Abstract
Expression of constructs encoding fusion proteins of ERK1 and ERK2 containing a C-terminal farnesylation motif (CAAX) is predominantly localized at the cell membrane and was activated by coexpression of constitutively active Ha-RasL61 and epidermal growth factor. Both fusion proteins significantly inhibit the transcriptional activation of a c-fos–chloramphenicol acetyltransferase reporter induced by RasL61, constitutively active MEK1, or constitutively active RafBXB. The corresponding SAAX chimeras or overexpression of the wild-type ERKs did not interfere with the transcriptional activation of c-fos. The inhibition of the Ras-mediated c-fos induction by ERK2-CAAX can in part be rescued by coexpression of a wild-type ERK2 but not by wild-type ERK1. We find that ERK1-CAAX acts in the same fashion, indicating that mitogen-activated protein kinase (MAPK)–CAAX chimeras interact in an isotype-specific manner. It is demonstrated that both ERK1-CAAX and ERK2-CAAX associate with the corresponding endogenous ERKs, which explains the isotype-specific inhibitory effects of the ERK-CAAX chimeras. Evidence is presented that expression of ERK-CAAX fusion proteins inhibits the nuclear translocation of the corresponding endogenous ERKs. Disruption of MAPK translocation by membrane targeting provides additional, independent proof that nuclear translocation of ERKs is essential for the transcriptional activation of c-fos.
The mitogen-activated protein kinase (MAPK) module consisting of a series of three protein kinases, a MAPK, a MAPK kinase (MAPKK), and a MAPKK kinase (MAPKKK), has been identified as one of the most important pathways for the transmission of signals from the plasma membrane to the nucleus (6, 12, 18, 49, 50, 58, 74, 79, 83, 92). In vertebrates, three MAPK pathways, comprising the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 MAPK pathways, have been well characterized (3, 19–22, 28, 30, 51, 70, 75, 77, 97). In the mammalian ERK1/2 pathway, Ras associates with Raf-1 and B-Raf, which upon phosphorylation act as MAPKKK and activate the dual-function threonine/tyrosine kinases MAPK/ERK kinases 1 and 2 (MEK1/2), which in turn activate ERK1 and ERK2, also termed p44 and p42 MAPK, respectively. Transformation by oncogenic Ras is blocked by a dominant negative mutant of MEK1, demonstrating that activation of the ERK pathway is required for transformation (13, 55, 69). Once phosphorylated, activated ERK1 and ERK2 translocate to the nucleus (9, 26, 53). Nuclear translocation is considered to be essential for the phosphorylation and activation of nuclear transcription factors (7, 8, 10, 23, 30, 32, 33, 35, 36, 42, 43, 60, 61, 81, 88), although definite proof for this assumption is still lacking. The activation of c-fos transcription is a paradigm for a gene regulated by the Ras pathway. The serum response element (SRE) represents a pivotal regulatory sequence of the fos promoter (39, 40, 86, 87). Two kinds of transcription factors are required for SRE activity: the serum response factor (SRF) and the ternary complex factors, which form ternary or, in some instances, quaternary complexes with the SRF. The ternary complex factors, which bind to the SRE, include Elk-1, SAP-1, and SAP-2, a subset of the Ets family of transcription factors (15, 25, 34). The N-terminal domains of Elk-1 and SAP-1 mediate DNA binding and ternary complex formation. The C-terminal domains of both Elk-1 and SAP-1 contain several MAPK phosphorylation sites. MAPK-mediated phosphorylation of Elk-1 results in a strong increase in transcriptional activity (23, 41, 56, 68, 71, 93).
Recently, we have demonstrated that the transcriptional activation of c-fos by oncogenic Ras requires the cooperative activities of three protein kinase C (PKC) isotypes (44). Evidence had been presented that the PKC isotypes act through the Raf-MAPK pathway (44). The exact mechanism by which the different PKC isotypes are implicated in this signaling pathway, however, had remained obscure.
Two of these PKC isotypes, PKC-ɛ and PKC-ζ, had been shown to act downstream of Raf and MEK1 (44), suggesting that they may be involved in the regulation of activation, the duration of the active state, or the translocation of the MAPKs from the cytosol to the nucleus. To address these questions, novel membrane-targeted MAPK chimeras have been constructed.
MAPK mutants have proven to be useful tools for studies concerned with the function or regulation of the MAPK pathway. The MAPK variants used so far contain amino acid substitutions in either the ATP binding site or the catalytic loop (1, 16, 29, 46, 67, 91). These kinase-defective chimeras have been shown to act as dominant negative MAPK inhibitors.
For our studies on the mechanism of signal transmission from oncogenic Ras to the c-fos promoter, we have decided to follow an alternative strategy by preparing MAPK chimeras carrying a C-terminal CAAX sequence. The rationale for this strategy was that the CAAX sequence as a farnesylation signal should anchor the chimeras to the plasma membrane and sequester MAPKKs and other MAPK binding proteins. Furthermore, as a translocation of activated MAPKs from the cytosol to the nucleus is considered essential for the MAPK-mediated activation of transcription factors, the trapping of upstream activators and/or dimerization with endogenous MAPKs (45) at the plasma membrane might lead to an inhibition of signal transmission from transforming Ras to the c-fos promoter.
The studies presented here demonstrate that this is indeed the case. Both ERK1-CAAX and ERK2-CAAX but not the corresponding SAAX chimeras block the transcriptional activation of a chloramphenicol acetyltransferase (CAT) reporter gene driven by a truncated human c-fos promoter consisting of the SRE and the putative upstream AP-1 binding site. The MAPK CAAX variants were found to act as isozyme-specific dominant negative mutants. The isotype-specific inhibitory effect is inferred to result from complex formation with endogenous MAPKs sequestered to the plasma membrane.
In a publication that appeared during the preparation of this report, Brunet et al. (5) demonstrated that sequestering p42/p44 MAPK in the cytoplasm by expression of a catalytically inactive mutant of cytoplasmic MAPK phosphatase (MKP-3) inhibits Elk-1-dependent transcription. The data presented here provide additional, independent evidence supporting the conclusion that the translocation of activated MAPKs to the nucleus is essential for the transcriptional activation of mitogen-induced genes like c-fos.
MATERIALS AND METHODS
Reagents and plasmids.
Epidermal growth factor (EGF), leupeptin, and aprotinin were purchased from Sigma (Vienna, Austria). d-threo[Dichloroacetyl-1,2-14C]chloramphenicol (58.4 mCi/mmol) and [γ-32P]ATP (10 mCi/ml; 3,000 mCi/mmol) were purchased from DuPont NEN (Vienna, Austria). Silica gel-coated high-performance thin-layer chromatography plates were supplied by Macherey-Nagel (Düren, Germany). Acetyl coenzyme A and mouse anti-hemagglutinin (HA) monoclonal antibody 12CA5 were obtained from Boehringer Mannheim (Mannheim, Germany). Ethylacetate was purchased from Fluka Chemicals (Buchs, Switzerland). Dulbecco’s modified Eagle’s medium (DMEM) and l-glutamine were obtained from BioWhittaker (Vienna, Austria). High-glucose (4.5 g/liter) DMEM containing N-acetyl-l-alanyl-l-glutamine was from Schöller Pharma (Vienna, Austria). Fetal bovine serum (FBS), Lipofectin transfection reagent, and Opti-MEM I were obtained from Life Technologies, Inc. (Vienna, Austria). Pansorbin was obtained from Calbiochem (Lucerne, Switzerland). PCR primers used for subcloning were obtained from Microsynth GmbH (Balgach, Switzerland). Rabbit polyclonal anti-ERK1, anti-ERK2, and anti-CAAX antibodies and rabbit monoclonal antibody 9E10 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Mouse monoclonal anti-green fluorescent protein (GFP) antibody was purchased from Clontech Laboratories Inc. (Palo Alto, Calif.). All other reagents were from Sigma (St. Louis, Mo.). Plasmids pEF-ERK2-CAAX and pEF-ERK2-SAAX, carrying the gene under control of the human EF1α promoter, were constructed from mouse ERK2 by PCR using pLNCAL7-HA-ERK2 as a template and primers 5′-GACTACGCGGCCGCCATGGGCCCGGAGATGGTCCG-3′ and 5′-CCCTTGTGCGGCCGCAGATCTGTATCCTGGCTGGAA-3′. NotI restriction sites (underlined) were generated at both ends of the cDNA. The resulting PCR fragment encoding amino acids 8 to 335 of murine ERK2 was digested with NotI overnight, gel purified, and subcloned into the NotI site of pEF-CAAX, a modified pEFneo plasmid expressing the carboxy-terminal 19 amino acids of wild-type c-Ha-Ras, which includes the Ha-Ras carboxy-terminal farnesylation signal. PCR was also used to eliminate the ERK2 stop codon to allow in-frame fusion to the farnesylation sequence. As control, the amplified ERK2 cDNA was also inserted into the NotI-digested pEF-SAAX vector. pEF-SAAX was obtained by replacing Cys-186 in the farnesylation sequence with a serine residue by PCR-directed mutagenesis. To obtain pEF-HA-ERK1, a 1.3-kb NotI fragment, encompassing the entire open reading frame of human ERK1 and sequences encoding the nine-amino-acid HA epitope (YPYDVPDYA) at the N terminus, was excised from pCEP4-HA-ERK1 and ligated to pEFneo previously digested with the same restriction enzyme. Plasmids pEF-HA-ERK1-CAAX and pEF-HA-ERK1-SAAX were generated by PCR with pEF-HA-ERK1 as the template and oligonucleotides 5′-CTCTAGGCGGCCGCCATGGCTTACCCATACGATGTTCCA-3′ and 5′-CAGCACTGCGGCCGCGAAGCGTGCTGTCTCCTGGAAG-3′. Both primers harbor a NotI restriction site (underlined). The PCR product was digested with NotI and cloned into the NotI sites of pEF-CAAX and pEF-SAAX, respectively. To construct pEF-GFP-CAAX and pEF-GFP-SAAX, amplification by PCR was performed from pGFP-N3 (Clontech) with the forward primer 5′-GGTACCGCGGCCGCGGGATCCATCGCCACCATG-3′ and the reverse primer 5′-GATTATGGCGGCCGCTCTAGATCCGGACTTGTATAGTTC-3′ (NotI sites are underlined). Subcloning was carried out as described above. All constructs were confirmed by DNA sequencing, and the expression of proteins was verified by immunoblotting.
