Abstract
Exposure to peer victimization is a traumatic stressor, with adverse consequences for mental and physical health. This prospective, multi-method, multi-informant study investigated how victimization “gets into the brain,” as reflected in neural dysregulation of emotion during adolescence. Moreover, we examined whether certain youth are particularly vulnerable to compromised neural function (i.e., a pattern of positive amygdala-right ventrolateral prefrontal cortex [rVLPFC] connectivity linked to poor emotion regulation [ER] and emotional distress) following victimization. 43 adolescent girls completed an implicit ER task during a functional brain scan, and reported on rejection sensitivity. In 6th – 9th grades, teachers and adolescents reported annually on victimization. Results revealed that a history of elevated victimization predicted less effective neural regulation of emotion (more positive amygdala-rVLPFC connectivity) in girls with high but not low rejection sensitivity. Consistent with a differential susceptibility model, high rejection sensitivity was associated with particularly effective neural regulation of emotion (more negative amygdala-rVLPFC connectivity) in girls with low-victimization histories. A parallel pattern emerged for a behavioral index of ER. This research provides insight into one pathway through which peer adversity undermines emotional development in ways that forecast compromised future health, and identifies youth who are at particularly high risk following peer adversity.
Keywords: peer victimization, emotion regulation, neural processing, fMRI, adolescent girls
Developmental science perspectives consider how exposure to early adversity undermines the normative development of self-regulatory processes in ways that compromise both short- and long-term health (Boyce & Ellis, 2005; O’Connor, 2003; Rudolph, Lansford, et al., 2016). Exposure to peer victimization (acts of physical, verbal, and psychological aggression) represents a particularly pernicious form of adversity. Not only is peer victimization common (Kochenderfer-Ladd & Wardrop, 2001), it forecasts a wide range of mental and physical health difficulties across the lifespan (McDougall & Vaillancourt, 2015). Given these pervasive and enduring effects, it is critical to understand processes through which victimization compromises development. Moreover, in line with differential susceptibility models of development (Boyce & Ellis, 2005; Ellis et al., 2011), it is important to determine which youth are particularly vulnerable to these adverse effects of peer adversity.
To address these goals, this study examined how a history of exposure to peer victimization and one potential marker of differential susceptibility— psychological sensitivity to rejection— jointly predict neural regulation of emotion. In particular, we focused on a pattern of amygdala-right ventrolateral prefrontal cortex (rVLPFC) connectivity that is linked to compromised emotion regulation (ER; e.g., rumination; Fowler et al., 2017; Hare et al., 2008) and emotional distress (Davis et al., 2019; Fowler et al., 2017; Hare et al., 2008; Monk et al., 2008). Because adolescence is a stage of heightened social and emotional sensitivity (Crone & Dahl, 2012; Silk et al., 2014; Somerville, 2013; Spear, 2009), especially in girls (Charbonneau et al., 2009; Guyer et al., 2012), we focused on understanding the interactive contribution of peer victimization and rejection sensitivity to neural regulation of emotion in adolescent girls.
Neural Regulation of Emotion
ER refers to modifying the nature, intensity, and expression of emotions (Gross, 2015). Effective ER typically refers to responses that aid individuals to modulate emotions in a way that helps them to manage environmental demands and/or meet internal goals (Gross, 2015). ER can encompass both the up- and down-regulation of negative and positive emotions (McRae & Gross, 2020). In the present study, we were particularly interested in how exposure to peer adversity and rejection sensitivity contribute to down-regulation of negative emotions; thus, we focused on this aspect of ER, but also examined the specificity of our findings to negative versus positive emotions.
Research examining the neural correlates of ER focuses on connectivity between brain networks involved in top-down control, mainly the prefrontal cortex, and a primary subcortical region involved in emotion reactivity, the amygdala (Kanske et al., 2011; Phillips et al., 2008). At the neural level, effective down-regulation of negative emotions would be reflected in the ability of control regions to modulate activation in regions involved in emotion reactivity (Gyurak et al., 2011). Most work on the neural correlates of ER focuses on intentional ER (Goldin et al., 2008; Schaefer et al., 2002), which involves purposefully changing an emotionally evocative stimulus in a conscious manner to alter the associated emotional response (Gross, 1998). Meta-analyses reveal heightened activation in prefrontal regions during intentional ER (e.g., cognitive reappraisal; Buhle et al., 2014; Frank et al., 2014; Kalisch, 2009; Kohn et al., 2014), accompanied by reductions in amygdala activation, supporting the notion that frontal cortical regions are involved in modulating amygdala reactivity. Indeed, functional connectivity analyses show that activation in frontal regions covaries with activation in the amygdala, and the strength of such coupling predicts how well negative affect is attenuated after reappraisal (Banks et al., 2007). In particular, this research implicates the VLPFC, which has strong anatomical connections to the amygdala (Etkin et al., 2015), as playing an inhibitory role during reappraisal (Ochsner et al., 2012), as reflected in a negative correlation between activity in the VLPFC and the amygdala (Banks et al., 2007; Ochsner et al., 2002).
ER also includes automatic processes occurring outside of conscious awareness (Gross & Thompson, 2007). In particular, affect labeling (verbalizing an emotion by placing a label on it; Torre & Lieberman, 2018) elicits reductions in self-reported negative affect similar to explicit ER strategies and is believed to share similar mechanisms of action (Burklund et al., 2014; Lieberman et al., 2011). Moreover, the neural correlates of affect labeling are quite similar to reappraisal (Berkman & Lieberman, 2009). Among prefrontal regions, the rVLPFC specifically plays an active role in reducing amygdala activation (Duncan & Own, 2000; Torre & Lieberman, 2018). Not only is negative amygdala-rVLPFC connectivity consistently observed during affect labeling (Hariri et al., 2000; Lieberman et al., 2007; Payer et al., 2012), but dynamic causal modeling implicates the rVLPFC as playing a particularly strong role in attenuating amygdala activation during affect labeling (Torrisi et al., 2013). Moreover, relative to passive viewing of emotionally evocative stimuli, the reduction in amygdala activity during affect labeling and reappraisal are positively correlated, and common regions of the VLPFC are activated during the two types of ER (Payer et al., 2012), supporting overlapping neural substrates of intentional and automatic down-regulation of negative emotion.
Adolescent Development of Individual Differences in Neural Regulation of Emotion
During adolescence, neural systems subserving emotion processing undergo significant remodeling (Tamnes et al., 2017). This reorganization may heighten susceptibility to environmental input, providing the opportunity for changes in brain function that promote either increases in emotional vulnerability or healthy emotional growth (Ernst et al., 2006; Spear, 2009). Seminal developmental neuroscience theories suggested that emotional vulnerability may stem from maturational asynchrony in brain regions guiding reactivity versus regulation (Casey et al., 2008; Ernst et al., 2006). Specifically, subcortical regions implicated in emotion reactivity (e.g., amygdala) show acute increases in sensitivity during adolescence (Ernst et al., 2006; Somerville et al., 2010), whereas prefrontal regions implicated in ER mature more gradually (Casey et al., 2008; Somerville et al., 2010; Steinberg, 2008). Consistent with this view, mid-adolescents experience more negative emotions and emotional lability than younger children, late adolescents, or adults (Somerville et al., 2010) and attend more to emotion cues (Hare et al., 2008; Silk et al., 2009).
However, more recent perspectives suggest that protracted development of regulatory regions allows for a less automatic and more flexible regulatory system (Crone & Dahl, 2012; Schriber & Guyer, 2016), leaving open the possibility that challenges during adolescence are met with positive growth in ER. Indeed, across normative development, task-dependent amygdala-PFC connectivity shifts from more positive in childhood to more negative by mid-adolescence (Gee et al., 2013) to young adulthood (Silvers et al., 2015). This shift is thought to reflect neural maturity that leads to improved ER as the PFC more effectively downregulates the amygdala (Gee et al., 2013; Hare et al., 2008). Consistent with this idea, resting state negative connectivity between the VLPFC and subcortical regions predicts better self-control (Lee & Telzer, 2016), whereas task-dependent positive amygdala-ventral PFC connectivity in adolescence is linked to less effective neural and psychological regulation of emotion, as reflected in weaker habituation of amygdala activity (Hare et al., 2008) and more stress-reactive rumination (i.e., tendency to ruminate in response to an in vivo peer stressor; Fowler et al., 2017). Moreover, positive amygdala-ventral PFC connectivity in adolescence also predicts concurrent (Fowler et al., 2017; Hare et al., 2008; Monk et al., 2008) and future (Davis et al., 2019) internalizing symptoms. Collectively, this research highlights the possibility of emerging individual differences in the development of neural systems involved in ER during adolescence, such that more negative amygdala-ventral PFC connectivity reflects a more effective pattern (i.e., better down-regulation of negative emotions) and more positive amygdala-ventral PFC connectivity reflects a less effective pattern (i.e., compromised down-regulation of negative emotions), with associated implications for psychological and emotional well-being.
