Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with unknown brain etiology. Our knowledge to date about structural brain development across the lifespan in ASD comes mainly from cross-sectional studies, thereby limiting our understanding of true age effects within individuals with the disorder that can only be gained through longitudinal research. The present study describes FreeSurfer-derived volumetric findings from a longitudinal dataset consisting of 607 T1-weighted magnetic resonance imaging (MRI) scans collected from 105 male individuals with ASD (349 MRIs) and 125 typically developing male controls (258 MRIs). Participants were six to forty-five years of age at their first scan, and were scanned up to 5 times over a period of 16 years (average inter-scan interval of 3.7 years). Atypical age-related volumetric trajectories in ASD included enlarged gray matter volume in early childhood that approached levels of the control group by late childhood, an age-related increase in ventricle volume resulting in enlarged ventricles by early adulthood and reduced corpus callosum age-related volumetric increase resulting in smaller corpus callosum volume in adulthood. Larger corpus callosum volume was related to a lower (better) ADOS score at the most recent study visit for the participants with ASD. These longitudinal findings expand our knowledge of volumetric brain-based abnormalities in males with ASD, and highlight the need to continue to examine brain structure across the lifespan and well into adulthood.
Keywords: Autism spectrum disorder, longitudinal development, MRI, brain volumes, ventricles, corpus callosum
1. Introduction
Autism spectrum disorder (ASD) is a lifetime disorder with unknown neurobiological etiology and poorly characterized lifelong clinical course in most cases. Despite earlier diagnosis and interventions, many individuals remain functionally impaired throughout their lives (Howlin et al., 2013). In order to understand the etiology of ASD and the mechanisms involved and design treatments and therapies that will improve outcome in children, adolescents and adults, a further understanding of brain changes in ASD throughout the lifespan is needed.
There are a number of research groups focusing on longitudinal brain development in ASD during infancy and childhood that show abnormalities early in life. As early as 6 months of age, increased extra-axial CSF is present and remains elevated into early childhood in those who later develop ASD (Shen et al., 2017; Shen et al., 2013). Longitudinal and cross-sectional studies also show enlarged brain volumes by 2 years of age (Hazlett et al., 2017; Hazlett et al., 2011) although this may be attributed to subgroups of children within the disorder (Nordahl et al., 2011; Ohta et al., 2016). A small (n=18 ASD) longitudinal study reported atypical decreases in gray matter volume during childhood (Hardan, Libove, et al., 2009), however, the majority of our knowledge about brain development during childhood and adolescence in ASD is inferred from cross-sectional research. As a result, some early childhood studies report increased brain volumes in ASD (Courchesne et al., 2003; Lucibello et al., 2019; Sparks et al., 2002) that may normalize by later childhood (Courchesne et al., 2001) or decrease below typical samples (Herbert et al., 2003; McAlonan et al., 2005); while studies in adolescence report enlarged gray matter volumes in ASD (Freitag et al., 2009; Hazlett et al., 2006). A recent publication of a large cross-sectional sample (n=456) of ASD and typically developing control (TDC) participants age 6–25 years found persistently increased global brain volumes (total, gray matter, white matter, ventricles) in ASD, suggesting that early brain overgrowth does not normalize and remains larger into adulthood (Yankowitz et al., 2020). This contrasts with other cross-sectional studies from adolescence into adulthood showing no atypical global brain structure in ASD (Aylward et al., 2002; Hallahan et al., 2009; Lin et al., 2015; Maier et al., 2018), regionally decreased volumes (Ecker et al., 2012; Toal et al., 2010) or absence of regional gray matter decrease found in a typical comparison group (Raznahan et al., 2010).
The lack of a clear volumetric brain development trajectory in ASD could be due to a number of factors. Discrepant volumetric findings can result from different image processing methods (Katuwal et al., 2016), error introduced by combining datasets collected from different research sites and scanner hardware (Martinez-Murcia et al., 2017), between-site heterogeneity in ASD and TDC samples, unknown sex differences in brain morphology (Bedford et al., 2020), unknown details about change in clinical course over time, and differences in analytic methods employed. Cross-sectional studies infer brain changes from age-related differences across individuals. Longitudinal studies, although challenging to conduct as they require collecting multiple time points of data and retaining participants, often over years, directly measure change within individuals and can more accurately describe how the brain changes during development, maturation, and aging (Farrington, 1991). Longitudinal studies also remove additional sources of variance in the data due to a consistent site and equipment and allow the ability to track individual differences in clinical course over time that may be related to brain changes.
In this paper, we describe volumetric findings from the Interdisciplinary Science to Learn about Autism (ISLA) project with all data collected at the University of Utah. This longitudinal study of late brain development in autism began in 2003 and is currently in its 6th wave of data collection. Participant ages ranged from childhood to early adulthood at study enrollment, providing the strength of an accelerated longitudinal design and ability to examine brain changes over a large developmental period in a shorter timeframe than a single longitudinal cohort (Galbraith et al., 2017; Harezlak et al., 2005; Willett et al., 1998). Our group previously described volumetric brain changes in a subgroup of male individuals age 6–35 years, with up to 3 longitudinal scans collected over an 8-year period (n=100 ASD, n=56 TDC). We found volumetric developmental trajectories that differed in our ASD group in whole brain, white matter, lobes and ventricles (Lange et al., 2015). Since that publication we have collected two additional imaging timepoints and increased our sample sizes of ASD and TDC participants. The purpose of this study is to examine how additional longitudinal datapoints enhance our understanding of volumetric brain development in males with autism and extend our previous study into mid-adulthood. We also describe volumetric differences between two subgroups of our ASD sample identified at the most recent time point (those who continued to meet criteria for ASD based on ADOS-2 algorithm and expert clinical consensus classification vs those who did not) to examine how clinical heterogeneity within the disorder is related to longitudinal brain trajectories.
2. Methods
2.1. Participants
Participants included 105 male individuals with ASD and 125 male participants without ASD (TDC) recruited from a longitudinal neuroimaging study at the University of Utah (see Table 1). A subgroup of this sample (n=90 ASD, n=55 TDC) overlaps with the participants from our previous volumetric publication (Lange et al., 2015). Participants were scanned up to 5 times over a 16-year period from 2003 – 2019 with the first wave of data collection between 2003 – 2007. Although new participants were recruited at subsequent timepoints, participant retention of those recruited during the initial study period has remained high. Our study includes 82 individuals with ASD and 52 individuals with TDC who were enrolled during this initial period. Of this original cohort, 75 ASD and 47 TDC had at least 2 MRIs, representing 91% retention, and 68 ASD and 36 TDC had at least 3 MRIs (84% and 69% retention respectively). At timepoint 5 (2017 – 2019), 73% of the initial ASD sample (n=68) and 50% of the TDC sample (n=26) returned for scanning. Fifty-four percent (54%) of the ASD sample reported psychotropic medication use at some point during the longitudinal study: antidepressants = 45%, stimulants = 24%, antipsychotics = 9%, anti-anxiety = 8%, mood stabilizers = 6%, anti-insomnia = 5%, anticonvulsants = 1%, multiple medications = 30%. At timepoint 5, two participants, typically developing at study entry, reported new psychotropic medication use (anti-insomnia and antidepressant).
