Abstract
Background
Hypotension occurs frequently during surgery and may be associated with adverse complications. Vasopressor titration is frequently used to correct hypotension, but requires considerable time and attention, potentially reducing the time available for other clinical duties. To overcome this issue, we have developed a closed-loop vasopressor (CLV) controller to help correct hypotension more efficiently. The aim of this randomised controlled study was to evaluate whether the CLV controller was superior to traditional vasopressor management at minimising hypotension in patients undergoing abdominal surgery.
Methods
Thirty patients scheduled for elective intermediate-to high-risk abdominal surgery were randomised into two groups. In the CLV group, hypotension was corrected automatically via the CLV controller system, which adjusted the rate of a norepinephrine infusion according to MAP values recorded using an advanced haemodynamic device. In the control group, management of hypotension consisted of standard, manual adjustment of the norepinephrine infusion. The primary outcome was the percentage of time that a patient was hypotensive, defined as MAP <90% of their baseline value, during surgery.
Results
The percentage of time patients were hypotensive during surgery was 10 times less in the CVL group than in the control group (1.6 [0.9–2.3]% vs 15.4 [9.9–24.3]%; difference: 13 [95% confidence interval: 9–19]; P<0.0001). The CVL group also spent much less time with MAP <65 mm Hg (0.2 [0.0–0.4]% vs 4.5 [1.1–7.9]%; P<0.0001).
Conclusions
In patients undergoing intermediate- to high-risk surgery under general anaesthesia, computer-assisted adjustment of norepinephrine infusion significantly decreases the incidence of hypotension compared with manual control.
Clinical trial registration
Keywords: closed-loop, haemodynamic, hypertension, hypotension, intraoperative monitoring, norepinephrine, safety, vasopressor
Editor's key points.
-
•
A closed-loop vasopressor (CLV) controller may help to correct hypotension and reduce hypotensive episodes during anaesthesia.
-
•
This randomised trial investigated whether use of CLV could reduce the number of hypotensive episodes during general anaesthesia compared with manual norepinephrine adjustments.
-
•
The percentage of time with hypotension was about 10 times lower in the CVL group than in the control group.
-
•
A large RCT is required to evaluate the impact of this strategy on relevant outcomes.
Hypotension occurs frequently during surgery and likely has a negative impact on postoperative outcomes.1,2 Several authors have documented a significant association between hypotension and adverse postoperative events, including myocardial infarction, acute kidney injury, stroke, and death.3, 4, 5, 6, 7, 8, 9, 10 Two large RCTs have also suggested that interventions directed at limiting hypotension can reduce the risk of complications after surgery.11,12 Futier and colleagues11 published the most popular of these in JAMA in 2017. In this study, the authors determined that maintaining systolic arterial pressure within 10% of a patient's preoperative value during surgery prevented end-organ damage compared with routine care. They achieved this target using a norepinephrine infusion from anaesthesia induction through the end of surgery.
However, tight control of arterial pressure requires frequent manual infusion rate adjustments and constant vigilance, which are both time consuming and can be extremely difficult for human providers. To complicate this, many anaesthesia providers can be intermittently distracted with other tasks in the operating theatre (OT). As a result, it seems difficult, if not completely infeasible, for anaesthesia providers to dedicate sufficient attention to vasopressor infusion adjustments to ensure optimal haemodynamic control. We have previously demonstrated that patients receiving continuous vasopressor infusions in the OT may spend approximately 50% of treatment time outside an MAP range of 60–80 mm Hg.13
To overcome this issue, we recently developed a closed-loop vasopressor (CLV) controller with the goal of decreasing the incidence of hypotension via continuous adjustments of a norepinephrine infusion with the aim of keeping arterial pressure within a narrow range. The initial development stages have already been completed, with engineering and animal studies strongly supporting the efficacy of the controller when coupled with an arterial line or a noninvasive continuous arterial pressure monitoring device.14, 15, 16 Last year, our group published a proof-of-concept study demonstrating the efficacy of the controller in minimising hypotension for a cohort of 20 patients undergoing various major surgical procedures.17 In view of these positive results, we designed an RCT to evaluate the superiority of the CLV controller compared with manual management (administration of norepinephrine by standard infusion pump with the rate adjusted by bedside providers) to minimise hypotension in patients undergoing intermediate- and high-risk abdominal surgery.
Methods
Ethics approval and trial design
This single-centre, two-arm, parallel, randomised controlled superiority study was approved on August 21, 2019, by the Ethics Committee of Erasme Hospital, Brussels, Belgium under the number P2019/347/B406201940690 and registered with ClinicalTrials.gov (NCT04089644; principal investigator: Alexandre Joosten) on September 13, 2019. The study was conducted from September 17, 2019 until March 5, 2020. Written informed consent was obtained from each patient before their inclusion in the study.
Patient inclusion and exclusion
Adult (≥18 yr) patients undergoing intermediate- to high-risk abdominal surgery (open or laparoscopic assisted), who required advanced cardiac output monitoring (EV1000™; Edwards Lifesciences, Irvine, CA, USA) and careful arterial pressure control, were considered for inclusion. Exclusion criteria were severe cardiac arrhythmias and severe chronic kidney disease (serum creatinine >2 mg dl−1 or dialysis). For safety reasons, the principal investigator (AJ) supervised the primary anaesthesia provider throughout the intraoperative study period for the CLV group.