The mammalian expression vector containing the BXB mutant of c-Raf-1 kinase lacking amino acids 26 to 203 was kindly provided by H. Mischak. pSRαII-HaRasL61, containing an amino acid substitution at position 61 of Phe to Leu, which converts the Ha-Ras protein in the constitutively active GTP-bound form, was a generous gift from M. Karin. pFC-MEK1(dN3/S218E/S222D), which represents the constitutively active form of MEK1 (55), was purchased from Stratagene. This mutant was made by deletion of amino acid residues 32 to 51 and replacement of Ser-218 by glutamic acid and Ser-222 by aspartic acid.
Cell culture and transient transfection.
African green monkey kidney fibroblasts (COS-1 or COS-7), obtained from the American Type Culture Collection, were maintained in high-glucose DMEM containing 10% FBS and 1.028 g of N-acetyl-l-alanyl-l-glutamine/liter. NIH 3T3 cells, obtained from the European Collection of Animal Cell Cultures, were cultured in DMEM supplemented with 10% FBS and 2 mM l-glutamine. All cells were grown in 100-mm-diameter culture dishes (Falcon) at 37°C in a humid atmosphere (5% CO2, 95% air). Transient transfections were performed on 70 to 80% confluent monolayers in 100-mm-diameter dishes with the combinations of plasmids indicated in the figure legends, using Lipofectin (GIBCO-BRL) according to the manufacturer’s instructions. The parent vector, pEFneo, was added to transfections as needed to equalize the total amount of transfected DNA. At 16 h posttransfection, cells were washed once in serum-free medium and incubated for 24 h at 37°C in DMEM–10% FBS prior to either serum starvation in DMEM containing 0.5% FBS for 10 to 15 h or harvesting. For immunocomplex kinase assays, serum-starved COS-1 cells were subjected to a 5-min EGF (10 nM) treatment at 37°C. For CAT reporter assays, NIH 3T3 cells were transfected by Lipofectin as described above except that the transfection medium was replaced by DMEM supplemented with 0.5% FBS and cells were deprived of serum for 40 h before harvesting.
Subcellular fractionation.
The method was adapted from that of Chen et al. (9), with minor modifications. Cells were washed once with cold phosphate-buffered saline (pH 7.2) and scraped into 0.5 ml of hypotonic lysis buffer (1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 1 mM Na3VO4, 2 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol, 40 μg of phenylmethylsulfonyl fluoride/ml, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). The cell suspension was incubated on ice for 20 min to allow swelling. All subsequent manipulations were performed at 4°C on ice. The cells were then homogenized with 40 strokes in a Dounce homogenizer with a tight-fitting pestle and centrifuged at 500 × g for 10 min at 4°C to pellet the nuclei. To prepare the cytosolic fraction, the supernatant was centrifuged at 100,000 × g for 30 min at 4°C, whereas the nuclear pellet was resuspended in 100 μl of hypotonic lysis buffer, loaded onto 1 ml of 1 M sucrose in lysis buffer, and centrifuged at 1,600 × g for 10 min. Both the sucrose-purified nuclei and the membrane pellet obtained from the 100,000 × g centrifugation step were solubilized in hypotonic lysis buffer containing 1% NP-40 for 1 h on ice and centrifuged at 20,000 × g for 10 min to remove insoluble material. After extensively washing of the sedimented material, cytoskeletal proteins were extracted by solubilizing the NP-40-insoluble membrane pellet in 5× sodium dodecyl sulfate (SDS) sample buffer (300 mM Tris-HCl [pH 6.8], 50% glycerol, 10% [vol/wt] SDS, 25% [vol/vol] β-mercaptoethanol, 0.4 mg of bromphenol blue/ml) at 95°C until the pellet was dissolved. Equal proportions of each extract were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) on a 14% gel and then immunoblotted with a rabbit polyclonal anti-CAAX antibody (C-20; Santa Cruz). This antibody recognizes an Ha-Ras-specific epitope between residues 170 and 189, corresponding to the farnesylation sequence of c-Ha-Ras. Since this antibody specifically interacts with the CAAX and SAAX motifs of the MAPK chimeras, it was suitable for immunoprecipitation and immunoblotting in all cell lines expressing MAPK-CAAX and MAPK-SAAX chimeras.
For our translocation studies, cytosolic and nuclear fractions were immunoprecipitated with an anti-HA antibody, the immunoprecipitates were subjected to SDS-PAGE (14% gel) and blotted, and the membranes were probed with a polyclonal anti-ERK1 antibody.
Western blot analysis.
Western blotting was carried out on subcellular fractions prepared as described above. To verify expression levels of MAPK chimeras expressed in COS-1 cells, whole-cell lysates containing approximately 50 μg of total cellular protein were prepared. Protein concentrations were determined by the Bradford method. Samples were boiled with 5× SDS sample buffer for 5 min, resolved on an SDS–14% polyacrylamide gel, and transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Vienna, Austria) in 25 mM Tris-HCl (pH 7.5)–193 mM glycine–20% (vol/vol) methanol–0.1% SDS at 300 mA (constant) for 1 h. Membranes were blocked overnight in 1× TBST (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% [vol/vol] Tween 20) containing 5% (wt/vol) nonfat milk powder and then probed with polyclonal anti-ERK1 antibodies (diluted at 1:200 in blocking solution), anti-ERK2 antibodies (1:200 dilution), anti-CAAX antibodies (1:200 dilution) or monoclonal anti-GFP antibodies (1:500 dilution) for 1 h at room temperature. After washes with TBST (once for 15 min and twice for 10 min), the blots were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G (1:2,000 dilution in TBST containing 5% [wt/vol] nonfat milk powder) for 45 min at room temperature. The membranes were washed again in TBST (once for 15 min and four times for 5 min each), and immunoreactive bands were visualized by enhanced chemiluminescence as described by Überall et al. (90).
Immunocomplex kinase assay.
Transfected cells were washed once with ice-cold phosphate-buffered saline and then lysed in 0.5 ml of NP-40 lysis buffer (50 mM Tris-HCl [pH 7.3], 50 mM NaCl, 5 mM Na4P2O7 · 10H2O, 5 mM NaF, 5 mM Na3VO4, 5 mM EDTA, 50 mg of aprotinin/ml, 50 mg of leupeptin/ml, 2% NP-40) for 30 min on ice. Lysates were clarified by centrifugation at 13,000 × g for 10 min at 4°C. After preclearing of the supernatants with Pansorbin for 30 min, the lysates were incubated with anti-CAAX antibodies or c-Myc monoclonal antibody 9E10 (1 μg/10 μl; Santa Cruz) overnight at 4°C on a rotating wheel. Immune complexes were subsequently precipitated by adding 25 μl of Pansorbin and continuous rotation for 1 to 2 h at 4°C. Immunoprecipitates were collected by centrifugation (2 min, 10,000 × g) and washed three times with lysis buffer and twice with kinase buffer (25 mM HEPES-NaOH [pH 7.5], 2 mM MnCl2, 20 mM MgCl2). MAPK activities were assayed by resuspending the pellets in a total volume of 20 μl of kinase buffer containing 9 μg of glutathione S-transferase (GST)–Elk-1, 10 mM disodium ATP, and 0.4 μCi of [γ-32P]ATP, followed by incubation for 30 min at 30°C. The reactions were terminated by the addition of 6 μl of 5× SDS sample buffer. After boiling for 5 min, the samples were separated on an SDS–10% polyacrylamide gel and transferred to an Immobilon-P membrane (Millipore).
Phosphorylated GST–Elk-1 was visualized by autoradiography, or phosphorylation analysis was done by phosphorimaging of the corresponding Immobilon membranes.
CAT assay.
CAT assays were performed as described previously (89).
RESULTS
In vivo expression of ERK1 and ERK2 variants containing a C-terminal CAAX or SAAX sequence.
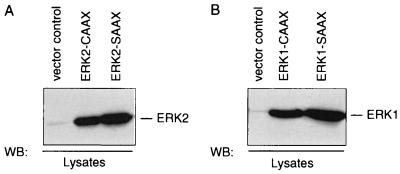
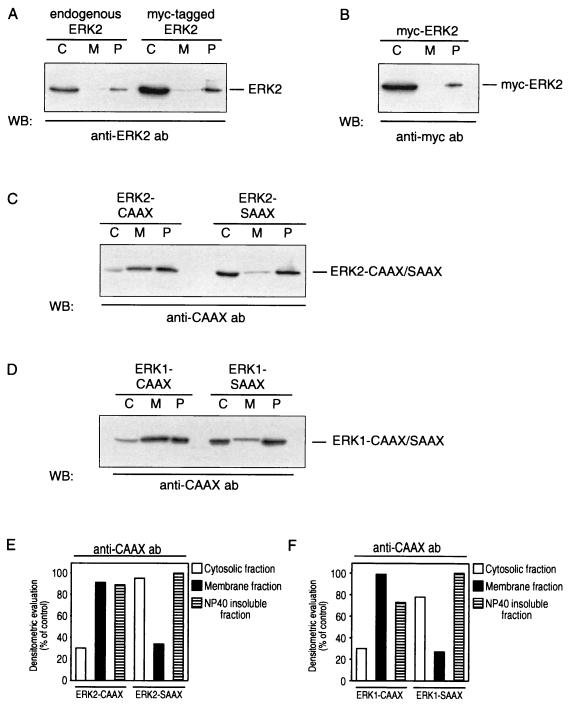
To discriminate between endogenous ERKs and the overexpressed ERK1/2 variants, we used an anti-CAAX antibody which recognizes an epitope between residues 170 and 189 of Ha-Ras, corresponding to the farnesylation sequence. As shown in Fig. 1, the constructs encoding the ERK1 and ERK2 variants containing a C-terminal CAAX or SAAX sequence are highly expressed in transiently transfected COS-1 cells. The subcellular distribution of endogenous or Myc-tagged ERK2 and of the ERK2-CAAX and -SAAX chimeras is demonstrated in Fig. 2. In agreement with the findings of others (63, 72), endogenous ERK2 is predominantly found in the cytosol and in part associated with the NP-40-insoluble, particulate fraction representing mainly cytoskeletal elements like microtubules and F-actin filaments. The same distribution is seen following expression of wild-type ERK2 and a Myc-tagged ERK2 (Fig. 2A and B).
