Abstract
Identification of carotid artery atherosclerosis is conventionally based on measurements of luminal stenosis. However, histopathologic studies demonstrate considerable differences between plaques with identical degrees of stenosis and indicate that certain plaque features are associated with increased risk for ischemic events. As a result of the rapid technological evolution in medical imaging, several important steps have been taken in the field of carotid plaque imaging allowing us to visualize the carotid atherosclerotic plaque and its composition in great detail. For computed tomography, magnetic resonance imaging, positron emission tomography, and ultrasound scan, evidence has accumulated on novel imaging-based markers that confer information on carotid plaque vulnerability, such as intraplaque hemorrhage and lipid-rich necrotic cores. In terms of the imaging-based identification of individuals at high risk of stroke, routine assessments of such imaging markers are the way forward for improving current clinical practice. The current review highlights the main characteristics of the vulnerable plaque indicating their role in the etiology of ischemic stroke as identified by intensive plaque imaging.
Keywords: Carotid, Plaque, Stenosis
Background and basic information
The word “atherosclerosis” is derived from the two Greek words “athera” (mush) and “sclerosis” (hardening), indicating hardening of the vascular wall. Ischemic stroke is one of the most important causes of morbidity and mortality worldwide, and it is responsible of 5% of global disability-adjusted life years and over 10% of deaths worldwide. The current incidence of stroke in Europe and the USA is approximately 200 per 100,000 population per year of which 80% of strokes are ischemic.1,2 Severe carotid artery stenosis is a well-established risk factor and accounts for approximately 20% of all ischemic strokes. Approximately 20% of patients with either stroke or a transient ischemic attack have an ipsilateral carotid stenosis of >50%.3
In the last 30 years, significant advances have been made in the knowledge of the atherosclerotic disease and its treatment. First, newer surgical strategies have reduced the risk of procedural complications during carotid endarterectomy.4, 5, 6 Second, new trials showing the positive impact of new drugs for the treatment of carotid disease7, 8, 9 and, finally, advancements in imaging technology allow us to gather information regarding the composition of the atherosclerotic plaque and the morphology of the carotid wall.10,11
The results of clinical trials performed in the eighties,12,13 before the advent of morphologic plaque analyses based on vessel wall magnetic resonance imaging (MRI) and high-resolution computed tomography (CT) angiography, should be considered outdated. These were based on the degree of stenosis alone. Our current guidelines regarding the treatment and management of both symptomatic and asymptomatic atherosclerotic plaque must incorporate information regarding plaque morphology and their composition.
This is important because the vessel wall is a complex dynamic structure that is involved in the regulation of blood flow both in physiologic and pathologic states. Our previous understanding was that atherosclerosis was a process that resulted in progressive loss of lumen caliber and thereby of blood flow. It has now become clear that there are multiple complex cellular and molecular processes driven by the endothelium that influence the condition of the remaining vessel wall elements. Early atherosclerotic processes result in a relatively predictable asymptomatic progression of disease, followed later by unpredictable manifestations that can result in downstream ischemic sequelae.14
The assessment of risk of stroke using only one population-based parameter, that is, the degree of stenosis, is not sufficient. In fact, the most frequently used methods for quantifying the degree of stenosis (North American Symptomatic Carotid Endarterectomy trial and European Carotid Surgery trial), often deliver different results.12,13 This alone suggests that the incorporation of additional imaging parameters based on the actual plaque pathophysiology can no longer be ignored for a more accurate analysis of the risk of an ischemic stroke. Such comprehensive parameters on single individuals are the aim of precision medicine that allows for tailored diagnostics and treatment. This will lead to improved management of the single patient and is more effective than the application of the previous “one-size-fits-all” management strategy.
The impact of plaque imaging biomarker is now being incorporated in the consensus recommendation and guidelines. Recently the European Society for Vascular Surgery and European Society of Cardiology developed recommendations in asymptomatic patients, which requires for the first time inclusion of plaque morphology features in deciding optimal management.15,16
Moreover, a growing impact is emerging from the application of artificial intelligence.17 The field of machine learning and its subset—deep learning—has started to penetrate the health care, especially, the field of biomedical imaging. Using machine learning, we can learn about the grayscale features and covariates or risk predictors using an offline training system, which can then be applied on test patients to predict these vulnerable plaque features.18 These systems can then be generalized based on ethnicity because the disease of atherosclerosis is ethnicity dependent. Furthermore, the vulnerable plaque can be characterized using deep learning by extracting the vulnerable features in training set using higher set of convolutional and pooling layers and generating the training weights.19,20 The training weights can then be used for predicting the granularity of risk of vulnerable plaque in the form of probabilities or stratification of vulnerable risk in the form of labels. These deep learning tools are automated and can be optimized even if the data are small using augmentation strategies.21 Lastly, the power of training can be avoided using “transfer-learning” improving speed and accuracy.