Table 1.
Participant characteristics
| ASD N=105 |
TDC N=125 |
||||
|---|---|---|---|---|---|
| Total number of scans | 349 | 258 | |||
| Mean (sd) | Range | Mean (sd) | Range | Group Comparison | |
| Age at first scan (years) | 16.4 (8.1) | 6.0 – 45.4 | 22.4 (8.7) | 6.0 – 44.4 | t=5.4, p<.001 |
| Age across all scans (years) | 21.2 (8.7) | 6.1 – 47.1 | 22.5 (8.5) | 6.0 – 46.9 | t=1.9, p=.05 |
| Number MRIs per person | 3.3 (1.5) | 1 – 5 | 2.0 (1.4) | 1 – 5 | t=6.4, p<.001 |
| Interscan Interval (years) | 3.7 (1.9) | 1.6 – 13.4 | 3.8 (2.3) | 1.5 – 14.7 | t=0.5, ns |
| FSIQ | 103.7 (18) | 60 – 150 | 118.3 (11) | 87 – 144 | 6.7 p<.001 |
| NVIQ | 104.8 (17) | 67 – 150 | 116.7 (13) | 87 – 150 | 5.2 p<.001 |
| VIQ | 102.7 (19) | 61 – 145 | 116.7 (12) | 74 – 149 | 5.8 p<.001 |
Notes: sd=standard deviation; FSIQ: Full Scale IQ; NVIQ: Nonverbal IQ; VIQ: Verbal IQ
At study enrollment, ASD was diagnosed using the Autism Diagnostic Interview-Revised (ADI-R (Lord et al., 1994)), the Autism Diagnostic Observation Schedule (ADOS (Lord et al., 2000; Lord et al., 2012)), DSM-IV (American Psychiatric Association, 1994) and ICD-10 criteria. Enrollment of both the ASD and TDC groups has been described previously (Alexander et al., 2007; Lange et al., 2015; Zielinski et al., 2014). Participant consent, assent for those age 7 – 18 and parental permission for all participants under age 18 was obtained at each timepoint. All study procedures were approved by the Institutional Review Board.
Intelligence (IQ) was assessed at study enrollment and subsequent timepoints as part of a comprehensive cognitive battery. IQ was assessed with the Differential Abilities Scale (Elliott, 1990), Wechsler Intelligence Scale for Children-Third Edition (Wechsler, 1991), Wechsler Adult Intelligence Scale-Third Edition (Wechsler, 1997) or Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), depending on participant’s age and verbal ability, providing indices of Full Scale, Verbal and Nonverbal IQ. Group means and ranges for the most recent IQ scores obtained from study participants are presented in Table 1.
ASD Subgroup Classification.
At timepoint 5 the ADOS-2 Module 4 (Lord et al., 2012) was administered to all ASD participants (n=82). Our approach to evaluate Persistence and Remission in ASD was to investigate dimensional outcome based on symptom severity rather than categorical DSM 5 diagnoses. We defined remitted ASD as falling below ADOS-2 algorithm criteria for a current classification of ASD and below expert clinical consensus for a current classification of ASD based on all information available about the participant’s current behavior. Our remitted ASD group was comprised of individuals who at the time of the study entry met full ADI-R, ADOS-2, and DSM criteria for ASD, and thus had a Lifetime diagnosis of ASD. But at Time 5, the level of their ASD symptoms fell below the threshold for a current classification of ASD.
2.2. Imaging Protocol
At each timepoint, whole-brain T1 weighted magnetic resonance images were acquired on a Siemens Trio 3.0 T scanner. At timepoint 1, an 8-channel receive-only RF head coil was used to acquire the sagittal 3D MPRAGE images (TI=1100 ms, TR=1800 ms, TE=2.93 ms, flip angle=12°, FOV=256 mm, slice thickness=1mm, 160 slices). At timepoints 2 through 5, a 12-channel, receive only RF head coil was used to acquire the sagittal 3D MPRAGE images (TI=900 ms, TR=2300 ms, TE=2.91 ms, flip angle=9°, FOV=256 mm, slice thickness=1.2 mm, 160 slices). To control for imaging head coil differences between timepoints 1 and 2, a head coil covariate was included in all analyses.
2.3. Volumetric Estimation
Volumes were estimated using the longitudinal stream (Reuter et al., 2012) in the FreeSurfer image analysis suite v6.0 (http://surfer.nmr.mgh.harvard.edu; (Fischl et al., 2002; Fischl et al., 2004)). All MRI scans were processed on the same workstation. The longitudinal pipeline creates a within subject template space and image from all of the images collected for each participant. This processing results in increased reliability and statistical power because processing steps, such as skull stripping, Talairach transforms, atlas registration and parcellations utilize common information from the within subject template (Reuter et al., 2012). Although this project included a subset of MRI images described previously, all images were reprocessed using the updated FreeSurfer v6.0 and were included in the longitudinal pipeline for each participant. All images were visually inspected and edited blind to diagnosis by the lead author (M.P.) under the supervision of the senior author (B.Z.). A total of 635 MRIs were available for analysis. After initial FreeSurfer processing, quality control involved inspection of the following volumetric and surface files in Freeview: T1, brainmask, wm, aseg, rh.white, lh.white, rh.pial, and lh.pial. A total of 28 MRIs were excluded: 25 images (8 TDC, 17 ASD) had segmentation errors resulting from poor image quality due to motion or image artifacts and 3 images (3 TDC) had FreeSurfer processing errors. After excluding these images, 8 participants had no usable MRIs (5 TDC, 3 ASD). Of the remaining 607 datasets described in this paper, 370 MRIs passed quality control with no editing. The remaining 237 MRIs were reprocessed after manual edits to brainmask.mgz files. Fifteen MRIs had edits to cross-sectional images only (8 TDC, 7 ASD) and 156 images (62 TDC, 94 ASD) were reprocessed after edits to that participant’s base image. After base edits, longitudinal edits were made to 45 images (21 TDC, 24 ASD) and 21 longitudinal images were edited with no prior base or cross-sectional edits (11 TDC, 10 ASD). The following regions were examined: whole brain (FreeSurfer variable SupraTentorialNotVent), total gray matter (cortical and subcortical), total white matter, corpus callosum, thalamus, caudate, cerebellum, ventricles (sum of lateral, inferior lateral, 3rd, 4th, and 5th ventricles), and lobes (frontal, temporal, parietal, occipital). We also examined ventricle to whole brain ratio and total gray matter to whole brain ratio.
