Abstract
Live, oral rotavirus vaccines are more effective at preventing rotavirus disease in countries with low child mortality compared to high child mortality. Among several hypotheses, poorer protection in malnourished children, who are more prevalent in countries with high child mortality, may partially explain this difference. We conducted a literature search to identify articles with a laboratory-confirmed rotavirus endpoint that evaluated differences by malnutrition status in rotavirus vaccine effectiveness and efficacy (VE) or the prevalence of rotavirus infection or illness among children <5 years old. We identified 7 analyses from 11 countries published from 2007–2019 that stratified rotavirus VE by malnutrition status. Among well-nourished children, VE point estimates ranged from 71–84% in observational studies and 26–61% in clinical trials. Among malnourished children, they ranged from −28–45% in observational studies and −3–61% in clinical trials. The relative difference between VE in well-nourished and malnourished children by length-for-age ranged from 37–64%, by weight-for-age ranged from 0–107%, and by weight-for height ranged from −65–137%. We identified 3 cohort and 6 cross-sectional studies of natural rotavirus infection and illness and none reported that malnourished children were more susceptible to rotavirus infection or illness than well-nourished children. Overall, rotavirus vaccines may offer less protection to children with malnutrition than well-nourished children. As malnourished children often have worse outcomes from diarrhea, high rotavirus vaccine coverage and a better understanding the performance of oral rotavirus vaccines in this population is important, though our finding that malnourished children may be less susceptible to rotavirus provides important context and information for vaccine evaluation design.
Keywords: Rotavirus, rotavirus vaccine, vaccine effectiveness, literature review, malnutrition
Introduction
Despite the availability of rotavirus vaccines since 2006, rotavirus is estimated to cause approximately 200,000 deaths worldwide among children <5 years old (1, 2). In 2009, the World Health Organization (WHO) expanded the recommendation for 2 live, oral rotavirus vaccines (Rotarix (GlaxoSmithKline Biologicals, Rixensart, Belgium) and RotaTeq (Merck & Co., West Point, PA, USA)) to all countries (1) and 2 additional live, oral rotavirus vaccines, Rotasiil (Serum Institute of India Pvt. Ltd., Pune, India), and ROTAVAC (Bharat Biotech International Ltd., Hyderabad, India) were prequalified in 2017 (3). To date, >100 countries have introduced a rotavirus vaccine into their routine, infant immunization schedule (4). In observational studies and clinical trials, a difference of about 20 percentage points in Rotarix and RotaTeq performance by a country’s <5 year old child mortality level has been documented (5, 6). While there are several hypotheses for lower rotavirus vaccine performance in higher mortality countries, including interference by oral polio vaccine, differences in gut microbiome and prevalence of malnutrition, the contribution to reduced effectiveness by these possible factors is unknown (7, 8).
Malnutrition in children has a high burden of disability and mortality and long-term malnutrition before a child’s 2nd birthday has irreversible, lifelong impacts (9–12). Though child malnutrition has been declining worldwide, progress has been uneven and more than 1 in 3 children in Sub-Saharan African and South Asia have chronic malnutrition (9, 13). There are 3 common anthropometric indicators of malnutrition in children: length-for-age (stunting), weight-for-length (wasting), and weight-for-age (a combination of wasting and stunting). Low length-for-age, that is length-for-age with a Z-score of <−2, is considered a measure of long-term malnutrition while low weight-for-length suggests acute malnutrition (14). Additionally, malnutrition can be characterized by deficiencies in micronutrients, such as zinc, vitamin A and iron, and can be measured by serum levels of these micronutrients (10, 13, 15). Related to malnutrition, environmental enteric dysfunction (EED) is an asymptomatic condition characterized by malabsorption, increased permeability, and increased inflammation in the small intestine due to frequent enteropathogen infections (11, 16). EED can be measured with fecal and serum biomarkers and has been linked to poor weight gain and stunting (11, 16–19). Malnutrition is associated with increased susceptibility to disease and there is limited evidence that it also impairs oral and parenteral vaccine performance (8, 20, 21). There is insufficient evidence from a limited number of evaluations to determine if EED impairs oral vaccine performance, including oral rotavirus vaccine performance (22).
In this review of the literature, we aim to summarize the available data of natural rotavirus infection and illness by nutritional status and quantify rotavirus vaccine performance among well-nourished and malnourished children <5 years old.