Randomisation, blinding, and data collection
Patients were randomised (1:1 allocation) to CLV or manual management by an independent person not involved in the study using internet-based randomisation software (http://www.randomization.com). Allocation was concealed in sequentially numbered opaque envelopes. Anaesthesia providers were not blinded to group allocation, but subjects and the investigator collecting the outcome data were. Importantly, all CLV cases were done by the principal investigator, whilst all subjects in the control group were done by members of the anaesthesia staff not involved in the current study.
Anaesthetic protocol
The subjects had standard monitoring devices in place, including a five-lead electrocardiogram, noninvasive pulse oximetry, upper arm blood pressure cuff, end-tidal CO2 monitoring device, rectal temperature probe, and a bispectral (BIS™) monitor (Aspect Medical Systems, Natick, MA, USA). A radial arterial catheter was inserted before induction and connected via the FloTrac sensor (Edwards Lifesciences, Irvine, CA, USA) to an advanced haemodynamic monitor (EV1000; Edwards Lifesciences). A triple-lumen central venous catheter was also inserted in all patients post-induction.
Anaesthesia was induced with propofol (2 mg kg−1) combined with remifentanil administered via a target-controlled infusion system using the pharmacokinetic models of Minto and colleagues.18 Sevoflurane and remifentanil were used for maintenance of anaesthesia with adjustments made to maintain the BIS between 40 and 60. Rocuronium (0.6 mg kg−1) was administered for tracheal intubation, and muscle relaxation was maintained with additional 10 mg boluses to keep the train-of-four ratio <2 using ToFscan® technology (IDMED, Marseille, FRANCE). The lungs were mechanically ventilated with FiO2 of 50% (Infinity® C700 anaesthesia machine; Dräger Medical GmbH, Lübeck, Germany), a tidal volume of 8 ml kg−1 of predicted body weight, and a PEEP of 5–7 cm H2O. Recruitment manoeuvres were allowed when deemed necessary. Ventilatory frequency was set to achieve an end-tidal carbon dioxide pressure of between 32 and 36 mm Hg. Prophylactic antibiotics were administered before skin incision. Postoperative pain was prevented with of spinal morphine 200 μg injected before induction. An additional dose of morphine 0.05 mg kg−1 was injected intravenously 1 h before the end of the procedure along with paracetamol with or without non-steroidal anti-inflammatory agents. A forced-air warming system (3M™ Bair Hugger™; St Paul, MN, USA) and a blood–fluid warming system (3M Ranger™) were used to avoid hypothermia throughout surgery.
Fluid administration was standardised in both groups and consisted of a baseline infusion of a balanced isotonic crystalloid solution (PLASMA-LYTE®; Baxter, Lessines, Belgium) set at 3 ml kg−1 h−1. Additional 100 ml mini-fluid challenges of PLASMA-LYTE were allowed in both groups and manually delivered using a goal-directed haemodynamic algorithm to maintain stroke volume variation (SVV) <13% during surgery. If necessary, anaesthetists could compensate blood loss with modified fluid gelatin 3% (Geloplasma®; Fresenius Kabi GmbH, Bad Homburg, Germany) titrated per 100 ml instead of PLASMA-LYTE. Packed red blood cells were administered perioperatively to maintain the haemoglobin concentration between 7 and 9 g dl−1, depending on each subject's clinical condition and comorbidities.
Closed-loop vasopressor controller
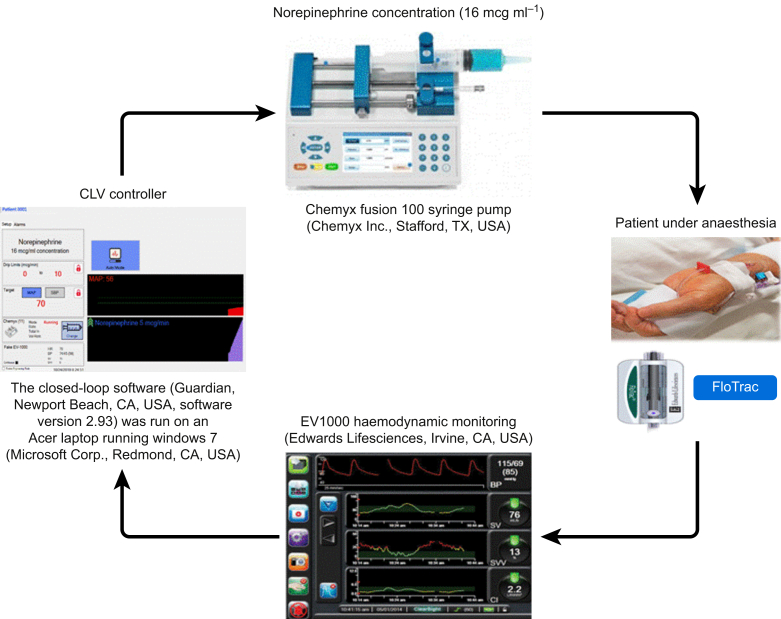
The CLV controller used in this study has been described previously14, 15, 16, 17 and more completely in Supplementary Appendix 1. Briefly, the system collects real-time MAP values from the EV1000 monitor and, through a combination of proportional–integral–derivative (PID) and rules-based control modules, titrates a norepinephrine infusion (16 μg ml−1) to maintain the predefined target MAP at (5) mm Hg of the predefined target through automated adjustments of the infusion rate. The PID element enables adjustment of both current and anticipated future errors, and the rules-based component provides additional safety features and functionality, such as rate and rate-of-change limits. The algorithm was coded in Microsoft Visual C (Microsoft Corp., Redmond, WA, USA). Version 2.93 of the CLV controller software was used for all the patients in this study. The controller software was run on an Acer laptop using Windows 7 (Microsoft Corp.). It was connected to a serial output on an EV1000 monitor and a Chemyx Fusion 100 syringe pump (Chemyx Inc., Stafford, TX, USA). Fig. 1 schematically represents our CLV system. Importantly, the norepinephrine infusion was administered via a different i.v. line (proximal line) than that used for the central venous catheter.
