Abstract
Patients with neuromuscular diseases, during their illness are more susceptible to respiratory infections due to predisposing factors. Ineffective cough and the presence of atelectasis and hypoventilation, dysphagia and drooling can represent risk factors for the development of respiratory infection and fatal respiratory failure.
Infections of respiratory tract with acute respiratory failure are the most common reason for hospitalizations, and pneumonia is among the leading causes of morbidity and mortality worldwide. The setting in which pneumonia is acquired heavily influences diagnostic and therapeutic choices. We will focus on aetiopathogenesis, diagnosis and treatment of pneumonia in these subjects, particularly considering the disease severity, rates of antibiotic resistance and the possible complications. In this case consultations with specialized physicians are strongly recommended.
Key words: neuromuscular disorders, pneumonia, respiratory infections
Introduction
Neuromuscular disorders (NMDs) are a large group of inherited and acquired diseases that affect a number of neural structures including motor nerves, neuromuscular junctions and muscles themselves. Despite most of them are rare diseases, however the number of individuals requiring hospital care, often related to cardiac or respiratory complications, is significant.
Sleep disordered breathing, daytime hypoventilation, coughing and swallowing are the most frequent observed symptoms 1,2. In these cases, non-invasive support with night ventilation with a mask and pressure support device, can be extraordinarily useful to delay daytime ventilatory failure 3.
Dysphagia and difficulty in managing secretions are also common symptoms in neuromuscular diseases due to muscle weakness. Dysphagia may lead to medical complications, such as malnutrition, dehydration, aspiration pneumonia, and other pulmonary complications, causing social isolation and reduced overall quality of life 4. In particular, patients with Duchenne Muscular Dystrophy (DMD), Myotonic Dystrophy type 1 (DM1), Spinal Muscular Atrophy (SMA), Pompe disease 5 and Amyotrophic Lateral Sclerosis (ALS) 6 develop severe progressive respiratory muscle weakness, with consequent impaired cough and secretion clearance, restrictive lung disease, dysphagia and aspiration 7. Recurrent respiratory infections, airway obstruction and disordered sleep breathing leading to alveolar hypoventilation and respiratory failure, are a frequent cause of death 1,2.
Sialorrhoea is a common and problematic symptom that arises from a number of neurological conditions associated with bulbar or facial muscle dysfunction. Drooling can significantly affect quality of life both for physical complications such as oral chapping, and psychological complications such as embarrassment, and social isolation.
When saliva is excessive and thick, it can be sucked in and cause cough. If the cough is ineffective, or the patient also has gastroesophageal reflux, it can lead to aspiration pneumonia. These symptoms can be silent for a long time and result in a severe respiratory failure and pneumonia 7-9.
Respiratory tract infections can affect patients with NMDs with severe exacerbations, due to ventilatory insufficiency that results in impaired alveolar ventilation and stagnation of secretions. Furthermore, they can get worse hypoxemia favoring the onset of acute respiratory failure in subjects often suffering from chronic respiratory failure, or dysphagia. Even patients on non-invasive mechanical ventilation can develop severe respiratory failure, sometimes requiring emergency intubation or tracheostomy.
Coordinated multidisciplinary care has led to better survival outcomes over the past decades.
Epidemiology of pneumonia in NMDs
The context in which pneumonia is acquired, heavily influences diagnostic and therapeutic choices. As the causative organism is typically unknown early on, timely administration of empiric antibiotics is a cornerstone of pneumonia management 11.
From an epidemiological point of view, Streptococcus pneumoniae is still the most relevant pathogen agent, immediately followed by Haemophilus influenzae and Moraxella catharralis.
A multiplicity of infectious agents, of a viral and bacterial nature, circulates in the human population and causes, with particular epidemiological relevance, manifestations of respiratory infection in the period between October and April. In other seasonal periods, respiratory forms mostly caused by so-called atypical respiratory pathogens, such as Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella spp. (which probably also exert a predisposing function for a second bacterial infection), occupy – in a hypothetical etiological top ten – the positions of immediate reinforcement. About 10% of hospitalized community acquired pneumonia (CAP) requires hospitalization in intensive care units (ICUs), especially for forms mainly sustained by S. pneumoniae and Legionella spp.