FIG. 1.
Expression patterns of ERK-CAAX and ERK-SAAX chimeras in COS-1 cells. COS-1 cells were transiently transfected with 10 μg of the indicated plasmids, using a Lipofectin transfection procedure according to the manufacturer’s instructions. At 48 h posttransfection, whole-cell lysates containing approximately 50 μg of protein were separated on SDS–14% polyacrylamide gels, transferred to PVDF membranes, and immunoblotted (Western blotted [WB]) with rabbit polyclonal anti-CAAX antibody C-20 (Santa Cruz). Shown are representative expression patterns out of four independent experiments performed.
FIG. 2.
Subcellular distribution of MAPKs in COS-1 cells. Shown are the subcellular distributions of endogenous ERK2 versus Myc-tagged ERK2 (A and B), ERK2-CAAX versus ERK2-SAAX (C), and ERK1-CAAX versus ERK1-SAAX (D) and densitometric evaluation of the gels (E and F). At 48 h posttransfection, cytosol (lanes C), membrane (lanes M), and cytoskeletal (NP-40-insoluble particulate; P) fractions were prepared from cells transfected with the ERK chimeras and nontransfected, exponentially growing COS-1 cells. Equal amounts of proteins of each fraction were separated by SDS-PAGE (14% gel), transferred to a PVDF membrane, and probed with rabbit polyclonal anti-ERK2 antibody (ab) (A), with mouse monoclonal anti-Myc antibody 9E10 (B), and with rabbit polyclonal anti-CAAX antibody targeted to the CAAX/SAAX motif of the ERK chimeras. Shown are representative Western blots (WB) out of four independent experiments performed.
In contrast to the behavior of the wild-type ERK2, the ERK2-CAAX mutant is predominantly found in the membrane and the particulate fraction (Fig. 2C). Similar results were obtained with the ERK1-CAAX mutant (Fig. 2D). Consistent with our working hypothesis, ERK1-SAAX and ERK2-SAAX chimeras were found, like endogenous MAPKs, predominantly in the cytosolic and nonsoluble fractions (Fig. 2C and D).
Intracellular activation of the ERK1 and ERK2 chimeras.
To check whether the chimeric ERK1 and ERK2 proteins containing the C-terminal CAAX or SAAX sequence are functionally active in vivo, we determined whether EGF is able to activate these MAPK chimeras expressed in COS-1 cells.
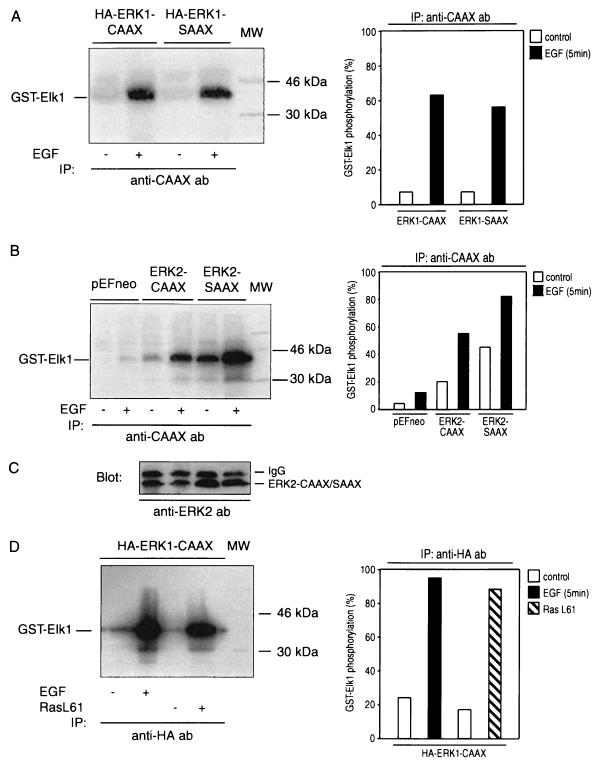
As shown in Fig. 3, addition of EGF to COS-1 cells expressing the ERK1 or ERK2 chimeras results in a marked activation of both CAAX and SAAX variants. The higher basal activity of ERK2-SAAX than of ERK2-CAAX is explained by the higher expression level of the SAAX variant as shown in the Western blot (Fig. 3C).
FIG. 3.
MAPK chimeras can be activated by EGF. Shown are the intracellular activation patterns of ERK1-CAAX/SAAX (A), ERK2-CAAX/SAAX (B), and ERK1-CAAX-HA (D). Loading controls were done by blotting lysates from panels A and B with anti-ERK1 (not shown) and anti-ERK2 (C) antibodies (ab). Briefly, COS-1 cells were transiently transfected with pEFneo-ERK2-CAAX/SAAX, pEFneo-ERK1-CAAX/SAAX, or control vector pEFneo (10 μg of each cDNA expression plasmid per 10-cm-diameter dish). Following transfection, the cells were incubated in 10% FBS for 24 h, serum starved in DMEM containing 0.5% FBS for 24 h, and left unstimulated or stimulated with 10 ng of EGF/ml for 5 min. Cell extracts were prepared as described in Materials and Methods. MAPK chimeras were immunoprecipitated (IP) with anti-CAAX or anti-HA antibodies, and kinase activities were determined by an immunocomplex kinase assay, using purified bacterially expressed GST–Elk-1 as a substrate. Panels A, B, and D show representative autoradiograms out of three independent kinase assays performed. MW, molecular weight markers.
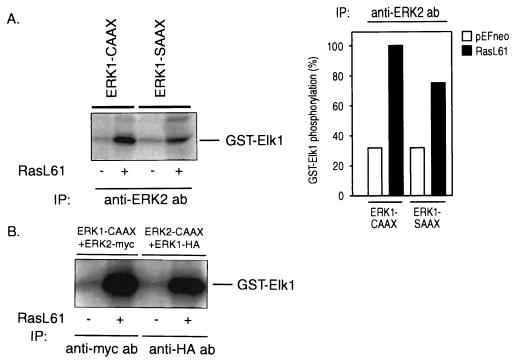
Given that the anti-CAAX antibody is targeted to an epitope of Ras, a coprecipitation of Ras-associated kinases could confuse results obtained from Ras-overexpressing cells. To avoid this problem, cells were transfected with HA-tagged ERK1-CAAX. After stimulation with EGF or RasL61, the tagged ERK-CAAX chimera was selectively precipitated by an anti-HA antibody, and the kinase activity was determined with GST–Elk-1 as a substrate. As shown in Fig. 3D, the results are similar to those in Fig. 2 and 7, where ERK-CAAX had been isolated by using an anti-CAAX antibody. These data clearly demonstrate that both RasL61 and EGF activate ERK1-CAAX and that the elevated phosphorylation of Elk-1 is not due to some other coprecipitated Elk-1 kinases. Identical results were obtained with HA-tagged ERK2-CAAX (data not shown).
FIG. 7.
Immunodepletion of cells from the ERK-CAAX chimeras is accompanied by a dramatic decrease of the amount and activity of wild-type MAPKs caused by association of endogenous ERKs with membrane-anchored MAPK chimeras activated by oncogenic Ha-Ras. (A) NIH 3T3 cells were transiently transfected with ERK2-CAAX or -SAAX chimera or with HA-tagged ERK1-CAAX or -SAAX (4 μg of each plasmid) together with 2 μg of either activated pSR-αII-Ha-RasL61 or 2 μg of empty expression vector pEFneo for 8 h, using Lipofectin as the transfection reagent. Following transfection, the cells were incubated in DMEM supplemented with 10% FBS overnight and then deprived of serum (0.5%) for 24 h prior to cell lysis. The ERK chimeras were immunoprecipitated (IP) from cell lysates containing 300 μg of total protein with rabbit polyclonal anti-CAAX antibodies. Enzyme activities were determined by an immunocomplex kinase assay using GST–Elk-1 as a substrate. The phosphorylated proteins were separated on an SDS–10% polyacrylamide gel, blotted on PVDF membranes, and visualized by autoradiography. The position of GST–Elk-1 is indicated on the left. MW, molecular weight markers. (B) The cell extracts obtained in panel A were precleared from the CAAX and SAAX chimeras by two successive immunoprecipitation steps using the anti-CAAX antibody, targeted to the CAAX/SAAX motif. After depletion of the ERK chimeras, the endogenous ERKs were precipitated from these cleared lysates by anti-ERK1 or anti-ERK2 antibody, and their activities were determined with GST–Elk-1 as a substrate. The kinase reaction mixtures were separated by SDS-PAGE (10% gel), blotted, autoradiographed, and then stained with anti-ERK2 antibody. As a control, NIH 3T3 cells were transiently transfected with either pEFneo or pSR-αII Ha-RasL61. After 24 h, cells were serum starved in medium supplemented with 0.5% FBS for 24 h. The lysates were precleared with the anti-CAAX antibody and then divided into two portions for immunoprecipitation of endogenous ERK1 and ERK2, using the antibodies described above. Shown are representative results out of three experiments done in duplicate. IgG, immunoglobulin G. (C) NIH 3T3 fibroblasts were cotransfected with a Myc-tagged ERK2 (100 ng) and Ha-RasL61 or an empty vector (pEFneo) together with an expression vector encoding ERK2-CAAX or ERK2-SAAX as indicated. After immunoprecipitation with Myc-specific antibody 9E10, immunocomplex kinase assays were performed with GST–Elk-1 as a substrate. The autoradiographed blot was subsequently probed with an anti-ERK2 antibody. Shown are a representative autoradiogram and immunoblot out of three experiments performed.