Differential Susceptibility Models of Development
Understanding the relative balance of emerging emotional risks (i.e., heightened emotion reactivity, resulting in emotional vulnerability) versus resources (i.e., heightened neural flexibility and maturity, resulting in positive growth in ER) across adolescence requires identifying factors that distinguish youth who develop more or less effective neural regulation of emotion during this stage. In particular, developmental scientists emphasize the need to consider the interaction between social context and personal attributes to better understand the development of individual differences in neural activation to social and emotional cues (Schriber & Guyer, 2016), yet little research directly examines such interactions (for one exception, see Jarcho et al., 2019). To address this gap, the present study drew from differential susceptibility models of development (Boyce & Ellis, 2005; Ellis et al., 2011), which propose that individual differences in psychological (e.g., temperament) or biological (e.g., genetic, adrenocortical) susceptibility to the environment can confer both disadvantages (in the context of unfavorable environments) or advantages (in the context of favorable environments). Thus, the same attributes that heighten vulnerability to adversity also may promote optimal adjustment in the face of positive social environments. To elucidate the interactive contribution of social experiences and personal attributes of youth to neural regulation of emotion, this study examined whether exposure to high (or, alternatively, low) levels of peer adversity differentially predicted patterns of neural regulation of negative emotion (i.e., functional connectivity between the amygdala and the rVLPFC) in adolescent girls with high versus low levels of psychological sensitivity to rejection.
Peer victimization.
Given the increasing social reorientation that occurs across adolescence (Nelson et al., 2005), the peer landscape may play a particularly prominent role in shaping individual differences in neural regulation of emotion. In the past two decades, scientists, practitioners, and policymakers are paying increasing attention to peer victimization as a deleterious form of peer adversity that poses a critical public health threat. Not only does victimization occur at alarming rates, but the adverse effects of victimization are far-ranging (Card et al., 2007) and long-lasting (McDougall & Vaillancourt, 2015).
Peers play a particularly salient role in adolescent development as they begin to assume stronger roles as socialization agents (Furman & Buhrmester, 1992; Rose & Rudolph, 2006). Healthy peer relationships can foster the growth of effective self-regulatory skills by modeling and providing feedback about emotional reactions and serving as support systems that scaffold responses to emotional challenges (Bukowski, 2003; Rudolph, Lansford, et al., 2016). When youth are marginalized through victimization, they fail to receive these regulatory benefits. Persistent exposure to victimization also may serve as a traumatic stressor, sensitizing emotional systems and overwhelming adolescents’ developing capacity for managing emotions, tipping the balance toward emotion dysregulation (Rudolph et al., 2009). Collectively, these emotional costs of victimization may undermine adolescents’ self-efficacy, motivation, and ability to engage in adaptive ER efforts. Indeed, exposure to peer victimization predicts less effortful engagement with stressors and negative emotions and heightened self-reported and observed emotional arousal, sensitivity, and dysregulation (Adrian et al., 2019; Herts et al., 2012; Kochenderfer-Ladd, 2004; Rudolph et al., 2009). In turn, disruptions in ER (Adrian et al., 2019; McLaughlin et al., 2009; Monti et al., 2017) and stress responses (Troop-Gordon et al., 2015) account for the contribution of peer victimization to internalizing symptoms, implicating emotion dysregulation as one pathway through which peer victimization confers risk for adverse mental health outcomes over time.
Recurring or chronic exposure to victimization also may sensitize biological systems involved in emotion reactivity and compromise those involved in ER (Rudolph et al., 2021). In this way, victimization may “get inside the brain” by shaping neural function. Emerging research supports the idea that exposure to peer victimization and related adversity can shape brain function, specifically in regions involved in emotion processing. In the context of receiving negative feedback in the lab, peer-rejected youth, compared to their non-rejected counterparts, show heightened neural activity in regions implicated in emotional reactivity (i.e., the amygdala; Lee et al., 2014). Examining neural responses to social exclusion, three studies revealed that youth exposed to chronic peer victimization (McIver et al., 2018; Rudolph, Miernicki, et al., 2016) and rejection (Will et al., 2016) show heightened activation in brain regions involved in emotion processing (e.g., amygdala, dorsolateral anterior cingulate cortex [dACC], inferior fusiform gyrus) relative to non-victimized/rejected youth.
Rejection sensitivity.
Rejection sensitivity is conceptualized as a cognitive-affective processing disposition that heightens the tendency to defensively expect, readily perceive, and overreact to implied or overt interpersonal rejection (Downey & Feldman, 1996). Adolescents with high levels of rejection sensitivity are likely to be particularly attuned to social cues that convey signals of rejection, potentially amplifying the adverse effects of peer victimization on ER. Consistent with this idea, rejection sensitivity predicts difficulties in regulating emotional responses to aversive stimuli (Silvers et al., 2012), attentional interference by rejection-related stimuli (Berenson et al., 2009), and attentional biases toward negative emotional stimuli (i.e., sad facial expressions) following peer exclusion (Kraines et al., 2018), and moderates the effect of lab-induced (Downey et al., 1998) and naturally occurring (Chango et al., 2012) peer stressors on emotional distress. Moreover, rejection sensitivity predicts more activation in emotion processing regions of the brain (e.g., dACC; Burklund et al., 2007; Masten et al., 2010) and less activation in regulatory regions (Kross et al., 2007) in the face of rejection-related stimuli.
Consistent with a differential susceptibility model, however, rejection sensitivity not only may confer costs to youth exposed to high victimization, but also may confer benefits to youth exposed to low victimization. That is, rejection sensitivity may instill a general sensitivity to social cues and feedback that allows youth to benefit from favorable social environments. In these environments, rejection-sensitive youth may be readily attuned to cues of acceptance and engage in deeper and more complex processing of social situations (Schriber & Guyer, 2016), increasing the emotional benefits they receive from a supportive peer context. Supporting this idea, attributes related to rejection sensitivity (e.g., social-evaluative concerns, social avoidance motivation, anxious attachment, interpersonal dependency) show some adaptive advantages (Cooper et al., 1998; Cross & Madson, 1997; Leary et al., 1995; Rudolph & Conley, 2005), including predicting emotional well-being in low-victimization contexts (Llewellyn & Rudolph, 2014).
Study Overview
Drawing from a differential susceptibility model, this research used a prospective, multi-method, multi-informant design to examine the interactive contribution of peer victimization and rejection sensitivity to neural regulation of negative emotion in adolescent girls. Relative to boys, adolescent girls show more emotion sensitivity, lability, and reactivity to social stressors (Charbonneau et al., 2009; Rudolph, 2009), are more likely to ruminate (Jose & Brown, 2008), show less amygdala habituation to emotional faces (Thomas et al., 2001), and display heightened neural sensitivity in social-evaluative contexts (Guyer et al., 2012). Thus, victimization and rejection sensitivity may be particularly likely to disrupt neural connections that support ER in adolescent girls.
During an fMRI scan, girls completed an implicit ER (affect labeling) task (Lieberman et al., 2007). Prior research implicates functional connectivity between the amygdala and rVLPFC during this task as an indicator of ER (Lieberman at al., 2007; Torre & Lieberman, 2018). We predicted that (a) a history of victimization would predict less effective ER (i.e., more positive amygdala-rVLPFC connectivity) in girls with high compared to low rejection sensitivity; and (b) in low-victimization contexts, girls with high rejection sensitivity would show more effective ER (i.e., more negative amygdala-rVLPFC connectivity) than girls with low rejection sensitivity. To examine the specificity of effects based on emotional valence, we compared patterns of activation during labeling of negative versus positive emotions. We anticipated that the effects would be particularly salient in the context of negative emotions, which may require stronger regulatory activity. To examine whether these effects replicated with a behavioral index of ER, we conducted a parallel set of analyses using girls’ task performance as reflected in accuracy during the labeling condition.
Methods
Participants and Procedures
Participants included 43 adolescent girls (M age = 15.44, SD = .39; 67.4% White; 25.6% African American; 2.3% Asian; 2.3% Latina; 2.3% Native American/Alaskan) from a larger longitudinal study. Participants and their teachers completed questionnaires annually from 6th to 9th grade. For their participation, teachers received monetary compensation and youth received a small gift. During the summer following the 9th grade1, a subset of girls from this larger sample was recruited to participate in a laboratory visit during which they completed questionnaires as well as an ER task while undergoing fMRI. Participants received monetary compensation for completion of the questionnaires and fMRI scan. All procedures were approved by the university’s Institutional Review Board.