Similar considerations deserve viral infections that are sustained by a large number of agents such as influenza A and B virus, respiratory syncytial virus (RSV), Rhinovirus (HRV), coronavirus (hCoV), meta-pneumovirus (hMPV), parainfluenza virus (hPIV), adenovirus (ADV), measles virus.
Healthcare-related infections
Healthcare-related infections can be distinguished in a) nosocomial, with onset 48 hours after hospitalization, as the “community infection pneumonia” in patients without previous care contacts; b) non-nosocomial, with onset within 48 h of hospitalization in patients with previous care contacts such as nursing or home IV therapy, wound care, hemodialysis, IV chemotherapy in the last 30 days, hospitalization in the previous 90 days, residence in a nursing home or long-term care.
The pattern of etiological agents is much broader and represented by species resident in the nosocomial environment, with complex resistance profiles.
Pseudomonas aeruginosa and methicillin-resistant S. aureus are the most frequent microorganisms, followed by Enterobacteriaceae, such as A. baumannii. Episodes of pneumonia caused by multidrug-resistant microorganisms (MDRs) - including enterobacteria and mainly Klebsiella pneumoniae carbapenemase-producing (KPC) - are increasingly common in long-term care and hospitals.
Furthermore, the risk of nosocomial pneumonia related to exogenous colonization by environmental pathogens, mainly contaminating the water systems, inhaled after aerosol, should not be overlooked.
Legionella spp represents the archetype of this category of pathogens, but others such as P. aeruginosa, Aspergillus spp and fast-growing mycobacteria have non-secondary roles. The mode of transmission in these cases is due to aspiration maneuvers, use of bronchoscopes, contamination of nebulizers, humidifiers and ventilation circuits.
Pulmonary complications are the leading cause of respiratory deaths. Hypoxemia is common and results from both hypoventilation and micro atelectasis.
In patients with NMDs, over 90% of pneumonias are triggered by upper respiratory tract infections. Chest infections pose a serious problem for the treatment of vulnerable patients with muscle weakness and ineffective cough 12. Pneumonia is the main complication in these patients due to both inefficient respiratory mechanics and a lack of mucociliary clearance capacity. Though the latter is theoretically preserved, ciliary function is nevertheless often impaired due to chronic aspiration and mucopurulent bronchitis.
Overtime, patients can be colonized by opportunistic pathogens such as Pseudomonas aeruginosa and methicillin resistant Staphylococcus aureus. In some cases, irreversible lung damage can occur with the development of bronchiectasis and pulmonary fibrosis 13.
Pneumonia etiology
The etiology of pneumonia can be multifactorial but essentially arises from compromised lung and chest wall function, which produces obstructive and restrictive lung diseases.
The first point is to understand when to treat the patient by distinguishing when he is colonized from when he is in an acute infection; clinical evaluation usually involves measurement of temperature, tracheobronchial secretion volume, culture and purulence assessment of tracheobronchial secretions, evaluation for chest radiograph resolution, white blood cell count, arterial oxygen tension/inspiratory oxygen fraction (PaO2/FiO2) 14.
The most frequent pathogen agents in patients with NMD are MRSA, Pseudomonas aeruginosa and MDR pathogens 15. Prolonged antibiotic treatment is unlikely to prevent this secondary pneumonia but may select for more MDR pathogens. Anaerobes are the etiology in only 0.2 to 0.3% of all patients.
The microbiology of pneumonia after macro aspiration has changed over the last 60 years from an anaerobic infection to an aerobic and nosocomial infection 16.
It has long been known that micro aspiration is the dominant pathophysiologic mechanism behind CAP. Supporting evidence includes the finding that most common CAP causing microorganisms colonize the oropharynx or nasopharynx in non-hospitalized patients. The distinct microbiology of HAP derives from the micro aspiration that occurs after hospitalized patients are colonized with the virulent organisms found in ICUs and hospital settings 16.