Methods
Literature search methods and inclusion criteria
The literature search was conducted in 3 parts and completed on October 2, 2020. First, we reviewed articles identified as part of a systematic review of rotavirus vaccine post-licensure effectiveness for any analyses that stratified vaccine effectiveness (VE) by well-nourished and malnourished children <5 years old (5). Detailed methods and exclusion criteria for the initial search have been previously described (5). We completed a second PubMed search of abstracts including “rotavirus” and the terms “*nutrition”, “*nourished”, or “environmental enter*” to identify articles on rotavirus prevalence or rotavirus vaccine efficacy (VE) or immunogenicity in clinical trials. Finally, we reviewed the references and citing articles of key publications because analyses of natural rotavirus infection stratified by nutritional status were often secondary objectives or rotavirus was one of many enteropathogens tested and both were not always indicated in the title or abstract.
We included articles that had a laboratory-confirmed rotavirus endpoint and categorized malnutrition among children <5 years old using anthropometric indicators. Laboratory-confirmed rotavirus endpoints included rotavirus-positive diarrhea, rotavirus-positive stool specimens (rotavirus infection), presence of rotavirus antibodies in serum specimens (seropositivity), and an increase in the concentration of rotavirus antibodies in serum specimens (seroconversion). Articles were excluded if nutritional status was defined by feeding method only, for example exclusively breastfed or duration of breastfeeding. Among evaluations of natural rotavirus infection prevalence, we excluded articles where the relationship being investigated was diarrhea as the cause of malnutrition or malnutrition as a predictor diarrhea illness outcomes. We limited VE articles to those published in 2006 or later; we did not limit articles about natural infection by date of publication.
Finally, articles that measured vaccine performance by EED status were excluded as we determined no additional analyses were published since the publication of another a recent literature review and that the variation in methods limited our ability to meaningfully aggregate the results (22). Two rotavirus prevalence articles that otherwise met our inclusion criteria used the same dataset, with 1 year of overlap in data collection period; however, the earlier article included more types of anthropometric indicators of malnutrition, so we chose to include both articles. For a multi-country study of malnutrition and intestinal infections in children in low-income countries (the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development or MAL-ED study), we chose to include overall results, rather than published findings from any individual country.
Analytic methods
We divided the articles into two groups: rotavirus prevalence and performance of rotavirus vaccines; we further categorized vaccine performance evaluations by VE and immunogenicity. For each group, we describe the study population, design, and analytic methods as well as summarize key, common findings across studies. Data were abstracted using a Microsoft Excel database, analyzed using R v.6.3.1, and figures generated using the ggplot2 package (23).
Results
Vaccine efficacy and effectiveness by nutritional status
We identified 7 analyses from 11 countries published from 2007–2019 that stratified rotavirus vaccine efficacy or effectiveness by malnutrition status using anthropometric indicators (Table 1a and Table 1b). Five studies used Rotarix and 1 used RotaTeq, which included separate analyses for 2 groups of countries. One Rotarix and both RotaTeq analyses were post-hoc VE analyses of clinical trial data, which measured malnutrition at time of receipt of the first rotavirus vaccine dose; the 4 case-control VE evaluations of Rotarix measured malnutrition at the time of the child’s diarrhea illness. Overall among well-nourished children, the VE point estimates ranged from 71–84% (median: 77%) for observational studies and 26–61% (median: 32%) for clinical trials (Figure 1). Among malnourished children, the VE point estimates ranged from −28–45% (median: 20%) for observational studies and −3–61% (median: 21%) for clinical trials. The range of relative difference between VE in well-nourished and malnourished children was 37–64% (median: 61%) in the 4 studies that classified malnutrition by length-for-age, 0–107% (median: 62%) in the 4 studies that classified malnutrition by weight-for-age, and −65–137% (median: 111%) in the 3 studies that classified malnutrition by weight-for height (Figure 2). The post-hoc clinical trial analyses found a smaller relative difference between malnourished and well-nourished children than the case-control evaluations of rotavirus vaccine under conditions of routine use.
Table 1a.