Fig 1.
Schematic representation of the closed-loop vasopressor system. CLV, closed-loop vasopressor.
For subjects in the CLV group, a second norepinephrine syringe (16 μg ml−1) was prepared and connected via a separate infusion pump in case there were errors in the CLV system or the primary anaesthesia team was not satisfied with the management. Additionally, no bolus of any other vasopressor (e.g. ephedrine and phenylephrine) was allowed intraoperatively.
Importantly, whilst the insertion of the arterial catheter was placed just before anaesthesia induction, the connection to the EV1000 monitor was made just after induction of anaesthesia. Additionally, as all patients had a triple-lumen central venous catheter placed post-induction, the study protocol was started when the dedicated proximal lumen of this catheter became available.
Study measurements and objectives
Haemodynamic variables (MAP, heart rate, stroke volume index, cardiac index, and SVV) were recorded every 20 s by the EV1000 monitor, and subsequently averaged. A novel index was measured using a Foley catheter called IKORUS® (Vygon, Écouen, France); this Foley catheter is equipped with a photoplethysmographic sensor enabling continuous monitoring of the urethral perfusion index,19 with which we could assess the impact of vasopressor infusion on peripheral tissue perfusion. Mean intraoperative haemodynamic and urethral perfusion index values and the average values over the first and last 15 min were calculated for each subject.
The primary objective was the percentage of case time during which the subjects were hypotensive, defined as MAP <90% of the baseline value. Of note, baseline MAP values were defined as the MAP taken during the anaesthesia preoperative consultation. Secondary objectives included percentage of case time with MAP <65 mm Hg, percentage of case time ‘in target’ (MAP [10] mm Hg of the baseline MAP), volume of fluid and vasopressor administered, haemodynamic variables, and overall fluid balance. The incidence of major and minor complications (as described20,21) was recorded at 30 days post-surgery. Lengths of stay in the PACU, the ICU, and the hospital were also recorded.
Study power
Preliminary data at Erasme Hospital showed that patients in whom norepinephrine was manually adjusted spent 12 (8)% of case time with MAP <90% of their baseline value with standard management vs 2.6 (2.2)% with our CLV controller.17 As such, with a power of 95% and an alpha risk of 0.05, we needed to include 12 patients per group to detect a statistically significant difference between groups. We therefore decided to include 15 subjects per group (30 subjects in total) to take into account potential dropout.
Statistical analysis
Data were analysed using an intention-to-treat approach. The distribution of continuous data was tested for normality using a Kolmogorov–Smirnov test. Normally distributed variables were compared using Student's t-test and expressed as mean (standard deviation), and those not normally distributed were compared using Mann–Whitney U-test and expressed as median [25–75%] percentiles. Discrete data were expressed as a number and percentage, and compared using χ2 or Fisher's exact test when indicated. Significance was set at a 0.05 level. Data were analysed using Minitab (Minitab, Paris, France).
Results
Characteristics of the patients
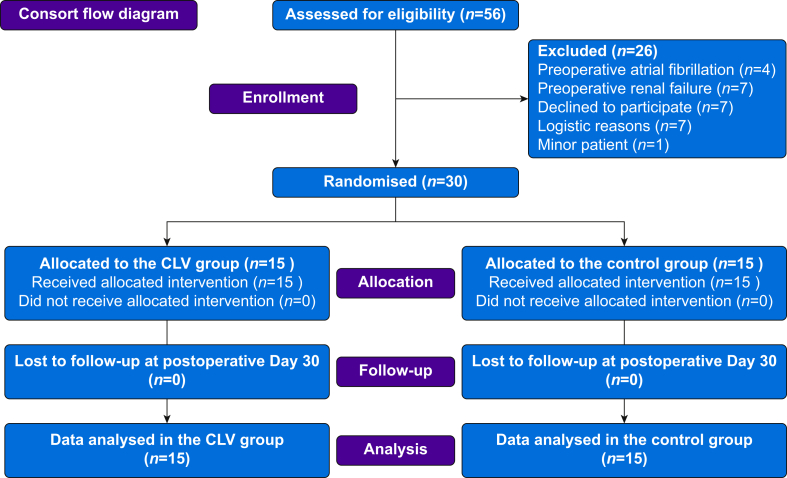
Between September 17, 2019 and March 5, 2020, 56 patients were screened for eligibility and 30 patients were enrolled and randomised (Fig. 2). The urethral perfusion index was not available in one patient because of a technical problem. Baseline characteristics are shown in Table 1 and intraoperative data are summarised in Table 2.