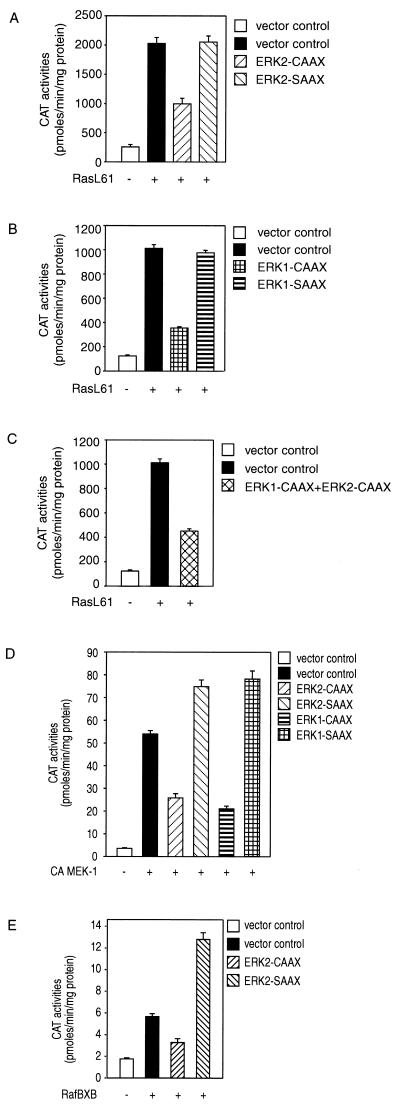
Expression of the CAAX chimeras of ERK1 or ERK2 but not of the corresponding SAAX variants inhibits transcriptional activation of c-fos by RasL61, Raf-1, and MEK1.
Expression of RasL61 results in a transcriptional activation of the c-fos–CAT construct (Fig. 4). The induction of c-fos by transforming Ras is depressed in cells expressing the ERK1- or ERK2-CAAX chimera but not by expression of the corresponding SAAX variants (Fig. 4A and B). Coexpression of ERK1-CAAX and ERK2-CAAX did not enhance the inhibitory effect (Fig. 4C), suggesting that Ras-mediated c-fos induction requires both ERK1 and ERK2 or that the noninhibitable fraction reflects activation by an ERK-independent pathway.
FIG. 4.
The induction of c-fos by transforming Ras, constitutively active MEK1, or RafBXB is depressed in cells expressing the MAPK-CAAX chimeras but not by the corresponding SAAX variants. Coexpression of ERK1-CAAX and ERK2-CAAX did not enhance the inhibitory effect. ERK2- and ERK1-CAAX chimeras significantly block transcriptional activation of a c-fos–CAT reporter induced by transforming Ras (A and B). Coexpression of ERK1-CAAX and ERK2-CAAX did not enhance the inhibitory effect (C). MAPK-CAAX chimeras inhibit the transcriptional activation of c-fos induced by constitutively active MEK1 or RafBXB (D and E). Subconfluent NIH 3T3 cells were transfected with 2 μg of pEFneo (vector control) or 2 μg of pSR-αII-Ha-RasL61, pEF-RafBXB, or pFC-MEK1(dN3/S218E/S222D) alone or together with 4 μg of pEF plasmid expressing an ERK1,2-CAAX or -SAAX variant; 1 μg of the c-fos–CAT reporter plasmid (-711-TK-CAT) was added to all combinations. The total amount of plasmid DNA was adjusted to 6 μg with empty pEFneo vector. Following transfection and serum starvation (0.5% FBS) for 48 h, a CAT reporter kinase assay was done as described by Kampfer et al. (44). CAT activities are representative of three independent experiments done in triplicate (bars indicate means ± standard errors, n = 9).
To confirm that the ERK-CAAX variants indeed interfere with the Ras/Raf pathway, we determined the effect of the ERK-CAAX variants on the transcriptional activation of the c-fos–CAT construct by constitutively active mutants of MEK1 or Raf (RafBXB). As shown in Fig. 4D, both ERK1-CAAX and ERK2-CAAX inhibit the transcriptional activation of c-fos by constitutively active MEK1 [plasmid pFC-MEK1(dN3/S218E/S222D)], whereas the SAAX chimeras exert even a superinduction. Expression of ERK2-CAAX also significantly depresses the transcriptional activation of c-fos by RafBXB (Fig. 4E; the effect of ERK1-CAAX was not determined in this experiment).
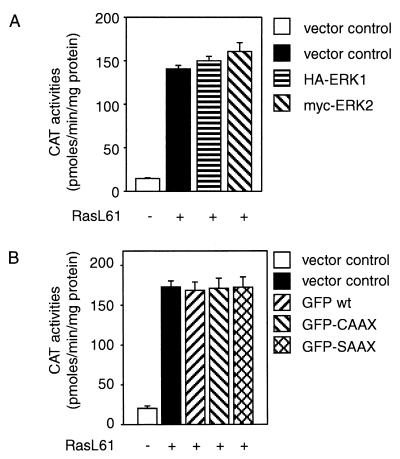
Overexpression of wild-type ERK1 or ERK2 does not interfere with Ras-mediated c-fos induction, excluding nonspecific effects caused by enzyme overexpression as responsible for the inhibitory activities of the CAAX variants of ERK1 and ERK2 (Fig. 5A). As the overexpression of a protein containing the CAAX farnesylation signal may interfere with the prenylation and activation of Ras, we investigated whether overexpression of a GFP-CAAX protein would affect the RasL61-mediated transcriptional activation of c-fos. As shown in Fig. 5B, this was not the case.
FIG. 5.
Overexpression of wild-type ERK2 and ERK1 and expression of membrane-targeted GFP-tagged CAAX and SAAX chimeras does not interfere with L61-Ha-Ras-mediated c-fos–CAT transactivation, excluding nonspecific effects caused by enzyme overexpression or inhibition of Ras prenylation. The experiments were designed as described in the legend to Fig. 4. (A) NIH 3T3 cells were transiently transfected with the empty control plasmid pEFneo (2 μg) or constitutively active pSR-αII-Ha-RasL61 (2 μg) alone or together with either pEFneo-ERK2-myc (4 μg) or pEFneo-ERK1-HA (4 μg); 1 μg of the c-fos–CAT reporter plasmid (-711-TK-CAT) was added to all combinations. DNA concentrations were kept constant by adding the empty control vector pEFneo. In both panels, experiments were designed as described in the legend to Fig. 4, and CAT activities are representative of three independent experiments done in triplicate (bars indicate means ± standard errors, n = 9). (B) NIH 3T3 cells were transiently transfected as follows: control vector pEFneo (2 μg) alone or with pSR-αII-Ha-RasL61 (2 μg) or 4 μg of pEFneo plasmid expressing GFP (pEFneo-GFP-CAAX, pEFneo-GFP-SAAX, or pEFneo-GFP wt [wild type]); 1 μg of the c-fos–CAT reporter plasmid (-711-TK-CAT) was added to all combinations.
ERK-CAAX chimeras associate selectively with corresponding wild-type ERKs.
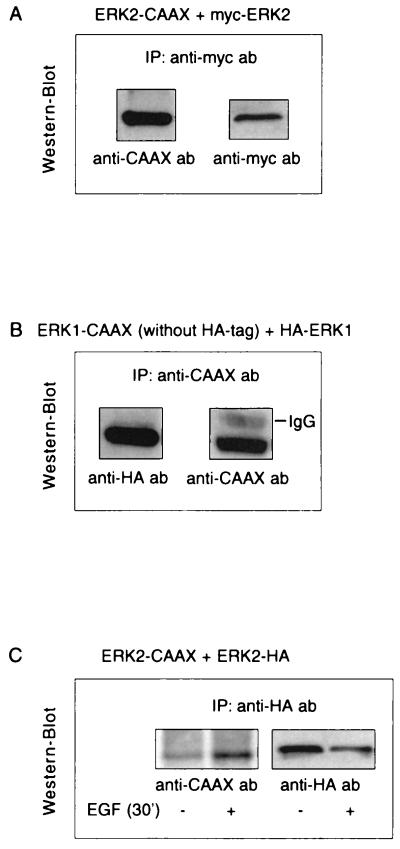
It has recently been shown that ERKs form homodimers in vivo and that phosphorylation of one monomer is sufficient for dimer stabilization (45). Dimerization of an ERK-CAAX chimera with the corresponding endogenous ERK would offer an explanation for the inhibitory effects of the ERK-CAAX chimeras on c-fos induction. We therefore investigated whether such complexes consisting of an ERK-CAAX and a corresponding tagged wild-type ERK are formed in vivo. For this purpose, cells were cotransfected with either ERK2-CAAX and Myc-tagged ERK2 or ERK1-CAAX and HA-tagged ERK1. ERK-CAAX mutants and the corresponding wild-type ERKs were immunoprecipitated with specific antibodies targeted to the CAAX, Myc, and HA epitopes, respectively. Immunoprecipitates were separated by SDS-PAGE, transferred to a PVDF membrane, and probed with a rabbit polyclonal anti-CAAX antibody, a mouse monoclonal anti-HA antibody, or a mouse monoclonal anti-Myc antibody. As shown in Fig. 6, in lysates from cells coexpressing ERK2-CAAX and Myc-tagged ERK2, anti-Myc antibody coprecipitates ERK2-CAAX together with Myc-tagged ERK2. Similarly, in lysates from cells cotransfected with ERK1-CAAX and HA-tagged ERK1, anti-CAAX antibody coprecipitates HA-tagged ERK1 together with ERK1-CAAX (Fig. 6). The same results are obtained if the precipitating antibody is targeted to the other binding partner; i.e., anti-CAAX coprecipitates ERK2-Myc in cells expressing ERK2-CAAX and Myc-tagged ERK2 and anti-HA antibody coprecipitates ERK1-CAAX together with HA-tagged ERK1 in cells coexpressing these constructs (data not shown). Since these coimmunoprecipitation studies were performed under conditions where MAPKs are fully activated, further experiments were conducted to determine whether complex formation between the ERKs is phosphorylation (activation) dependent as described in the recent study (45). COS-1 cells transiently cotransfected with ERK2-CAAX and ERK2-HA were serum starved for 16 h in DMEM with 0.5% fetal calf serum followed by serum-free medium for 6 h and left untreated or stimulated with 10 ng of EGF/ml for 30 min. ERK2-CAAX was immunoprecipitated with the anti-CAAX antibody, and coimmunoprecipitated HA-ERK2 was analyzed by immunoblotting with an anti-HA antibody. As shown in Fig. 6C, while in resting cells only a weak interaction was visible, complex formation between ERK2-CAAX and HA-ERK2 increased significantly in response to EGF, indicating that ERK homodimerization is clearly activation dependent. Coexpression of ERK2-CAAX with HA-tagged ERK1 or ERK1-CAAX with Myc-tagged ERK2 yielded no evidence of heterodimerization (data not shown).