Diagnosis
For the microbiological diagnosis, the samples should be collected from respiratory tract, sputum, pleural fluid, endotracheal aspirate, or bronchoalveolar lavage, and blood within 24 hours of hospitalization. The biomarkers determinations may include C-reactive protein (CRP), procalcitonin (PCT), copeptin and adrenomedullin peptide (ADM).
A very important role is now played by the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) which has become a gold standard for microbial identification in clinical microbiology laboratories. In order to speed up the microbiological diagnostic, the typing of single strains as well as the resistance tests to antibiotics and antimycotics has come into focus, in addition to the identification of microorganisms.
A rapid identification of pathogens is important in the survival of patients undergoing a septic event because it has allowed to shorten the time of access to appropriate antibiotic therapy and has contributed to the improvement of patient outcomes.
Another very important role for the rapid diagnosis of upper and lower respiratory tract infections is now played by the latest generation molecular tests, in particular, Biofire® respiratory panel RP2.1 plus for the upper respiratory tract and Biofire® Pneumonia Plus (Pneumoplus) for lower respiratory tract. RP2.1 plus allows to collect a nasopharyngeal on viral transport, sample volume 300 μL which collects all respiratory viruses including Sars-Cov-2 as well as atypical pathogens responsible for pneumonia (Bordetella, Chlamidia, Mycoplasma).
The Pneumo plus panel, on the other hand, which provides a sample of about 200 microL of sputum and brochoalveolar lavage (BAL), identifies bacteria, viruses, atypical bacteria and antibiotic resistance genes; this last point is very important for those patients who over time undergo multiple antibiotic therapies 17.
For fungal infections, blood cultures are always important, even those tested with Biofire® film array panels that allow the identification of a broad spectrum of yeasts, not least Candida auris, as well as antimicrobial resistance genes on the blood. Sputum culture and research of mannan, galactomannan and betaDglucan markers in the blood are of additional help in the diagnostic process.
Treatment
Several trails in the field of hospital pneumonia suggest using a 7-8 days course of antibiotic therapy in patients with VAP without immunodeficiency, and with cystic fibrosis, empyema, lung abscess, cavitation pneumonia or necrosis with a good clinical response to therapy.
Patients with NMDs can be included in these categories as the highest percentage of infection is determined by forms ab ingests, including pneumonia due to continuous aspirations and difficulty in swallowing.
Community-acquired pneumonia (CAP)
CAP has S. Pneumoniae, atypical and mycoplasma, in particular Haemophilus, Moraxella and in 30% cases to virus Influenza, Parainfluenza, RSV (Fig. 1) and in the last year 2020 Sars-CoV-2 as its prevalent etiologies 18.
Figure 1.
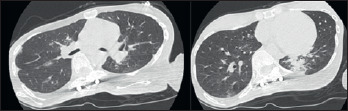
Lung CT scan. Typical picture of pulmonary involvement caused by respiratory syncytial virus, in a patient with congenital myopathy and acute respiratory failure.
Oropharyngeal aspiration is an important etiological factor leading to pneumonia in the elderly and patients with neuromuscular diseases. These disorders are associated with dysphagia and an impaired cough reflex which in turn increases the likelihood of oropharyngeal aspiration. Aspiration pneumonia is difficult to distinguish from other pneumonia syndromes. More than 90% of hospitalized patients have a risk for aspiration; however, some studies do not recommend the routine use of an anti-anaerobic antibiotic coverage 19.
Primary regimen therapy for CAP is summarized in Table I. It is important to discontinue antibiotics with normalization of procalcitonin to 0.1-0.2 mcg/ mL.
Table I.