3. Statistical analysis
Linear mixed-effects models (Laird & Ware, 1982; Lange & Laird, 1989) were employed to describe longitudinal volumetric brain changes over time in the ASD participants in comparison to the TDC group. This method allowed for the inclusion of individual random intercepts and slopes and varying number of data points per participant. All models contained group, linear age and scanner headcoil effects. The following covariates were examined: age2, group*age, group*age2 and FSIQ. For each region of interest, best fitting models were chosen by application of the Akaike Information Criterion (Akaike, 1974). In the volumetric models, the ASD group was the reference group, thus volumes and age effects are represented in the intercepts and slopes; any differences in our TDC sample are interpreted through the group effects and group*age interactions. In our initial analysis, age was mean-centered at 21 years. In the regions where significant group*age interactions were found, we performed a post-hoc analysis to identify the age at which the groups differed by re-centering the age variable. We also employed mixed-effects models to compare mean brain volume and age related changes between the ASD subgroups identified at the most recent study visit. Due to the number of regions being analyzed, FDR correction at a family wise error rate of p<.05 was applied (Benjamini & Hochberg, 1995) and all reported findings are corrected p-values. Between-group differences in demographic variables were examined using t-tests. R version 3.6.2 (R Core Team, 2019) was used and the R package nlme (Pinheiro et al., 2020) for mixed-effects models.
4. Results
4.1. Participant Characteristics
At the initial study enrollment (2003 – 2007), there were no significant differences between ages of the ASD and TDC participants (ASD n=82, mean age=15.8 years, sd=8 years, range 6 – 45 years; TDC n=52, mean=16.0 years, sd=6 years, range 6 – 29 years; between group difference t=.14, p=ns; all males). Participants were recruited at subsequent timepoints to increase our sample and account for attrition, resulting in similar age ranges but younger mean age in the ASD participants at each participant’s first scan and across all scans (see Table 1). A total of 349 MRIs was collected from the ASD group and 258 MRIs from the TDC group (see Figure 1). The ASD group had more MRIs per person but interscan interval did not differ between groups (see Table 1).
Figure 1.
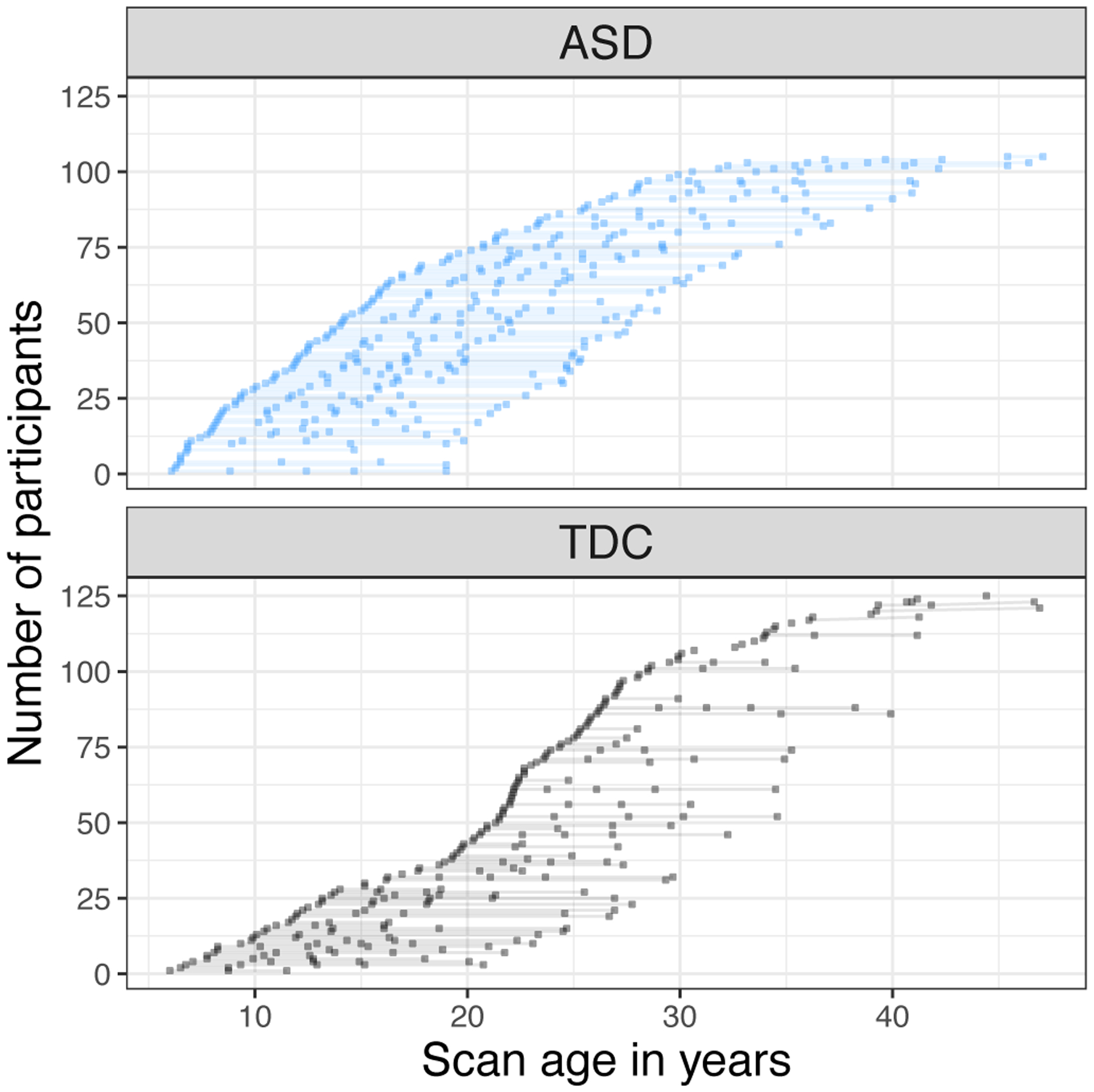
Longitudinal Imaging Dataset
4.2. Longitudinal Volumetric Development in ASD
Results from the best fitting mixed-effects models are summarized in Table 2. Linear and non-linear volumetric age-related changes are presented for the ASD group and significant differences from the TDC comparison sample are captured in the group effect and group by age interactions. Figure 2 shows the individual volumetric datapoints and linear mixed model-derived predicted age curves for each group.