Rotavirus vaccine efficacy and effectiveness evaluations stratified by nutritional status, 2006–2020
| Vaccine | Type of study | Year of publication | Country | Age group | Recruitment setting | Rotavirus endpoint | Indicator of malnutrition | Malnourished VE (95% CI) | Well-nourished VE (95% CI) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Rotarix | Randomized trial | 2007 | Brazil, Mexico, Venezuela | <12 months | Community | Rotavirus positive diarrhea | Weight-for-age | 61 (10, 83) | 61 (37, 75) | (27) |
| Rotarix | Case-control | 2016 | Botswana | 4–59 months | Emergency department, inpatient | Rotavirus positive diarrhea | Weight-for-length | −28 (−309, 60) | 75 (41, 89) | (28) |
| Rotarix | Case-control | 2016 | Malawi | <32 months | Emergency department, inpatient | Rotavirus positive diarrhea | Length-for-age | 28 (−100, 74) | 78 (6, 95) | (29) |
| Rotarix | Case-control | 2019 | Kenya | 1–32 months | Inpatient | Rotavirus positive diarrhea | Weight-for-age | 10 (−134, 66) | 84 (62, 93) | (30) |
| Length-for-age | 28 (−118, 76) | 75 (48, 88) | ||||||||
| Weight-for-length | −9 (−224, 63) | 84 (64, 93) | ||||||||
| Rotarix | Case-control | 2019 | Zimbabwe | 6–11 months | Emergency department, inpatient | Rotavirus positive diarrhea | Length-for-age | 45 (−148, 88) | 71 (29, 88) | (31) |
| RotaTeq | Randomized trial | 2017 | Bangladesh, Vietnam | <24 months | Community | Rotavirus positive diarrhea | Weight-for-age | −3 (−256, 70) | 45 (24, 60) | (32) |
| RotaTeq | Randomized trial | 2017 | Ghana, Kenya, Mali | <24 months | Community | Rotavirus positive diarrhea | Weight-for-age | 21 (−34, 53) | 32 (17, 43) | (32) |
| Length-for-age | 13 (−58, 52) | 32 (18, 44) | ||||||||