Measures
Table 1 provides descriptive and reliability data about the measures.
Table 1.
Descriptive and Psychometric Information
| 6th grade | 7th grade | 8th grade | 9th grade | |||||
|---|---|---|---|---|---|---|---|---|
| Measure | M (SD) | a | M (SD) | a | M (SD) | a | M (SD) |
a
|
| Victimization | ||||||||
| Youth | 1.85 (.82) | .95 | 1.76 (.78) | .96 | 1.81 (.85) | .97 | 1.76 (.78) | .96 |
| Teacher | 1.48 (.58) | .97 | 1.63 (.58) | .96 | 1.41 (.49) | .96 | 1.35 (.53) | .97 |
| Rejection Sensitivity | 7.67 (3.46) | .75 | ||||||
| Labeling Accuracy: Negative Emotions | .92 (.10) | |||||||
| Labeling Accuracy: Positive Emotions | .96 (.07) | |||||||
Victimization.
To assess peer victimization, youth and teachers completed a revised version (Rudolph et al., 2011) of the Social Experiences Questionnaire (Crick & Grotpeter, 1996) annually from 6th through 9th grade. This measure assesses overt victimization (11 items; e.g., “How often do you get hit by another kid?” “How often do you get teased by another kid?”) and relational victimization (10 items; e.g., “How often does another kid say they won’t like you unless you do what they want you to do?”), resulting in a 21-item measure administered in 6th and 7th grade. A 5-item cybervictimization subscale (e.g., “How often has another kid made a rude or mean comment to you online?”) was later added to the child report version, resulting in a 26-item questionnaire administered to youth in 8th and 9th grade. Other than this subscale, items on the child and teacher report versions were identical other than altering the wording as relevant (e.g., substituting “you” with “this child”). Youth and teachers rated on a 5-point scale (Never to All the Time) how often youth experience each type of victimization. This measure has well-established reliability, temporal stability, and validity (Rudolph et al., 2011).
Given high within-wave correlations between overt and relational victimization (rs = .77 - .91, ps < .001) and between overt/relational and cyber victimization (rs = .68 - .85, ps < .001), we collapsed across types of victimization. Composite scores representing overall levels of victimization were computed by averaging ratings across waves within informant. Youth and teacher reports on this composite score were strongly correlated (r = .68, p < .001); thus, we standardized by informant and then averaged across youth and teacher report. Prior research suggests that both self- and teacher reports of victimization are reliable and provide overlapping and unique information (Ladd & Kochenderfer-Ladd, 2002). Moreover, multi-informant reports of victimization are better predictors of outcomes than single-informant reports and reduce single-informant measurement bias (Ladd & Kochenderfer-Ladd, 2002). Thus, a composite score of youth- and teacher-reported victimization was used to capture a more comprehensive view of victimization.
Rejection sensitivity.
To assess sensitivity to rejection, participants completed a subset of items from the Children’s Rejection Sensitivity Questionnaire (CRSQ; Downey et al., 1998) prior to the scan session. The original measure includes twelve vignettes; given the focus of this study on peer victimization, youth completed six vignettes that were specific to potential peer rejection (e.g., “Imagine you are in your classroom, and everyone is splitting up into groups to work on a special project together. You sit there and watch lots of other kids getting picked. As you wait, you wonder if the kids will want you for their group”). After each vignette, youth reported on their anticipated level of anxiety (e.g., “How nervous would you feel, right then, about whether or not they will choose you?”), anger (e.g., “How mad would you feel, right then, about whether or not they will choose you?”), and expectations of rejection (e.g., “Do you think the kids in your class will choose you for their group?”) in that situation. Items were rated on a 6-point scale (Not Nervous to Very, Very Nervous; Not Mad to Very, Very Mad; and Yes…No). Separate scores for anxiety sensitivity and anger sensitivity were computed by multiplying the anxiety/anger ratings by the rejection ratings across each vignette to create mean anxious-rejection and anger-rejection scores. A composite rejection sensitivity score was then computed by averaging the mean anxious-rejection and anger-rejection scores, with higher scores indicating more sensitivity to rejection. This measure has established convergent and discriminant validity (Downey et al., 1998; London et al., 2007).
Emotion regulation task.
During the summer following the 9th grade, participants completed a modified implicit ER task (Lieberman et al., 2007) while undergoing fMRI. For each trial, participants are presented with a negative (anger, fear, sadness) or positive (happy, surprise, calm) emotional face and are instructed to either passively view the emotional face (observe; Figure 1a) or match the emotional face to one of two emotion word labels presented below the image (label; Figure 1b). Participants completed two blocks of each emotional valence for the observe condition and two blocks of each emotional valence for the label condition, for a total of eight blocks. Blocks were presented by valence and block order was randomized across participants. Each block included six trials, and each trial lasted six seconds (including the inter-trial interval) with a 10-second rest period between blocks. For both trial types, faces were on display for 3900 ms, during which participants either passively observed or labeled the emotional face. Accuracy scores for the label condition were calculated separately for the positive and negative trials by taking the correct number of matches over the total number of label trials (12) within each valence. The photos were all young women (ages 21-30) of European and African American descent and were selected from a standardized collection of faces (the NimStim; Tottenham et al., 2009).
Figure 1.
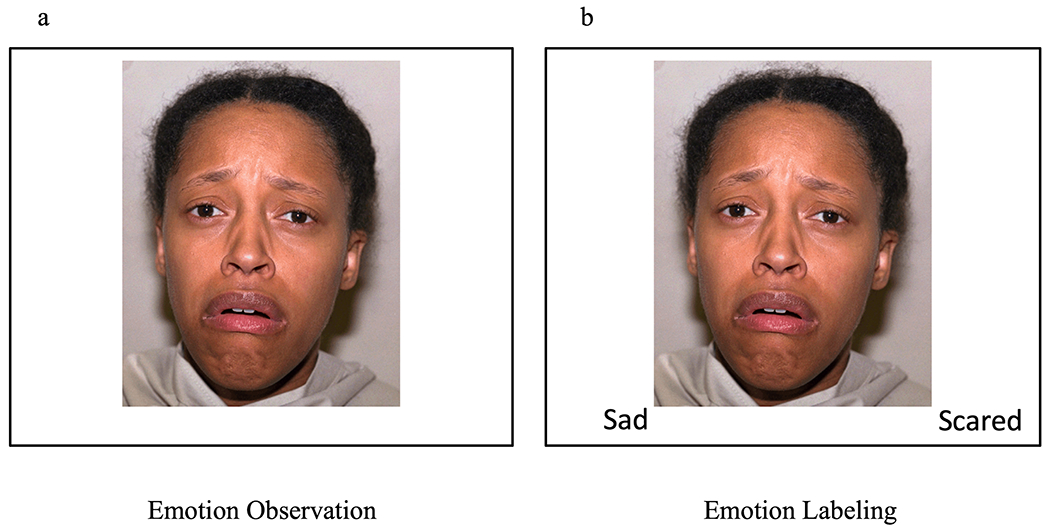
Observation and labeling conditions of the emotion regulation task.
Data Acquisition and Analysis
fMRI data acquisition.
Imaging data were collected during the implicit ER task using a 3 Tesla Siemens Trio MRI scanner. The task included T2*-weighted echoplanar images (EPI) [slice thickness = 3 mm; 38 slices; TR = 2 sec; TE = 25 msec; matrix = 92 x 92; FOV = 230 mm; voxel size 2.5 x 2.5 x 3 mm3]. Structural scans consisted of a T2*weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4 sec; TE = 64 msec; matrix = 192 x 192; FOV=230; slice thickness=3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 sec; TE = 2.3 msec; matrix = 256 x 256; FOV = 230; sagittal plane; slice thickness = 1 mm; 192 slices).
fMRI data analysis.
The fMRI data were preprocessed using statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images were spatially realigned to correct for head motion. Volumes that were greater than 2.5 mm of motion in any direction were dropped from analyses. Realigned functional data were coregistered to the structural MPRAGE, which was then segmented into cerebrospinal fluid, gray matter, and white matter. Structural and functional images were then transformed into standardized stereotactic space as defined by the Montreal Neurological Institute. The normalized functional data were smoothed by applying an 8mm Guassian kernel, full-width-at-half-maximum, to increase signal-to-noise ratio.
In each participant’s fixed-effects analysis, a general linear model (GLM) was created using regressors that corresponded to the task conditions: observe and label, separately for negative and positive emotions. High-pass temporal filtering with a cutoff of 128 seconds was applied to remove low-frequency drift in the data. Parameter estimates resulting from the GLM were then used to create linear contrasts. We focused on the label > observe contrast given evidence that affect labeling reduces emotion reactivity to emotional stimuli (Torre & Lieberman, 2018), whereas passively observing emotional stimuli elicits emotion reactivity (Lieberman et al., 2007). Contrasts were created separately for negative and positive emotions to examine the specificity of neural regulation to negative versus positive emotions.
