were crossed between vials within the same replicate line in all the treatments to avoid sibling pairing and reduce inbreeding depression. After the period in which males and females were allowed to interact, females were moved to new vials (without males) for oviposition for 24 h. The eggs from these vials formed the next generation. Note that although we can study precopulatory sexual selection by experimentally excluding postcopulatory sexual selection, the reverse is not the case. Therefore, the flies in the regime with postcopulatory sexual selection also—by necessity—experienced precopulatory sexual selection.
The time we allowed males and females to interact is based on previous studies that have shown that in D. melanogaster copulations occur within the first 2 h of pairing males and females (García-Roa et al. 2019). Moreover, seminal accessory glands that reduce female receptivity take 8–10 h to take effect (Scott 1987), and within this time females mate on average 1.6–1.8 times (García-Roa et al. 2019). Therefore, we expect that females exposed to males for 2 h mated on average only once, whereas females exposed to males for 24 h had mostly mated more than once.
Fitness assays
To investigate if experimental lines exposed to increasing temperatures had adapted, and if so through which mechanisms of sexual selection, we performed a fitness assay. After 6 generations of selection we allowed females from the stable temperature treatment to oviposit in 2 different vials in 2 consecutive periods of 24 h. The first replicate (first 24 h) was transferred from 25°C to 29°C (hereafter sudden change) to compare with females of the gradual change treatment. The second replicate (second 24-h period) was kept at 25°C temperature (stable temperature) and used as a control.
Increased temperature can have an effect on fecundity if exposed during larvae and adult stages (Cohert and David 1978). However, emerged individuals had experienced high temperatures only during larvae but not during adulthood. Therefore, individuals from the sudden change, gradual change and stable temperature treatments were allowed to emerge. From these emergences, we moved 10 randomly chosen virgin males and 10 females to a new vial and allowed them to mate. After 24 h, we moved the females to new vials, 1 female per vial, to oviposit for 24 h. These individuals have then experienced high temperatures during larvae and adult stages. Female fitness was quantified as number of adults emerged per female during the first 24 of oviposition. Moreover, by equalizing male and female adult densities (10 males and 10 females), we avoid any possible effect of perceived densities on male sperm production (Bretman et al. 2009; Moatt et al. 2014).
Body size
Individual fitness is closely related to size (Blanckenhorn 2000; Kingsolver and Huey 2008), and size can be affected by temperature (Kingsolver and Huey 2008). To test the effect of temperature on body size, and of body size on fitness, we froze all the males and females used in the fitness assay at −20°C for 10 days, after which we measured the full body length in mm of each individual, from head to abdomen. We used a reticule attached to the eye piece of a stereoscopic microscope, measuring length to the nearest 0.05 mm.
Statistical analysis
To analyze female fitness, estimated as the number of emerging adults per female, and given overdispersion (dispersion ratio of Poisson model was larger than 1), we used a negative binomial mixed model. Temperature regime (stable, sudden, and gradual change), mating regime (no choice, precopulatory, and postcopulatory sexual selection) and their interaction were included as fixed factors in a fully factorial model. Each replicate line was coded with an ID (differentiating replicates within the different combinations of temperature and mating regime) and included as random effect. This way replicate ID takes into account that the replicate line is nested within each combination of experimental treatment. We expected a main overall negative effect of temperature on fitness, resulting in higher female fitness in the stable temperature treatment, compared with the sudden and gradual change temperature treatments. A significant difference between sudden and gradual change temperatures would indicate local adaptation to increasing temperature. Because adaptation to increasing temperature can occur in some mating regimes and not in others (e.g., only in sexual selection regimes), we compared female fitness between the temperature treatments within the different mating regimes. This comparison will tell us if thermal adaptation occurs in the different mating regimes.
To investigate how body size changed in our different experimental treatments, we fitted a linear mixed model assuming a normal distribution for the dependent variable (body length) and evaluated how different temperature regimes affected male and female body size. Our full model included temperature treatment, sex, mating regime, and all 2- and 3-way interactions were fixed factors in this fully factorial model. Like in the previous mixed model, we included replicate line ID as a random factor. To find the best-fit model we perform a series of models removing factors from the full model in every possible combination and compared the AIC score. The model with the lowest AIC value is the model with the best fit. All analyses were performed using the packages “emmeans” for Tukey post hoc tests (Lenth 2018), “lme4” (Bates et al. 2015), and “car” (Fox and Weisberg 2011) for linear models in R (R Development Core Team 2018).