Histopathologic studies
Most patients with high-grade stenosis remain asymptomatic, whereas a significant number with minor stenosis become symptomatic.22 Therefore, factors other than the degree of stenosis must be important determinants or markers of an “active” carotid lesion. The search for these factors began with attempts to correlate plaque characteristics identified primarily during surgery or postmortem examinations in patients with the presence or absence of ischemic symptoms and events.
Carotid artery plaques are considered “hard” when their composition is predominantly collagenous or calcified, or “soft,” in case of intraplaque hemorrhage or when they contain atheromatous debris.23 Soft plaques are more commonly associated with symptoms than are hard plaques,23,24 even though certain specific features such as the presence of intraplaque hemorrhage have been reported to be a marker of recently symptom-producing plaques by some23,25,26 but not by others.27, 28, 29, 30 Similarly, the presence of calcification has been associated with asymptomatic carotid plaques by some23 and not by others.31
These conflicting findings are likely a result of differences in the populations studied (different timing between onset of symptoms for differing degrees of carotid stenosis), differences in plaque assessment methodology (microscopic or macroscopic examination), nonstandardized terminology, and differences in parameters measured (eg, intraplaque hemorrhage can be old or new and mural thrombi can vary in size from less than 1 mm to those that occupy 50%-75% of the lumen, and ulceration of plaques can be either smooth-lined and free of thrombotic material or not32). Inconclusive findings can also be explained by inadequate comparison with controls (asymptomatic or nonstenosed arteries), small sample sizes, and lack of clear statistical comparisons.
Biological variability in plaque morphology plays a role with presentation of multiple different features (calcium, intra plaque hemorrhage [IPH] inflammation) within the plaque with significant variation in its longitudinal representation. In particular, the plaques could have a significant degree of heterogeneity33 with problems similar to the “biopsy sampling error”34 where the analysis of a specific part of the plaque is not representative of the complete one. In addition, the pathology of asymptomatic and symptomatic carotid plaques overlaps considerably. Recent and old hemorrhages, ulceration, and organized thrombi are commonly found in both symptomatic and asymptomatic stenotic plaques examined pathologically25,27, 28, 29, 30, 31,35, 36, 37 (Fig 1). Intraplaque hemorrhage may well be important in the production of cerebrovascular symptoms, but its mere presence does not make it directly a cause of symptoms.27 Apparently, plaque complications occur frequently and usually heal without giving rise to symptoms.38
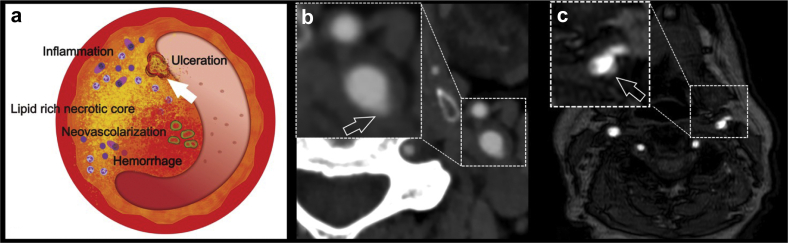
Fig 1.
(a) Schematic diagram showing the features of vulnerable plaques and the white arrow shows the presence of an ulceration. In panel (b), a computed tomography angiography axial image obtained in a 63-year-old man with transient ischemic attack shows the presence of an ulceration in the left common carotid artery (white open arrow). (c) The time-of-flight (TOF) magnetic resonance (MR) of a 59-year-old man with ischemic stroke shows the presence of small ulcers in the left bifurcation (white open arrow).
Following a comprehensive literature review, Golledge et al39 were able to pool data from 10 studies in which carotid plaque histology in symptomatic and asymptomatic patients with high-grade (>50%) stenosis were compared. Studies in which the degree of stenosis was not stated or was not comparable were excluded because carotid plaque complications (such as surface ulceration, intraplaque hemorrhage, and lumen thrombus) are more likely to be present with higher degree of stenosis.29,30,37 It is described that surface ulceration or plaque rupture occurred in 48% of 157 symptomatic plaques compared with 31% of 113 asymptomatic (P < .001), indicating that these are features of unstable plaques. By contrast, intraplaque hemorrhage (found in 48% of 216 symptomatic lesions and 50% of 144 asymptomatic lesions; P = .73) conferred no discriminative value regarding plaque instability. The importance of carotid plaque disruption in the pathogenesis of cerebral symptoms parallels findings with respect to acute coronary artery syndromes.40, 41, 42, 43 Using a subset of these articles, Golledge et al also identified a thin fibrous cap (FC; <65 μm44), a necrotic core closer to the plaque surface, and infiltration of the lesion by a larger number of inflammatory cells, as markers of a recently symptomatic carotid plaque.