Fig 2.
Consolidated Standards of Reporting Trials (CONSORT) participant flow. Flow diagram illustrating patient enrolment and reasons for exclusion. CLV, closed-loop vasopressor.
Table 1.
Baseline subject characteristics. Data are listed as number and (%), mean (standard deviation), or median and [25th–75th] percentiles. CLV, closed-loop vasopressor.
| Variable | Control group (n=15) | CLV group (n=15) |
|---|---|---|
| Age (yr) | 64 (9) | 64 (13) |
| Male, n(%) | 10 (67) | 7 (47) |
| Weight (kg) | 80 [68–90] | 75 [67–80] |
| Height (cm) | 173 (13) | 169 (9) |
| BMI (kg m−2) | 26 (7) | 27 (5) |
| ASA physical status 2/3 | 7/8 | 6/9 |
| Baseline haemoglobin (g dl−1) | 13.4 (1.8) | 12.5 (1.6) |
| Baseline creatinine (mg dl−1) | 0.90 (0.25) | 0.85 (0.24) |
| Baseline lactate (mM) | 0.7 [0.6–0.9] | 0.7 [0.7–0.9] |
| Baseline MAP (mm Hg) | 80 [80–85] | 85 [80–85] |
| Medication, n (%) | ||
| Aspirin | 5 (33) | 4 (27) |
| Beta blocker | 5 (33) | 7 (47) |
| Angiotensin-converting enzyme inhibitor | 4 (27) | 3 (20) |
| Statin | 5 (33) | 4 (27) |
| Calcium blocker | 4 (27) | 1 (7) |
| Hypoglycaemic agent | 4 (27) | 3 (20) |
| Comorbidities, n (%) | ||
| Myocardial injury | 1 (7) | 1 (7) |
| Arterial hypertension | 10 (67) | 9 (60) |
| Hyperlipidaemia | 5 (33) | 6 (40) |
| Heart failure | 2 (13) | 2 (13) |
| Diabetes | 4 (27) | 3 (20) |
| Chronic obstructive pulmonary disease | 0 (0) | 1 (7) |
| Type of surgery, n (%) | ||
| Oesophagectomy | 2 (13) | 1 (7) |
| Gastrectomy | 1 (7) | 1 (7) |
| Duodenopancreatectomy | 8 (53) | 8 (53) |
| Hepatobiliary surgery | 0 (0) | 3 (20) |
| Other∗ | 4 (27) | 2 (13) |
∗Included combined surgery (total mesothelial excision and liver radio frequency, abdomino-perineal rectal amputation, and robotic nephrectomy).
Table 2.
Intraoperative data. ‘Total IN’ is the sum of crystalloid, colloid, and blood product administration, and ‘Total OUT’ is the sum of estimated blood loss, urine output, and gastric suction. Net fluid balance is the difference of Total IN–Total OUT. Data are expressed as mean (standard deviation) or median and [25th–75th] percentiles. Of note, only one subject received packed red blood cells in each group (272 and 307 ml, respectively). CLV, closed-loop vasopressor.
| Variable | Control group (n=15) | CLV group (n=15) | P-value |
|---|---|---|---|
| Anaesthesia duration (min) | 415 (132) | 434 (122) | 0.670 |
| Surgery duration (min) | 321 (134) | 329 (117) | 0.850 |
| Baseline crystalloid (ml) | 1600 [1000–1900] | 1300 [900–2500] | 0.917 |
| Bolus of crystalloid (ml) | 1300 [1000–6200] | 1100 [700–2400] | 0.361 |
| Bolus of colloid (ml) | 750 [500–1000] | 500 [500–1000] | 0.884 |
| Total IN (ml) | 3325 (1739) | 3004 (1308) | 0.573 |
| Estimated blood loss (ml) | 550 [400–1700] | 672 [300–1300] | 1.000 |
| Urine output (ml) | 350 [260–550] | 500 [380–1000] | 0.101 |
| Gastric suction | 88 [35–138] | 200 [100–300] | 0.009 |
| Total OUT (ml) | 1100 [725–1810] | 1522 [800–2525] | 0.419 |
| Net fluid balance (ml) | 1879 (917) | 1318 (896) | 0.101 |
Outcome measures
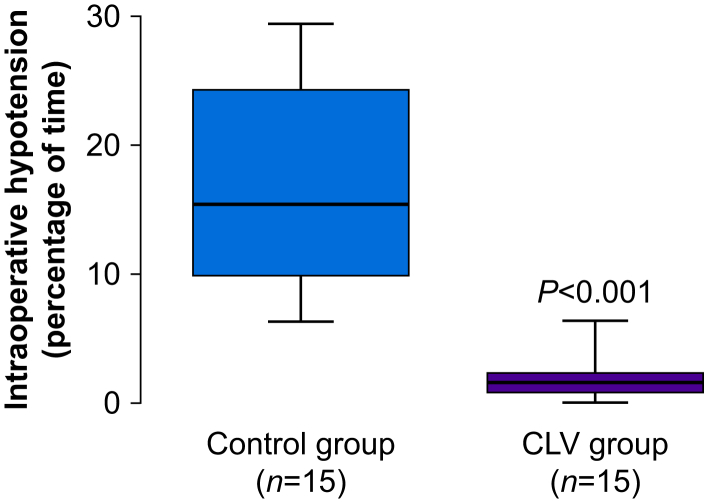
