Abstract
Background
Fasting C-peptide (FCP) has been shown to play an important role in the pathophysiology of mood disorders including depression and schizophrenia, but it is unknown whether it also predicts post-stroke depression (PSD). This study examined the association between FCP and PSD at 6 months after acute ischemic-stroke onset among Chinese subjects.
Methods
A total of 656 stroke patients were consecutively recruited from three hospitals of Wuhan city, Hubei province. Clinical and laboratory data were collected on admission. PSD status was evaluated by DSM-V criteria and 17-item Hamilton Rating Scale for Depression (HAMD-17) at 6 months after acute ischemic stroke. The χ2-test, Mann-Whitney U-test, and t-test were used to check for statistical significance. Multivariate logistic regression model was used to explore independent predictor of PSD.
Results
In the univariate analysis, significant differences were found between the PSD and non-PSD groups in terms of FCP level (p = 0.009). After multivariate adjustments, FCP remained a significant independent predictor of PSD, with an adjusted odds ratio of 1.179 (95%CI: 1.040–1.337, p = 0.010).
Conclusions
Higher FCP levels on admission were found to be associated with PSD at 6 months after acute ischemic-stroke onset. For stroke patients, doctors should pay attention to the baseline FCP for screening high-risk PSD in clinical practice.
Keywords: Fasting C-peptide, Post-stroke depression, Acute ischemic-stroke
Background
Post-stroke depression is the most common and serious affective disorder after stroke, affecting approximately one-third of stroke patients [1–3]. It is generally known that PSD is linked with higher stroke morbidity, mortality, and recurrence, exerts negative effects on the quality of life of stroke survivors as well as brings heavy burden to caregivers [4, 5]. Although the significance of PSD has been well documented and there are validated screening tools for PSD, a body of PSD patients cannot be diagnosed and treated promptly [6]. One reason is that there are no reliable objective biomarkers for diagnosing and predicting PSD.
So far, the pathogenesis of PSD remains unclear [7]. Previous studies have demonstrated that PSD is associated with an increase in the serum levels of some inflammatory cytokines, such as interleukins IL-1β, IL-6, IL-18, intracellular adhesion molecule 1, tumor necrosis factor-α(TNF-α) and c-reactive protein (CRP) [8–10]. Other molecular markers, including brain-derived neurotrophic factor (BDNF) [11], homocysteine, triiodothyronine [12], uric acid [13, 14], total bilirubin [15] and leptin [16] have also been associated with the development of depression in stroke.
C-peptide, one of the lysis products of proinsulin, is secreted into the circulation in equal amounts to insulin [17]. Compared with insulin, C-peptide is more stable because it is less easily destroyed by the liver [18] and has a longer half-life [19]. Therefore, measuring C-peptide levels can better reflect the function of islet β cell synthesis and the secretion of endogenous insulin, which is widely used in clinical practice. Initially, C-peptide was considered as an inactive molecule. However, currently it is well established that this peptide has significant biological functions, which actually has both anti-inflammatory and pro-inflammatory effects in the body [20–22].
The role of fasting C-peptide level in mood disorders in non-stroke subjects has been explored, with lower fasting C-peptide level was found to be associated with depression [16]. Similarly, Jong et al. [23] found a negative correlation between plasma C-peptide and the self-administered Beck Depression Inventory score in patients with maintenance hemodialysis. Besides, increased concentration of C-peptide was found in schizophrenia patients [24]. To date, however, no study has examined the relationship between fasting C-peptide level and PSD. The lack of data in this field provided the impetus for the study reported herein.