| Weight-for-length | 43 (15, 62) | 26 (10, 40) |
Table 1b.
Anti-rotavirus immunogenicity evaluations including nutritional status as a predictor, 2006–202
| Vaccine | Type of study | Year of publication | Country | Age group | Study setting | Rotavirus endpoint | Indicator of malnutrition | Key finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rotarix | Randomized trial | 2016 | Bangladesh | <6 months | Community | IgA (seroconversion) | Length-for-age | No association | (33) |
| Weight-for-length | No association | ||||||||
| Rotarix | Randomized trial | 2020 | Zimbabwe | <18 months | Community | IgA (seroconversion) | Weight-for-age | No association | (34) |
| Length-for-age | Normal associated with seroconversion | ||||||||
| Weight-for-length | No association | ||||||||
| IgA (seropositivity) | Weight-for-age | Normal associated with seroconversion in univariate model only | |||||||
| Length-for-age | Normal associated with seroconversion | ||||||||
| Weight-for-length | No association |
Figure 1.
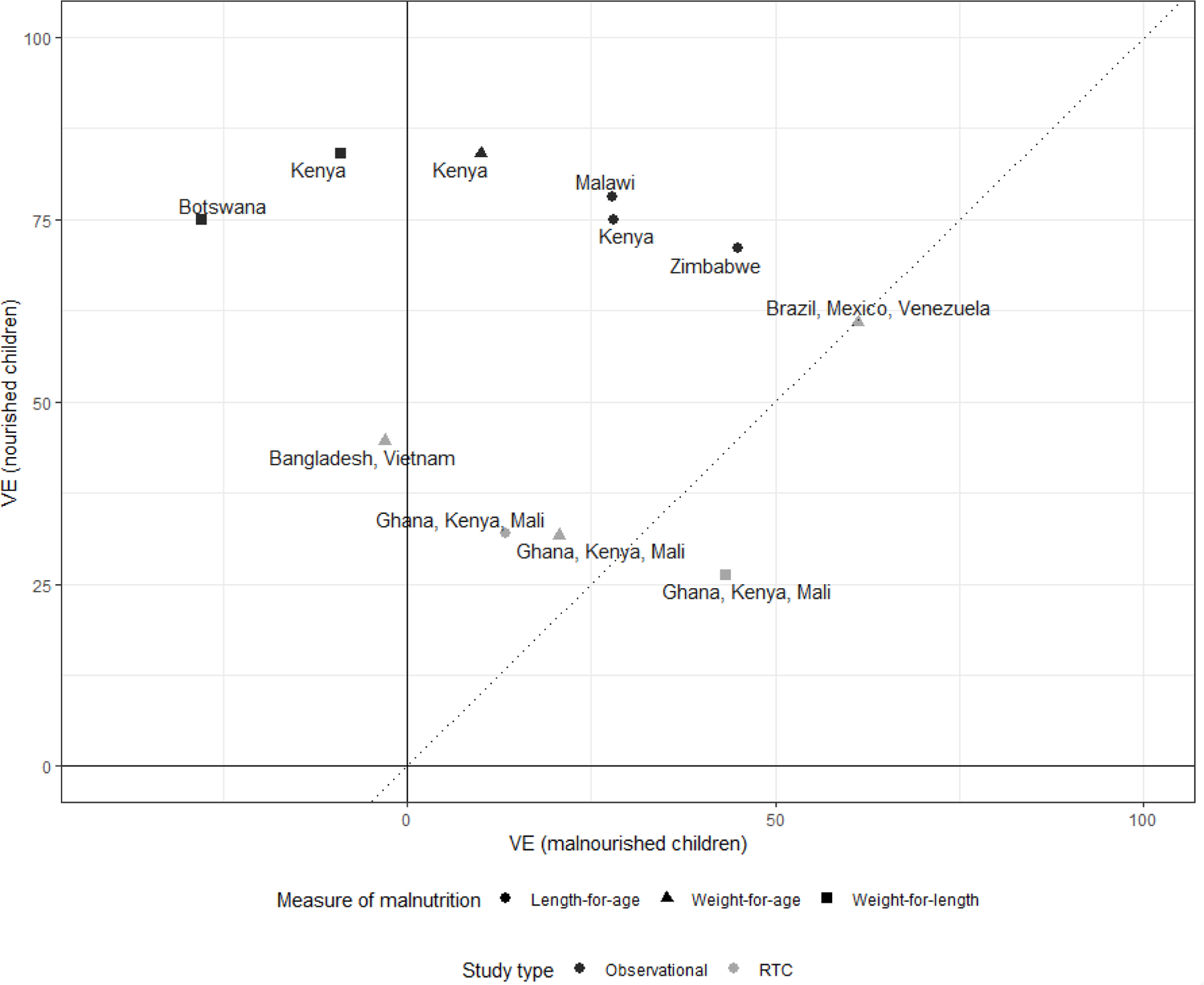
Vaccine efficacy and effectiveness estimates for well-nourished and malnourished children by study design and anthropometric indicator of malnutrition
Figure 2.
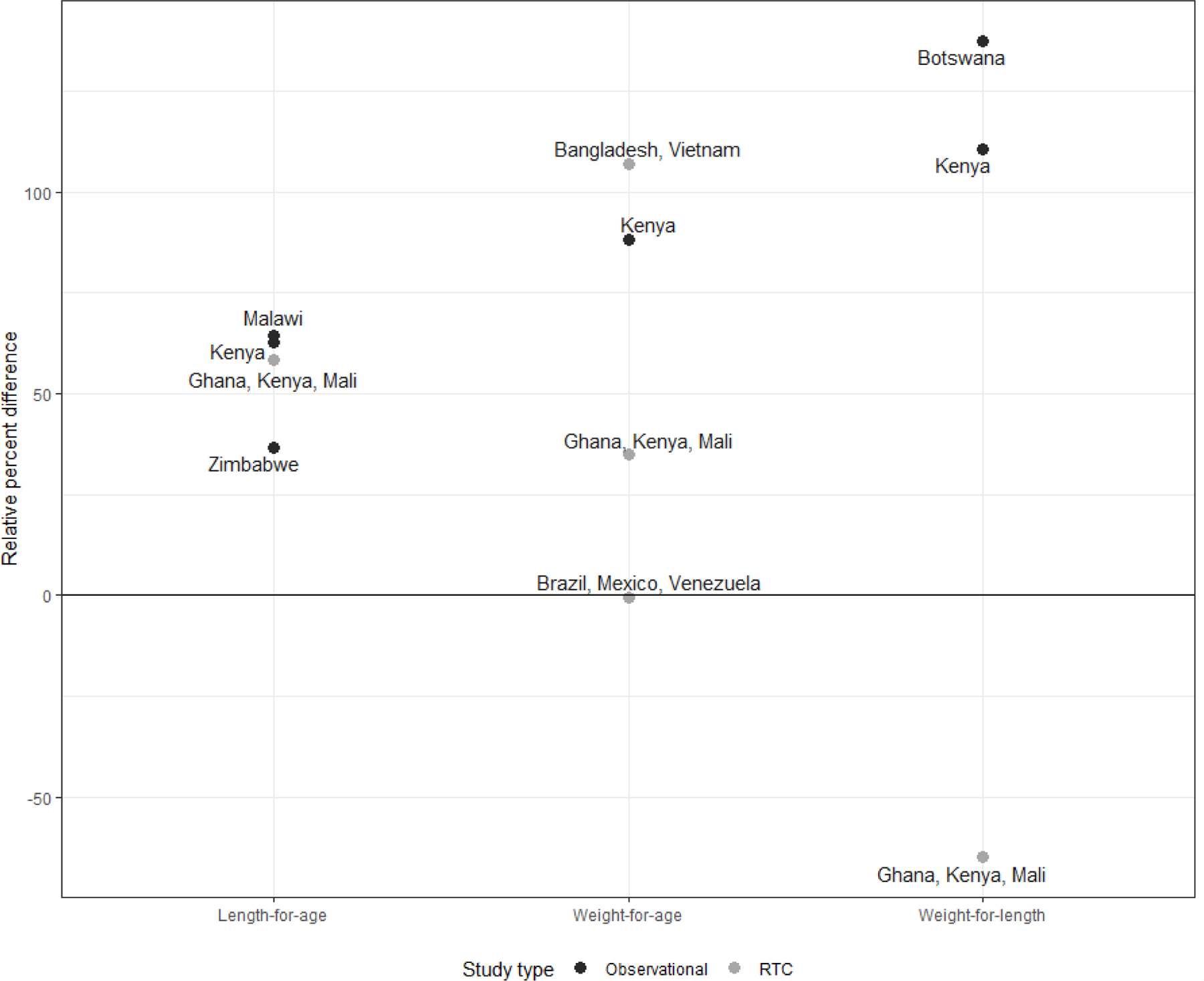
Relative difference in rotavirus vaccine efficacy or effectiveness estimates between well-nourished and malnourished children by anthropometric indicator of malnutrition, study design, and rotavirus vaccine
We identified two additional clinical trials that measured immunogenicity after vaccination with Rotarix. One found that normal length-for-age was predictive of anti-rotavirus IgA seroconversion and seropositivity. The other found no association between anti-rotavirus IgA seroconversion and any anthropometric indicator of malnutrition. The age groups for these two studies were quite different, making any summary measures a challenge.
Rotavirus prevalence by nutritional status
We identified 3 cohort and 6 cross-sectional studies comparing natural rotavirus infection and illness in well-nourished and malnourished children published 1990–2019 (Table 2). In 1 of the 9 studies, data was collected in 8 countries and rotavirus vaccine was available in half of the countries during part or all of the data collection period; in all other studies, rotavirus vaccines were not available through the national immunization program at the time of data collection. Overall, of the 12 countries that are represented, 11 are low- or middle-income countries. Study populations and design were variable in these evaluations; malnutrition was assessed at different timepoints relative to testing for rotavirus, making comparisons across studies challenging. In two studies that considered rotavirus infection as the endpoint, there was no association found with length-for-age, weight-for-age, or weight-for-length. In the 7 studies where rotavirus illness was the outcome of interest, 4 found malnourished children were less likely to have rotavirus diarrhea than well-nourished children and 3 found no association. Finally, 1 study in a pre-vaccine setting found lower IgG titers in weight-for-age malnourished children than well-nourished children and no differences in IgM titers by anthropometric indicators of malnutrition. Overall, none of the articles found malnourished children were more likely to have rotavirus than well-nourished children.
Table 2.