Because we were interested in ER, we focused on connectivity between the amygdala and rVLPFC. Psychophysiological interactions (PPI) were used to examine neural connectivity, with the bilateral amygdala as the seed region. The amygdala seed region was defined by combining the left and right anatomically defined amygdala in the AAL atlas of the WFU PickAtlas. The automated gPPI toolbox in SPM (gPPI; McLaren et al., 2012) was used (1) to extract the deconvolved times series from the bilateral amygdala for each participant to create the physiological variables, (2) to convolve each trial type with the canonical HRF to create the psychological regressor, and (3) to multiply the time series from the psychological regressors with the physiological variable to create the PPI interaction. Given our a priori hypotheses and prior research using an affect labeling task (Burklund et al., 2014; Hariri et al., 2000; Lieberman et al., 2007; Payer et al., 2012), we restricted our PPI analyses to the rVLPFC, which was defined as the Pars Triangularis and Pars Orbitalis using the AAL atlas in the WFU PickAtlas (Maldjian et al., 2003; Tzourio-Mazoyer et al., 2002). Using the MarsBaR toolbox, parameter estimates of signal intensity were extracted from the rVLPFC, which represents amygdala-rVLPFC functional connectivity.
Overview of Analyses
First, we conducted correlation analyses to examine the pattern of associations among the variables. Next, we conducted two sets of analyses (for the neural and behavioral indexes of ER) to examine whether findings were consistent with a differential susceptibility model. To support this model, several criteria need to be met: (1) there should be a significant interaction between social context (victimization) and sensitivity (rejection sensitivity) predicting ER (amygdala-rVLPFC connectivity and labeling accuracy); (2) the association between victimization and ER should be significant in girls with high but not low sensitivity; (3) within adverse social contexts (high victimization), ER should be significantly worse in girls with high than low sensitivity; and (4) within favorable social contexts (low victimization), ER should be significantly better in girls with high than low sensitivity (Ellis et al., 2011; Roisman et al., 2012).
To test criterion (1), separate hierarchical multiple regression analyses were conducted to examine the interactive contribution of victimization and rejection sensitivity to amygdala-rVLPFC connectivity and labeling accuracy during the ER task. Prior to analysis and calculation of the interaction terms, each variable was standardized. The main effects of victimization and rejection sensitivity were entered at the first step, and the two-way Victimization x Rejection Sensitivity interaction term was entered at the second step. To test criterion (2), we conducted simple slope analyses predicting amygdala-rVLPFC connectivity and accuracy from victimization at low (−1 SD) and high (+1 SD) levels of rejection sensitivity (Aiken & West, 1991) and we depicted the results graphically.
To test criteria (3) and (4), we examined (1) the SD differences in connectivity and labeling accuracy between high versus low levels of rejection sensitivity at low and high levels of victimization (to quantify the size of the differences); (2) the Regions of Significance (RoS) with respect to victimization (i.e., the values of victimization at which the differences between high versus low levels of rejection sensitivity are significant; when the lower-bound and upper-bound RoS fall within −2 SD and + 2 SD, there is support for a differential susceptibility model; Roisman et al., 2012); (3) the Proportion of Interaction (PoI) with respect to victimization, which reflects the proportion of the total area represented on either side of the crossover of regression lines in an interaction plot; suggested cut-offs for differential susceptibility range from .40 - .60 (Roisman et al., 2012) to .20 - .80 (Del Giudice, 2017); and (4) the Proportion Affected (PA) with respect to victimization, which reflects the proportion of youth who experience the benefits of especially low levels of victimization; values close to .50 support differential susceptibility. To discount the possibility that significant interactions were accounted for by a nonlinear association (Roisman et al., 2012), we estimated a model including the quadratic term (i.e., victimization squared) and its interaction with the moderator (rejection sensitivity X victimization squared).
Results
Intercorrelations among the Variables
Table 2 presents the intercorrelations among the variables. Victimization was significantly positively correlated with amygdala-rVLPFC connectivity in the context of negative emotions and was significantly negatively correlated with labeling accuracy in the context of negative emotions. Labeling accuracy in the context of negative and positive emotions were significantly positively correlated.
Table 2.
Intercorrelations among the Variables
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Victimization | -- | |||||
| 2. Rejection Sensitivity | .21 | -- | ||||
| 3. Connectivity: Negative Emotions | .44** | .20 | -- | |||
| 4. Connectivity: Positive Emotions | .04 | .06 | .07 | -- | ||
| 5. Labeling Accuracy: Negative Emotions | −.31* | −.19 | −.19 | −.02 | -- | |
| 6. Labeling Accuracy: Positive Emotions | −.08 | −.11 | .01 | .14 | .42** | -- |
p < .05
p < .01.
Victimization and Rejection Sensitivity Predicting Neural Regulation of Emotion
Separate hierarchical linear regressions examined the interactive contribution of victimization and rejection sensitivity to amygdala-rVLPFC connectivity in the context of negative and positive emotions during the ER task (Table 3).
Table 3.
Victimization and Rejection Sensitivity Predicting Neural Regulation of Emotion
| Negative Emotions | Positive Emotions | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Predictors | b | SE | t | b | SE | t |
| Step 1 | ||||||
| Victimization | .32 | .11 | 2.88** | .03 | .18 | .19 |
| Rejection Sensitivity | .09 | .11 | .77 | .06 | .18 | .33 |
| Step 2 | ||||||
| Victimization | .32 | .10 | 3.12** | .03 | .18 | .19 |
| Rejection Sensitivity | .04 | .10 | .43 | .07 | .18 | .37 |
| Victimization x Rejection Sensitivity | .27 | .10 | 2.67* | −.05 | .18 | −.28 |
p < .05.
p < .01.
Negative emotions.
The regression predicting amygdala-rVLPFC connectivity in the context of negative emotions revealed a significant main effect of victimization, a nonsignificant main effect of rejection sensitivity, and a significant Victimization X Rejection Sensitivity interaction (Table 3). As shown in Figure 2, decomposition of this interaction revealed that victimization significantly predicted more positive amygdala-rVLPFC connectivity in girls with high, b = .59, SE = .15, t = 4.07, p < .001, but not low, b = .05, SE = .15, t = .34, p = .74, levels of rejection sensitivity. At low levels of victimization, girls with high rejection sensitivity had amygdala-rVLPFC connectivity scores .57 SD lower (i.e., more negative) than girls with low rejection sensitivity. At high levels of victimization, girls with high rejection sensitivity had amygdala-rVLPFC connectivity scores .79 SD higher (i.e., more positive) than girls with low rejection sensitivity. The lower-bound and upper-bound RoS were at −1.87 SD and .68 SD, respectively. The PoI was 66% to the right of the crossover and 34% to the left of the crossover, and the PA was 53%. The addition of victimization squared and rejection sensitivity X victimization squared terms resulted in a nonsignificant ∆R2, F(2, 37) = 2.08, p = .14. Collectively, these indexes provide support for a differential susceptibility model (Del Giudice, 2017; Roisman et al., 2012).
Figure 2.
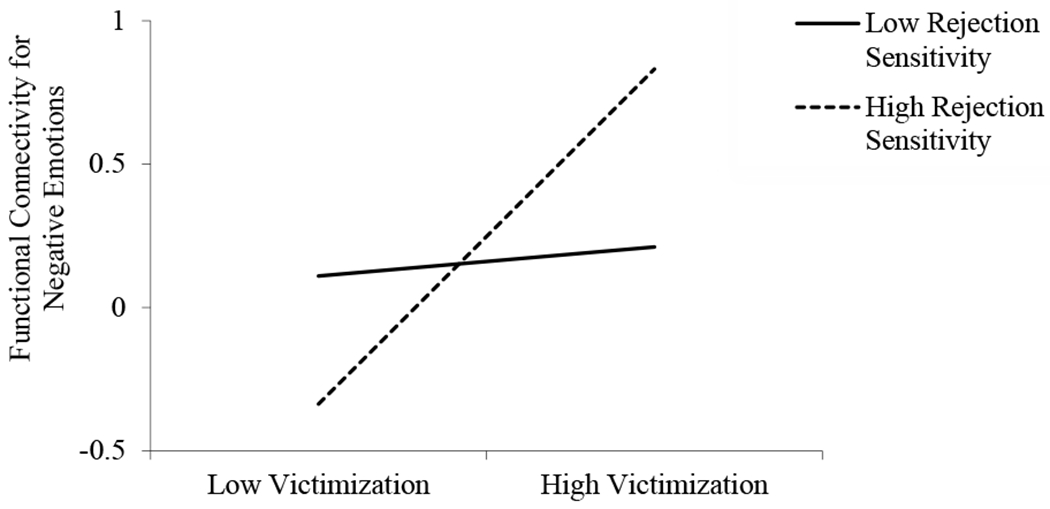
The interactive contributions of victimization and rejection sensitivity predicting amygdala-rVLPFC functional connectivity for negative emotions.