Methods
Subjects
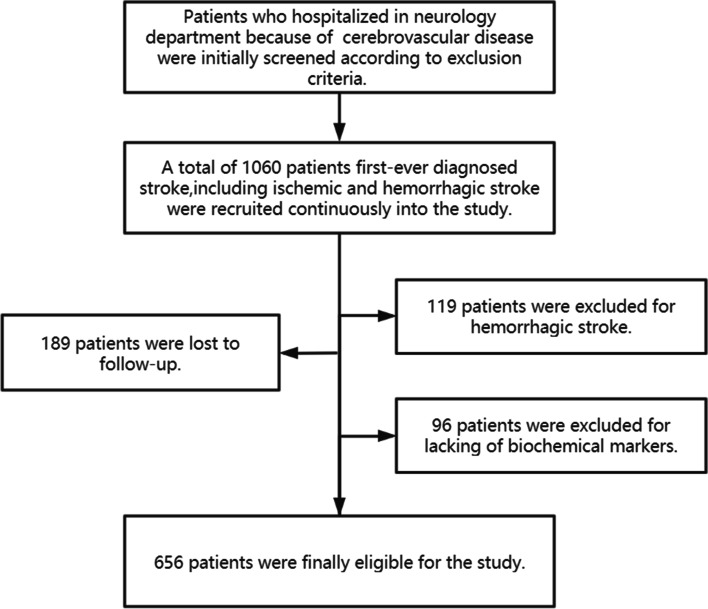
As a multicenter prospective cohort study, our research was conducted at three independent hospitals (Tongji Hospital, Wuhan First Hospital and Wuhan Central Hospital) between August 2018 and June 2019. Inclusion and exclusion criteria for enrollment of patients has been previously reported [14]. A total of 1060 patients first-ever diagnosed stroke, including ischemic as well as hemorrhagic stroke, were consecutively enrolled and 656 patients were finally eligible for the research (Fig. 1).
Fig. 1.
The enrollment flow chart of this study
This study was approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology (Approved No. of ethic committee: TJ-IRB20171108). According to the Declaration of Helsinki, all subjects signed an informed consent form.
Data collection
A standardized questionnaire was used Upon admission to collect detailed information about each patient’s demographic and medical history, including age, gender, height, weight, sleep time, educational level, smoking history, drinking history, hypertension, diabetes mellitus (DM), hyperlipemia and history of coronary heart disease (CHD). Hospitalization days were also included into the variables, which derived from the patient’s discharge records.
Blood samples were obtained from the medial cubital vein in the sitting position at fasting in the early morning. We measured fasting C-peptide (FCP), adrenocorticotropic hormone (ACTH), c-reactive protein (CRP), cortisol, total WBC count (WBC), neutrophil count (NEU), lymphocyte count (LYM), monocyte count (MONO), interleukin (IL-1β, IL-6, IL-10, IL-18), tumor necrosis factor (TNF-α), and interferon (IFN-γ).
The National Institutes of Health Stroke Scale (NIHSS), Barthel index (BI), Modified Rankin scale (mRS), Mini-Mental State Examination (MMSE), Conner-Davidson Resilience Scale (CD-RISC) and Neuroticism scale (subscale of Eysenck Personality Questionnaire) were evaluated within 3 days of the patient’s admission. The 17-item Hamilton Rating Scale for Depression (HAMD-17) had been proved to have good reliability and validity in Chinese population [25], which therefore was used to measure the severity of depressive symptoms at 6 months after ischemic stroke onset.
Participants who met DSM-V diagnostic criteria (depression caused by other medical conditions) along with HAMD-17 score > 7 were assigned to depression group (PSD) [14], otherwise, they were assigned to non-depression group (NPSD). The reason why we chose 6 months after ischemic stroke onset for depression assessment is mainly to avoid the period of transient emotional adjustment to the disability caused by the stroke.
Statistical analysis
Data analysis were performed using the Statistical Program for Social Sciences (SPSS) statistical software (version 25, Chicago, IL, USA). Categorical variables were expressed as percentages and were compared using χ2-test. Continuous variables were presented as medians [interquartile range (IQR)] or means ± standard deviation (S.D.) depending on the normal or nonnormal distribution of data by Kolmogorov-Smirnov test and were compared using t-test or Mann–Whitney U-test (when continuous variables had skewed distributions) [14]. Every variable was analyzed by univariate analysis to cover all potentially important predictors. Variables with P < 0.05 were considered statistically significant.