Antibiotic therapy regimens for community-acquired pneumonia.
| CAP | Drug | Dose | Route | Duration |
|---|---|---|---|---|
| Mild CAP | Amoxicillin | 1000 mg TID | Oral | 5 days |
| Mild CAP (penicillin allergy) | Doxycycline | 100 mg BID | Oral | 5 days |
| Intermediate CAP (comorbidities) | Amoxicillin/clavulanic + Azithromycin OR levofloxacin |
875/125 mg BID 500 mg QD 750 mg QD |
Oral Oral Oral |
7-10 days |
| Severe CAP (acute respiratory failure, mechanical ventilation) | III generation cefalosporin | Intravenous | 7-10 days |
Hospital Acquired Pneumonia (HAP)
Antibiotic therapy regimens for hospital-acquired pneumonia are shown in Table II. Each regimen is selected with specific therapy after culture results (sputum, blood, pleural fluid etc.) (Fig. 2).
Table II.
Antibiotic therapy regimens for hospital-acquired pneumonia.
| HAP | Drug | Dose | Route | Duration |
|---|---|---|---|---|
| First line | Cefepime OR piperacillin/tazobactam OR meropenem + macrolide |
2000 mg BID 4500 mg TID-QID 1000 mg TID |
Intravenous | 10-14 days |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Vancomycin OR Linezolid + macrolide |
1000 mg BID 600 mg BID |
Intravenous | 10-14 days |
| Vancomycin-resistant Staphylococcus aureus (VRSA) | Ceftarolina OR ceftobiprolo + macrolide |
600 mg BID | Intravenous | 10-14 days |
| Pseudomonas A. | IV generation cefalosporin + ciprofloxacin OR amikacin |
400 mg BID 15 mg/kg DIE |
Intravenous | 10 days |
Fig. 2.
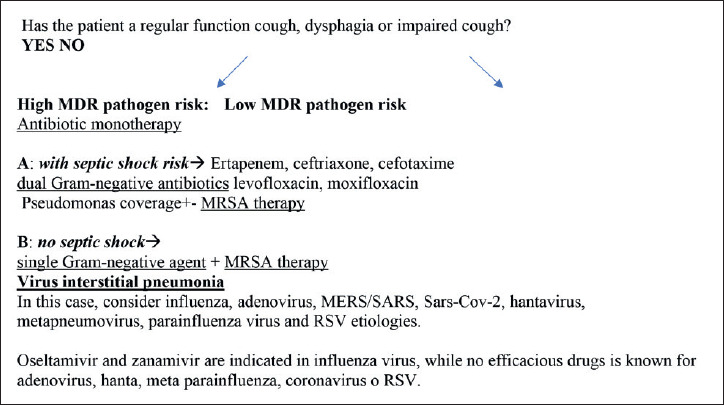
CAP or HAP in patients with NMDs. Risk assessment for MDR pathogens and mortality (from 2017 European HAP/ VAP guideline, mod.). 23.
Aspiration community acquired pneumonia (ACAP)
ACAP is frequent in patients with NMDs; dysphagia, lack of cough reflex, esophageal motility disorders, typical in neurological diseases are considered the most important risk factors for aspiration pneumonia.
Etiologies anaerobes and viridians streptococci group-related are predominant. Primary therapy regimen is with Amp/sulb 3 gr ev/6 h or ceftriaxone 1 gr in 24 h plus metronidazole 500 mg ev every 6 hours 20,21. Infections associated with anaerobes do not evidence pathogenic role 21 and Gram-Negative bacteria were more prevalent in patients with severe ACAP, with higher prevalence of Pseudomonas aeruginosa and Enterobacteriaceae (Other GNB). Oral cavity is considered the principal source of pathogens responsible for aspiration pneumonia. Microbiology data from patients with other comorbidities suggested that P. aeruginosa, K. pneumoniae and E. Coli were frequently isolated from oral samples (Figs. 3-4). For the more appropriate therapeutic choice, it is essential to keep always in mind the local epidemiology of antimicrobic resistance, which varies according to geographic areas 22,23.
Figure 3.
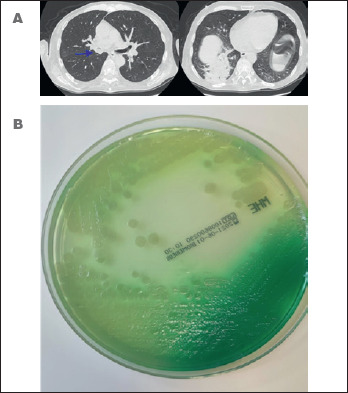
A) Lung CT scan. Typical picture of pulmonary involvement caused by Pseudomonas aeruginosa (sputum isolation) in a patient with amyotrophic lateral sclerosis, dysphagia and respiratory failure. Evidence of food ingestions in the middle lobe bronchus and right lower lobe; B) Pseudomonas aeruginosa in Mueller-Hinton agar plate. Evidence of Pyocyanin or fluorescin production.