Features of plaque vulnerability
Most patients with high-grade stenosis are, in fact, asymptomatic, and among patients in this asymptomatic cohort, the rate of symptomatic conversion is very low at less than 1% per year.45 Conversely, there exist a substantial proportion of patients with <50% stenosis who have anterior circulation strokes.22 A number of studies have demonstrated that up to 20% of patients with cryptogenic stroke or embolic stroke of undetermined source have only mild carotid stenosis. Among these patients, the risk of recurrent ipsilateral stroke is as high as 8% at 3 years.46 Thus, imaging technologies that better capture morphologic details of plaque morphology, such as the presence of a lipid-rich necrotic core (LRNC), the thickness and eventual rupture of an FC, the presence of IPH (Fig 2), plaque neovascularization, and calcifications, are important to improve risk prediction, selection of patients for intervention, and ultimately also for the development of preventive plaque stabilizing pharmacotherapy. The recently published results of the Carotid Plaque Imaging in Acute Stroke (CAPIAS) trial showed that the IPH is more prevalent at the symptomatic side in patients with carotid plaques and <50% stenosis22 (Fig 3).
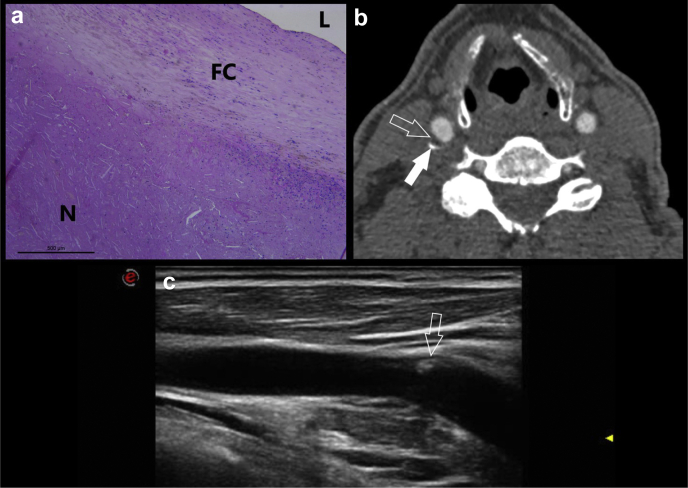
Fig 2.
A 67-year-old-male patient who underwent right carotid endarterectomy. The histopathologic figure (a) shows an intact and thick fibrous cap (FC) separating the necrotic core (N) from the lumen (L). In panel (b), the computed tomography angiography (CTA) axial slice is shown, and the white arrows show the presence of a mixed plaque (open white arrow) with thin calcifications (open arrow). The ultrasound image (c) obtained at the same day of the CTA confirm the presence of a mild carotid artery plaque with focal area of hyperechogenicity related to the presence of calcium (open white arrow).
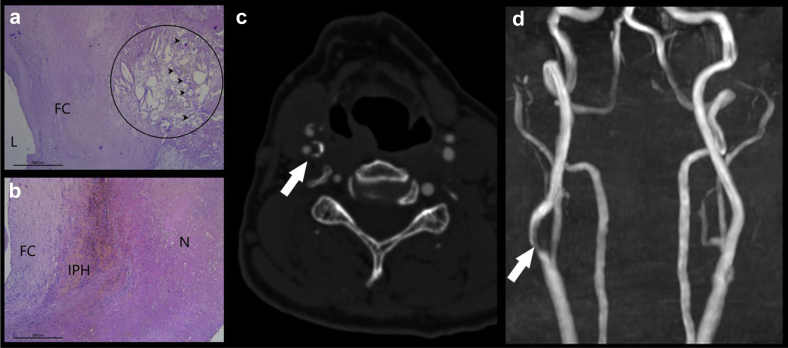
Fig 3.