Subsequently, baseline variables that have been proved to be clinically relevant or that showed a univariate relationship with PSD at 6 months after stroke were entered into multivariate logistic regression model. Variables for inclusion were carefully chosen, given the number of events available, to ensure parsimony of the final model. Collinearity of variables that entered the multivariate logistic regression analysis was assessed by variation inflation factors (< 5 being considered nonsignificant) and tolerance (> 0.2 being considered nonsignificant). The multivariable logistic regression using a backward stepwise method with input of variables if p-value < 0.05 and backward elimination if p-value > 0.05. All p-values were two-sided, p < 0.05 was considered statistically significant.
Results
Baseline characteristics
In total, 656 patients with acute ischemic stroke were consecutively recruited in this study. Of all these potential subjects, 236 (36%) finally developed PSD at 6 months after ischemic stroke onset. Table 1 shows a comparison of baseline information between the NPSD and PSD groups. The PSD and non-PSD groups did not differ in terms of age or BMI, but the PSD group had a higher proportion of female (p = 0.013), lower education level (p = 0.004), longer hospitalized time (p = 0.002), shorter sleep time (p = 0.002), higher NIHSS score (p < 0.001), lower BI score (p < 0.001), higher m RS score (p = 0.002), lower CD-RISC score (p = 0.008) and higher neuroticism score (p = 0.027). As for serum biochemicals, the PSD group showed higher level of FCP (p = 0.009), higher white blood cell count (p = 0.037), higher neutrophil count (p = 0.018) and higher monocyte count (p = 0.006).
Table 1.
Demographic and clinical characteristics of patients without and with PSD
| Parameter | NPSD(N = 420) 64% |
PSD(N = 236) 36% |
P |
|---|---|---|---|
| Demographic information | |||
| Age, median (IQR) | 58(51, 66) | 60(52, 67) | 0.143 |
| Females, n (%) | 82(19.50) | 66(28.00) | 0.013* |
| BMI, median (IQR) | 24.45(22.50, 26.90) | 24.20(22.50,26.73) | 0.387 |
| hospitalization days, median (IQR) | 10(8, 13) | 11(8, 15) | 0.002* |
| Sleep time < 5 h, n (%) | 44(10.50) | 45(19.1) | 0.002* |
| Education level, n (%) | 189(45.00) | 79(33.50) | 0.004* |
| Smoking, n (%) | 190(45.20) | 99(41.90) | 0.415 |
| Drinking, n (%) | 195(46.40) | 93(39.40) | 0.082 |
| Hypertension, n (%) | 249(59.30) | 136(57.60) | 0.679 |
| Diabetes mellitus, n(%) | 120(28.60) | 67(28.40) | 0.961 |
| Hyperlipemia, n (%) | 106(25.20) | 73(30.90) | 0.116 |
| CHD, n (%) | 43(10.20) | 26(10.50) | 0.755 |
| Serum biochemicals | |||
| FCP, median (IQR) | 1.82(1.14, 2, 49) | 2.00(1.38, 2.70) | 0.009* |
| ACTH, median (IQR) | 30.90(16.64, 44.43) | 32.40(18.61,47.95) | 0.229 |
| CRP, median (IQR) | 1.80(0.80, 5.48) | 2.50(0.93, 5.89) | 0.087 |
| Cortical, median (IQR) | 13.00(10.33, 15.70) | 13.50(10.93,16.20) | 0.077 |
| WBC, median (IQR) | 6.57(5.39, 8.08) | 6.89(5.66, 8.50) | 0.037* |
| NEU, median (IQR) | 4.11(3.25, 5.46) | 4.36(3.47, 5.80) | 0.018* |
| MONO, median (IQR) | 0.50(0.39, 0.62) | 0.53(0.44, 0.67) | 0.006* |
| LYM, median (IQR) | 1.58(1.27, 2.09) | 1.65(1.23, 1.99) | 0.904 |
| IL.1β, median (IQR) | 66.47(28.05, 165.07) | 63.60(26.05,167.02) | 0.894 |
| IL.6, median (IQR) | 6.00(2.76,9.54) | 6.00(2.60,10.06) | 0.242 |
| IL.10, median (IQR) | 9.13(2.89,22.96) | 7.78(2.57,19.73) | 0.286 |