Positive emotions.
The regression predicting amygdala-rVLPFC connectivity in the context of positive emotions revealed nonsignificant main effects of victimization and rejection sensitivity and a nonsignificant Victimization X Rejection Sensitivity interaction (Table 3).
Victimization and Rejection Sensitivity Predicting Behavioral Performance
Separate hierarchical linear regressions examined the interactive contribution of victimization and rejection sensitivity to labeling accuracy in the context of negative and positive emotions during the ER task (Table 4).
Table 4.
Victimization and Rejection Sensitivity Predicting Labeling Accuracy
| Negative Emotions | Positive Emotions | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Predictors | b | SE | t | b | SE | t |
| Step 1 | ||||||
| Victimization | −.03 | .02 | −1.87+ | −.00 | .01 | −.38 |
| Rejection Sensitivity | −.01 | .02 | −.87 | −.01 | .01 | −.57 |
| Step 2 | ||||||
| Victimization | −.03 | .01 | −2.02+ | −.00 | .01 | −.39 |
| Rejection Sensitivity | −.01 | .01 | −.55 | −.00 | .01 | −.41 |
| Victimization x Rejection Sensitivity | −.04 | .01 | −2.55* | −.01 | .01 | −1.07 |
p < .10.
p < .05.
Negative emotions.
The regression predicting labeling accuracy in the context of negative emotions revealed a significant main effect of victimization, a nonsignificant main effect of rejection sensitivity, and a significant Victimization X Rejection Sensitivity interaction (Table 4). As shown in Figure 3, decomposition of this interaction revealed that victimization significantly predicted worse accuracy in girls with high, b = −.07, SE = .02, t = −3.21, p = .003, but not low, b = .01, SE = .02, t = .36, p = .72, levels of rejection sensitivity. At low levels of victimization, girls with high rejection sensitivity had accuracy scores .56 SD higher than girls with low rejection sensitivity. At high levels of victimization, girls with high rejection sensitivity had accuracy scores .58 SD lower than girls with low rejection sensitivity. The lower-bound and upper-bound RoS were at −2.38 SD and .69 SD, respectively. The PoI was 71% to the right of the crossover and 29% to the left of the crossover, and the PA was 51%. The addition of victimization squared and rejection sensitivity X victimization squared terms resulted in a nonsignificant ΔR2, F(2, 37) = 1.37, p = .27. Collectively, these indexes provide support for a differential susceptibility model (Del Giudice, 2017; Roisman et al., 2012).
Figure 3.
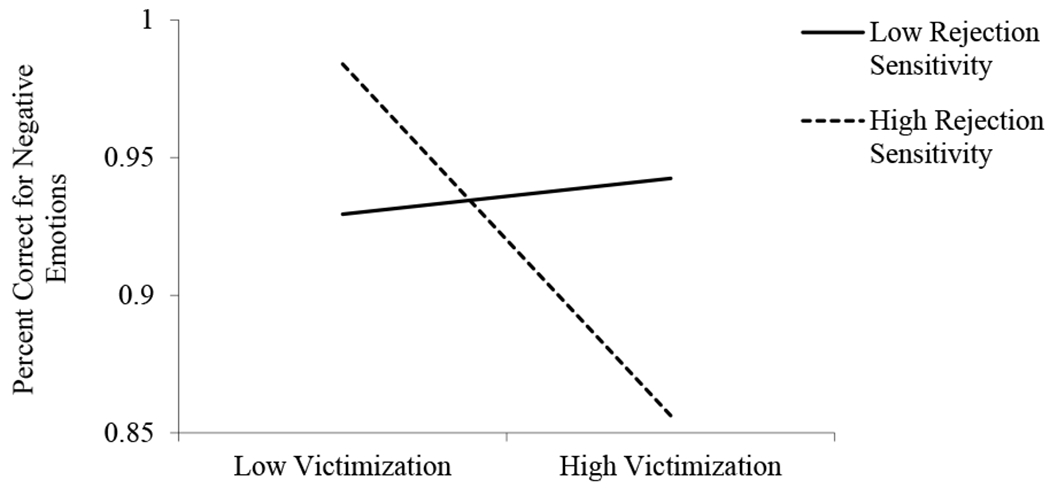
The interactive contributions of victimization and rejection sensitivitity predicting labeling accuracy for negative emotions.
Positive emotions.
The regression predicting labeling accuracy in the context of positive emotions from victimization and rejection sensitivity revealed nonsignificant main effects of victimization and rejection sensitivity, and a nonsignificant Victimization X Rejection Sensitivity interaction (Table 4).
Discussion
Understanding the development of individual differences in neural regulation of emotion during adolescence is critical given the significant reorganization in brain networks involved in emotion processing across this stage (Nelson et al., 2005). The extent to which this reorganization translates into disrupted or enhanced ER is likely contingent on the joint influence of personal attributes and the contexts in which youth develop (Jarcho et al., 2019; Schriber & Guyer, 2016). Drawing from theories of biological embedding of experience (Juster et al., 2010; Shonkoff & Bales, 2011) and differential susceptibility (Boyce & Ellis, 2005; Ellis et al., 2017), the present study examined the novel hypothesis that individual differences in susceptibility to social cues, as reflected in rejection sensitivity, would moderate the contribution of peer victimization to neural regulation of negative emotion during an implicit ER task. Results supported this person x environment interaction, such that elevated victimization predicted less effective neural regulation of negative emotions in adolescent girls with high relative to low rejection sensitivity; however, this same sensitivity predicted more effective neural regulation within low-victimization contexts. Examination of a behavioral index of ER (accuracy in labeling of negative emotions) yielded the same pattern of results.
Victimization x Rejection Sensitivity Predicting ER
Developmental programming theories (e.g., O’Connor, 2003) propose that early adversity may disrupt maturing biological systems in ways that undermine subsequent adjustment. Consistent with this biological embedding of experiences (Juster et al., 2010; Shonkoff & Bales, 2011), girls exposed to higher levels of peer victimization across several years showed more positive amygdala-rVLPFC connectivity in the context of negative emotional stimuli during an implicit ER task in high school. Prior research implicates the rVLPFC as playing an active role in reducing amygdala activation during ER (Duncan & Own, 2000; Torre & Lieberman, 2018), including affect labeling (Torrisi et al., 2013). Moreover, positive amygdala-rVLPFC functional connectivity is associated with less effective ER (e.g., rumination; Fowler et al., 2017; Hare et al., 2008) and more emotional distress (Davis et al., 2019; Fowler et al., 2017; Hare et al., 2008; Monk et al., 2008). Thus, this pattern of positive connectivity suggests that peer-victimized girls show less effective neural regulation of emotion than less-victimized girls. Supporting this interpretation, peer victimization also predicted lower accuracy in labeling of negative emotions.
Exposure to peer victimization may disrupt the development of ER through several pathways. Girls exposed to chronic peer victimization may fail to receive the benefits of healthy relationships, which can include support that helps to scaffold and socialize the management of negative emotions. Moreover, victimization may serve as a stressor that increases girls’ sensitivity to negative emotions (Kochenderfer-Ladd, 2004) and promotes rumination (Monti et al., 2017), thereby interfering with regulatory efforts. These findings are consistent with prior research demonstrating that peer adversity predicts heightened activation in emotion processing regions of the brain in the context of negative feedback (Lee et al., 2014) and social exclusion (McIver et al., 2018; Rudolph, Miernicki, et al., 2016; Will et al., 2016), but extend this work to suggest that victimization may compromise neural regulation of general emotional cues, as reflected in facial expressions, as well. Future work is needed, however, that involves repeated assessments of ER over time. Although a prospective design strengthened our study, allowing us to avoid recall biases and capture several years of exposure to victimization and subsequent ER, girls completed only one brain scan. Thus, we cannot establish definitively that exposure to victimization preceded the emergence of poor ER. Difficulty regulating emotions may mark youth as targets of peer victimization (Riley et al., 2019), suggesting the possibility of reciprocal associations between victimization and emotion dysregulation across development. It would be beneficial for future work to include larger samples that would enable more sophisticated analytic approaches examining within-person changes in victimization and neural function over time. Moreover, we assessed peer victimization at a macrolevel with annual reports; this may limit our understanding of the day-to-day experiences of youth. Thus, incorporating other methods, such as experience sampling, into this line of work may provide useful microlevel information about victimization experiences and their role in emotion regulation.