Table 2.
Linear Mixed Effects Models of Volumetric Brain Development in ASD
| ASD Intercept B (SE) |
Group Effect B (SE) |
Age B (SE) |
Age2 B (SE) |
Group × Age B (SE) |
Group × Age2 B (SE) |
FSIQ B (SE) |
|
|---|---|---|---|---|---|---|---|
| Whole Brain | 1122.18 (9.7) |
−3.9 (14.1) |
−4.5*** (.25) |
.04* (.01) |
1.24** (.42) |
−.10** (.02) |
1.0* (.41) |
| Gray Matter Total | 633.8 (5.3) |
−3.5 (7.7) |
−6.3*** (.18) |
.14*** (.01) |
1.0** (.30) |
−.06** (.02) |
.61** (.21) |
| Gray Matter: Cortical | 565.7 (4.9) |
−2.3 (7.1) |
−6.1*** (.17) |
.14*** (.01) |
.96** (.29) |
−.06** (.02) |
.54* (.19) |
| Gray Matter: Subcortical | 66.8 (.52) |
−1.0 (.76) |
−.11*** (.01) |
−.006*** (.001) |
.07* (.02) |
−.005** (.001) |
.06** (.02) |
| White Matter | 381.9 (18.8) |
−2.6 (6.0) |
1.08*** (.10) |
−.07*** (.006) |
.21 (.17) |
−.02* (.01) |
.37* (.17) |
| Ventricles | 18.6 (.73) |
−2.0* (.98) |
.25*** (.02) |
- | −.10** (.03) |
- | - |
| Cerebellum | 148.3 (1.4) |
−.25 (2.0) |
−.17** (.05) |
−.02*** (.002) |
.04 (.08) |
- | .15* (.06) |
| Thalamus | 16.4 (.14) |
−.07 (.20) |
−.04*** (.005) |
−.002*** (.0003) |
.02* (.009) |
- | .01* (.005) |
| Caudate | 8.6 (.11) |
−.24 (.16) |
−.03*** (.002) |
−.0005** (.0001) |
- | - | .01* (.004) |
| Corpus Callosum | 3.56 (.05) |
.05 (.07) |
.01*** (.002) |
−.001*** (.0001) |
.008* (.003) |
- | - |
| Lobes | |||||||
| Frontal | 213.5 (2.1) |
−1.1 (3.1) |
−2.6*** (.08) |
.06*** (.005) |
.32* (.13) |
−.02** (.008) |
.28** (.08) |
| Temporal | 129.0 (1.2) |
.39 (1.6) |
−.95*** (.03) |
.01*** (.002) |
.15* (.06) |
−.01* (.004) |
- |
| Parietal | 148.5 (1.4) |
−.13 (2.05) |
−1.99*** (.06) |
.05*** (.004) |
.39*** (.10) |
−.02** (.007) |
.14* (.05) |
| Occipital | 59.3 (.67) |
−.02 (.9) |
−.48*** (.02) |
.012*** (.001) |
.12*** (.032) |
−.006* (.002) |
- |
| TGM over WBV | .56 (.001) |
−.001 (.002) |
−.003*** (.00007) |
.00008*** (.00001) |
.0001 (.0001) |
- | - |
| VBR | .016 (.0006) |
−.0018* (.0008) |
.0003*** (.00002) |
- | −.0001** (.00003) |
- | - |
corrected p<.001,
corrected p<.01,
corrected p<.05;
B: Beta value; SE: standard error; TGM: total gray matter; WBV: whole brain volume; VBR: ventricle to whole brain ratio; - effect not included in best fitting model.
Figure 2.
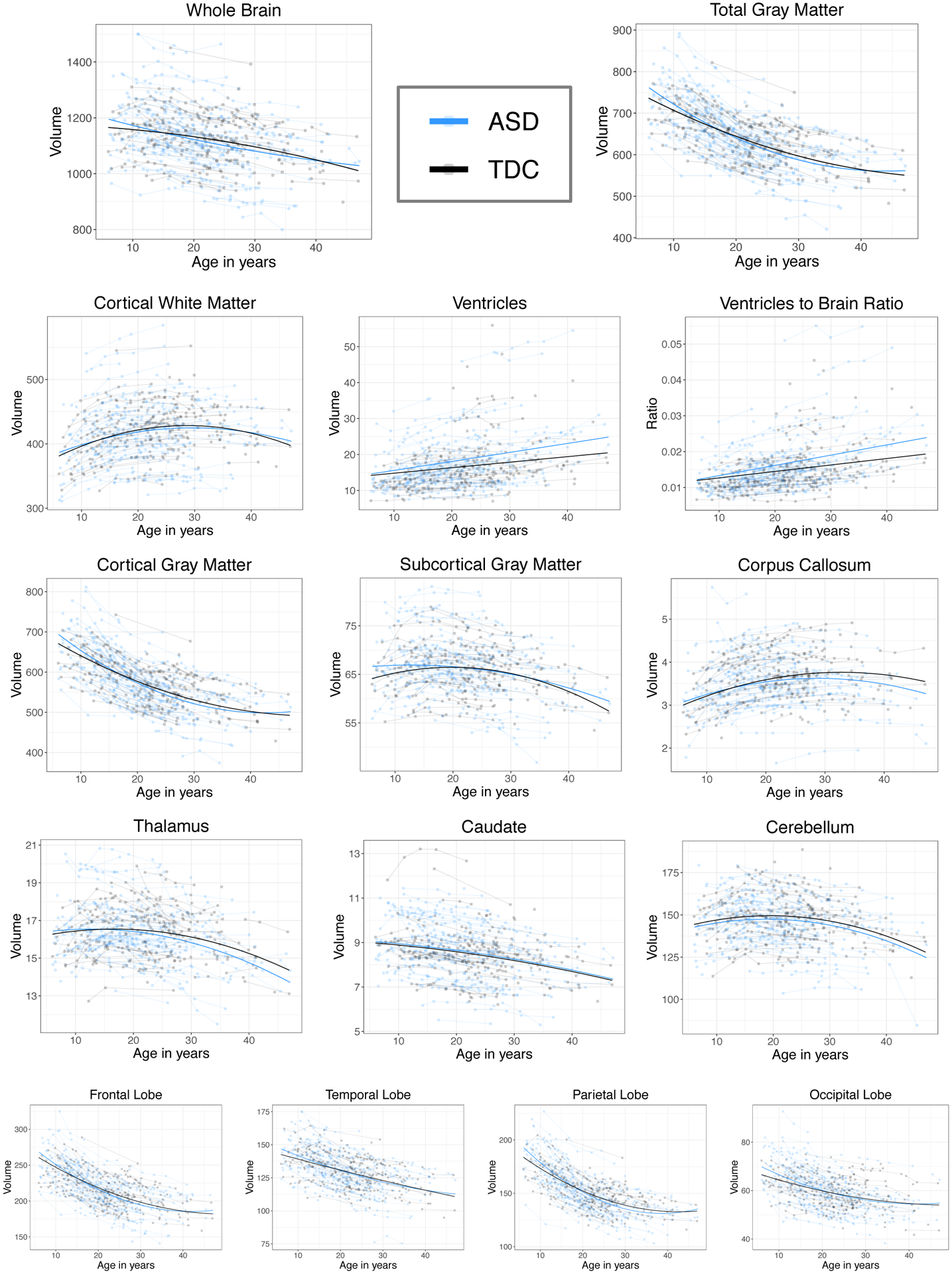
Longitudinal volumetric trajectories in ASD and TDC
4.2.1. Volumetric Age-Related changes
The ASD group showed greater volumetric decline with age compared to the TDC sample in whole brain (linear age*group p=.006, age2*group p<.001), gray matter (linear age*group p=.001, age2*group p=.001), and lobar volumes (see Table 2 and Figure 2). Whole brain and occipital lobe findings replicate those reported previously in Lange et al. (2015), yet the gray matter findings and the following are new to this expanded dataset. Total white matter volume showed a significant age2*group interaction only (p=.04). Ventricular volume and ventricle to brain ratio (VBR) increased at a greater rate (ventricle age*group p=.006; VBR age*group p=.002) and thalamic volume decreased at a greater rate in ASD (age*group p=.04). Corpus callosum volume increased at a greater rate in the TDC sample (p=.01; see Figure 2). We did not find any ASD vs TDC differences in age-related volumetric changes in the caudate or cerebellum.
4.2.2. Mean Volumetric Differences