Results
We found a significant effect of temperature (χ2 = 51.7, P < 0.001) and of the interaction between temperature treatment and mating regime (χ2 = 9.96, P = 0.041), but no main effect of mating regime (χ2 = 1.39, P = 0.49; Table 1) on female fitness. Post hoc tests show that populations at stable temperature had the highest fitness (41.44 offspring) compared with the gradual (5.68 offspring, P < 0.001) and sudden change treatments (2.81 offspring P < 0.001). On average, in the gradual change treatments females were twice as productive as in the sudden change (5.8 and 2.8 offspring, respectively), although this difference was not statistically significant (P = 0.19; Table 1).
Table 1.
We found an effect of temperature and the interaction of temperature and mating regime in female fitness
| Term | χ2 | df | P-value | |
|---|---|---|---|---|
| Main models | ||||
| Temperature | 51.706 | 2 | <0.001 | |
| Mating regime | 1.3949 | 2 | 0.497 | |
| Temp: mating | 9.9665 | 4 | 0.041 | |
| Random effect variance (SD) = 1.09 (1.04) | ||||
| Post-hoc | ||||
| Contrast | Estimate | SE | z ratio | P-value |
| Gradual—Sudden | 0.92 | 0.529 | 1.739 | 0.1908 |
| Gradual—Stable | −2.8 | 0.505 | −5.533 | <0.001 |
| Sudden—Stable | −3.72 | 0.526 | −7.066 | <0.001 |
N = 270.
Tukey post hoc tests show that overall, female fitness was higher in the stable temperature treatment than in gradual and sudden change treatments. Although gradual change treatments had twice as much fitness than sudden change, the difference between these 2 treatments was not significant.
We found a consistent, although not statistically significant, trend for females of the mating regime with pre- and postcopulatory sexual selection to have higher fitness (15.8 offspring per female) than females of the precopulatory sexual selection (15.1 offspring) and no choice regimes (15.6 offspring). This trend was consistent in the gradual (postcopulatory = 9.1, precopulatory = 4.2, no choice = 3.6) and sudden temperature change (postcopulatory = 6.2, precopulatory = 0.4, no choice = 1.7) treatments (Table 2).
Table 2.
Planned comparisons between temperature treatments in the different mating regime treatments showed evidence of increased thermal adaptation in the precopulatory sexual selection treatment
| Planned comparisions | |||||
|---|---|---|---|---|---|
| Contrast | Mating | Estimate | SE | z ratio | P-value |
| Gradual—Sudden | No choice | 1.100 | 0.900 | 1.224 | 0.4386 |
| Gradual—Stable | No choice | −2.710 | 0.861 | −3.142 | 0.0048 |
| Sudden—Stable | No choice | −3.810 | 0.894 | −4.258 | <0.001 |
| Gradual—Sudden | Postcopulatory | −1.180 | 0.906 | −1.306 | 0.3919 |
| Gradual—Stable | Postcopulatory | −3.310 | 0.903 | −3.668 | <0.001 |
| Sudden—Stable | Postcopulatory | −2.130 | 0.858 | −2.485 | 0.0346 |
| Gradual—Sudden | Precopulatory | 2.840 | 0.963 | 2.947 | 0.0090 |
| Gradual—Stable | Precopulatory | −2.370 | 0.857 | −2.762 | 0.0159 |
| Sudden—Stable | Precopulatory | −5.210 | 0.959 | −5.428 | <0.001 |
| Observations |
|||
|---|---|---|---|
| Temperature | Mating regime | Female fitness | SE |
|
| |||
| Gradual | No choice | 3.63 | 1.34 |
| Gradual | Postcopulatory | 9.17 | 2.91 |
| Gradual | Precopulatory | 4.27 | 1.31 |
| Sudden | No choice | 1.70 | 1.13 |
| Sudden | Postcopulatory | 6.27 | 2.25 |
| Sudden | Precopulatory | 0.47 | 0.31 |
| Stable | No choice | 42.48 | 3.00 |
| Stable | Postcopulatory | 41.03 | 2.80 |
| Stable | Precopulatory | 40.83 | 2.55 |
|
| |||
N = 270.