Consistent with a differential susceptibility model (Ellis et al., 2011, 2017), not all adolescent girls were equally susceptible to these deleterious effects of peer victimization. Specifically, a history of victimization predicted less effective regulation of negative emotions at both the neural and behavioral levels in adolescent girls with high but not low levels of rejection sensitivity. Rejection-sensitive girls are likely to process victimization in ways that amplify its threat value, thereby creating greater impairment in ER. Interestingly, this heightened attunement to social cues predicted a pattern suggesting more effective neural (i.e., more negative amygdala-rVLPFC connectivity) and behavioral (i.e., better labeling accuracy) regulation in the context of negative emotions in girls with low-victimization histories. Thus, rejection sensitivity seems to exert both risk-augmenting and risk-protective effects contingent on the social context, such that rejection-sensitive girls suffer when living in threatening peer environments but benefit when living in favorable peer environments. These results parallel prior research indicating that neural sensitivity to rejection can serve as a differential susceptibility factor, increasing emotional sensitivity to both stressful and supportive family contexts (Rudolph et al., 2020a). These findings therefore add to a growing body of research suggesting that both psychological and biological susceptibilities contribute to context-dependent developmental outcomes.
Notably, the joint effect of victimization and rejection sensitivity on ER emerged in the context of processing negative but not positive emotions, suggesting that negative emotional stimuli may specifically overwhelm the regulatory resources of at-risk girls or, alternatively, there is less need for regulation of positive emotions. Also, the right hemisphere shows greater sensitivity for processing negative than positive emotions (Dolcos et al., 2004), perhaps increasing the likelihood of finding individual differences in patterns of amygdala-rVLPFC connectivity in the context of negative than positive emotions. However, a similar pattern of results (significant differential susceptibility for negative but not positive emotions) emerged for the behavioral index of task performance, decreasing the plausibility of this explanation. Nevertheless, because affect labeling can serve as an affect attenuator regardless of emotional valence (Lieberman et al., 2011), future research should continue to better understand contributors to individual differences in adolescent neural regulation of positive emotion.
Implications and Future Directions
This study highlights the critical importance of integrating person x environment interactions into efforts aimed at elucidating the development of neural systems across adolescence. Specifically, these findings suggest that stressful or supportive peer environments can be instantiated in specific patterns of neural function, but the nature of this biological embedding differs depending on psychological differences in youth. Because positive amygdala-ventral PFC connectivity in adolescence is associated with less emotional competence, including lower levels of mindfulness (Creswell et al., 2007) and higher levels of rumination (Fowler et al., 2017), as well as with heightened emotional distress, including anxiety (Davis et al., 2019; Hare et al., 2008; Monk et al., 2008) and depression (Fowler et al., 2017), this pattern of neural regulation of emotion may help to account for why some victimized youth are at heightened risk for emotional disorders, such as anxiety and depression, during adolescence (Forbes et al., 2019; Stapinski et al., 2014). In this study, we chose to focus on girls given their elevated reactivity to social stressors (Rudolph, 2019) and increasing neural sensitivity (Guyer et al., 2012) during adolescence. It is possible that peer victimization would be less disruptive to ER in boys because they are less reactive and less inclined to ruminate in response to social stressors; however, other stress responses (e.g., avoidance) also may interfere with the development of effective ER. Thus, further research is needed to determine whether similar patterns of emotion dysregulation emerge in peer-victimized adolescent boys.
Given that victimization differentially predicts ineffective ER across youth, it will be critical to identify personal attributes or external resources that can serve as buffers against the development of maladaptive patterns of ER during adolescence among peer-victimized youth, particularly those amenable to change in the context of prevention efforts. For example, encouraging youth to adopt mastery-oriented social goals (i.e., a focus on developing relationships) rather than performance-oriented social goals (i.e., a focus on demonstrating competence in relationships; Rudolph et al., 2011; Ryan & Shim, 2008) may refocus peer-victimized youth toward developing meaningful relationships and lessen the impact of victimization. Moreover, research reveals that high-quality parent-child relationships can attenuate the impact of peer victimization on social and emotional risks (Rudolph et al., 2020b); this protective effect may operate in part by providing parental support and scaffolding for the development of effective emotion management. Indeed, greater maternal emotional resources (e.g., secure attachment, high levels of emotional clarity) predict more effective neural regulation of emotion in adolescent girls (Modi et al., in press). Thus, it may be fruitful to examine whether supportive parent-child relationships can buffer peer-victimized youth against the development of ineffective patterns of neural regulation of emotion.
It is important to note that this research focuses specifically on implicit regulation of emotions that occurs in the context of affect labeling (Burklund et al., 2014; Torre & Lieberman, 2018). Research focused on family adversity suggests that youth exposed to parental maltreatment are able to use intentional ER strategies (i.e., distancing) to effectively down-regulate amygdala activation to negative emotional stimuli, but this success requires greater cognitive effort (McLaughlin et al., 2015). Although research supports an overlap in the psychological correlates and neural substrates of explicit and implicit ER (Berkman & Lieberman, 2009; Burklund et al., 2014; Lieberman et al., 2011; Payer et al., 2012), it will be important to examine directly whether rejection-sensitive girls exposed to high levels of victimization show disrupted neural and behavioral regulation of emotion in the context of more explicit ER processes (e.g., cognitive reappraisal). Moreover, recent research reveals structural differences in the brains of youth with and without exposure to peer victimization (du Plessis et al., 2018; Quinlan et al., 2018), highlighting the need to understand the structural changes through which person x environment interactions are translated into functional differences in emotion processing.
Conclusion
Overall, this research provides novel insight into the contribution of peer adversity to developing neural systems during adolescence and highlights how individual differences in youth influence patterns of biological embedding, creating sensitivity to both unfavorable and favorable social contexts. Elucidating the neural processes underlying the emergence of emotional risk and resilience during adolescence has significant implications both for developing conceptual models of adolescent emotional development as well as for informing efforts to redirect adolescents toward healthy developmental pathways.
Acknowledgments.
We would like to thank the families and schools who participated in this study. We are grateful to Samirah Ali, Suravi Changlani, Inge Karosevica, Yuji Kim, Michelle Miernicki, and Heather Ross for their assistance in data collection and management. This work was supported by a University of Illinois Research Board Award to KDR, National Institute of Mental Health Grants [MH105655] to KDR and EHT and [MH68444] to KDR, and a NARSAD Young Investigator Award to EHT.
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Two adolescents completed the laboratory visit during the summer following 10th grade due to prior ineligibility (i.e., metal braces) for the fMRI scan. One additional girl completed the ER task but did not complete the measure of rejection sensitivity.