The percentage of case time subjects had hypotension (defined as MAP <90% of MAP baseline) during surgery was 10 times less in the CVL group compared with the patients in the control group (1.6 [0.9–2.3]% vs 15.4 [9.9–24.3]%; difference: 13 [95% confidence interval {CI}: 9–19]; P<0.0001) (Fig. 3). The percentage of time with MAP <65 mm Hg during surgery was also lower in the CVL patients than in the controls (0.2 [0.0–0.4]% vs 4.5 [1.1–7.9]%; difference: 4 [95% CI: 1–6]; P<0.0001), and these subjects had fewer episodes of hypotension, defined as MAP <65 mm Hg for >1 min (0 [0–1] episodes vs 3 [0–7] episodes; P=0.004). Subjects in the CVL group were within the target range ([10] mm Hg of their baseline MAP value) for a greater percentage of time than those in the control group (94 [5]% vs 70 [13]%; difference: 21 [95% CI: 18–26]; P=0.000). The percentage of time with hypertension (defined as MAP >10 mm Hg of the MAP target) was also lower in the CLV group than in the control group (3.5 [0.6–6.7]% vs 14.3 [8.9–22]%; P=0.001). The total dose of norepinephrine was not significantly different between the groups. However, it seems that this is likely just a matter of (insufficient) sample size, as the automated system administered more than double the amount compared with manual administration by the anaesthesiologist (1807 [672–3741] μg in the closed-loop group vs 750 [398–1395] μg in the manual group; P=0.078). Outcomes measures are shown in Table 3.
Fig 3.
Primary outcome representation. Box plot shows the incidence of intraoperative hypotension (defined as MAP <90% of patient's baseline MAP value) in the two groups. CLV, closed-loop vasopressor.
Table 3.
Outcome data. Data are expressed as number and (%), mean (standard deviation), or median and [25th–75th] percentiles. CLV, closed-loop vasopressor; SVV, stroke volume variation.
| Variable | Control group (n=15) | CLV group (n=15) | P-value |
|---|---|---|---|
| MAP target∗ (mm Hg) | 72 [72–76] | 76 [72–76] | 0.272 |
| Primary outcome | |||
| Percentage of case time with MAP <90% of MAP target (%) | 15.4 [9.9–24.3] | 1.6 [0.9–2.3] | 0.000 |
| Secondary outcomes | |||
| Percentage of case time in target (MAP [10] mm Hg) (%) | 69.7 (12.6) | 94.2 (4.8) | 0.000 |
| Percentage of case time with MAP >10 mm Hg above MAP target (%) | 14.3 [8.9–22.1] | 3.5 [0.6–6.7] | 0.001 |
| Percentage of case time with MAP <65 mm Hg (%) | 4.5 [1.1–7.9] | 0.2 [0.0–0.4] | 0.000 |
| Number of episodes with MAP <65 mm Hg for ≥1 min | 3 [0–7] | 0 [0–1] | 0.004 |
| Longest episode with MAP <65 mm Hg (min) | 4 [0–7] | 0 [0–1] | 0.004 |
| Percentage of case time with MAP <60 mm Hg (%) | 1.7 [0.0–2.7] | 0.0 [0.0–0.2] | 0.021 |
| Mean urethral perfusion index | 11.4 (7.3) | 8.0 (4.7) | 0.149 |
| Mean urethral perfusion index during the first 15 min | 12.8 (10.2) | 9.5 (4.5) | 0.282 |
| Mean urethral perfusion index during the last 15 min | 5.7 [3.2–15.1] | 8.5 [3.7–11.2] | 0.810 |
| Stroke volume index (ml m−2) | 37 [34–42] | 39 [35–45] | 0.756 |
| Cardiac index (L min−1 m−2) | 2.8 (0.7) | 3.1 (0.6) | 0.199 |
| SVV (%) | 9 [8–11] | 8 [6–9] | 0.051 |
| Percentage of case time with SVV <13% (%) | 81 [65–94] | 90 [75–97] | 0.206 |
| Percentage of case time with cardiac index <2.5 L min−1 m−2 (%) | 34 [14–67] | 5 [2–22] | 0.028 |
| Total dose of norepinephrine during surgery (μg) | 750 [398–1395] | 1807 [672–3741] | 0.078 |
| Mean rate of norepinephrine infusion (μg min−1) | 2.1 [1.1–2.9] | 4.2 [2.0–8.8] | 0.078 |
| Number of subjects with major complications | 5 (33) | 2 (13) | 0.195 |
| Number of subjects with minor complications | 4 (27) | 6 (40) | 0.439 |
| Number of subjects with major and minor complications | 6 (40) | 6 (40) | 1.000 |
| Length of stay in the ICU/PACU (h) | 19 [18–20] | 19 [18–20] | 0.576 |
| Length of stay in the hospital (days) | 10 [9–15] | 9 [7–15] | 0.576 |
| Postoperative haemoglobin concentration (g dl−1) | 10.7 (1.9) | 10.5 (1.7) | 0.849 |
| Postoperative creatinine concentration (mg dl−1) | 0.7 [0.6–0.9] | 0.7 [0.6–0.8] | 0.330 |
| Postoperative lactate concentration (mM) | 1.3 [0.9–1.9] | 1.3 [0.8–1.6] | 0.507 |
∗MAP target is the MAP at baseline–10%.