As evident in Table 2, the only ASD vs TDC mean differences present at age 21 years (mean centered age) were increased ventricles (p=.04) and VBR (p=.03) in ASD. Some of the longitudinal differences described above resulted in volumetric differences between the ASD and TDC groups at different stages of development. Total gray matter volume was increased in ASD during childhood up to age 12 years (p=.04, 3.1% larger in ASD); cortical GM was larger up to age 11 (p=.04, 3.3% larger in ASD) and subcortical GM up to age 15 (p=.03, 2.9% larger). Frontal volume was increased in ASD until age 8 (4% larger, p=.02) and parietal and occipital lobes were increased into adolescence (parietal 3% larger at age 12, p=.03; occipital 3.4% larger at age 9, p=.03). By age 21 years, ventricular volume was larger in ASD (p=.03, 11% larger) and by age 36, corpus callosum volume was smaller in ASD (p=.03, 5% smaller).
4.2.3. Timepoint 5: ASD ADOS-2 Outcome Classification and Brain Volumes
We examined longitudinal volumetric changes between the “remitted ASD” subgroup versus the “persistent ASD” group (i.e., those still meeting ADOS-2 criteria and expert clinical consensus classification for ASD based on current behaviors). All of the remitted ASD participants were under 30 years of age, thus to maintain equal age ranges between ASD subgroups, we restricted the statistical analysis to those participants age 30 and younger, resulting in 78 participants (persistent ASD n=61, remitted ASD n=17). We did not find longitudinal age-related differences between the ASD subgroups but did find persistently smaller corpus callosum volume in the subgroup with persistent ASD at timepoint 5 (group effect B=−.37, p=.03, see Figure 3). We also found larger TGM/TBV ratios up until age 18 years in the persistent ASD group (group effect B=.01, uncorrected p=.04 at age 18, see Figure 3; this finding was no longer significant at p<.05 after FDR correction). A greater percentage of the persistent ASD group reported psychotropic medication use at timepoint 5 (54% vs remitted ASD 33%). The persistent ASD group had significantly lower mean Full Scale and Verbal IQ scores (Persistent ASD: FSIQ 101.9, VIQ 99.2, NVIQ 104.5; Remitted ASD: FSIQ 114.0, VIQ 114.5, NVIQ 110.3; group differences: FSIQ t=3.0, p=.005, VIQ t=3.4 p=.002, NVIQ t=1.7 p=.09, uncorrected p-values) but controlling for FSIQ did not improve model fit for any of the volumetric comparisons.
Figure 3.
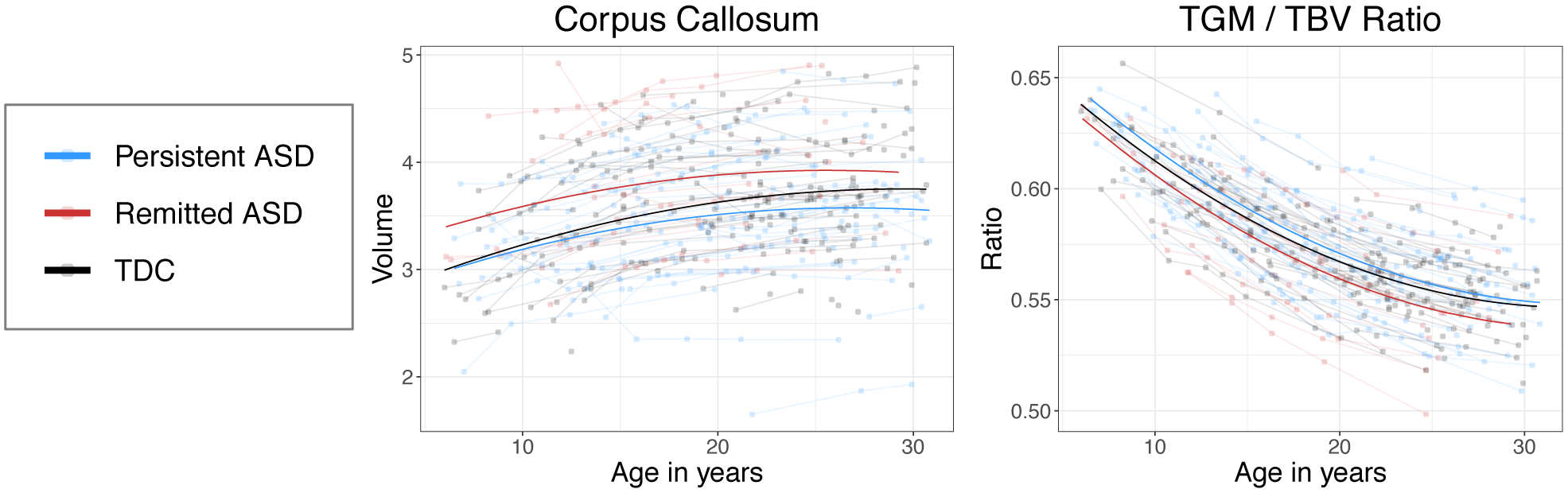
Volumetric brain differences in ASD subgroups based on Time 5 classification
To test whether ASD severity differences were present in these subgroups prior to timepoint 5, we compared ADOS calibrated severity scores (CSS; (Gotham et al., 2009; Hus & Lord, 2014)) obtained at study entry and ADI algorithm scores. The persistent ASD group had higher ADOS CSS scores at study entry (persistent ASD CSS=8.6, sd=1.3; remitted ASD CSS=7.5, sd=1.2; t=3.0, p<.01), but a comparison of ADI algorithm scores from the “Most Abnormal age 4–5” items showed similar Social and Communication scores at age 4–5 (ADI A: persistent ASD = 18.5, sd=5.5; remitted ASD = 18.7, sd=6.6; t=.08, p=ns; ADI B: persistent ASD = 9, sd=3; remitted ASD 9.25, sd=3; t=.26, p=ns), suggesting similar autism behaviors in childhood.