References
- Adrian M, Jenness JL, Kuehn KS, Smith MR, & McLaughlin KA (2019). Emotion regulation processes linking peer victimization to anxiety and depression symptoms in adolescence. Development and Psychopathology, 31(3), 999–1009. 10.1017/S0954579419000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, & West SG (1991). Multiple regression: Testing and interpreting interactions. Sage Publications, Inc. [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson KR, Gyurak A, Ayduk O, Downey G Garner MJ, Mogg K, Bradley BP, & Pine DS (2009). Rejection sensitivity and disruption of attention by social threat cues. Journal of Research in Personality, 43(6), 1064–1072. 10.1016/j.jrp.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, & Lieberman MD (2009). Using neuroscience to broaden emotion regulation: Theoretical and methodological considerations. Social and Personality Psychology Compass, 3(4), 475–493. 10.1111/j.1751-9004.2009.00186.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17(2), 271–301. 10.1017/S0954579405050145 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, & Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski WM (2003). Peer relationships. In Bornstein MH, Davidson L, Keyes CLM, & Moore KA (Eds.), Crosscurrents in contemporary psychology. Well-being: Positive development across the life course (221–233). Lawrence Erlbaum. [Google Scholar]
- Burklund LJ, Creswell JD, Irwin MR, & Lieberman MD (2014) The common and distinct neural bases of affect labeling and reappraisal in healthy adults. Frontiers in Psychology, 5, 10. 10.3389/fpsyg.2014.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, & Lieberman MD (2007). The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience, 2(3–4), 238–253. 10.1080/17470910701391711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card NA, Isaacs J, & Hodges EVE (2007). Correlates of school victimization: Implications for prevention and intervention. In Zins JE, Elias MJ, & Maher CA, (Eds), Bullying, victimization, and peer harassment: A handbook of prevention and intervention (pp. 339–366). Haworth. [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chango JM, McElhaney KB, Allen JP, Schad MM, & Marston E (2012) Relational stressors and depressive symptoms in late adolescence: Rejection sensitivity as a vulnerability. Journal of Abnormal Child Psychology, 40(3), 369–379. 10.1007/s10802-011-9570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau AM, Mezulis AH, & Hyde JS (2009). Stress and emotional reactivity as explanations for gender differences in adolescents’ depressive symptoms. Journal of Youth and Adolescence, 38(8), 1050–1058. 10.1007/s10964-009-9398-8 [DOI] [PubMed] [Google Scholar]
- Cooper ML, Shaver PR, & Collins NL (1998). Attachment styles, emotion regulation, and adjustment in adolescence. Journal of Personality and Social Psychology, 74(5), 1380–1397. 10.1037/0022-3514.74.5.1380 [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, & Lieberman MD (2007). Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine, 69(6), 560–565. 10.1097/PSY.0b013e3180f6171f [DOI] [PubMed] [Google Scholar]
- Crick NR, & Grotpeter JK (1996). Children’s treatment by peers: Victims of relational and overt aggression. Development and Psychopathology, 8(2), 367–380. 10.1017/S0954579400007148 [DOI] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Cross SE, & Madson L (1997) Models of the self: Self-construals and gender. Psychological Bulletin, 122(1), 5–37. 10.1037/0033-2909.122.1.5 [DOI] [PubMed] [Google Scholar]
- Davis MM, Miernicki ME, Telzer EH, & Rudolph KD (2019) The contribution of childhood negative emotionality and cognitive control to anxiety-linked neural dysregulation of emotion in adolescence. Journal of Abnormal Child Psychology, 47, 515–527. 10.1007/s10802-018-0456-0 [DOI] [PubMed] [Google Scholar]
- Del Giudice M (2017). The evolution of interaction shape in differential susceptibility. Child Development, 88(6), 1897–1912. 10.1111/cdev.12710 [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, & Cabeza R (2004). Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage, 23(1), 64–74. 10.1016/j.neuroimage.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Downey G, & Feldman SI (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology, 70(6), 1327–1343. 10.1037/0022-3514.70.6.1327 [DOI] [PubMed] [Google Scholar]
- Downey G, Lebolt A, Rincon C, & Freitas AL (1998). Rejection sensitivity and children’s interpersonal difficulties. Child Development, 69(4), 1074–1091. 10.1111/j.14678624.1998.tb06161.x [DOI] [PubMed] [Google Scholar]
- du Plessis MR, Smeekens S, Cillessen AH, Whittle S, & Güroǧlu B (2019). Bullying the brain? Longitudinal links between childhood peer victimization, cortisol, and adolescent brain structure. Frontiers in Psychology, 9, 9. 10.3389/fpsyg.2018.02706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, & Own AM (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience, 10, 475–483. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, & van Ijzendoorn MH (2011). Differential susceptibility to the environment: An evolutionary- neurodevelopmental theory. Developmental Psychopathology, 23(1), 7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, & Shirtcliff EA (2017). The adaptive calibration model of stress responsivity: Concepts, findings, and implications for developmental psychopathology. In Beauchaine TP & Hinshaw SP (Eds.), Child and adolescent psychopathology (3rd ed., pp. 237–276). Wiley. [Google Scholar]
- Ernst PM, Pine DS, & Harden M (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36(3), 299–312. 10.1017/S0033291705005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C, & Gross JJ (2015) The neural bases of emotion regulation. Nature Reviews Neuroscience, 16(11), 693–700. 10.1038/nm4044 [DOI] [PubMed] [Google Scholar]
- Forbes MK, Fitzpatrick S, Magson NR, & Rapee RM (2019). Depression, anxiety, and peer victimization: Bidirectional relationships and associated outcomes transitioning from childhood to adolescence. Journal of Youth and Adolescence, 48(4), 692–702. 10.1007/s10964-018-0922-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CH, Miernicki ME, Rudolph KD, & Telzer EH (2017). Disrupted amygdala prefrontal connectivity during emotion regulation links stress-reactive rumination and adolescent depressive symptoms. Developmental Cognitive Neuroscience, 27, 99–106. 10.1016/j.dcn.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, & Sabatinelli D (2014). Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews, 45, 202–211. 10.1016/j.neubiorev.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Furman W, & Buhrmester D (1992). Age and sex differences in perceptions of networks of personal relationships. Child Development, 63, 103–115. 10.2307/1130905 [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, & Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15463. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W & Gross JJ (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–586. 10.1016/j.biopsych.2007.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2(3), 271–299. 10.1037/1089-2680.2.3.271 [DOI] [Google Scholar]
- Gross JJ (2015). Emotion regulation: Current status and future prospects. Psychological Inquiry, 26(1), 1–26. 10.1080/1047840X.2014.940781 [DOI] [Google Scholar]
- Gross JJ, & Thompson RA (2007). Emotion regulation: Conceptual foundations. In Gross JJ (Ed.), Handbook of emotion regulation (pp. 3–24). Guilford. [Google Scholar]
- Guyer AE, Choate VR, Pine DS, & Nelson EE (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. 10.1093/scan/nsr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, & Etkin A (2011). Explicit and implicit emotion regulation: A dual-process framework. Cognition and Emotion, 25(3), 400–412. 10.1080/02699931.2010.544160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, & Mazziotta JC (2000). Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport, 11(1), 43–48. 10.1097/00001756-200001170-00009 [DOI] [PubMed] [Google Scholar]
- Herts KL, McLaughlin KA, & Hatzenbuehler ML (2012). Emotion dysregulation as a mechanism linking stress exposure to adolescent aggressive behavior. Journal of Abnormal Child Psychology, 40(7), 1111–1122. 10.1007/s10802-012-9629-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Grossman HY, Guyer AE, Quarmley M, Smith AR, Fox NA, Leibenluft E, Pine DS, & Nelson EE (2019). Connecting childhood wariness to adolescent social anxiety through the brain and peer experiences. Journal of Abnormal Child Psychology, 47(7), 1153–1164. 10.1007/s10802-019-00543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose P, & Brown I (2008). When does the gender difference in rumination begin? Gender and age differences in the use of rumination by adolescents. Journal of Youth and Adolescence, 37, 180–192. 10.1007/s10964-006-9166-y [DOI] [Google Scholar]
- Juster RP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009/10.002 [DOI] [PubMed] [Google Scholar]
- Kalisch R (2009). The functional neuroanatomy of reappraisal: Time matters. Neuroscience Biobehavioral Review, 33(8), 1215–1226. 10.1016/j.neubiorev.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S Bongers A, & Wessa M (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21(6), 1379–1388. 10.1093/cercor/bhq216 [DOI] [PubMed] [Google Scholar]
- Kochenderfer-Ladd B (2004). Peer victimization: The role of emotions in adaptive and maladaptive coping. Social Development, 13(3), 329–349. 10.1111/j.14679507.2004.00271.x [DOI] [Google Scholar]
- Kochenderfer-Ladd B, & Wardrop JL (2001) Chronicity and instability of children’s peer victimization experiences as predictors of loneliness and social satisfaction trajectories. Child Development, 72(1), 134–151. 10.1111/1467-8624.00270 [DOI] [PubMed] [Google Scholar]
- Kohn N Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014). Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraines MA, Kelberer LJA, & Wells TT (2018). Rejection sensitivity, interpersonal rejection, and attention for emotional facial expression. Journal of Behavior Therapy and Experimental Psychiatry, 59, 31–39. 10.1016/j.jbtep.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, & Downey G (2007). Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience, 19(6), 945–956. 10.1162/jocn.2007.19.6.945 [DOI] [PubMed] [Google Scholar]
- Ladd GW, & Kochenderfer-Ladd B (2002). Identifying victims of peer aggression from early to middle childhood: Analysis of cross-informant data for concordance, estimation of relational adjustment, prevalence of victimization, and characteristics of identified victims. Psychological Assessment, 14(1), 74–96. 10.1037/10403590.14.1.74 [DOI] [PubMed] [Google Scholar]
- Leary MR, Tambor ES, Terdal SK, & Downs DL (1995). Self-esteem as an interpersonal monitor: The sociometer hypothesis. Journal of Personality and Social Psychology, 68(3), 518–530. 10.1037/0022-3514.68.3.518 [DOI] [Google Scholar]