The closed-loop system typically made four adjustments of the infusion rate per minute across all cases (median: 4.5 [4.1–4.9]). The maximum rate change per minute in any case was six (the limit of the controller) and the minimum was zero. Rate changes by the manual group were not tracked in the anaesthesia record.
As fluid administration was standardised in the two groups, the total amount of i.v. fluid received and the net fluid balance were similar in both groups. Additionally, haemodynamic variables did not differ between groups. The percentage of time during surgery with an SVV <13% was similar in the two groups. However, the percentage of time during surgery with a CI <2.5 L min−1 m−2 was lower in the CLV group than in the control group (5 [2–22]% vs 34 [14–67]%; P=0.028). There were no significant differences in the mean urethral perfusion index between the groups at the beginning, end, or overall during the case (Table 3).
The incidences of postoperative complications and lengths of stay (PACU, ICU, or hospital) were also similar (Table 3).
There were no system errors during use of the CLV system. The primary anaesthesia providers in the CLV group never needed to use backup vasopressor options during management.
Discussion
We show that in patients undergoing intermediate- to high-risk surgery, automated closed-loop titration of a norepinephrine infusion was associated with significantly fewer hypotensive episodes, a smaller percentage of time with hypotension during surgery, and a greater percentage of time spent within the MAP target range compared with manual adjustments. The percentage of time with hypotension was about 10 times lower in the CVL group, making this a clinically relevant effect. When using the traditional definition of hypotension as any blood pressure episode <65 mm Hg, the subjects in the CLV group had virtually no hypotension. This system therefore represents a significant improvement over current manually adjusted arterial pressure management. It has been shown that even a single minute of hypotension may increase the risk of cardiac and renal complications,3 so the tight arterial pressure control obtained with our CLV system could have a significant impact on postoperative morbidity in patients undergoing intermediate- to high-risk surgery. It is important to note that the present study did not reveal statistically significant differences in the postoperative outcomes between the two groups, most likely because of the small sample size and underpowering. However, as a reduction in postoperative complications would be the main reason to routinely use the automated CLV system, our long-term goal would be to investigate whether a CLV system can actually improve outcome.
Closed-loop titration of vasopressor infusions is not a novel concept. However, the recent increased evidence of an association between perioperative hypotension and worsened postoperative clinical outcome3, 4, 5, 6, 7, 8, 9, 10 has led to renewed enthusiasm for this approach.22,23 In addition, the academic community has recently highlighted the importance of personalising arterial pressure management.24 In a multicentre RCT, Futier and colleagues11 showed that ‘individualised’ arterial pressure management (systolic blood pressure within 10% of a patient's baseline level) was associated with less postoperative organ dysfunction than was routine therapy. These results highlight the importance of maintaining arterial pressure within a narrow, personalised range. One might argue that using a norepinephrine infusion throughout surgery to target a personalised arterial pressure close to baseline MAP may potentially decrease peripheral perfusion. However, there were no differences between groups in microvascular perfusion, as assessed using the urethral perfusion index. This is of major importance, as organ perfusion can be low, even when systemic haemodynamics (MAP) appear normal. As a result, monitoring the urethral perfusion index may provide a real ‘added’ value to the target of resuscitation, as this index relies not only on arterial-pressure-based endpoints, but also on tissue-perfusion-based endpoints. It is important to note that this study was underpowered to detect any significant change in this index. Therefore, on the one hand, using a CLV system to target a personalised MAP value may reduce tissue perfusion as a result of local vasoconstriction, but on the other hand, low tissue perfusion pressures may also contribute to an impaired tissue perfusion and oxygen delivery, which could potentially be avoided by improving MAP and cardiac index. Our future studies will investigate the impact of CLV on sublingual and urethral microcirculation to further explore this hypothesis.
Many techniques can be used to avoid hypotension and maintain tight personalised perioperative arterial pressure control, but we believe that automated closed-loop controllers may represent the most efficient and effective approach moving forward. In addition to appropriate fluid management, vasopressors are frequently used for patients experiencing vasoplegia or other types of fluid-unresponsive hypotension. As manually titrated vasopressor infusions can be unpredictable and require a high level of practitioner involvement, patients may spend a significant amount of time with inappropriately low (or high) blood pressures. At least two studies have demonstrated that the presence of arterial blood pressure variability contributes to morbidity and mortality.25,26 Our controller not only decreases the incidence of hypotension, but also better maintains arterial pressure within a narrow target range ([10] mm Hg) compared with manual management.
Today, at least four international teams are developing closed-loop systems for vasopressor infusions.27, 28, 29, 30 Two teams from China and Singapore have published data from studies in women undergoing Caesarean section under spinal anaesthesia in whom a CLV system was used to titrate phenylephrine alone or in association with ephedrine based on continuous and noninvasive blood pressure monitoring.29,30 These groups demonstrated the superiority of the automated system over manual management. To the best of our knowledge, however, no other published clinical data are available on a CLV system that can automatically titrate a norepinephrine infusion using an arterial line coupled to an advanced haemodynamic monitor in patients undergoing intermediate-to high-risk surgical procedures under general anaesthesia.