| IL.18, median (IQR) | 2158.84(1012.72,4753.75) | 1896.82(954.22,4913.12) | 0.439 |
| TNF-α, median (IQR) | 37.11(21.85,57.62) | 41.42(23.20,59.97) | 0.260 |
| IFN-γ, median (IQR) | 4.33(1.50,8.27) | 5.10(2.00,9.72) | 0.063 |
| Clinical characteristics | |||
| NIHSS, median (IQR) | 2(1,5) | 3(2,7) | <0.001* |
| BI, median (IQR) | 95(65,100) | 80(40,100) | <0.001* |
| MRS, median (IQR) | 2(1,3) | 2(1,4) | 0.002* |
| CD-RISC, median (IQR) | 65(54,77) | 61(50,73) | 0.008* |
| N, median (IQR) | 8(4,11) | 9(6,13) | 0.027* |
PSD Post-stroke depression, IQR Interquartile range, BMI Body mass index, CHD Coronary heart disease, FCP Fasting C-peptide, ACTH Adrenocorticotropic hormone, CRP C-reactive protein, WBC White blood count, NEU Neutrophil count, LYM Lymphocyte count, MONO Monocyte count, IL Interleukin, TNF-α Tumor necrosis factor-α, IFN Interferon, NIHSS The National Institutes of Health Stroke Scale, BI Barthel Index, m RS Modified Rankin Scale, CD-RISC Conner-Davidson Resilience Scale, N Neuroticism
*Statistically significant at p < 0.05 level, two-sided
Independent predictors of PSD
Baseline variables that showed p<0.05 in univariate analysis (Table 1) or that have been proved to be clinically relevant were finally included in multivariate logistic regression model. Variance Inflation Factor (VIF) was used to check for multicollinearity among each variable and no significant statistical collinearity was observed for these variables. After adjusting by potential confounders, educational level (OR = 0.677,95%CI:0.479–0.958, p = 0.028), sleep time (OR = 1.998, 95%CI: 1.250–3.192, p = 0.004), hospitalization days (OR = 1.029,95%CI: 1.001–1.059, p = 0.042), FCP (OR = 1.179,95%CI: 1.040–1.337, p = 0.010), NIHSS (OR = 1.100, 95% CI: 1.045–1.157, p = 0.000) and CD-RISC (OR = 0.987, 95% CI: 0.977–0.997, p = 0.011) remained independently and significantly related with PSD at 6 months after the onset of ischemic stroke (Table 2).
Table 2.
Multivariate logistic regression model for PSD
| Parameter | β | SE | P | OR (95%CI) |
|---|---|---|---|---|
| Educational level | −0.389 | 0.177 | 0.028* | 0.677(0.479–0.958) |
| Sleep time | 0.692 | 0.239 | 0.004* | 1.998(1.250–3.192) |
| Hospitalization days | 0.029 | 0.014 | 0.042* | 1.029(1.001–1.059) |
| FCP | 0.165 | 0.064 | 0.010* | 1.179(1.040–1.337) |
| NIHSS | 0.095 | 0.026 | 0.000* | 1.100(1.045–1.157) |
| CD-RISC | −0.013 | 0.005 | 0.011* | 0.987(0.977–0.997) |
Variables for inclusion:age, gender, hospitalization days, educational level, sleep time, hypertension, diabetes mellitus, hyperlipemia, CHD, FCP, ACTH, cortical, CRP, WBC, NEU, LYM, MONO, NIHSS, MRS, BI, CD-RISC, N
PSD Post-stroke depression, FCP Fasting C-peptide, NIHSS National Institutes of Health stroke scale, CD-RISC Conner-Davidson Resilience Scale, SE Standard Error, OR Odds Ratio, CI Confidence Interval
*Statistically significant at p < 0.05 level, two-sided
Discussions
In this multicenter prospective cohort study, 36% of acute ischemic stroke patients were diagnosed as PSD at 6 months after stroke onset, which is consistent with the results of previous researches [2, 26]. Our study suggested that higher level of C-peptide, higher NIHSS score, longer hospital stay and shorter sleep time were risk factors for PSD. Furthermore, higher CD-RISC score and higher educational level were protective factors lowering the risk of PSD.