FIG. 6.
Isotype-specific complex formation of the ERK-CAAX chimeras with their endogenous counterparts. (A and B) COS-7 cells were cotransfected with either ERK2-CAAX and Myc-tagged ERK2 or ERK1-CAAX and HA-tagged ERK1. Following transfection, cells were grown in DMEM plus 10% FCS for 40 h. ERK-CAAX mutants and the corresponding wild-type ERKs were immunoprecipitated (IP) with specific antibodies (ab) targeted to the CAAX, Myc, or HA epitope, respectively. Immunoprecipitates were separated by SDS-PAGE, transferred to PVDF membranes, and probed with a rabbit polyclonal anti-CAAX antibody, mouse monoclonal anti-HA antibody, or mouse monoclonal anti-Myc antibody. As can be seen in lysates from cells coexpressing ERK2-CAAX and Myc-tagged ERK2, anti-Myc antibody coprecipitates ERK2-CAAX together with Myc-tagged ERK2. Similarly, in lysates from cells cotransfected with ERK1-CAAX and HA-tagged ERK1, anti-CAAX antibody coprecipitates HA-tagged ERK1 together with ERK1-CAAX. The same results are obtained if the precipitating antibody is targeted to the other binding partner; i.e., anti-CAAX coprecipitates ERK2-Myc in cells expressing ERK2-CAAX and Myc-tagged ERK2 and anti-HA antibody coprecipitates ERK1-CAAX together with HA-tagged ERK1 in cells coexpressing these constructs (data not shown). IgG, immunoglobulin G. (C) To examine an activation-dependent association between MAPKs, COS-7 cells were cotransfected with ERK2-CAAX and HA-tagged ERK2, serum starved for 16 h in DMEM containing 0.5% fetal calf serum followed by serum-free medium for 6 h, and then stimulated with 10 ng of EGF/ml for 30 min or left untreated prior to cell lysis. ERK2-CAAX was immunoprecipitated with the anti-CAAX antibody, and coimmunoprecipitated wild-type ERK2 was analyzed by Western blotting with a mouse monoclonal anti-HA antibody. No evidence for heterodimerization was observed.
Immunodepletion of cells from the CAAX chimeras concomitantly decreases the amount and activity of endogenous ERKs.
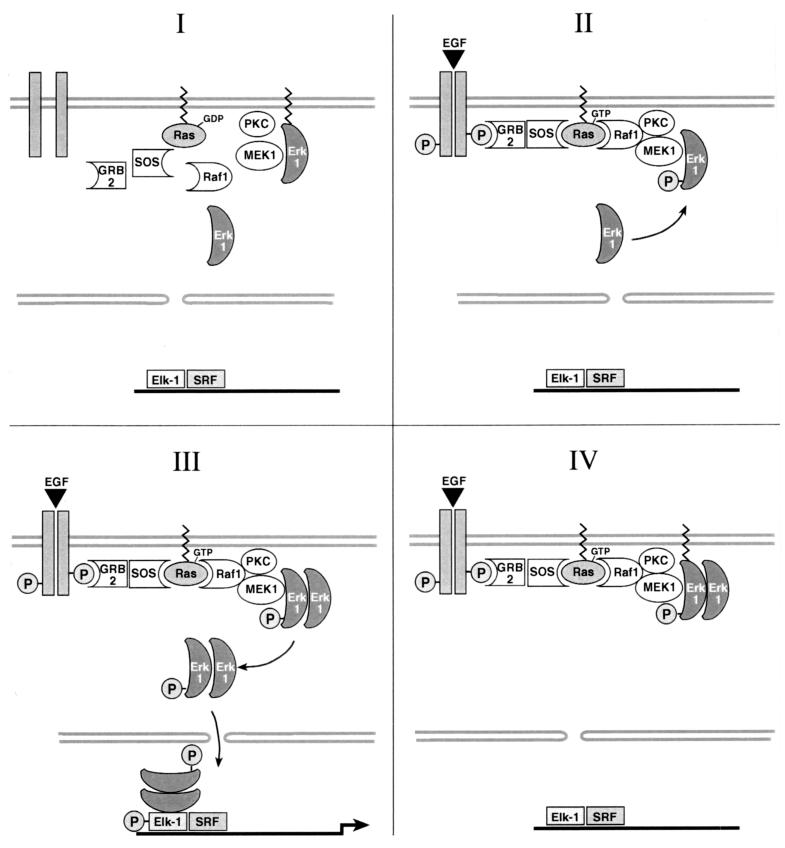
As ERK-CAAX chimeras are localized at the plasma membrane and homodimerize in an activation-dependent manner with their corresponding endogenous counterparts, a model can be proposed whereby the activated ERK-CAAX fusion proteins sequester endogenous MAPKs to the cell membrane, thereby inhibiting their nuclear translocation and transcriptional activation of c-fos (see Fig. 12). To test this hypothesis, we first determined whether expression of an ERK-CAAX chimera reduces the level of free (non-ERK-CAAX-bound) endogenous ERK. NIH 3T3 cells were cotransfected with either ERK1/2-CAAX or ERK1/2-SAAX along with RasL61 or a control plasmid (pEFneo). After serum starvation for 24 h, the ERK-CAAX variants were immunoprecipitated and their activities were measured by an in vitro kinase assay using GST–Elk-1 as a substrate.
FIG. 12.
Schematic presentation of the postulated mechanism of novel membrane-anchored ERK signal transduction. In accordance with our working hypothesis, we propose a model whereby the activated ERK-CAAX fusion proteins sequester endogenous MAPKs to the cell membrane, thereby inhibiting their nuclear translocation and transcriptional activation of c-fos.
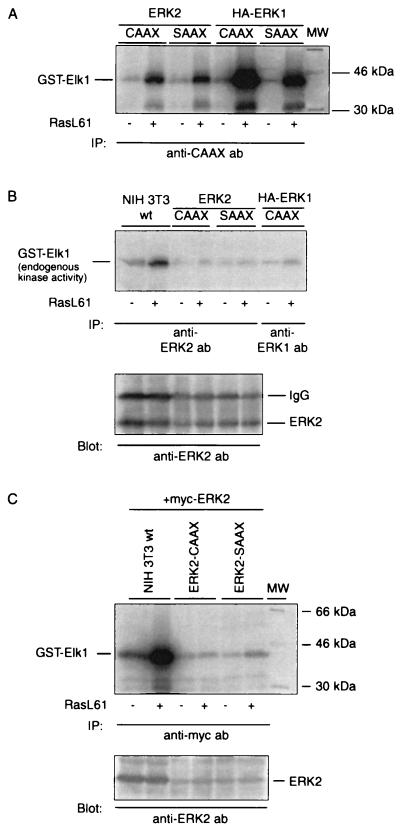
In accordance with the data shown in Fig. 3, demonstrating that the CAAX and SAAX chimeras of both ERK1 and ERK2 are functionally active in intact COS-1 cells, Fig. 7A depicts the activation of these ERK chimeras by transforming RasL61 in NIH 3T3 cells. The apparent stronger activation of ERK1-CAAX and -SAAX is explained by a higher expression level of the ERK1 variants.
The cell lysates obtained in Fig. 7A were cleared from the ERK fusion proteins by two successive immunoprecipitation steps using the anti-CAAX antibody. As a control, NIH 3T3 cells were transiently transfected with RasL61 or pEFneo and serum starved for 24 h, and the cell extracts were incubated with the anti-CAAX antibody to exclude the possibility that the Ras-specific antibody pulls down Ras-associated kinases, thereby reducing the amount of endogenous MAPKs in the absence of coexpressed ERK-CAAX. After treatment with the anti-CAAX antibody, the endogenous ERKs were precipitated by anti-ERK1 or anti-ERK2 antibodies, their expression levels were examined by Western blot analysis, and their activities were determined with GST–Elk-1 as a substrate. Consistent with the model described above, the amount of wild-type ERKs declined markedly in the CAAX and SAAX precleared lysates of cells expressing the ERK chimeras in comparison to the control cells (Fig. 7C). Accordingly, as shown in Fig. 7B, almost no kinase activity could be detected in the immunocomplexes from the ERK-CAAX-depleted lysates. No significant reduction of endogenous ERKs after treatment with anti-CAAX antibody was observed in lysates from Ras-transfected but not ERK-CAAX/SAAX-expressing cells. To further support the notion that homodimerization of membrane-targeted CAAX chimeras with their endogenous counterparts is induced in cells transfected with RasL61 in more detail, NIH 3T3 cells were cotransfected with RasL61 and a Myc-tagged ERK2 (100 ng of DNA per well was determined by titration to correspond approximately to endogenous ERK2 expression) along with either ERK2-CAAX, ERK2-SAAX, or a control plasmid (pEFneo). Cell extracts were precleared with the anti-CAAX antibody from the ERK2-CAAX/SAAX variants, and Myc-tagged ERK2 was immunoprecipitated with an anti-Myc antibody. It is demonstrated in Fig. 7D and E that although Myc-tagged ERK2 is highly expressed and activated by RasL61 in the control cells, neither Myc-ERK2 nor kinase activity was recovered above the background levels from cells expressing the ERK2-CAAX or -SAAX chimera.