There are limitations of our study that require discussion. First, this was a single-centre study with a relatively small sample size. Second, we also had a dedicated anaesthetist to manage the CLV for every patient in the study. This is obviously not realistic moving forward. However, the technology is relatively easy to use, so providers will become more comfortable with it and with simple troubleshooting of its set-up. As it is streamlined and incorporated into protocols, the need for a dedicated anaesthesiologist will therefore be progressively eliminated. Not allowing practitioners to administer boluses of vasoactive agents may have increased the hypotension time for both groups slightly because the effects of changes in the norepinephrine infusion rate can take time to appear. A standard fluid management strategy was used for all patients, which is an important consideration for any automated haemodynamic management system in the future, because fluid status and vasopressor administration are intimately linked. Third, we did not record the percentage of case time each patient was hypotensive during anaesthesia induction because our connection with the EV1000 monitor was done just after induction. Recording these data would have allowed us to distinguish if treatment of hypotension by the CLV system works well during anaesthesia induction in addition to the intraoperative period. This point is important to consider in future studies, as Maheshwari and colleagues31 recently reported that 30% of all hypotension during surgery occurs during anaesthesia induction. Fourth, we only included patients having intermediate-to high-risk abdominal surgery, and the results cannot be extrapolated to other types of surgery or clinical care settings (e.g. ICU), although research in those areas is currently ongoing by our team.
Future directions
There are multiple potential future directions for this work. A top priority is to maximise the benefits of the system whilst minimising the added risk of the automation (i.e. optimising the ‘risk transfer function’ from introduction of the new technology). Our team has begun working on aspects of meta-monitoring (i.e. checking the monitors themselves for sources of error) and on drug delivery monitoring towards this end. Additionally, the long-term vision has always been the integration of single systems into unified control schemes that can cross-coordinate activity; for example, vasopressor management alongside fluid management is a naturally cooperative combination. Finally, the role of predictive analytics in automation is as yet unknown, but could clearly provide opportunities to further improve performance through advanced strategic planning as opposed to the current state that is limited to reacting to the observed environment.
Conclusions
In patients undergoing intermediate- to high-risk surgery under general anaesthesia, computer-assisted adjustment of norepinephrine infusion significantly decreases the incidence of hypotension compared with manual control. A large RCT is now needed to evaluate the impact of this strategy on patient outcomes.
Authors' contributions
Study design: AJ, MC, JR
Patient recruitment: AJ, DC
Data collection: AJ, DC
Data analysis: all authors
Drafting of paper: AJ
Editing of paper: DC
Final drafting: PVdL, LB, BA
Final editing: JD, J-LV, MC, JR
All authors read and approved the final paper.
Acknowledgements
The authors wish to thank all the anaesthesiology and surgical teams of Erasme University Hospital for their support in this study, and Vygon (Écouen, France) for providing some Foley catheters.
Handling editor: Christa Boer
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.08.051.
Declarations of interest
AJ, MC, and JR are consultants for Edwards Lifesciences (Irvine, CA, USA). MC and JR have ownership interest in Sironis, and Sironis has developed a fluid closed-loop system that has been licensed to Edwards Lifesciences. The present closed-loop vasopressor system in this study is new, not owned, or supported by Edwards Lifesciences, Sironis, or any other commercial entity, and is the sole creation of the co-authors. Neither Edwards Lifesciences, Sironis, nor any other commercial entity provided any funding, directly or indirectly, in support of the current work, to the individual authors or any of their respective departments. The other authors have no conflicts of interest related to this study.