For most people, when it comes to C-peptide, the first thing that comes to mind is diabetes. However, a growing body of research suggests that C-peptide also plays an important role in the occurrence and development of other diseases. Several studies have shown positive correlation between C-peptide levels and the incidence of angio-cardiopathy, such as coronary artery disease and myocardial infarction [27–29]. In addition, studies on the relationship between C-peptide and depression have been increasing in recent years. Jong et al. found serum levels of C-peptide was negatively correlated with symptoms of depression in patients on maintenance hemodialysis [23]. Similarly, Kudo et al. found lower C-peptide level was associated with depression [16]. Contrary to their conclusions, our data suggests that higher fasting C-peptide level is related to depression in stroke patients.
Higher C-peptide levels may impact post-stroke depression indirectly through many mechanisms. The most plausible explanation is that high levels of C-peptide reflect insulin resistance and disrupted glucose metabolism, which contributes to depression. It is well known that chronic impairment of glucose metabolism is closely linked to depressive disorders [30] and the prevalence of depression is significantly higher in diabetes mellitus patients [31]. Depressive symptoms often appear at the prediabetes stage characterized by insulin resistance [32]. To a certain degree, as insulin is involved in depression, prevention and treatment insulin resistance may improve symptoms of depression. Considering there are many similarities between the pathogenesis of depression and post-stroke depression, it is reasonable to deem that high C-peptide levels as well as insulin resistance are associated with post-stroke depression. Another reasonable explanation is that C-peptide indirectly contributes to depression through its role in promoting atherosclerosis. A previous study has demonstrated that the deposit C-peptide on intima layer of the vessels promotes infiltration of monocytes/macrophages and lymphocytes CD4+, with impacts on the atherogenesis process [33–35]. Cerebrovascular atherosclerosis causes arterial stenosis, which leading to hemodynamic disturbance as well as low perfusion. Studies have shown that the hemodynamics changes of the middle cerebral artery are linked to the onset of depression in the elderly [36]. Besides, animal studies have also shown that depression-like behavior in rats was related to cerebral hypoperfusion [37]. Hence, cerebrovascular atherosclerosis could be a partial explanation for the link between C-peptide level and PSD. In summary, the underlying mechanism between C-peptide and post-stroke depression is not fully understood and needs further study.
The NIHSS score is regularly used as the measure of stroke severity upon admission. Consistent with the previous studies [38], we demonstrated that the NIHSS score serves as a strong risk factor for depression after acute ischemic stroke. Patients with high NIHSS scores tended to have more serious physical disability and therefore need to be stay in hospital more days receiving rehabilitation treatment. To a certain extent, longer hospital stay indicates higher medical costs, which brings serious psychological burden to patients and thus promotes the occurrence of depression.
The link between depression and poor sleep quality has been shown in numerous studies [39–42] . As many as 90% of depressed patients experience poor sleep. Depression can lead to sleep disturbances and, conversely, poor quality nighttime sleep can inhibit daytime function and produce depression. A vicious cycle may unfortunately exist between sleep and depression.
Our study suggests that higher education levels is protective against post-stroke depression. This is because, compared with patients with lower education levels, patients with higher education levels tend to have better self-regulation ability and more social resources to cope with negative life events [43].
Psychological resilience is defined broadly as positive emotional and/or behavioral adaptation to adversity. The higher the score of psychological resilience, the stronger the ability to adapt to stress [44, 45]. Stroke patients who can be able to face difficulties with an optimistic attitude are less likely to be depressed, which is consistent with previous studies.
Limitations
A limitation of this study is that patients with dysarthria, aphasia or other diseases were excluded, and these excluded patients might be suffered from depressive symptoms. This might cause a selection bias, resulting in a lower rate of PSD than actual data. What’s more, the loss rate of follow-up reduced some statistical power of the analyses. Finally, because this was a multicenter study, the accuracy of instruments varied from hospital to hospital.
Conclusions
Higher level of fasting C-peptide on admission is associated with the risk of PSD at 6 months after acute ischemic-stroke onset. Further investigations are needed to clarify the underlying pathophysiological link between fasting C-peptide level and PSD. For stroke patients, doctors should pay attention to the baseline FCP for screening high-risk PSD in clinical practice.
Acknowledgments
We would like to acknowledge all participants of this project and investigators for collecting data.