In the postcopulatory and no choice mating regime, females showed higher fitness in gradual over sudden change treatments, although this difference was not significant. Observed mean female fitness in all temperature treatments and mating regimes.
Moreover, we compared female fitness between temperatures within each mating regime. Interestingly, we found that although populations of all mating regimes in gradual change had higher fitness than in the sudden change, only the precopulatory sexual selection treatment was statistically significant (estimate = 2.84, P = 0.009). Although in the postcopulatory mating regime female fitness was higher in gradual (9.1 offspring) than in sudden temperature change (6.2 offspring), the difference was not statistically significant (estimate = −1.18, P = 0.39). A similar pattern was found in the no choice regime (Table 2, Figure 1).
Figure 1.

Experimental evidence of an evolutionary response in female fitness (no. offspring produced) was found in the precopulatory but not in the postcopulatory sexual selection or no choice mating regimes when we compared the “gradual” versus the “sudden” thermal treatments (Tables 1 and 2). Colored points show individual observations (number of emerging offspring per female), black points show mean fitness and error bars show standard errors around the mean.
Finally, we compared males and females from the different experimental regimes to analyze body size differences. From the total of 540 individuals, we removed 38 that lost body parts and measured a total of 502 (Table 3). The model with the best support included temperature, sex, and their interaction (the difference of AIC values was >20 with the second-best fit model; alternative models are shown in Supplementary Table S1). We found that in the gradual change treatments female body length decreased, compared with both the stable temperature treatments (3.5% smaller at gradual change treatments; P < 0.001) and the sudden change treatments (2.5% smaller at gradual change treatments; P < 0.001). Interestingly, gradual change treatments increased male body length compared with stable temperatures (2.6% larger at gradual change treatment; P < 0.001) and the sudden change treatments (3% larger at gradual change treatment; P < 0.001). The combined result of these opposite body length trends in the 2 different sexes effectively resulted in reduced sexual size dimorphism under gradual changes in temperatures (Figure 2, Table 3).
Table 3.
Mean body length (measure to the nearest 0.05 mm) of males and females from the 3 different temperature treatments
| Observations | |||||
|---|---|---|---|---|---|
| Temperature | Sex | Body length (SD) | n | ||
|
| |||||
| Gradual | F | 2.89 (0.10) | 89 | ||
| Gradual | M | 2.35 (0.12) | 86 | ||
| Stable | F | 2.99 (0.08) | 77 | ||
| Stable | M | 2.28 (0.09) | 80 | ||
| Sudden | F | 2.97 (0.09) | 86 | ||
| Sudden | M | 2.28 (0.08) | 84 | ||
|
| |||||
| Main model | |||||
|
| |||||
| Term | χ2 | df | P-value | ||
|
| |||||
| Sex | 5633.147 | 1 | <0.001 | ||
| Temperature | 1.170 | 2 | 0.557 | ||
| Sex:Temperature | 73.067 | 2 | <0.001 | ||
|
| |||||
| Post-hoc | |||||
|
| |||||
| Contrast | Sex | Estimate | SE | t ratio | P-value |
|
| |||||
| Gradual—Stable | F | −0.101 | 0.022 | −4.695 | <0.001 |
| Gradual—Sudden | F | −0.075 | 0.021 | −3.555 | 0.002 |
| Stable—Sudden | F | 0.025 | 0.022 | 1.187 | 0.468 |
| Gradual—Stable | M | 0.063 | 0.022 | 2.927 | 0.015 |
| Gradual—Sudden | M | 0.068 | 0.021 | 3.187 | 0.007 |
| Stable—Sudden | M | 0.005 | 0.022 | 0.223 | 0.972 |
N = 502.
Analysis of body size was performed with a generalized linear mixed model, the model with the best fit included temperature, sex and their interaction as fixed factors (a). Tukey post hoc tests between the different temperature treatments and sexes are shown that sexual size dimorphism decreased in the gradual change temperature treatment (B).
Figure 2.