Scientific articles showing prevalence of rotavirus stratified by nutritional status
| Vaccine | Type of study | Year of publication | Country | Age group | Recruitment setting | Rotavirus endpoint | Indicator of malnutrition | Key finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Rotarix (4 countries) / none (4 countries) | Cohort | 2017 | Bangladesh, Brazil, India, Nepal, Peru, Pakistan, South Africa, Tanzania | <24 months old | Community | Rotavirus positive diarrhea, rotavirus infection | Length-for-age, weight-for-age, weight-for-length | No association | (35) |
| None | Cohort | 2016 | Bangladesh | <3 years old | Community | Rotavirus positive diarrhea | Length-for-age, weight-for-age, weight-for-length | Associated with better nourishment | (36) |
| None | Cohort | 2019 | Malawi | 6–18 months old | Community | Rotavirus infection | Length-for-age (excluded children with wasting) | No association | (37) |
| None | Cross sectional | 1990 | Israel | <3 years old | Outpatient | Rotavirus positive diarrhea | Weight-for-length | No association | (38) |
| None | Cross sectional | 1995 | Ecuador | 12–59 months | Community | IgM, IgG titers | Length-for-age, weight-for-age, weight-for-length, Zinc, Hemoglobin, Vitamin A | Higher IgG associated with normal weight-for-age | (39) |
| None | Cross sectional | 1998 | Bangladesh | <5 years old | Inpatient | Rotavirus positive diarrhea | Length-for-age, weight-for-age, weight-for-length | Associated with better nourishment | (40) |
| None | Cross sectional | 2011 | Burkina Faso | <5 years old | Outpatient, inpatient | Rotavirus positive diarrhea | Length-for-age, weight-for-age, weight-for-length | Associated with better nourishment | (26) |
| None | Cross sectional | 2013 | Bangladesh | <5 years old | Inpatient | Rotavirus positive diarrhea | Weight-for-age | Associated with better nourishment | (41) |
| None | Cross sectional | 2017 | Tanzania | <5 years old | Outpatient | Rotavirus positive diarrhea | Weight-for-length | No association | (42) |
Discussion
We found in 9 of 11 analyses that the rotavirus VE point estimate was lower among malnourished children compared to well-nourished children. The VE among well-nourished children was remarkably consistent across studies, with a range of <20 percentage points for each vaccine and were slightly higher than those found by a meta-analysis of post-introduction VE (5), which included both well-nourished and malnourished children, supporting the hypothesis that rotavirus vaccines provide better protection to well-nourished children than malnourished children. Overall, rotavirus VE among malnourished children was consistently lower than for well-nourished children in studies that measured malnutrition at the time of the child’s illness and mostly lower in the clinical trials, where malnutrition was measured at the time of vaccination (~6 weeks of age). However unlike well-nourished children, there was a wide range of VE estimates among malnourished children, even among studies using the same anthropometric indicators of malnutrition, though the overall medians were similar between observational and trial data. Although we didn’t formally include sample size in this evaluation due to inconsistent reporting, fewer children were malnourished than well-nourished, likely making the VE estimates for malnourished children less precise and more unstable.
The relative difference between VE among chronically malnourished (low length-for-age) and well-nourished children was consistent; this relative difference was considerably more variable when using other indicators of malnutrition which relied on the child’s weight, though the median percent differences in length-for-age and weight-for-age were similar. As rotavirus diarrhea is dehydrating, weight at the time of illness, which was used in several of the case-control evaluations, may be especially susceptible to misclassification. The 2 studies that presented VE for all 3 measures of malnutrition found variation between anthropometric indicators of malnutrition, and the trends in this variation were not consistent. There was also no obvious pattern in these findings by anthropometric indicators of malnutrition in the articles showing natural rotavirus infection and illness. More generally, anthropometric indicators of malnutrition may be caused by an array of conditions (13, 24). Studies included in this literature review also varied in when illness occurred relative to when malnutrition was measured and anthropometric indicators of malnutrition at 6 weeks of age including low birth weight and prematurity are correlated with, but distinct from, malnutrition later in infancy or in the second year of life (13). Future research evaluating rotavirus vaccine performance should carefully consider which indicators of malnutrition and when they are measured.