If the supposition that heterodimerization between MAPKs ERK1 and ERK2 does not occur in vivo, as reported previously (45), is correct, catalytic activity of ERK2 in ERK1-CAAX-expressing cells and of ERK1 in ERK2-CAAX-expressing cells should not be affected by immunodepleting the cells from the CAAX chimeras. To check this, NIH 3T3 cells were either transiently transfected with ERK1-CAAX or ERK1-SAAX or cotransfected with ERK1-CAAX and Myc-ERK2 or ERK2-CAAX and HA-ERK1 together with transforming Ras or an empty vector. After preclearing of the cell extracts with the anti-CAAX antibody, endogenous ERK2, Myc-ERK2, and HA-ERK1 were immunoprecipitated with anti-ERK2, anti-Myc, or anti-HA antibodies. Consistent with the model mentioned above, ERK1-CAAX does not affect the enzyme activity of endogenous or Myc-tagged ERK2 after depletion of ERK1-CAAX with an anti-CAAX antibody (Fig. 8). Similar results are obtained when the catalytic activity of HA-tagged wild-type ERK1 is examined after immunoprecipitation of ERK2-CAAX in cells expressing ERK2-CAAX (Fig. 8B).
FIG. 8.
The interaction of ERK-CAAX chimeras with wild-type ERKs is isoenzyme specific. (A) NIH 3T3 cells were transiently transfected with either ERK1-SAAX or ERK1-CAAX together with pSR-αII-Ha-RasL61 or the empty control vector pEFneo. After removal of the CAAX chimeras, endogenous ERK2 was immunoprecipitated (IP) by anti-ERK2 antibody (ab), and enzyme activity was measured with GST–Elk-1 as a substrate. (B) NIH 3T3 cells were cotransfected with pSR-αII-Ha-RasL61 or an empty vector along with ERK1-CAAX and Myc-tagged ERK2 or ERK2-CAAX and HA-tagged ERK1. After preclearing of the lysates from the CAAX variants, the tagged wild-type enzymes were immunoprecipitated with anti-Myc or anti-HA antibody, and enzyme activities were determined by an immunocomplex kinase assay with GST–Elk-1 as a substrate.
Taken together, these studies are consistent with the model that the CAAX chimeras of ERK1 and ERK2 form homodimers with their endogenous counterparts probably at the plasma membrane in cells transfected with transforming Ras, thereby reducing the levels of the free endogenous ERKs.
RasL61-induced association of the ERK-CAAX chimeras with their endogenous counterparts at the plasma membrane attenuates the nuclear translocation of wild-type MAPKs.
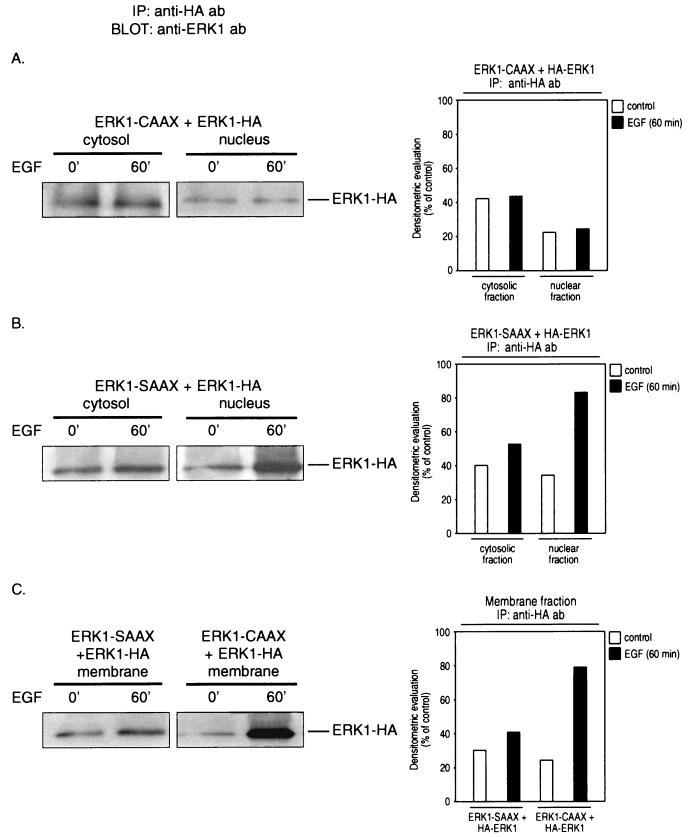
The observations described above prompted us to further investigate whether the in vivo association of membrane-anchored ERK-CAAX fusion proteins with endogenous enzymes inhibits the nuclear translocation of the wild-type ERKs. For this purpose, NIH 3T3 cells were cotransfected with HA-ERK2 together with either ERK1-CAAX or, as a control, ERK1-SAAX, serum starved for 24 h, and then stimulated with 10 ng of EGF/ml for 60 min or left untreated. Cytosol, membrane, and nuclear fractions were prepared, each fraction was immunoprecipitated with a monoclonal anti-HA antibody, and the immunoprecipitates were analyzed by immunoblotting with a polyclonal anti-ERK1 antibody. As shown in Fig. 9, upon EGF treatment, nuclear translocation of HA-tagged ERK1 was almost completely abolished in cells expressing ERK1-CAAX, whereas in cells expressing the ERK1-SAAX chimera, accumulation of wild-type ERK1 in the nucleus increased markedly in response to EGF, in agreement with our model described above. Also, accumulation of HA-tagged ERK1 in membrane fractions of cells expressing ERK1-CAAX could be detected, further supporting the postulated mechanism. Similar results were obtained when the subcellular distribution of wild-type ERK2 in response to EGF was examined in ERK2-CAAX-expressing cells.
FIG. 9.
Isotype-specific association of membrane-anchored ERK-CAAX chimeras with their endogenous counterparts at the plasma membrane inhibits the nuclear translocation of wild-type enzymes. Subconfluent NIH 3T3 cells were transiently transfected with either 2 μg of ERK1-CAAX or 2 μg of ERK1-SAAX along with 0.5 μg of HA-ERK1. After serum starvation for 24 h, the cells were stimulated with 10 ng of EGF/ml for 60 min or left untreated. Cytosol, membrane, and nuclear fractions were immunoprecipitated (IP) with monoclonal anti-HA antibodies (ab) to pull down wild-type HA-tagged ERK1, and the immunoprecipitates were subsequently analyzed by using a polyclonal ERK1 antibody. Shown are representative immunoblots and bar graphs out of three independent experiments performed.
In conclusion, these results clearly provide evidence that the phosphorylation-dependent in vivo association of the ERK-CAAX variants with endogenous ERKs sequesters the wild-type enzymes to the plasma membrane, thereby abrogating their nuclear translocation, which probably explains the inhibition of Ras-mediated c-fos induction in cells expressing the CAAX chimeras.
The ERK-CAAX variants act as isozyme-specific, dominant negative chimeras.
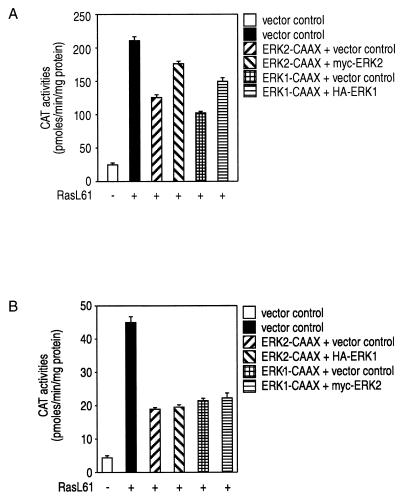
Figure 10A demonstrates that the significant reduction of Ras-mediated transcriptional activation of c-fos by ERK1-CAAX or ERK2-CAAX can be in part overcome by coexpression of the corresponding wild-type enzymes. Coexpression of wild-type ERK1 in cells expressing ERK2-CAAX or of wild-type ERK2 together with ERK1-CAAX does not affect the inhibitory effect of the CAAX variants (Fig. 10B). These data suggest that the ERK-CAAX chimeras act as isotype-specific ERK inhibitors.
FIG. 10.
The MAPK-CAAX chimeras act as isoenzyme-specific, dominant negative mutants. Shown is isoenzyme-specific rescue of ERK1-CAAX- or ERK2-CAAX-mediated suppression of Ras-induced c-fos transactivation by coexpression of the corresponding wild-type enzyme (A). NIH 3T3 cells were transiently transfected with plasmids pEFneo-ERK2-CAAX, pEFneo-ERK2-myc, pEFneo-ERK1-CAAX, and pEFneo-ERK1-HA (3 μg of each). pEFneo together with 2 μg of pSR-αII-Ha-RasL61 or 2 μg of pSR-αII-Ha-RasL61 alone or pEFneo vector alone was added as indicated; 1 μg of the c-fos–CAT reporter plasmid was added to all combinations. Coexpression of HA-tagged wild-type ERK1 in cells expressing ERK2-CAAX or of Myc-tagged wild-type ERK2 together with ERK1-CAAX does not affect the inhibitory effect of the CAAX variants (B). CAT activities are representative of three independent experiments done in triplicate (bars indicate means ± standard errors, n = 9).
A fraction of the ERK-CAAX mutant remains in the cytosol and is activated by RasL61.
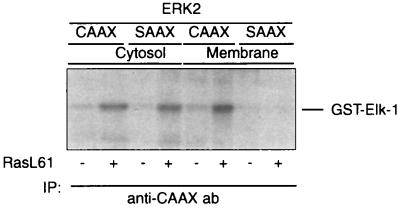
As shown above, the CAAX variants of both ERK1 and ERK2 reduce the Ras-mediated transcriptional activation of c-fos. In contrast to the marked reduction of Ras-mediated ERK activation, only a partial inhibition of the transcriptional activation of c-fos by RasL61, RafBXB, or constitutively active MEK1 could be observed. Although the CAAX chimeras are predominantly membrane bound, a part remains in the cytosol and is also activated by RasL61 (Fig. 11). This portion of the total ERK-CAAX mutant may, presumably, dimerize with the endogenous counterpart, translocate to the nucleus, and mediate the transcriptional activation of the c-fos–CAT reporter.
FIG. 11.