Funding
National Heart, Lung, and Blood Institute of the National Institutes of Health (U54HL119893); National Institutes of Health/National Center for Advancing Translational Sciences (UL1 TR00001414).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sessler D.I., Bloomstone J.A., Aronson S. PeriOperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–574. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Wesselink E.M., Kappen T.H., Torn H.M. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Walsh M., Devereaux P.J., Garg A.X. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 4.Sessler D.I., Khanna A.K. Perioperative myocardial injury and the contribution of hypotension. Intensive Care Med. 2018;44:811–822. doi: 10.1007/s00134-018-5224-7. [DOI] [PubMed] [Google Scholar]
- 5.Sessler D.I., Meyhoff C.S., Zimmerman N.M. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death: a substudy of the POISE-2 trial. Anesthesiology. 2018;128:317–327. doi: 10.1097/ALN.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 6.Sun L.Y., Wijeysundera D.N., Tait G.A. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 7.Sun L.Y., Chung A.M., Farkouh M.E. Defining an intraoperative hypotension threshold in association with stroke in cardiac surgery. Anesthesiology. 2018;129:440–447. doi: 10.1097/ALN.0000000000002298. [DOI] [PubMed] [Google Scholar]
- 8.Mascha E.J., Yang D., Weiss S. Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 2015;123:79–91. doi: 10.1097/ALN.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 9.Roshanov P.S., Sheth T., Duceppe E. Relationship between perioperative hypotension and perioperative cardiovascular events in patients with coronary artery disease undergoing major noncardiac surgery. Anesthesiology. 2019;130:756–766. doi: 10.1097/ALN.0000000000002654. [DOI] [PubMed] [Google Scholar]
- 10.van Waes J.A., van Klei W.A., Wijeysundera D.N. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124:35–44. doi: 10.1097/ALN.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 11.Futier E., Lefrant J.Y., Guinot P.G. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X., Jiang Z., Ying J. Optimal blood pressure decreases acute kidney injury after gastrointestinal surgery in elderly hypertensive patients: a randomized study: optimal blood pressure reduces acute kidney injury. J Clin Anesth. 2017;43:77–83. doi: 10.1016/j.jclinane.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Rinehart J., Ma M., Calderon M.D. Blood pressure variability in surgical and intensive care patients: is there a potential for closed-loop vasopressor administration? Anaesth Crit Care Pain Med. 2019;38:69–71. doi: 10.1016/j.accpm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Rinehart J., Ma M., Calderon M.D. Feasibility of automated titration of vasopressor infusions using a novel closed-loop controller. J Clin Monit Comput. 2018;32:5–11. doi: 10.1007/s10877-017-9981-6. [DOI] [PubMed] [Google Scholar]
- 15.Rinehart J., Joosten A., Ma M. Closed-loop vasopressor control: in-silico study of robustness against pharmacodynamic variability. J Clin Monit Comput. 2019;33:795–802. doi: 10.1007/s10877-018-0234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joosten A., Delaporte A., Alexander B. Automated titration of vasopressor infusion using a closed-loop controller: in vivo feasibility study using a swine model. Anesthesiology. 2019;130:394–403. doi: 10.1097/ALN.0000000000002581. [DOI] [PubMed] [Google Scholar]
- 17.Joosten A., Alexander B., Duranteau J. Feasibility of closed-loop titration of norepinephrine infusion in patients undergoing moderate- and high-risk surgery. Br J Anaesth. 2019;123:430–438. doi: 10.1016/j.bja.2019.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minto C.F., Schnider T.W., Egan T.D. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86:10–23. doi: 10.1097/00000542-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Depret F., Leone M., Duclos G. Monitoring tissue perfusion: a pilot clinical feasibility and safety study of a urethral photoplethysmography-derived perfusion device in high-risk patients. J Clin Monit Comput. 2020;34:961–969. doi: 10.1007/s10877-019-00414-9. [DOI] [PubMed] [Google Scholar]
- 20.Joosten A., Delaporte A., Ickx B. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128:55–66. doi: 10.1097/ALN.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 21.Joosten A., Coeckelenbergh S., Delaporte A. Implementation of closed-loop-assisted intra-operative goal-directed fluid therapy during major abdominal surgery: a case-control study with propensity matching. Eur J Anaesthesiol. 2018;35:650–658. doi: 10.1097/EJA.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 22.Joosten A., Rinehart J. Part of the steamroller and not part of the road: better blood pressure management through automation. Anesth Analg. 2017;125:20–22. doi: 10.1213/ANE.0000000000002201. [DOI] [PubMed] [Google Scholar]
- 23.Michard F., Liu N., Kurz A. The future of intraoperative blood pressure management. J Clin Monit Comput. 2018;32:1–4. doi: 10.1007/s10877-017-9989-y. [DOI] [PubMed] [Google Scholar]
- 24.Godet T., Grobost R., Futier E. Personalization of arterial pressure in the perioperative period. Curr Opin Crit Care. 2018;24:554–559. doi: 10.1097/MCC.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 25.Aronson S., Stafford-Smith M., Phillips-Bute B. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology. 2010;113:305–312. doi: 10.1097/ALN.0b013e3181e07ee9. [DOI] [PubMed] [Google Scholar]
- 26.Jinadasa S.P., Mueller A., Prasad V. Blood pressure coefficient of variation and its association with cardiac surgical outcomes. Anesth Analg. 2018;127:832–839. doi: 10.1213/ANE.0000000000003362. [DOI] [PubMed] [Google Scholar]
- 27.Libert N., Chenegros G., Harrois A. Performance of closed-loop resuscitation of haemorrhagic shock with fluid alone or in combination with norepinephrine: an experimental study. Ann Intensive Care. 2018;8:89. doi: 10.1186/s13613-018-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uemura K., Kawada T., Zheng C. Computer-controlled closed-loop drug infusion system for automated hemodynamic resuscitation in endotoxin-induced shock. BMC Anesthesiol. 2017;17:145. doi: 10.1186/s12871-017-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sng B.L., Tan H.S., Sia A.T. Closed-loop double-vasopressor automated system vs manual bolus vasopressor to treat hypotension during spinal anaesthesia for caesarean section: a randomised controlled trial. Anaesthesia. 2014;69:37–45. doi: 10.1111/anae.12460. [DOI] [PubMed] [Google Scholar]
- 30.Ngan Kee W.D., Khaw K.S., Ng F.F. Randomized comparison of closed-loop feedback computer-controlled with manual-controlled infusion of phenylephrine for maintaining arterial pressure during spinal anaesthesia for caesarean delivery. Br J Anaesth. 2013;110:59–65. doi: 10.1093/bja/aes339. [DOI] [PubMed] [Google Scholar]
- 31.Maheshwari K., Turan A., Mao G. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–1228. doi: 10.1111/anae.14416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.