5. Discussion
The inclusion of the 4th and 5th waves of longitudinal imaging data improve our understanding of brain changes from childhood into adulthood in males with ASD. This expanded dataset replicated our previous study describing whole brain volumetric age-related decline in ASD above that found in TDC but also newly identified greater age-related decline in cortical and subcortical gray matter volumes in ASD. We also found developmental differences in ventricles and the corpus callosum resulting in larger ventricles and reduced corpus callosum volume in adulthood in ASD.
Atypical longitudinal gray matter trajectories in ASD were accompanied by enlarged gray matter during early childhood that reached levels in the typical group by later childhood and adolescence: cortical gray matter by age 11, subcortical gray matter by age 15. We found significantly greater volumetric decline with age present in all four lobes; parietal and occipital lobes remained larger up until late childhood. Our results suggest nonuniform, regional gray matter volumetric differences persistent into late childhood in males with ASD. Atypical volumetric development can be driven by a number of underlying factors, such as regional thickness and surface area changes (Ecker et al., 2013; Hazlett et al., 2011). Future work is needed to examine additional structural changes underlying volumetric gray matter.
Ventricular volume and ventricle to brain ratio increased at a greater rate in our ASD sample throughout the wide age range studied. This atypical rate of increasing ventricular volume resulted in significantly increased volume by 21 years of age. Whereas infants who develop ASD tend to have increased subarachnoid extra-axial CSF (Shen et al., 2017; Shen et al., 2013), older individuals with ASD have now well-replicated volumetric ventricular differences (Haar et al., 2016; Richards et al., 2020; Turner et al., 2016; van Rooij et al., 2018). To these convergent findings we add a very important novel detail: for the first time, using longitudinal data collected over 16 years, we show evidence that ventricular volume is increasing at an atypical rate in males with ASD.
Although we anticipated finding atypical development of cortical white matter in ASD, we only found nonlinear cortical white matter differences. The relationship between atypically increased ventricles and age-related changes in gray and white matter remains unknown. In traumatic brain injury, ventricular enlargement is believed to be related to periventricular white matter loss (Bigler et al., 2013). How white matter abnormalities in ASD reported through other imaging modalities, such as diffusion tensor imaging (DTI; (Ecker et al., 2016; Itahashi et al., 2015)), are related to ventricle expansion is not yet known but will be important to examine as markers of brain health and function across the lifespan.
Corpus callosum volume increased at a greater rate in TDC and was smaller in ASD by adulthood (significant at age 36 years and older). Smaller corpus callosum volume or area is a repeated finding in cross-sectional studies of individuals as young as 2 years of age through adulthood (Boger-Megiddo et al., 2006; Frazier et al., 2012; Freitag et al., 2009; Hardan, Pabalan, et al., 2009; Levman et al., 2018; Piven et al., 1997; Wolff et al., 2015) with few exceptions (Kucharsky Hiess et al., 2015). Besides our previous publication, the only other research groups to report longitudinal corpus callosum development have been in very young children (Wolff et al., 2015) or only during late childhood and early adolescence (Frazier et al., 2012). Interestingly, when we examined our ASD sample further when grouped by ADOS-2 and expert clinical consensus classification based on current behaviors at their most recent study visit, it became apparent that smaller callosal volumes were driven by the participants still meeting criteria for ASD, or those with more prevalent ASD behaviors. These findings underscore the importance of incorporating the clinical heterogeneity of ASD into imaging studies (Bedford et al., 2020; Boger-Megiddo et al., 2006; Chen et al., 2019). Although the microstructural properties of the atypical corpus callosum volume are unknown, atypical corpus callosum structure and development has widespread implications for behavioral functioning that relies on interhemispheric communication. Accordingly, our group has reported previously on processing speed differences in ASD related to callosal area or diffusion tensor properties (Alexander et al., 2007; Prigge et al., 2013). Future investigation into the longitudinal development of microstructural properties, such as myelination, that may explain functional differences are warranted.
There are a number of limitations in our study. The first limitation is the focus on male participants at the outset of the longitudinal study to decrease heterogeneity and have adequate power in the face of limited resources. As a result our findings may not be generalizable to brain structure and development in females. A small pilot sample of ASD and TDC females was recruited at the 4th wave of data collection, and will continue to be followed. Studies continue to identify sex differences in brain morphology (Bedford et al., 2020), thus the inclusion of female participants is essential to understand how sex may be related to and independent from brain structural abnormalities reported in previous studies of ASD individuals. Not all participants were able to complete all 5 imaging timepoints. Our participant retention of the original cohort has been 73% but we recruited additional ASD and TDC participants at later timepoints with fewer visits; our mixed models are able to accommodate missing data but more complete longitudinal data will allow individual tracking. Although we excluded images with significant motion, it is unknown how small amounts of motion may be biasing our results. Finally, this analysis is limited to volumetric data and specific regions. As our data analysis is ongoing, we will continue to examine other regions and imaging features that will, in conjunction with the current volumetric data, help describe longitudinal brain development across the lifespan in ASD.