A fraction of the ERK-CAAX mutant remains in the cytosol and is activated by RasL61. Transfection procedures, lysate preparation, assay conditions, and abbreviations are as specified in the legend to Fig. 7. Shown is a representative autoradiogram out of three independent experiments performed.
DISCUSSION
This report describes effects obtained by the expression of constructs encoding fusion proteins consisting of ERK1 or ERK2 and the 20-amino-acid C-terminal sequence of Ha-Ras including the CAAX box at their carboxy termini. As these ERK-CAAX variants contain the Ras farnesylation signal, it was assumed that they would be anchored at the plasma membrane and therefore unable to translocate to the cell nucleus. If these ERK-CAAX chimeras are overexpressed relative to the levels of the endogenous wild-type enzymes, they may trap upstream activating proteins like MEK1/2 and/or sequester endogenous ERKs by dimerization (45) and thereby interfere with the Ras/MAPK pathway.
As controls, the corresponding proteins in which the cysteine of the CAAX box had been replaced by a serine (ERK-SAAX) were used. It is shown that these constructs are expressed in vivo and activated in intact cells by EGF or oncogenic Ras. As expected, the ERK-CAAX variants were predominantly localized in the membrane fraction, whereas the corresponding SAAX chimeras were preferentially found in the cytosol.
Expression of either ERK1-CAAX or ERK2-CAAX inhibits the transcriptional activation of a c-fos–CAT reporter by RasL61, whereas the corresponding SAAX variants did not depress the induction of c-fos by oncogenic Ras. It is demonstrated that these effects are indeed caused by an interference with the Ras/Raf/ERK pathway, as both ERK-CAAX chimeras reduce to the same extent the transcriptional activation of the c-fos–CAT reporter by constitutively active MEK1 or constitutively active RafBXB.
The ERK-CAAX fusion proteins were found to suppress the transactivation of c-fos in an isozyme-specific fashion and obviously act as transdominant negative mutants. This conclusion is based on the observation that the inhibition of Ras-mediated c-fos induction by ERK1-CAAX can be overcome by expression of wild-type ERK1 but not of wild-type ERK2. Similarly, the depression exerted by ERK2-CAAX can be compensated for by wild-type ERK2 but not by wild-type ERK1.
These data suggest that the activation of c-fos SRE by RasL61 requires the combined activities of both ERK1 and ERK2. In view of the high sequence homology between these two MAPK isozymes, it might be expected that they compensate for each other. It should be noted, however, that from the nine potential MAPK phosphorylation sites of Elk-1, only five have been shown to be phosphorylated by ERK1 (23). It is possible, therefore, that the phosphorylation patterns generated by ERK1 and ERK2, respectively, are overlapping but not identical and that activation of both MAPKs is necessary to stimulate SRE-controlled transcription. The observation that the ERK-CAAX fusion proteins are able to discriminate between ERK1 and ERK2, i.e., act as isozyme-specific inhibitors, also emerged rather unexpectedly. During the course of this study, however, it was demonstrated by others that ERKs as well as other MAPKs form homodimers in vivo and that phosphorylation reduces the dimer KD more than 3,000-fold (45). Phosphorylation of one monomer proved to be sufficient for dimer stabilization (45). As the membrane-anchored ERK-CAAX mutants are activated by EGF as well by Ras, they should be able to associate with nonphosphorylated and phosphorylated endogenous ERKs. The data shown here demonstrate that the transfection with the ERK-CAAX encoding constructs in which the ERK-CAAX fusion protein is under control of the strong EF1α promoter generates remarkably high intracellular expression levels of the corresponding protein. As the ERK-CAAX chimeras are predominantly localized at the cell membrane and activated by constitutively active Ras, it appears reasonable to postulate that the phosphorylated ERK-CAAX can form stable dimers with the corresponding endogenous enzyme in RasL61-expressing cells.
The data presented here demonstrate that this homodimerization does indeed occur. As the ERK-CAAX chimeras are present in excess compared to the endogenous ERKs, they will trap the corresponding wild-type MAPKs. Since the ERK-CAAX fusion proteins are anchored to the membrane, the trapped ERKs are sequestered at the cell membrane and unable to translocate. This model is supported by the observation that in lysates of ERK-CAAX-expressing cells, the levels and activities of the corresponding free (i.e., not ERK-CAAX-bound) endogenous ERK or cotransfected tagged wild-type enzyme are substantially reduced after immunoprecipitation of the CAAX variants (Fig. 7)—a phenomenon which is not explained by a nonspecific coprecipitation of Ras-associated kinases by the anti-CAAX antibody. Finally, a significant reduction of Ras-induced translocation to the nuclei in ERK-CAAX-expressing cells could be demonstrated.
The ERK-SAAX chimeras, which are also activated by RasL61, should also form dimers with the corresponding endogenous ERKs, a supposition which is supported by a decrease of endogenous ERK after depletion of the SAAX variant by immunoprecipitation. However, as the ERK-SAAX variants are not membrane bound, they may freely translocate to the nucleus and stimulate transcription of the c-fos–CAT reporter. In fact, dimerization has been shown to be a prerequisite for nuclear translocation (45). Thus, the formation of dimers between the ERK-SAAX chimeras and the endogenous enzymes or homodimerization of two corresponding ERK-SAAX fusion proteins explains why expression of the ERK-SAAX chimeras does not interfere with Ras-mediated c-fos induction.
Alternatively, the inhibitory effects of the ERK-CAAX variants may also be explained by a model in which the membrane-anchored ERK-CAAX chimeras trap MEK1 and MEK2 and thereby interfere with the activation of the endogenous ERKs. The difficulty with this model is that MEK1 and MEK2 have both been shown to activate both ERK1 and ERK2 (14, 54, 73, 80, 94, 98, 99). It should be mentioned, however, that evidence for separate activation pathways for ERK1 and ERK2 has been presented (47, 48, 62). Furthermore, an ERK-specific scaffold protein termed MEK partner 1 has recently been cloned; this protein interacts with ERK1 and MEK1 but not with ERK2, functionally linking a subset of components of the MAPK pathway by facilitating specific protein-protein interactions, which determines signaling specificity in the absence of overexpression of MEK1 and MEK2, respectively (78). In view of our immunodepletion studies and subcellular fractionation, it seems unlikely that this mechanism explains the isozyme-specific action of the ERK1- and ERK2-CAAX chimeras.
Considering the fact that the ERK-CAAX fusion proteins are mainly bound to the plasma membrane and homodimerize with endogenous wild-type enzymes, it is surprising that the ERK variants cause only a partial inhibition of the Ras-mediated transcriptional activation of c-fos. In addition to stimulating the Raf/ERK cascade, Ras has been shown to stimulate the JNK/SAPK and p38 MAPK pathways, which also lead to the activation of Elk-1 and SAp-1a (7, 37, 38, 59–61, 71, 84, 93, 94). Rac, Rho, RalGDS, Rlf, and Rgl have also been discussed as mediating signals from Ras to the c-fos promoter (11, 31, 64, 66, 76, 95).
It cannot be excluded, therefore, that the ERK-CAAX refractory part of the Ras-mediated c-fos induction is mediated by one or more of these alternative pathways. It should be noted, however, that although the CAAX variants are predominantly found in the membrane fraction, a minor part remains detectable in the cytosol and, as shown in Fig. 11, is accessible to activation by Ras. This cytosolic ERK-CAAX fusion protein is translocated upon activation by Ras (not shown) and able to activate transcription of c-fos. The reason why not all of the CAAX fusion proteins are bound to the membrane is probably due to the high expression levels of these proteins. Farnesylated Ha-Ras has been shown to be targeted to high-affinity saturable binding sites at the plasma membrane, a process which in addition to prenylation requires additional posttranslational modifications (82).
As these ERK-CAAX chimeras are expressed in cells which also overexpress oncogenic Ha-Ras, it is reasonable to assume that the corresponding Ha-Ras binding sites at the membrane are saturated. This conclusion is supported by the observation that in cells transfected with the RasL61 expression vector, elevated levels of Ha-Ras are detectable in the cytosol (not shown). It may also be argued that according to the data, both ERK1 and ERK2 may in principle be capable of activating the transcription of the c-fos SRE-regulated reporter but that both isotypes are required to obtain maximal activation and that therefore inhibition of one isotype should lead only to a partial reduction. This possibility can, however, be excluded, as coexpression of ERK1-CAAX and ERK2-CAAX does not enhance the inhibitory effect (Fig. 4C).
In conclusion, the data presented here are consistent with a model by which the ERK-CAAX chimeras inhibit Ras-mediated transcriptional activation of c-fos by inhibiting the nuclear translocation of endogenous ERKs. Endogenous ERKs are trapped at the plasma membrane by dimerization with membrane-anchored activated ERK-CAAX fusion proteins (Fig. 12). Recently, it has also been reported that phosphorylation of MAPK induces its dimerization, which is required for its nuclear translocation (45). Until recently, however, direct evidence in support of the notion that an inhibition of MAPK translocation to the nucleus inhibits transcriptional activation was lacking.
During the preparation of this report, Brunet et al. (5) published data demonstrating that enforced cytoplasmic retention of ERKs by coexpression of an inactive mutant of the cytoplasmic phosphatase MKP-3 inhibits Elk-1-dependent gene expression and blocks activation of the c-fos promoter. The data presented here, which were obtained by an entirely different strategy, provide additional independent evidence indicating that nuclear translocation is indeed essential for the transcriptional activation of c-fos by a Ras-dependent pathway as proposed by others (4, 53, 57, 65, 85).
ACKNOWLEDGMENTS
We are grateful to M. Weber (University of Virginia Health Sciences Center, Charlottesville) and M. Cobb (University of Texas Southwestern Medical Center, Dallas) for providing plasmids pEF-myc-ERK2, pLNCAL7-HA-ERK2, and pCEP4-HA-ERK1. Reporter plasmid p(DSE)tk-CAT was obtained from H. König, Kernforschungszentrum Karlsruhe, Karlsruhe, Germany. We also thank Eugen Preuss (Presentation, Documentation, and Learning Systems) for illustration.