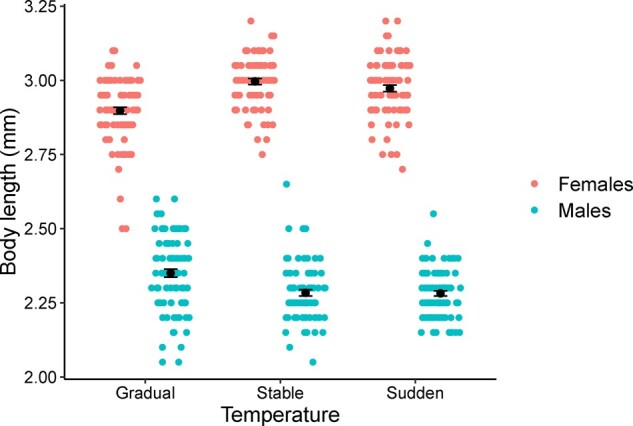
Sexual size dimorphism was present in the 3 temperature treatments (stable, sudden, and gradual change). However, in the gradual temperature change regime, female body size decreased while male increased, effectively reducing sexual size dimorphism (Table 3). Colored points show individual body length (to the nearest 0.05 mm), black points show means and error bars standard errors around the mean.
Discussion
There is a growing debate on whether sexual selection can facilitate species adaptation to environmental change. We studied the effect of pre- and postcopulatory sexual selection in adaptation to gradually increasing temperatures. We show that precopulatory sexual selection promoted thermal adaptation to gradually increasing temperatures in D. melanogaster by increasing female fitness, compared with a sudden change. This significant increase in female fitness was not observed in the no choice mating regime (where presumably only natural selection operated) or under a regime with both pre- and postcopulatory sexual selection. These results suggest that precopulatory sexual selection alone might be sufficient to promote rapid thermal adaptation in D. melanogaster, whereas we have no evidence in this study for any role of natural selection alone or a combination of pre- and postcopulatory sexual selection. Adaptation by precopulatory sexual selection can be caused by female mate choice (Servedio and Boughman 2017) or by male–male competition, allowing only males in high condition to get access to females (Gomez-Llano et al., 2020). An alternative explanation is that precopulatory sexual selection has a negative effect on female fitness when exposed to a sudden temperature change. However, there is no biological reason, that we are aware of, by which precopulatory sexual selection can decrease fitness.
We found a consistent trend in which the mating regimes with pre- and postcopulatory sexual selection had consistently higher female fitness than precopulatory sexual selection alone and no choice in the gradual and sudden temperature change treatments. These suggests a role of postcopulatory sexual selection acting as a buffer, limiting the negative effects of high temperatures and facilitating population recovery more than promoting local adaptation. This interpretation agrees with the biology of D. melanogaster. At high temperatures, male flies can approach their thermal fertility limit (Walsh et al. 2019). As sperm production is especially sensitive to heat stress, males become sterile as the temperature approaches 30°C (David et al. 2005; Hoffmann 2010; Pedersen et al. 2011). The maximum temperature (29°C) in our experiment is likely to result in increased variance among male genotypes, and multiple mating in postcopulatory sexual selection could reduce the costs of mating unfertile males. However, local adaptation might be hindered by the effect of sexual conflict in this mating regime (Long et al., 2009; Chenoweth et al., 2015; Yun et al., 2018).