6. Conclusions
Autism is a heterogenous disorder. As a result, a reliance on cross-sectional samples, which infer developmental brain changes from age-related differences across different individuals, can provide contradictory and only indirect information about age-related group differences and when they appear during development. Longitudinal studies that examine biological changes within affected individuals in relation to clinical changes across time are well-poised to help elucidate the developmental neuropathologies of ASD across the lifespan, potential neurobiological subtypes, and brain mechanisms involved in variations in core features clinical course, severity, and outcome.
Acknowledgements:
The research reported in this publication was supported by the National Institutes of Mental Health of the National Institutes of Health under Award Numbers R01MH080826 (JEL) and K08MH100609 (BAZ) and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD090256). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Alyson Froehlich, Jared Nielsen, Jubel Morgan and Carolyn King for their assistance with data collection.
References
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 716–723. [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, … Lainhart JE, (2007). Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage, 34(1), 61–73. doi: 10.1016/j.neuroimage.2006.08.032 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, & Singh N (2002). Effects of age on brain volume and head circumference in autism. Neurology, 59(2), 175–183. doi: 10.1212/wnl.59.2.175 [DOI] [PubMed] [Google Scholar]
- Bedford SA, Park MTM, Devenyi GA, Tullo S, Germann J, Patel R, … Chakravarty MM (2020). Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol Psychiatry, 25(3), 614–628. doi: 10.1038/s41380-019-0420-6 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of tthe Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, … Owen Yeates K (2013). Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology, 27(4), 438–451. doi: 10.1037/a0032837 [DOI] [PubMed] [Google Scholar]
- Boger-Megiddo I, Shaw DW, Friedman SD, Sparks BF, Artru AA, Giedd JN, … Dager SR (2006). Corpus callosum morphometrics in young children with autism spectrum disorder. J Autism Dev Disord, 36(6), 733–739. doi: 10.1007/s10803-006-0121-2 [DOI] [PubMed] [Google Scholar]
- Chen H, Uddin LQ, Guo X, Wang J, Wang R, Wang X, … Chen H (2019). Parsing brain structural heterogeneity in males with autism spectrum disorder reveals distinct clinical subtypes. Hum Brain Mapp, 40(2), 628–637. doi: 10.1002/hbm.24400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Carper R, & Akshoomoff N (2003). Evidence of brain overgrowth in the first year of life in autism. JAMA, 290(3), 337–344. doi: 10.1001/jama.290.3.337 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, … Courchesne RY (2001). Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology, 57(2), 245–254. doi: 10.1212/wnl.57.2.245 [DOI] [PubMed] [Google Scholar]
- Ecker C, Andrews D, Dell’Acqua F, Daly E, Murphy C, Catani M, … Murphy DG (2016). Relationship Between Cortical Gyrification, White Matter Connectivity, and Autism Spectrum Disorder. Cereb Cortex, 26(7), 3297–3309. doi: 10.1093/cercor/bhw098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC, … Consortium MA (2013). Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry, 70(1), 59–70. doi: 10.1001/jamapsychiatry.2013.265 [DOI] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, … Consortium MA (2012). Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry, 69(2), 195–209. doi: 10.1001/archgenpsychiatry.2011.1251 [DOI] [PubMed] [Google Scholar]
- Elliott CD (1990). Differential Ability Scales: Introductory and Technical Handbook. New York: The Psychological Corporation. [Google Scholar]
- Farrington DP (1991). Longitudinal research strategies: advantages, problems, and prospects. J Am Acad Child Adolesc Psychiatry, 30(3), 369–374. doi: 10.1097/00004583-199105000-00003 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. doi: 10.1016/s0896-6273(02)00569-x [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1), 11–22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Frazier TW, Keshavan MS, Minshew NJ, & Hardan AY (2012). A two-year longitudinal MRI study of the corpus callosum in autism. J Autism Dev Disord, 42(11), 2312–2322. doi: 10.1007/s10803-012-1478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, … Konrad C (2009). Total brain volume and corpus callosum size in medication-naive adolescents and young adults with autism spectrum disorder. Biol Psychiatry, 66(4), 316–319. doi: 10.1016/j.biopsych.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith S, Bowden J, & Mander A (2017). Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Stat Methods Med Res, 26(1), 374–398. doi: 10.1177/0962280214547150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, & Lord C (2009). Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord, 39(5), 693–705. doi: 10.1007/s10803-008-0674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Berman S, Behrmann M, & Dinstein I (2016). Anatomical Abnormalities in Autism? Cereb Cortex, 26(4), 1440–1452. doi: 10.1093/cercor/bhu242 [DOI] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O’Brien F, … Murphy DG (2009). Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med, 39(2), 337–346. doi: 10.1017/S0033291708003383 [DOI] [PubMed] [Google Scholar]
- Hardan AY, Libove RA, Keshavan MS, Melhem NM, & Minshew NJ (2009). A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry, 66(4), 320–326. doi: 10.1016/j.biopsych.2009.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Pabalan M, Gupta N, Bansal R, Melhem NM, Fedorov S, … Minshew NJ (2009). Corpus callosum volume in children with autism. Psychiatry Res, 174(1), 57–61. doi: 10.1016/j.pscychresns.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Ryan LM, Giedd JN, & Lange N (2005). Individual and population penalized regression splines for accelerated longitudinal designs. Biometrics, 61(4), 1037–1048. doi: 10.1111/j.1541-0420.2005.00376.x [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, … Statistical A (2017). Early brain development in infants at high risk for autism spectrum disorder. Nature, 542(7641), 348–351. doi: 10.1038/nature21369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, & Piven J (2006). Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry, 59(1), 1–6. doi: 10.1016/j.biopsych.2005.06.015 [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, … Piven J (2011). Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry, 68(5), 467–476. doi: 10.1001/archgenpsychiatry.2011.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, … Caviness VS Jr., (2003). Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain, 126(Pt 5), 1182–1192. doi: 10.1093/brain/awg110 [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, & Rutter M (2013). Social outcomes in mid- to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. J Am Acad Child Adolesc Psychiatry, 52(6), 572–581 e571. doi: 10.1016/j.jaac.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Hus V, & Lord C (2014). The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord, 44(8), 1996–2012. doi: 10.1007/s10803-014-2080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T, Yamada T, Nakamura M, Watanabe H, Yamagata B, Jimbo D, … Hashimoto R (2015). Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: a multimodal brain imaging study. Neuroimage Clin, 7, 155–169. doi: 10.1016/j.nicl.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katuwal GJ, Baum SA, Cahill ND, Dougherty CC, Evans E, Evans DW, … Michael AM (2016). Inter-Method Discrepancies in Brain Volume Estimation May Drive Inconsistent Findings in Autism. Front Neurosci, 10, 439. doi: 10.3389/fnins.2016.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharsky Hiess R, Alter R, Sojoudi S, Ardekani BA, Kuzniecky R, & Pardoe HR (2015). Corpus callosum area and brain volume in autism spectrum disorder: quantitative analysis of structural MRI from the ABIDE database. J Autism Dev Disord, 45(10), 3107–3114. doi: 10.1007/s10803-015-2468-8 [DOI] [PubMed] [Google Scholar]