This work was supported in part by funding from the Austrian Fond zur Förderung der wissenschaftlichen Forschung (project F208), SFB-Sonderforschungsbereich Biological Communication Systems (P10530-MED), and the Austrian Federal Bank (project 5734).
REFERENCES
- 1.Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell R G. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 2.Baier-Bitterlich G, Überall F, Bauer B, Fresser F, Wachter H, Grunicke H H, Utermann G, Altmann A, Baier G. Protein kinase C-θ isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol Cell Biol. 1996;16:1842–1850. doi: 10.1128/mcb.16.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. ERK’s: a family of protein serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 4.Brondello J M, McKenzie F R, Sun H, Tonks N K, Pouysségur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- 5.Brunet A, Roux D, Lenormand P, Dowd S, Keyse S, Pouysségur J. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 1999;18:664–674. doi: 10.1093/emboj/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cano E, Mahadevan L C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 7.Cavigelli M, Dolfi F, Claret F X, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R H, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng J T, Cobb M H, Baer R. Phosphorylation of the TAL1 oncoprotein by the extracellular signal-regulated protein kinase ERK1. Mol Cell Biol. 1993;13:801–808. doi: 10.1128/mcb.13.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chihara K, Amano M, Nakamura N, Yano T, Shibata M, Tokui T, Ichikawa H, Ikebe R, Ikebe M, Kaibuchi K. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J Biol Chem. 1997;272:25121–25127. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- 12.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;25:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 13.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 14.Crews C M, Alessandrini A, Erikson R L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science. 1992;258:478–480. doi: 10.1126/science.1411546. [DOI] [PubMed] [Google Scholar]
- 15.Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- 16.David M, Petricoin III E, Benjamin C, Pine R, Weber M J, Larner A C. Requirement for MAP kinase (ERK2) activity in interferon α- and interferon β-stimulated gene expression through STAT proteins. Science. 1995;269:1721–1723. doi: 10.1126/science.7569900. [DOI] [PubMed] [Google Scholar]
- 17.Davis R J. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 18.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 19.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 20.Derijard B B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 21.Freshney N M W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan E, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 22.Galcheva-Gargova Z, Derijard B, Wu I H, Davis R J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 23.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb M H, Shaw P E. ERK phosphorylation potentiates Elk-1 mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–416. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 25.Giovane A, Pintzas A, Maira S M, Sobieszczuk P, Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994;8:1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez F A, Seth A, Raden D L, Bowman D S, Fay F S, Davis R J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Cambell D, Derijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 28.Han J, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 29.Her J-H, Lakhani S, Zu K, Vila J, Dent P, Sturgill T W, Weber M J. Dual phosphorylation and autophosphorylation in mitogen-activated protein (MAP) kinase activation. Biochem J. 1993;296:25–31. doi: 10.1042/bj2960025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 31.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 32.Hipskind R A, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hipskind R A, Büscher D, Nordheim A, Baccarini M. Ras/MAP kinase-dependent and -independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994;8:1803–1816. doi: 10.1101/gad.8.15.1803. [DOI] [PubMed] [Google Scholar]
- 34.Hipskind R A, Rao V N, Mueller C G, Reddy E S, Nordheim A. Ets-related Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991;354:531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- 35.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAPK-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 36.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 37.Jahnknecht R, Hunter T. Activation of Sap-1a transcription factor by the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase. J Biol Chem. 1997;272:4219–4224. doi: 10.1074/jbc.272.7.4219. [DOI] [PubMed] [Google Scholar]
- 38.Jahnknecht R, Hunter T. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahnknecht R, Cahill M A, Nordheim A. Signal integration at the c-fos promoter. Carcinogenesis. 1995;16:443–450. doi: 10.1093/carcin/16.3.443. [DOI] [PubMed] [Google Scholar]
- 40.Jahnknecht R, Ernst W H, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216. [PubMed] [Google Scholar]
- 41.Jahnknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAPKs. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallunki T, Deng T, Hibi M, Karin M. c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell. 1996;87:929–939. doi: 10.1016/s0092-8674(00)81999-6. [DOI] [PubMed] [Google Scholar]
- 43.Kallunki T, Su B, Tsigelny I, Sluss H K, Derijard D, Moore G, Davis R J, Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 44.Kampfer S, Hellbert K, Villunger A, Doppler W, Baier G, Grunicke H H, Überall F. Transcriptional activation of c-fos by oncogenic Ha-Ras in mouse mammary epithelial cells requires the combined activities of PKC-λ, ɛ and ζ. EMBO J. 1998;17:4046–4055. doi: 10.1093/emboj/17.14.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khokhlatchev A V, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb M H. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 46.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kortenjann M, Shaw P E. Raf-1 kinase and ERK2 uncoupled from mitogenic signals in rat fibroblasts. Oncogene. 1995;11:2105–2112. [PubMed] [Google Scholar]
- 48.Kortenjann M, Thomae O, Shaw P E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase ERK2. Mol Cell Biol. 1994;14:4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 50.Kyriakis J M, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 51.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 52.Lavoie J N, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 53.Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk) but not of their activator MAP kinase kinase (p54mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macdonald S G, Crews C M, Wu L, Driller J, Clark R, Erikson R L, McCormick F. Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol. 1993;13:6615–6620. doi: 10.1128/mcb.13.11.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 56.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 57.Meloche S, Seuwen K, Pages G, Pouysségur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- 58.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 59.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and CDC42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 60.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 61.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R J, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994;14:6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mivechi N F, Giaccia A J. Mitogen-activated protein kinase acts as a negative regulator of the heat shock response in NIH 3T3 cells. Cancer Res. 1995;55:5512–5519. [PubMed] [Google Scholar]
- 63.Morishima-Kawashima M, Kosik K. The pool of MAP kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996;7:893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murai H, Ikeda M, Kishida S, Ishida O, Okazaki-Kishida M, Matsuura Y, Kikuchi A. Characterization of RalGDP dissociation stimulator-like (RGL) activities to regulate c-fos promoter and the GDP/GTP exchange of Ral. J Biol Chem. 1997;272:10483–10490. doi: 10.1074/jbc.272.16.10483. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen T T, Scimeca J C, Filloux C, Peraldi P, Carpentier J L, Van Oberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. 1993;268:9803–9810. [PubMed] [Google Scholar]
- 66.Okazaki M, Kishida S, Hinoi T, Hasegawa T, Tamada M, Kataoka T, Kikuchi A. Synergistic activation of c-fos promoter activity by Raf and RalGDP dissociation stimulator. Oncogene. 1997;14:515–521. doi: 10.1038/sj.onc.1200860. [DOI] [PubMed] [Google Scholar]
- 67.Pomerance M, Thang M N, Tocque B, Pierre M. The Ras-GTPase-activating protein SH3 domain is required for Cdc2 activation and Mos induction by oncogenic Ras in Xenopus oocytes independently of mitogen-activated protein kinase activation. Mol Cell Biol. 1996;16:3179–3186. doi: 10.1128/mcb.16.6.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price M, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 70.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 71.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reszka A A, Seger R, Diltz C D, Krebs E G, Fischer E H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;9:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robbins D J, Zhen E, Owaki H, Vanderbilt C A, Ebert D, Geppert T D, Cobb M H. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 74.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 75.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1995;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 76.Sahai E, Alberts A S, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 78.Schaeffer H J, Catling A D, Eblen S T, Collier L S, Krauss A, Weber M J. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science. 1998;281:1668–1671. doi: 10.1126/science.281.5383.1668. [DOI] [PubMed] [Google Scholar]
- 79.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 80.Seger R, Ahn N G, Posada J, Munar E S, Jensen J M, Cooper J A, Cobb M H, Krebs E G. Purification and characterization of MAP kinase(s) activators from epidermal growth factor stimulated A431 cells. J Biol Chem. 1992;267:14373–14381. [PubMed] [Google Scholar]
- 81.Seth A, Gonzalez F A, Gupta S, Raden D L, Davis R J. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992;267:24796–24804. [PubMed] [Google Scholar]
- 82.Siddiqui A A, Garland J R, Dalton M B, Sinensky M. Evidence for a high affinity, saturable, prenylation-dependent p21Ha-ras binding site in plasma membranes. J Biol Chem. 1998;273:3712–3717. doi: 10.1074/jbc.273.6.3712. [DOI] [PubMed] [Google Scholar]
- 83.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 84.Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb M J. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. EMBO J. 1996;15:4629–4642. [PMC free article] [PubMed] [Google Scholar]
- 85.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Treisman R. The serum response element. Trends Biochem Sci. 1992;17:423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- 87.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 88.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 89.Überall F, Kampfer S, Doppler W, Grunicke H H. Activation of c-fos expression by transforming Ha-ras in HC11 mouse mammary epithelial cells is PKC-dependent and mediated by the serum response element. Cell Signal. 1994;6:285–297. doi: 10.1016/0898-6568(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 90.Überall F, Giselbrecht S, Hellbert K, Fresser F, Bauer B, Gschwendt M, Grunicke H H, Baier G. Conventional PKC-α, novel PKC-ɛ and PKC-θ, but not atypical PKC-λ are MARCKS kinases in intact NIH3T3 fibroblasts. J Biol Chem. 1997;272:4072–4078. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 91.Weber J D, Hu W, Jefcoat S C, Raben D M, Baldassare J J. Ras-stimulated extracellular-signal related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27KIP1. J Biol Chem. 1997;272:32966–32971. doi: 10.1074/jbc.272.52.32966. [DOI] [PubMed] [Google Scholar]
- 92.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signaling transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 93.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–406. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 94.Whitmarsh A J, Yang S H, Su M S S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolthuis R M, deRuiter N D, Cool R H, Bos J L. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J, Harrison J K, Dent P, Lynch K R, Weber M J, Sturgill T W. Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol. 1993;13:4539–4548. doi: 10.1128/mcb.13.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 98.Zheng C F, Guan K L. Cloning and characterization of two distinct human extracellular signal-regulated activator kinases, MEK1 and MEK2. J Biol Chem. 1993;268:11435–11439. [PubMed] [Google Scholar]
- 99.Zheng C F, Guan K L. Properties of MEK’s, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993;268:23933–23939. [PubMed] [Google Scholar]