Experimental evolution studies in Drosophila that have investigated the role of natural and/or sexual selection in adaptation to novel environments (typically new food sources or temperature) have produced conflicting results (Holland 2002; Dolgin et al. 2006; Rundle et al. 2006; Correia et al. 2010; Chenoweth et al. 2015; Shenoi and Prasad 2016). Some of these studies suggested that sexual selection has low potential to increase adaptation to novel environments (Holland 2002; Candolin and Heuschele 2008), whereas others have shown a slight positive effect of sexual selection that is either aligned (Rundle et al. 2006) or opposed to natural selection (Chenoweth et al. 2015). Here, we demonstrated an overall positive fitness effect of precopulatory sexual selection in response to a gradually changing thermal environment and on a relatively fast time scale, after only 6 generations of sexual selection. Another recent experimental evolution study demonstrated similar rapid adaptation driven by sexual selection to increasing temperatures in only 8 generations (Parrett and Knell 2018). Given the limited number of generations in our experiment, it is not surprising that females from the stable temperature treatment have higher fitness than those in the changing temperature treatments (more than 8 times higher in stable over gradual and 20 times higher than sudden temperature changes), and the difference between gradual and sudden changes be smaller (2 times higher in gradual over sudden). Nevertheless, this would indicate that population recovery rate would be 2 times faster after only 6 generations of selection. Moreover, the fitness of the gradual change treatments is expected to increase, as well as the difference between gradual and sudden temperature changes, with more generations of selection. The rapid evolutionary response to sexual selection in response to increasing temperatures in this and the recent study by Parrett and Knell (2018), suggests that adaptation is likely to be a result of selection on standing genetic variation (Barrett and Schluter 2008), rather than through the emergence and selective fixation of novel mutations.
The effect of the different temperature treatments in male and female body size and the resulting sexual dimorphism is intriguing. Previous empirical evidence has shown that increased temperature can reduce insect body size, including in D. melanogaster (Partridge et al. 1994; Kingsolver and Huey 2008), but selection can also reverse this negative relationship between temperature and size, as shown in selection experiments in thorax size (Scheiner and Lyman 1991). A recent meta-analysis showed, somewhat unexpectedly, positive selection for larger body size even in warm thermal environments (Siepielski et al. 2019). In this study, we found a negative effect of temperature on female body length, whereas males instead increased body length at higher temperatures, effectively reducing sexual size dimorphism (Figure 2). These results are in broad agreement with theoretical predictions, where in harsh environments in which individuals are not well adapted, male and female phenotypes are pushed to a common optima, whereas in benign environments in which individuals are well adapted, the optima for males and females are expected to diverge (Connallon 2015). Therefore, reduced sexual size dimorphism could be expected in populations experiencing the early stages of adaptation (gradual change treatments). In contrast, increased sexual size dimorphism would be expected in populations that have not yet been exposed selection (sudden change) or in well adapted populations (stable temperature).
One caveat of our experiment is the small population size, which can increase stochasticity, and inbreeding effects. Stochastic effects could explain why even though gradual change treatments have fitness that is twice as high as sudden change, we were not able to detect statistically significant differences. Moreover, effective population size during selection is larger in the postcopulatory mating regime (due to multiple matings) than in the precopulatory and no choice treatments. Therefore, inbreeding is expected to be larger in the no choice and precopulatory sexual selection mating regime and smaller in the postcopulatory mating regime. Nevertheless, the fact that we found no effect of mating treatment on female fitness suggests that inbreeding effects (if any) were negligible. However, although these results are congruent with theoretical predictions, they should be interpreted with care.
Overall, our results suggest that precopulatory sexual selection can facilitate adaptation to changing environments, such as increasing temperatures. These results are in agreement with models suggesting that sexual selection can facilitate adaptive peak shifts and enable populations to cross fitness valleys (Bonduriansky 2011). Our results also have some direct applications, as information on reproductive behavior and sexual selection should ideally be taken into consideration when designing conservation and management plans, for example, in captive breeding programs.
Supplementary Material
Acknowledgments
Jessica Abbott provided the flies and lab supplies to carry out this study. Katrine Lund-Hansen provided feedback in the initial stages in the design of the experiments.
Funding
E.S. was financially supported by the Erasmus Programme. E.I.S. was financially supported by research grants from Stina Werners Fond, Gyllenstiernska Krapperupsstiftelsen, Olle Engqvist Byggmästare Foundation and the Swedish Research Council (VR; grant no. 2016-03356).
Authors’ Contributions
M.G.-L. conceived the study and designed the experiments. M.G.-L. and E.S. carried out the experiments and analyzed the data. E.I.S. provides conceptual support. M.G.-L. wrote the manuscript with substantial input from E.S. and E.I.S.
Data Accessibility
Data will be uploaded and available for download from a public repository (Dryad) upon acceptance.