While the findings from studies of prevalence rotavirus infection and illness stratified by nutritional status were mixed, none of the studies reported malnourished children were more likely to have rotavirus than well-nourished children. This is a noteworthy finding because malnourished children have been found to be more susceptible to other enteropathogens (20, 24, 25). Additional research could help better understand possible mechanisms for differences in susceptibility to natural rotavirus infection and disease, as it may help our understanding of live, oral rotavirus vaccine performance in malnourished children. If malnourished children are less susceptible to rotavirus infection and illness, this would also have important implications for rotavirus vaccine research design, for example in estimating appropriate sample size for stratified analyses to evaluate vaccine performance. Nonetheless, protection from rotavirus, which includes high vaccination coverage, is important for malnourished children, because if infected, malnourished children are more likely to have prolonged diarrhea and generally worse outcomes than well-nourished children (13, 26).
This literature review has several limitations. First, none of these studies were specifically powered to look at differences in VE among well-nourished and malnourished children. Thus, while the consistency of the findings of lower point estimate of VE among malnourished infants in most studies supports that this is a real phenomenon, the overlap in VE confidence intervals between the two groups preclude firm conclusions. Second, while we intended to be as comprehensive as possible, analyses of the association of rotavirus infection and illness with malnutrition were often secondary and it is possible we did not identify all published articles. However because the original VE literature review was extensive (5), we are confident all post-introduction VE articles stratified by nutritional status were included here. Third, there were a limited number of articles overall and no post-introduction VE evaluations of RotaTeq, Rotasiil, or ROTAVAC stratified by nutritional status. Finally, sample size was not well documented, making it challenging to assess quality and provide appropriate comparisons. Confidence intervals are quite wide and the sample sizes available indicate that these studies may have been underpowered for these analyses.
In this literature review, we found that rotavirus vaccines may offer less protection to children with malnutrition than well-nourished children, though likely offers some protection. As malnourished children often have worse outcomes from diarrhea, better understanding oral rotavirus vaccine performance in this population is important.
Footnotes
Conflicts of interest: The authors indicate that they have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.WHO. Rotavirus Vaccines WHO Position Paper. Weekly Epidemiological Record 2013;88:49–64.23424730 [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis 2016;62Suppl 2:S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis 2019;32:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccine in National Immunization Programme Update In: Immunization VaB, ed. https://www.who.int/immunization/monitoring_surveillance/en/: World Health Organization; 2020.
- 5.Burnett E, Parashar UD, Tate JE. Real-world effectiveness of rotavirus vaccines, 2006–19: a literature review and meta-analysis. Lancet Glob Health 2020;8:e1195–e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark A, van Zandvoort K, Flasche S, et al. Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials. Lancet Infect Dis 2019;19:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velasquez DE, Parashar U, Jiang B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: causes and contributing factors. Expert Rev Vaccines 2018;17:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker EP, Ramani S, Lopman BA, et al. Causes of impaired oral vaccine efficacy in developing countries. Future Microbiol 2018;13:97–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNICEF. Malnutrition March 2020 2020 Available at: https://data.unicef.org/topic/nutrition/malnutrition/. AccessedSeptember 15, 2020, 2020.
- 10.Haiti: WHO and UNICEF coverage estimates of immunization coverage: 2019 revision https://www.who.int/immunization/monitoring_surveillance/data/hti.pdf: World Health Organization;2020.