Figure 4.
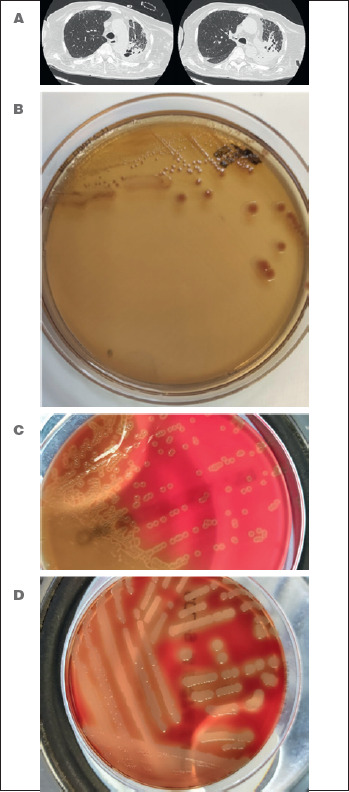
A) Lung CT scan. Picture of pulmonary involvement caused by Stenotrophomonas malthophilia Staphylococcus aureus and Streptococcus pneumoniae (bronchoaspirate isolation) in a patient with muscular dystrophy, tracheostomy and acute respiratory failure; B) Stenotrophomonas malthophilia in MacConkey agar plate; C) Streptococcus pneumoniae in blood agar plate. Evidence of mucoidal colonies and characteristic production of alpha-hemolysis zones; D) Staphylococcus aureus in blood agar plate. Evidence of characteristic production of clear beta-hemolysis zones.
Pulmunary Fungal infections (PFIs)
PFIs occur mostly as pulmonary nodules and aspergillosis is the most frequent cause. Interstitial pneumonia is caused by Pneumocystis. Histoplasmosis and aspergillosis can also be presented as ground glass pictures.
In this case, the first therapeutic choice is represented by echinocandins, especially if patients are unstable or have already taken azoles. Voriconazole is the drug of choice for aspergillosis. Amphotericin B is recommended as an alternative 24,25.
Recommendations
Infection surveillance should not be limited to the respiratory system, but other sites of possible infection such as generalized bacteremia or sepsis, urinary tract infections, catheter infections should also be considered; therefore, adequate source control with culture tests such as blood cultures, urine culture, nasal swabs and catheter culture tests is essential.
Other possible sites of first infection can be any bedsores, very common in patients with NMDs, often in a wheelchair, bedridden or on continuous non-invasive mechanical ventilation. If an infection is suspected, a pressure sore swab should be done.
In any case, an antimicrobial stewardship program is fundamental in the therapeutic choice of antibiotics according to local microbiological epidemiology, to prevent antimicrobial resistance. It should be noted that combination therapy was associated with a significantly lower risk of death compared to monotherapy.
Conclusions
Diagnostic tests play a fundamental role in healthcare: they help to further improve antimicrobial stewardship by optimizing the use of antibiotics, a more delicate therapy in vulnerable patients; they also help prevent the appearance of bacterial resistance. However, it should be remembered that antibiotic administration can also adversely affect respiratory muscle function. Numerous studies have assessed that penicillin-type drugs are rarely associated with the development of a myopathy. It is therefore very important to choose a targeted antibiotic therapy, which, on the one hand, must eradicate infections, and, on the other, must not cause damage to the muscles or neuromuscular plaque. Disease severity and antibiotic resistance rates should carefully be considered, when choosing an empirical regimen. If complications occur, further investigation and consultation with a pulmonary specialist may be necessary.
The correct use of the available weapons can contribute to change the patients prognosis and quality of life.