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Angilletta MJ, 2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press. [Google Scholar]
- Angilletta MJ, Dunham AE, 2003. The temperature - size rule in ectotherms: simple evolutionary explanations may not be general. Am Nat 162:332–342. [DOI] [PubMed] [Google Scholar]
- Barrett R, Schluter D, 2008. Adaptation from standing genetic variation. TREE 23:38–44. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Soft 67:1–48. [Google Scholar]
- Baur J, Berger D, 2020. Experimental evidence for effects of sexual selection on condition-dependent mutation rates. Nat Ecol Evol 4:737–744. [DOI] [PubMed] [Google Scholar]
- Bell G, 2017. Evolutionary rescue. Annu Rev Ecol Evol Syst 48:605–627. [Google Scholar]
- Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F, 2012. Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckenhorn WU, 2000. The evolution of body size: what keeps organisms small? Quart Rev Biol 75:385–407. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, 2011. Sexual selection and conflict as engines of ecological diversification. Am Nat 178:729–745. [DOI] [PubMed] [Google Scholar]
- Bretman A, Fricke C, Chapman T, 2009. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc Biol Sci 276:1705–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cally JG, Stuart-Fox D, Holman L, 2019. Meta-analytic evidence that sexual selection improves population fitness. Nat Commun 10:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolin U, Heuschele J, 2008. Is sexual selection beneficial during adaptation to environmental change? TREE 23:446–452. [DOI] [PubMed] [Google Scholar]
- Ceballos G, Ehrlich PR, Dirzo R, 2017. Biological annihilation via the ongoing sixth mass extinction signalled by vertebrate population losses and declines. Proc Natl Acad Sci USA 114:6089–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenoweth SF, Appleton NC, Allen SL, Rundle HD, 2015. Genomic evidence that sexual selection impedes adaptation to a novel environment. Curr Biol 25:1860–1866. [DOI] [PubMed] [Google Scholar]
- Cohert Y, David J, 1978. Control of the adult reproductive potential by preimaginal thermal conditions. Oecologia 36:295–306. [DOI] [PubMed] [Google Scholar]
- Connallon T, 2015. The geography of sex-specific selection, local adaptation, and sexual dimorphism. Evolution 69:2333–2344. [DOI] [PubMed] [Google Scholar]
- Correia L, Yeaman S, Whitlock MC, 2010. Local adaptation does not always predict high mating success. J Evol Biol 23:875–878. [DOI] [PubMed] [Google Scholar]
- David JR, Araripe LO, Chakir M, Legout H, Lemos B. et al. , 2005. Male sterility at extreme temperatures: a significant but neglected phenomenon for understanding Drosophila climatic adaptations. J Evol Biol 18:838–846. [DOI] [PubMed] [Google Scholar]
- Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB. et al. , 2014. Defaunation in the Anthropocene. Science 345:401–406. [DOI] [PubMed] [Google Scholar]
- Dolgin ES, Whitlock MC, Agrawal AF, 2006. Male Drosophila melanogaster have higher mating success when adapted to their thermal environment. J Evol Biol 19:1894–1900. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S, 2011. An {R} Companion to Applied Regression. Thousand Oaks (CA: ): Sage publications. [Google Scholar]
- García-Roa R, Chirinos V, Carazo P, 2019. The ecology of sexual conflict: temperature variation in the social environment can drastically modulate male harm to females. Fun Ecol 33:681–692. [Google Scholar]
- Gomez-Llano M, Narasimhan A, Svensson E, 2020. Male-male competition causes parasite-mediated sexual selection for local adaptation. Am Nat 196:43. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, 2010. Physiological climatic limits in Drosophila: patterns and implications. J Experi Biol 213:870–880. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Sgrò CM, 2011. Climate change and evolutionary adaptation. Nature 479:479–485. [DOI] [PubMed] [Google Scholar]
- Holland B, 2002. Sexual selection fails to promote adaptation to a new environment. Evolution 56:721–730. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Huey RB, 2008. Size, temperature, and fitness: three rules. Evol Ecol Research 10:251–268. [Google Scholar]
- Lavergne S, Mouquet N, Thuiller W, Ronce O, 2010. Biodiversity and climate change: integrating evolutionary and ecological responses of species and communities. Annu Rev Ecol Evol and Syst 41:321–350. [Google Scholar]