Abbreviations
- FCP
Fasting C-peptide
- PSD
Post-stroke depression
- HAMD-17
17-item Hamilton Rating Scale for Depression
- TNF-α
Tumor necrosis factor-α
- CRP
C-reactive protein
- BDNF
Brain-derived neurotrophic factor
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- TIA
Transient ischemic attack
- BMI
Body mass index
- CHD
Coronary heart disease
- ACTH
Adrenocorticotropic hormone
- WBC
White blood count
- NEU
Neutrophil count
- LYM
Lymphocyte count
- MONO
Monocyte count
- IL
Interleukin
- IFN
Interferon
- NIHSS
The National Institutes of Health Stroke Scale
- BI
Barthel Index
- m RS
Modified Rankin Scale
- MMSE
Mini-Mental State Examination
- CD-RISC
Conner-Davidson Resilience Scale
- N
Neuroticism
Authors’ contributions
SZ and ZZ led the study. YW performed the data analysis and implemented the methodology. WS, JM, XQ, YL, GL, CP, and XZ collected the data. YW prepared the original draft. ZZ reviewed and edited the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Key R&D Program of China (grant number 2017YFC1310000), the Fundamental Research Funds for the Central Universities (grant number 2018KFYXMPT015), and Hubei Technological Innovation Special Fund (grant number 2019ACA132). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. ZZ and SZ had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Availability of data and materials
The de-identified database used in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All patients involved in this study or their family members gave written informed consents according to the Declaration of Helsinki. The approval of the study for experiments was obtained from the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanyan Wang, Email: wyy3308534100@163.com.
Wenzhe Sun, Email: sunwenzhe58@163.com.
Jinfeng Miao, Email: m201875948@hust.edu.cn.
Xiuli Qiu, Email: m201875947@hust.edu.cn.
Yan Lan, Email: LanYanswag@163.com.
Chensheng Pan, Email: pancs29@gmail.com.
Guo Li, Email: liguocat@126.com.
Xin Zhao, Email: 1024099129@qq.com.
Zhou Zhu, Email: zhouzhu@hust.edu.cn.
Suiqiang Zhu, Email: zhusuiqiang@163.com.
References
- 1.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 2.Hackett ML, Kohler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13(5):525–534. doi: 10.1016/S1474-4422(14)70016-X. [DOI] [PubMed] [Google Scholar]
- 3.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 4.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry. 2016;173(3):221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 5.Linden T, Blomstrand C, Skoog I. Depressive disorders after 20 months in elderly stroke patients: a case-control study. Stroke. 2007;38(6):1860–1863. doi: 10.1161/STROKEAHA.106.471805. [DOI] [PubMed] [Google Scholar]
- 6.Fournier LE, Beauchamp JES, Zhang X, Bonojo E, Love M, Cooksey G, et al. Assessment of the progression of Poststroke depression in ischemic stroke patients using the patient health Questionnaire-9. J Stroke Cerebrovasc. 2020;29(4):104561. [DOI] [PMC free article] [PubMed]
- 7.Huang G, Chen H, Wang Q, Hong X, Hu P, Xiao M, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. 2019;246:105–111. doi: 10.1016/j.jad.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Tang CZ, Zhang YL, Wang WS, Li WG, Shi JP. Serum levels of high-sensitivity C-reactive protein at admission are more strongly associated with Poststroke depression in acute ischemic stroke than Homocysteine levels. Mol Neurobiol. 2016;53(4):2152–2160. doi: 10.1007/s12035-015-9186-2. [DOI] [PubMed] [Google Scholar]
- 9.Narasimhalu K, Lee J, Leong YL, Ma L, De Silva DA, Wong MC, et al. Inflammatory markers and their association with post stroke cognitive decline. Int J Stroke. 2015;10(4):513–518. doi: 10.1111/ijs.12001. [DOI] [PubMed] [Google Scholar]