- 11.Korpe PS, Petri WA Jr. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med 2012;18:328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 2013;382:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–451. [DOI] [PubMed] [Google Scholar]
- 14.Blossner M dOM. Malnutrition: Quantifying the health impact at national and local levels. In: Nutrition for Health and Development WHO, ed. Geneva: World Health Organization; 2005. [Google Scholar]
- 15.Caulfield LE, Bose A, Chandyo RK, et al. Infant feeding practices, dietary adequacy, and micronutrient status measures in the MAL-ED study. Clin Infect Dis 2014;59Suppl 4:S248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl Trop Dis 2018;12:e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri WA Jr., Naylor C, Haque R. Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Med 2014;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg 2012;86:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoke MK, McCabe KA, Miller AA, McDade TW. Validation of endotoxin-core antibodies in dried blood spots as a measure of environmental enteropathy and intestinal permeability. Am J Hum Biol 2018;30:e23120. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of Childhood Malnutrition on Host Defense and Infection. Clin Microbiol Rev 2017;30:919–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc Lond B Biol Sci 2015;370. [DOI] [PMC free article] [PubMed]
- 22.Church JA, Parker EP, Kosek MN, et al. Exploring the relationship between environmental enteric dysfunction and oral vaccine responses. Future Microbiol 2018;13:1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickham H ggplot2: Elegant Graphics for Data Analysis: Springer International Publishing; 2016. [Google Scholar]
- 24.Walson JL, Berkley JA. The impact of malnutrition on childhood infections. Curr Opin Infect Dis 2018;31:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platts-Mills JA, Taniuchi M, Uddin MJ, et al. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr 2017;105:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitiema LW, Nordgren J, Ouermi D, et al. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int J Infect Dis 2011;15:e646–652. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis 2007;196:537–540. [DOI] [PubMed] [Google Scholar]
- 28.Gastanaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of Monovalent Rotavirus Vaccine After Programmatic Implementation in Botswana: A Multisite Prospective Case-Control Study. Clin Infect Dis 2016;62Suppl 2:S161–167. [DOI] [PubMed] [Google Scholar]
- 29.Bar-Zeev N, Jere KC, Bennett A, et al. Population Impact and Effectiveness of Monovalent Rotavirus Vaccination in Urban Malawian Children 3 Years After Vaccine Introduction: Ecological and Case-Control Analyses. Clin Infect Dis 2016;62Suppl 2:S213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khagayi S, Omore R, Otieno GP, et al. Effectiveness of monovalent rotavirus vaccine against hospitalization with acute rotavirus gastroenteritis in Kenyan children. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed]
- 31.Mujuru HA, Burnett E, Nathoo KJ, et al. Monovalent Rotavirus Vaccine Effectiveness Against Rotavirus Hospitalizations Among Children in Zimbabwe. Clin Infect Dis 2019;69:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber JF, Hille DA, Liu GF, et al. Heterogeneity of Rotavirus Vaccine Efficacy Among Infants in Developing Countries. Pediatr Infect Dis J 2017;36:72–78. [DOI] [PubMed] [Google Scholar]
- 33.Emperador DM, Velasquez DE, Estivariz CF, et al. Interference of Monovalent, Bivalent, and Trivalent Oral Poliovirus Vaccines on Monovalent Rotavirus Vaccine Immunogenicity in Rural Bangladesh. Clin Infect Dis 2016;62:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Church JA, Chasekwa B, Rukobo S, et al. Predictors of oral rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine 2020;38:2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan VR, Karthikeyan R, Babji S, et al. Rotavirus Infection and Disease in a Multisite Birth Cohort: Results From the MAL-ED Study. J Infect Dis 2017;216:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verkerke H, Sobuz S, Ma JZ, et al. Malnutrition Is Associated with Protection from Rotavirus Diarrhea: Evidence from a Longitudinal Birth Cohort Study in Bangladesh. J Clin Microbiol 2016;54:2568–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehto KM, Fan YM, Oikarinen S, et al. Presence of Giardia lamblia in stools of six- to 18-month old asymptomatic Malawians is associated with children’s growth failure. Acta Paediatr 2019;108:1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagan R, Bar-David Y, Sarov B, et al. Rotavirus diarrhea in Jewish and Bedouin children in the Negev region of Israel: epidemiology, clinical aspects and possible role of malnutrition in severity of illness. Pediatr Infect Dis J 1990;9:314–321. [DOI] [PubMed] [Google Scholar]
- 39.Brussow H, Sidoti J, Dirren H, Freire WB. Effect of malnutrition in Ecuadorian children on titers of serum antibodies to various microbial antigens. Clin Diagn Lab Immunol 1995;2:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewan N, Faruque AS, Fuchs GJ. Nutritional status and diarrhoeal pathogen in hospitalized children in Bangladesh. Acta Paediatr 1998;87:627–630. [DOI] [PubMed] [Google Scholar]
- 41.Das SK, Chisti MJ, Huq S, et al. Clinical characteristics, etiology and antimicrobial susceptibility among overweight and obese individuals with diarrhea: observed at a large diarrheal disease hospital, Bangladesh. PLoS One 2013;8:e70402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson ME, Elfving K, Shakely D, et al. Rapid Clearance and Frequent Reinfection With Enteric Pathogens Among Children With Acute Diarrhea in Zanzibar. Clin Infect Dis 2017;65:1371–1377. [DOI] [PubMed] [Google Scholar]