- Lenth R, 2018. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1. 1: 3. [Google Scholar]
- Long TAF, Pischedda A, Rice WR, 2010. Remating in Drosophila melanogaster: are indirect benefits condition dependent? Evolution 64:2767–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TAF, Pischedda A, Stewart AD, Rice WR, 2009. A cost of sexual attractiveness to high-fitness females. PLoS Biol 7:e1000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch PD, Proulx S, Rowe L, Day T, 2003. Condition-dependent sexual selection can accelerate adaptation. Evol Ecol Research 5:867–881. [Google Scholar]
- Moatt JP, Dytham C, Thom MDF, 2014. Sperm production responds to perceived sperm competition risk in male Drosophila melanogaster. Physiol Behav 131:111–114. [DOI] [PubMed] [Google Scholar]
- Parrett JM, Knell RJ, 2018. The effect of sexual selection on adaptation and extinction under increasing temperatures. Proc Biol Sci 285:20180303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrett JM, Mann DJ, Chung AYC, Slade EM, Knell RJ, 2019. Sexual selection predicts the persistence of populations within altered environments. Ecol Lett 22:1629–1637. [DOI] [PubMed] [Google Scholar]
- Partridge L, Barrie B, Fowler K, French V, 1994. Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48:1269–1276. [DOI] [PubMed] [Google Scholar]
- Pedersen LD, Pedersen AR, Bijlsma R, Bundgaard J, 2011. The effects of inbreeding and heat stress on male sterility in Drosophila melanogaster. Biol J Linn Soc 104:432–442. [Google Scholar]
- R Development Core Team, 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Radchuk V, Reed T, Teplitsky C, van de Pol M, Charmantier A. et al. , 2019. Adaptive responses of animals to climate change are most likely insufficient. Nat Commun 10:3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR, Linder JE, Friberg U, Lew TA, Morrow EH. et al. , 2005. Inter-locus antagonistic coevolution as an engine of speciation: assessment with hemiclonal analysis. Proc Natl Acad Sci USA 102: 6527–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Houle D, 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc Biol Sci 263:1415–1421. [Google Scholar]
- Rundle HD, Chenoweth SF, Blows MW, 2006. The roles of natural and sexual selection during adaptation to a novel environment. Evolution 60:2218–2225. [PubMed] [Google Scholar]
- Scheiner SM, Lyman RF, 1991. The genetics of phenotypic plasticity II. Response to selection. J Evol Biol 4:23–50. [Google Scholar]
- Scott D, 1987. The timing of the sperm effect on female Drosophila melanogaster receptivity. Anim Behav 35:142–149. [Google Scholar]
- Servedio MR, Boughman JW, 2017. The role of sexual selection in local adaptation and speciation. Annu Rev Ecol Evol Syst 48:85–109. [Google Scholar]
- Shenoi VN, Prasad NG, 2016. Local adaptation to developmental density does not lead to higher mating success in Drosophila melanogaster. J Evol Biol 29:2036–2042. [DOI] [PubMed] [Google Scholar]
- Siepielski AM, Morrissey MB, Carlson SM, Francis CD, Kingsolver JG. et al. , 2019. No evidence that warmer temperatures are associated with selection for smaller body sizes. Proc Biol Sci 286:20191332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ. et al. , 2004. Extinction risk from climate change. Nature 427:145. [DOI] [PubMed] [Google Scholar]
- Visser ME, 2008. Keeping up with a warming world: assessing the rate of adaptation to climate change. Proc Biol Sci 275:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller JT, Svensson EI, 2017. Body size evolution in an old insect order: no evidence for Cope’s Rule in spite of fitness benefits of large size. Evolution 71:2178–2193. [DOI] [PubMed] [Google Scholar]
- Walsh BS, Parratt SR, Hoffmann AA, Atkinson D, Snook RR. et al. , 2019. The impact of climate change on fertility. TREE 34:249–259. [DOI] [PubMed] [Google Scholar]
- Whitlock MC, Agrawal AF, 2009. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63:569–582. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol 14:e2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun L, Chen PJ, Kwok KE, Angell CS, Rundle HD. et al. , 2018. Competition for mates and the improvement of nonsexual fitness. Proc Natl Acad Sci USA 115:6762–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be uploaded and available for download from a public repository (Dryad) upon acceptance.