Practical suggestions
Dysphagia is often the “primum movens” of a number of complications.
Targeted dietary interventions for patients with dysphagia secondary to neurodegenerative diseases are currently available.
Once chronic damage has established, bronchiectasis and atelectasis may appear, which favor the triggering of infectious exacerbations.
The use of antiviral and antibacterial filters during mechanical ventilation is an indispensable safeguard for patients. In some cases, it may be necessary to sanitize the mechanical ventilator.
Vaccinations play an important role in disease management for prevention. The 23-valent pneumococcal vaccine, Haemophiles vaccine, annual influenza vaccine and respiratory syncytial virus antibody prophylaxis for infants are strongly recommended.
Acknowledgement
We thank the nursing staff for their hard work.
Ethical consideration
None.
Funding
None.
Conflict of interest
The Authors declare no conflict of interest to disclose.
Figures and tables
Footnotes
Author contributions
Conceptualization and preparation of the manuscript: NC, AA, A C, FS, AM, GF; data collection: AA, AC, AM, FS, MB, EP, PI data curation AM, PI, EP, AC, writing and editing NC, AA FS MB, PI, GF, revision of the manuscript NC, AA, AC, MB, PI, supervision NC, AA, FS, GF
References
- 1.David WS, Chad DA. Neuromuscular disorders. Semin Neurol 2015;35:325. https://doi.org/10.1055/s-0035-1558971 10.1055/s-0035-1558971 [DOI] [PubMed] [Google Scholar]
- 2.Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Curr Opin Neurol 2017;30:529-537. https://doi.org/10.1097/WCO.0000000000000470 10.1097/WCO.0000000000000470 [DOI] [PubMed] [Google Scholar]
- 3.Wenninger S, Jones HN. Hypoventilation syndrome in neuromuscular disorders. Curr Opin Neurol 2021;July6. https://doi.org/10.1097/WCO.0000000000000973. [Epub Ahead of Print] 10.1097/WCO.0000000000000973 [DOI] [PubMed] [Google Scholar]
- 4.Annunziata A, Coppola A, Polistina GE, et al. Daytime alternatives for non-invasive mechanical ventilation in neuromuscular disorders. Acta Myol 2021;40:51-60. https://doi.org/10.36185/2532-1900-042 10.36185/2532-1900-042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton D, Karam C, Schindler JS. Swallowing and secretion management in neuromuscular disease. Clin Chest Med 2018;39:449-457. https://doi.org/10.1016/j.ccm.2018.01.007 10.1016/j.ccm.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 6.Camela F, Gallucci M, Ricci G. Cough and airway clearance in Duchenne muscular dystrophy. Paediatr Respir Rev 2019;31:35-39. https://doi.org/10.1016/j.prrv.2018.11.001 10.1016/j.prrv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 7.Gozzer MM, Cola PC, Onofri SMM, et al. Fiberoptic endoscopic findings of oropharyngeal swallowing of different food consistencies in amyotrophic lateral sclerosis. Codas 2019;32:e20180216. https://doi.org/10.1590/2317-1782/20192018216 10.1590/2317-1782/20192018216 [DOI] [PubMed] [Google Scholar]
- 8.Annunziata A, Valente T, Cauteruccio R, et al. Silent dysphagia in two patients with Steinert disease and recurrent respiratory exacerbations. Acta Myol 2020;39:141-143. https://doi.org/10.36185/2532-1900-019 10.36185/2532-1900-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGeachan AJ, Mcdermott CJ. Management of oral secretions in neurological disease. Pract Neurol 2017;17:96-103. https://doi.org/10.1136/practneurol-2016-001515 10.1136/practneurol-2016-001515 [DOI] [PubMed] [Google Scholar]
- 10.Howards RS, Wiles CM, Hirsch NP, et al. Respiratory involvement in primary muscle disorders: assessment and management. Q J Med 1993;86:175-189. PMID [PubMed] [Google Scholar]
- 11.Perrin C, Unterborn JN, Ambrosio CD, et al. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve 2004;29:5-27. https://doi.org/10.1002/mus.10487 10.1002/mus.10487 [DOI] [PubMed] [Google Scholar]