- Laird NM, & Ware JH (1982). Random-effects models for longitudinal data. Biometrics, 38(4), 963–974. [PubMed] [Google Scholar]
- Lange N, & Laird NM (1989). The effect of covariance structure on variance estimation in balanced growth curve models with random parameters. J Amer Stat Assoc, 1989, 84. [Google Scholar]
- Lange N, Travers BG, Bigler ED, Prigge MB, Froehlich AL, Nielsen JA, … Lainhart JE (2015). Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Res, 8(1), 82–93. doi: 10.1002/aur.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levman J, Vasung L, MacDonald P, Rowley S, Stewart N, Lim A, … Takahashi E (2018). Regional volumetric abnormalities in pediatric autism revealed by structural magnetic resonance imaging. Int J Dev Neurosci, 71, 34–45. doi: 10.1016/j.ijdevneu.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Lin HY, Ni HC, Lai MC, Tseng WI, & Gau SS (2015). Regional brain volume differences between males with and without autism spectrum disorder are highly age-dependent. Mol Autism, 6, 29. doi: 10.1186/s13229-015-0022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, … Rutter M (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule, second edition (ADOS-2), manual (Part 1). Modules 1–4 Torrance, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Lucibello S, Verdolotti T, Giordano FM, Lapenta L, Infante A, Piludu F, … Battini R (2019). Brain morphometry of preschool age children affected by autism spectrum disorder: Correlation with clinical findings. Clin Anat, 32(1), 143–150. doi: 10.1002/ca.23252 [DOI] [PubMed] [Google Scholar]
- Maier S, Tebartz van Elst L, Perlov E, Duppers AL, Nickel K, Fangmeier T, … Riedel A (2018). Cortical properties of adults with autism spectrum disorder and an IQ>100. Psychiatry Res Neuroimaging, 279, 8–13. doi: 10.1016/j.pscychresns.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Martinez-Murcia FJ, Lai MC, Gorriz JM, Ramirez J, Young AM, Deoni SC, … Suckling J (2017). On the brain structure heterogeneity of autism: Parsing out acquisition site effects with significance-weighted principal component analysis. Hum Brain Mapp, 38(3), 1208–1223. doi: 10.1002/hbm.23449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, … Chua SE (2005). Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain, 128(Pt 2), 268–276. doi: 10.1093/brain/awh332 [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, … Amaral DG (2011). Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A, 108(50), 20195–20200. doi: 10.1073/pnas.1107560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Nordahl CW, Iosif AM, Lee A, Rogers S, & Amaral DG (2016). Increased Surface Area, but not Cortical Thickness, in a Subset of Young Boys With Autism Spectrum Disorder. Autism Res, 9(2), 232–248. doi: 10.1002/aur.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, & R Core Team. (2020). nlme: Linear and Nonlinear Mixed Effects Models: R package version 3.1–149 Retrieved from https://CRAN.R-project.org/package=nlme
- Piven J, Bailey J, Ranson BJ, & Arndt S (1997). An MRI study of the corpus callosum in autism. Am J Psychiatry, 154(8), 1051–1056. doi: 10.1176/ajp.154.8.1051 [DOI] [PubMed] [Google Scholar]
- Prigge MB, Lange N, Bigler ED, Merkley TL, Neeley ES, Abildskov TJ, … Lainhart JE (2013). Corpus Callosum Area in Children and Adults with Autism. Res Autism Spectr Disord, 7(2), 221–234. doi: 10.1016/j.rasd.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A Language and Environment for Statistical Computing. Vienna, Australia: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/ [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, … Murphy DG (2010). Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex, 20(6), 1332–1340. doi: 10.1093/cercor/bhp198 [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage, 61(4), 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R, Greimel E, Kliemann D, Koerte IK, Schulte-Korne G, Reuter M, & Wachinger C (2020). Increased hippocampal shape asymmetry and volumetric ventricular asymmetry in autism spectrum disorder. Neuroimage Clin, 26, 102207. doi: 10.1016/j.nicl.2020.102207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, … Infant Brain Imaging Study, N. (2017). Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants Who Later Develop Autism. Biol Psychiatry, 82(3), 186–193. doi: 10.1016/j.biopsych.2017.02.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, … Amaral DG (2013). Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain, 136(Pt 9), 2825–2835. doi: 10.1093/brain/awt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, … Dager SR (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology, 59(2), 184–192. doi: 10.1212/wnl.59.2.184 [DOI] [PubMed] [Google Scholar]
- Toal F, Daly EM, Page L, Deeley Q, Hallahan B, Bloemen O, … Murphy DG (2010). Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med, 40(7), 1171–1181. doi: 10.1017/S0033291709991541 [DOI] [PubMed] [Google Scholar]
- Turner AH, Greenspan KS, & van Erp TGM (2016). Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Res Neuroimaging, 252, 40–45. doi: 10.1016/j.pscychresns.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, … Buitelaar JK (2018). Cortical and Subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder and Healthy Individuals Across the Lifespan: Results From the ENIGMA ASD Working Group. Am J Psychiatry, 175(4), 359–369. doi: 10.1176/appi.ajp.2017.17010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1991). Wechsler Intelligence Scale for Children - Third Edition (WISC-III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale - Third Edition (WAIS-III). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio: The Psychological Corporation. [Google Scholar]
- Willett JB, Singer JD, & Martin NC (1998). The design and analysis of longitudinal studies of development and psychopathology in context: statistical models and methodological recommendations. Dev Psychopathol, 10(2), 395–426. doi: 10.1017/s0954579498001667 [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, … Network, I. (2015). Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain, 138(Pt 7), 2046–2058. doi: 10.1093/brain/awv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankowitz LD, Herrington JD, Yerys BE, Pereira JA, Pandey J, & Schultz RT (2020). Evidence against the “normalization” prediction of the early brain overgrowth hypothesis of autism. Mol Autism, 11(1), 51. doi: 10.1186/s13229-020-00353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, … Lainhart JE (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain, 137(Pt 6), 1799–1812. doi: 10.1093/brain/awu083 [DOI] [PMC free article] [PubMed] [Google Scholar]