- 10.de la Pena FR, Cruz-Fuentes C, Palacios L, Giron-Perez MI, Medina-Rivero E, Ponce-Regalado MD, et al. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psychiatry. 2020;10(8):175–186. doi: 10.5498/wjp.v10.i8.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang J, Cheng Q. Etiological mechanisms of post-stroke depression: a review. Neurol Res. 2009;31(9):904–909. doi: 10.1179/174313209X385752. [DOI] [PubMed] [Google Scholar]
- 12.Zhao JY, Ren WW, Lv DZ, Zhu ZY, Wang QZ, He JC. Low triiodothyronine syndrome is a predictor of post-stroke depression. Int J Geriatr Psychopharmacol. 2017;32(3):352–353. doi: 10.1002/gps.4639. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Xu W, Han K, Zhu L, Gao L, Shang X. Changes of serum uric acid and total bilirubin in elderly patients with major postischemic stroke depression. Neuropsychiatr Dis Treat. 2018;14:83–93. doi: 10.2147/NDT.S149712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Miao JF, Sun WH, Song XY, Lan Y, Zhao X, et al. Lower serum uric acid is associated with post-stroke depression at discharge. Front Psychiatry. 2020;11(52):1–9. [DOI] [PMC free article] [PubMed]
- 15.Tang WK, Liang HJ, Chu WCW, Mok V, Ungvari GS, Wong KS. Association between high serum total bilirubin and post-stroke depression. Psychiatry Clin Neurosci. 2013;67(4):259–264. doi: 10.1111/pcn.12051. [DOI] [PubMed] [Google Scholar]
- 16.Takekawa D, Kudo T, Saito J, Kimura F, Nikaido Y, Sawada K, et al. Higher plasma leptin and lower C-peptide levels are associated with depression: a cross-sectional study. J Affect Disord. 2019;243:70–74. doi: 10.1016/j.jad.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Robert-Cooperman CE, Carnegie JR, Wilson CG, Yang J, Cook JR, Wu J, et al. Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function. Diabetes. 2010;59(9):2209–2218. doi: 10.2337/db09-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yosten GL, Maric-Bilkan C, Luppi P, Wahren J. Physiological effects and therapeutic potential of proinsulin C-peptide. Am J Physiol Endocrinol Metab. 2014;307(11):E955–E968. doi: 10.1152/ajpendo.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lillo Urzua P, Nunez Murillo O, Castro-Sepulveda M, Torres-Quintana MA, Lladser Caldera A, Quest AFG, et al. Loss of Caveolin-1 is associated with a decrease in Beta cell death in mice on a high fat diet. Int J Mol Sci. 2020;21(15):5225. [DOI] [PMC free article] [PubMed]
- 20.Haidet J, Cifarelli V, Trucco M, Luppi P. Anti-inflammatory properties of C-peptide. Rev Diabet Stud. 2009;6(3):168–179. doi: 10.1900/RDS.2009.6.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasic D, Walcher D. C-peptide: a new mediator of atherosclerosis in diabetes. Mediat Inflamm. 2012;2012:858692. [DOI] [PMC free article] [PubMed]
- 22.Vasic D, Walcher D. Proinflammatory effects of C-peptide in different tissues. Int J Inf Secur. 2012;2012:932725. doi: 10.1155/2012/932725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jong IC, Tsai HB, Lin CH, Ma TL, Guo HR, Hung PH, et al. Close correlation between the ankle-brachial index and symptoms of depression in hemodialysis patients. Int Urol Nephrol. 2017;49(8):1463–1470. doi: 10.1007/s11255-017-1598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broedbaek K, Hilsted L. Chromogranin a as biomarker in diabetes. Biomark Med. 2016;10(11):1181–1189. doi: 10.2217/bmm-2016-0091. [DOI] [PubMed] [Google Scholar]
- 25.Zheng YP, Zhao JP, Phillips M, Liu JB, Cai MF, Sun SQ, et al. Validity and reliability of the Chinese Hamilton depression rating scale. Br J Psychiatry. 1988;152:660–664. doi: 10.1192/bjp.152.5.660. [DOI] [PubMed] [Google Scholar]
- 26.Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Brit J Psychiat. 2013;202(1):14–21. doi: 10.1192/bjp.bp.111.107664. [DOI] [PubMed] [Google Scholar]