- Lee HS, Lee JE, Lee KU, & Kim YH (2014) Neural changes associated with emotion processing in children experiencing peer rejection: A functional MRI study. Journal of Korean Medical Sciences, 29, 1293–1300. 10.3346/jkms2014.29.9.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, & Telzer EH (2016). Negative coupling between the right fronto-parietal and limbic resting state networks predicts increased self-control and later substance use onset in adolescence. Developmental Cognitive Neuroscience, 20, 35–42. 10.1016/j.dcn.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, & Way BM (2007). Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science, 18(5), 421–428. 10.111/j.14679280.2007.01916.x [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, & Crockett MJ (2011). Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion, 11(3), 468–480. 10.1037/a0023503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn N, & Rudolph KD (2014). Individual and sex differences in the consequences of victimization: Moderation by approach and avoidance motivation. Developmental Psychology, 50(9), 2210–2220. 10.1037/a0037353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London B, Downey G, Bonica C, & Paltin I (2007). Social causes and consequences of rejection sensitivity. Journal of Research on Adolescence, 17(3), 481–506. 10.1111/j.1532-7795.2007.00531.x [DOI] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, & Eisenberger NI (2010). Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience, 7(1), 106–114. 10.1093/scan/nsq098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, & Gross JJ (2020). Emotion regulation. Emotion, 20, 1–9. 10.1037/emo0000703 [DOI] [PubMed] [Google Scholar]
- McDougall P, & Vaillancourt T (2015). Long-term adult outcomes of peer victimization in childhood and adolescence: Pathways to adjustment and maladjustment. American Psychologist, 70(4), 300–310. 10.1037/a0039174 [DOI] [PubMed] [Google Scholar]
- McIver TA, Bosma RL, Sandre A, Goegan S, Klassen JA, Chiarella J, Booij L, & Craig W (2018). Peer victimization is associated with neural response to social exclusion. Merrill Palmer Quarterly, 64(1), 135–161. 10.13110/merrpalmquar1982.64.1.0135 [DOI] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, & Hilt LM (2009). Emotion dysregulation as a mechanism linking peer victimization to internalizing symptoms in adolescents. Journal of Consulting and Clinical Psychology, 77(5), 894–904. 10.1037/a0015760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi HH, Davis MM, Kim Y, Telzer EH, & Rudolph KD (in press). Maternal antecedents of adolescent neural regulation of emotion. Journal of Research on Adolescence. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, & Pine DS (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. 10.1001/archpsyc.65.5.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JD, Rudolph KD, & Miernicki ME (2017). Rumination about social stress mediates the association between peer victimization and depressive symptoms during middle childhood. Journal of Applied Developmental Psychology, 48, 25–32. 10.1016/j.appdev.2016.11.0030193-3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–174. 10.1017/S0033291704003915 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JD (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. 10.1162/089892902760807212 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. 10.111/j.17496632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG (2003). Early experiences and psychological development: Conceptual questions, empirical illustrations, and implications for intervention. Development and Psychopathology, 15(3), 671–690. 10.1017/S0954579403000336 [DOI] [PubMed] [Google Scholar]
- Payer DE, Baicy K, Lieberman MD, & London ED (2012). Overlapping neural substrates between intentional and incidental down-regulation of negative emotions. Emotion, 12(2), 229–235. 10.1037/a0027421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 829–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EB, Barker ED, Luo Q, Banaschewski T, Bokde AL, Bromberg U, Büchel C, Desrivières S, Flor H, Frouin V, Garavan H, Chaarani B, Gowland P, Heinz A, Brühl R, Martinot JL, Martinot MP, Nees F, Papadopoulos D, … Schumann G (2018). Peer victimization and its impact on adolescent brain development and psychopathology. Molecular Psychiatry, 12, 1–11. 10.1038/s41380-018-0297-9 [DOI] [PubMed] [Google Scholar]
- Riley TN, Sullivan TN, Hinton TS, & Kliewer W (2019). Longitudinal relations between emotional awareness and expression, emotion regulation, and peer victimization among urban adolescents. Journal of Adolescence, 72, 42–51. 10.1016/j.adolescence.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, & Haydon KC (2012). Distinguishing differential susceptibility from diathesis-stress: Recommendations for evaluating interaction effects. Developmental Psychopathology, 24(2), 389–409. 10.1017/S0954579412000065 [DOI] [PubMed] [Google Scholar]
- Rose AJ, & Rudolph KD (2006). A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin, 132(1), 98–131. 10.1037/0033-2909.132.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD (2009). The interpersonal context of adolescent depression. In Nolen-Hoeksema S & Hilt LM (Eds.), Handbook of Depression in Adolescents (pp. 377–418). Routledge. [Google Scholar]
- Rudolph KD, Abaied JL, Flynn M, Sugimura N, & Agoston AM (2011). Developing relationships, being cool, and not looking like a loser: Social goal orientation predicts children’s responses to peer aggression. Child Development, 82(5), 1518–1530. 10.111/j.1467-8624.2011.01631.x [DOI] [PubMed] [Google Scholar]
- Rudolph KD, & Conley CS (2005). The socioemotional costs and benefits of social evaluative concerns: Do girls care too much? Journal of Personality, 73(1), 115–138. 10.1111/j.1467-6494.2004.00306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Davis MM, Modi HH, Fowler C, Kim Y, & Telzer EH (2020a). Differential susceptibility to parenting in adolescent girls: Moderation by neural sensitivity to social cues. Journal of Research on Adolescence, 30, 177–191. 10.1111/jora.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Lansford JE, & Rodkin PC (2016). Interpersonal theories of developmental psychopathology (3rd ed.). In Cicchetti D (Ed.). John Wiley & Sons. [Google Scholar]
- Rudolph KD, Miernicki ME, Troop-Gordon W, Davis MM, & Telzer EH (2016). Adding insult to injury: Neural sensitivity to social exclusion is associated with internalizing symptoms in chronically victimized girls. Social Cognitive and Affective Neuroscience, 11(5), 829–842. 10.1093/scan/nsw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Monti JD, Modi H, Sze W, & Troop-Gordon W (2020b). Protecting youth against the adverse effects of peer victimization: Why do parents matter? Journal of Abnormal Child Psychology, 48, 163–176. 10.1007/s10802-019-00576-9 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Skymba HV, Modi HH, Davis MM, & Sze WY (2021). Biological embedding of peer experiences: The contribution of peer adversity to stress regulation. To appear in C van Lier PA, & Deckard KD (Eds.), Biosocial interplay in elementary school: Pathways toward maladaptation in young children. Springer. [Google Scholar]
- Rudolph KD, Troop-Gordon W, & Flynn M (2009). Relational victimization predicts children’s social-cognitive and self-regulatory responses in a challenging peer context. Developmental Psychology, 45(5), 1444. 10.1037/a0014858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AM, & Shim SS (2008). An exploration of young adolescents’ social achievement goals and social adjustment in middle school. Journal of Educational Psychology, 100(3), 672–687. 10.1037/0022-0663.100.3.672 [DOI] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, & Thompson-Schill SL (2002). Modulation of amygdala reactivity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience, 14(6), 913–921. 10.1162/089892902760191135 [DOI] [PubMed] [Google Scholar]
- Schriber RA, & Guyer AE (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/j.dcn.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, & Bales SN (2011). Science does not speak for itself: Translating child development research for the public and its policymakers. Child Development, 82(1), 17–32. 10.1111/j.1467-8624.2010.01538.x [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, & Dahl RE (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–1807. 10.1093/scan/nst175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, & Dahl RE (2009). Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Developmental Psychopathology, 21(1), 7–26. 10.1017/S0954579490000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, & Ochsner KN (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235–1247. 10.1037/a0028297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, & Ochsner KN (2015). Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science, 18(5), 771–784. 10.111/desc.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH (2013). The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–127. 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, & Casey BJ (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. 10.1016/j.bandc.2009/07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2009). The behavioral neuroscience of adolescence. W. W. Norton & Company. [Google Scholar]
- Stapinski LA, Araya R, Heron J, Montgomery AA, & Stallard P (2014). Peer victimization during adolescence: Concurrent and prospective impact on symptoms of depression and anxiety. Anxiety, Stress and Coping, 28(1), 105–120. 10.1080/10615806.2014.962023 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, & Mills KL (2017). Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. Journal of Neuroscience, 37, 3402–3412. 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, & Casey BJ (2001). Amygdala response to facial expressions in children and adults. Biological Psychiatry, 49(4), 309–316. 10.1016/S0006-3223(00)01066-0 [DOI] [PubMed] [Google Scholar]
- Torre JB, & Lieberman MD (2018). Putting feelings into words: Affect labeling as implicit emotion regulation. Emotion Review, 10(2), 116–124. 10.1177/1754073917742706 [DOI] [Google Scholar]
- Torrisi SJ, Lieberman MD, Bookheimer SY, & Altshuler LL (2013). Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage, 82, 481–488. 10.1016/j.neuroimage.2013.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troop-Gordon W, Rudolph KD, Sugimura N, & Little TD (2015). Peer victimization in middle childhood impedes adaptive responses to stress: A pathway to depressive symptoms. Journal of Clinical Child and Adolescent Psychology, 44(3), 432–445. 10.1080/15374416.2014.891225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, & Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Will GJ, van Lier PA, Crone EA, & Guroglu B (2016). Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of Abnormal Child Psychology, 44(1), 43–55. 10.1007/s108020159983-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