- 12.Benditt JO. Respiratory care of patients with neuromuscular disease. Respir Care 2019;64:679-688. https://doi.org/10.4187/respcare.06827 10.4187/respcare.06827 [DOI] [PubMed] [Google Scholar]
- 13.Lanks CW, Musani AI, Hsia DW. Community-acquired pneumonia and hospital-acquired pneumonia. Med Clin North Am 2019;103:487-501. https://doi.org/10.1016/j.mcna.2018.12.008 10.1016/j.mcna.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 14.Khatwa UA, Dy FJ. Pulmonary manifestations of neuromuscolar disease. Indian J Pediatr 2015;82:841-851. https://doi.org/10.1007/s12098-015-1814-3 10.1007/s12098-015-1814-3 [DOI] [PubMed] [Google Scholar]
- 15.Smith PE, Calverley PM, Edwards RH, et al. Practical problems in the respiratory care of patients with muscular dystrophy. N Engl J Med 1987;316:1197-205. https://doi.org/10.1056/NEJM198705073161906 10.1056/NEJM198705073161906 [DOI] [PubMed] [Google Scholar]
- 16.Campogiani L, Tejada S, Ferreira-Coimbra J, et al. Evidence supporting recommendations from international guidelines on treatment, diagnosis, and prevention of HAP and VAP in adults. Eur J Clin Microbiol Infect Dis 2020;39:483-491. https://doi.org/10.1007/s10096-019-03748-z 10.1007/s10096-019-03748-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa M, Hoshina T, Haro K, et al. The microbiological characteristics of lower respiratory tract infection in patients with neuromuscular disorders: an investigation based on a multiplex polymerase chain reaction to detect viruses and a clone library analysis of the bacterial 16S rRNA gene sequence in sputum samples. J Microbiol Immunol Infect 2019;52:827-830. https://doi.org/10.1016/j.jmii.2019.01.002 10.1016/j.jmii.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 2015;30:40-48. https://doi.org/10.1016/j.jcrc.2014.07.011 10.1016/j.jcrc.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 19.Buchan BW, Windham S, Balada-Llasat JM, et al. Pratical compararison of the Biofire Film array pneumonia Panel to routine diagnostic methods and potential impact on antimicrobial stewardship in adult hospitalized patients with lower respiratory tract infections. J Clin Microbiol 2020;58:e00135-20. https://doi.org/10.1128/JCM.00135-20 10.1128/JCM.00135-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aston SJ, Ho A, Jary H, et al. Etiology and risk factors for mortality in an adult community-acquired Pneumonia Cohort in Malawi. Am J Respir Crit Care Med 2019;200:359-369. https://doi.org/10.1164/rccm.201807-1333OC 10.1164/rccm.201807-1333OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin-Corral J, Pascual-Guardia S, Amati F, et al. Aspiration risk factors, microbiology, and empiric antibiotics for patients hospitalized with community-acquired pneumonia. Chest 2021;159:58-72. https://doi.org/10.1016/j.chest.2020.06.079 10.1016/j.chest.2020.06.079 [DOI] [PubMed] [Google Scholar]
- 22.Putman RK, Gudmundsson G, Axelsson GT, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med 2019;200:175-183. https://doi.org/10.1164/rccm.201809-1652OC 10.1164/rccm.201809-1652OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J 2017;50:1700582. https://doi.org/10.1183/13993003.00582-2017 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- 24.Terrero-Salcedo D, Powers-Fletcher MV. Updates in laboratory diagnostics for invasive fungal infections. J Clin Microbiol 2020;58:e01487-e01519. https://doi.org/10.1128/JCM.01487-19 10.1128/JCM.01487-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaragoza R, Maseda E, Pemán J. Individualized antifungal therapy in critically ill patients with invasive fungal infection]. Rev Iberoam Micol 2021;38:68-74. https://doi.org/10.1016/j.riam.2021.04.006 10.1016/j.riam.2021.04.006 [DOI] [PubMed] [Google Scholar]