- 27.Min JY, Min KB. Serum C-peptide levels and risk of death among adults without diabetes mellitus. Can Med Assoc J. 2013;185(9):E402–E4E8. doi: 10.1503/cmaj.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Leon AC, Garcia JGO, Rodriguez IM, Gonzalez DA, Sanchez JJA, Diaz BB, et al. C-peptide as a risk factor of coronary artery disease in the general population. Diab Vasc Dis Re. 2015;12(3):199–207. doi: 10.1177/1479164114564900. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li Y, Meng L, Zheng LS. Association between serum C-peptide as a risk factor for cardiovascular disease and high-density lipoprotein cholesterol levels in nondiabetic individuals. PLoS One. 2015;10(1):e112281. [DOI] [PMC free article] [PubMed]
- 30.Lang UE, Beglinger C, Schweinfurth N, Walter M, Borgwardt S. Nutritional aspects of depression. Cell Physiol Biochem. 2015;37(3):1029–43. [DOI] [PubMed]
- 31.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 32.Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance (vol 36, pg 480, 2013) Diabetes Care. 2013;36(5):1429. doi: 10.2337/dc13-er05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walcher D, Aleksic M, Jerg V, Hombach V, Zieske A, Homma S, et al. C-peptide induces chemotaxis of human CD4-positive cells: involvement of pertussis toxin-sensitive G-proteins and phosphoinositide 3-kinase. Diabetes. 2004;53(7):1664–1670. doi: 10.2337/diabetes.53.7.1664. [DOI] [PubMed] [Google Scholar]
- 34.Walcher D, Babiak C, Poletek P, Rosenkranz S, Bach H, Betz S, et al. C-peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res. 2006;99(11):1181–1187. doi: 10.1161/01.RES.0000251231.16993.88. [DOI] [PubMed] [Google Scholar]
- 35.Alves MT, Ortiz MMO, Dos Reis G, Dusse LMS, Carvalho MDG, Fernandes AP, et al. The dual effect of C-peptide on cellular activation and atherosclerosis: protective or not? Diabetes Metab Res Rev. 2019;35(1):e3071. doi: 10.1002/dmrr.3071. [DOI] [PubMed] [Google Scholar]
- 36.Tiemeier H, Bakker SLM, Hofman A, Koudstaal PJ, Breteler MMB. Cerebral haemodynamics and depression in the elderly. J Neurol Neurosur Ps. 2002;73(1):34–39. doi: 10.1136/jnnp.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SR, Choi B, Paul S, Seo JH, Bin Back D, Han JS, et al. Depressive-like behaviors in a rat model of chronic cerebral Hypoperfusion. Transl Stroke Res. 2015;6(3):207–214. doi: 10.1007/s12975-014-0385-3. [DOI] [PubMed] [Google Scholar]
- 38.Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A et al. Depression after stroke and lesion location: a systematic review. Lancet. 2000;356(9224):122–126. doi: 10.1016/S0140-6736(00)02448-X. [DOI] [PubMed] [Google Scholar]
- 39.Louie GH, Tektonidou MG, Caban-Martinez AJ, Ward MM. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Data from the 2007 National Health Interview Survey. Arthritis Care Res. 2011;63(2):247–260. doi: 10.1002/acr.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Li LQ, Wu CM, Gan Y, Qu XG, Lu ZX. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16:375. [DOI] [PMC free article] [PubMed]
- 42.Alvaro PK, Roberts RM, Harris JK. A systematic review assessing Bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen AW, Walton QL, Thomas C, Mouzon DM, Taylor HO. Social support from friends and depression among African Americans: the moderating influence of education. J Affect Disord. 2019;253:1–7. doi: 10.1016/j.jad.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Hou XL, Wang HZ, Hu TQ, Gentile DA, Gaskin J, Wang JL. The relationship between perceived stress and problematic social networking site use among Chinese college students. J Behav Addict. 2019;8(2):306–317. doi: 10.1556/2006.8.2019.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maatouk I, He S, Becker N, Hummel M, Hemmer S, Hillengass M, et al. Association of resilience with health-related quality of life and depression in multiple myeloma and its precursors: results of a German cross-sectional study. BMJ Open. 2018;8(7):e021376. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified database used in the current study are available from the corresponding author on reasonable request.