In this 76-year-old male patient who underwent right carotid endarterectomy, the histopathologic images show an area where within the lipid-rich necrotic core (LRNC) (circle) newly formed microvessels (arrowheads) are visible; L: lumen; FC: fibrous cap (a) and in another slice it is also visible the intraplaque hemorrhage (IPH), FC: fibrous cap; N: necrotic core (b). The computed tomography angiography (CTA) axial image confirms the presence of a vulnerable plaque with hypodense core. The time-of-flight (TOF) magnetic resonance (MR) confirm the presence of stenosis in the right internal carotid artery due to the plaque. In the panels c and d, the CTA axial image and the magnetic resonance angiography "time of flight" view are given.
Many of the prior studies of vessel wall disease have been based on patients with known carotid artery disease. Furthermore, the most commonly studied patients have been those with significant (>50%) stenosis as this group has, by definition, more advanced disease. They typically have more advanced pathologic features that are straightforward to detect including a thicker vessel wall that provides an easier imaging target. Less than 50% of patients with vessel wall disease are often considered to have mild or no disease. The designation of “no disease” is likely to be an underestimation resulting from comparative imaging using Doppler ultrasound (US), which commonly designates any measurement <50% as nonsignificant or disease-free. Regarding this point, a large plaque with a thin FC and large lipid core may be detected using US, which indicates increased clinical risk, but if the anatomy also shows <50% stenosis by NASCET criteria, it would be deemed clinically insignificant according to the current standard of practice. Regarding this latter point, the recently published meta-analyses by Schindler et al47 show that for carotid with less than 50% stenosis, the presence IPH plays an important role for the occurrence of cerebrovascular events. To date no outcome studies looking only at those vessels with less than 50% stenosis have been undertaken. Cross-sectional studies have however shown that vulnerable plaque features do exist at this stage of disease including LRNC or IPH (Fig 3), and these features were detected mainly by using MRI.48
An important point is that the plaques are dynamic in nature, and it is possible to observe progression and regression of the plaque over time. This is important in terms of risk stratification and therapeutic decisions. Moreover, the evidence of regression or progression has interesting implications in the occurrence of a cerebrovascular event. Progression of plaque morphology with increasing vessel wall volume and/or progression of plaque composition with increasing size of vulnerable plaque features are associated with increased risk of future cerebrovascular and cardiovascular events.49
In a meta-analysis of eight studies including 689 patients, the presence of IPH at baseline was associated with a sixfold higher risk for cerebrovascular events with an annualized event rate of 17.7% compared with 2.43% in patients with no IPH.50 A number of studies have examined rates and risk factors for carotid plaque progression. Among patients with a history of acute ischemic stroke, the rate of plaque progression in both symptomatic and asymptomatic carotid artery stenosis is approximately 5% per year. Progression is a major risk factor for development of future ischemic events as plaque progression is an imaging biomarker for a biologically active plaque.51
With regard to evidence of plaque regression, lipid-lowering treatment, predominately with statin therapy, has been shown to decrease carotid plaque size and change the composition. The authors showed a decrease in vessel wall thickness and vessel wall area,52 and subsequent observational studies have all used the 1-year time frame to study changes in vessel wall size.53 The effects in terms of variation in term of plaque composition after lipid-lowering treatment could play an important role for the plaque stratification could be not only based on the mere degree of stenosis but also on the plaque composition.
In the first longitudinal study using in vivo carotid plaque MRI, 18 asymptomatic hypercholesterolemic patients with documented carotid and/or aortic arch plaques were treated with statins and demonstrated no significant change in vessel wall area at 6 months, but showed significant reductions in vessel wall area (15% carotid plaque regression and 8% aortic plaque regression, both P < .0001) at 12 months.52 In the following sections, we will focus on the main features of plaque's vulnerability.
In the Table we suggest how the different imaging methods can explore the different features of vulnerability described in the next sections.
Table.
Current evidence of use of the imaging technique for the detection the features of vulnerability
| US | PET | MR | CT | |
|---|---|---|---|---|
| Carotid plaque volume | ± | ± | ++ | ++ |
| Maximum wall thickness | ++ | − | ++a | + |
| Plaque inflammation | ± | ++ | + | − |
| Neovascularization | + | ± | + | + |
| Ulceration | + | − | ++ | ++ |
| The LRNC | ± | − | ++ | ± |
| The FC | ± | − | ++ | − |
| Plaque calcification | ± | − | + | ++ |
| IPH | ± | − | ++ | + |
| Microembolism | ++ | − | − | − |
CT, Computed tomography; FC, fibrous cap; IPH, intraplaque hemorrhage; LRNC, lipid-rich necrotic core; MR, magnetic resonance; PET, positron emission tomography; US, ultrasound.
Strong supporting evidence ++.
Moderate supporting evidence +.
Conflicting supporting evidence ±.
Weak supporting evidence −.
With dedicated coils to increase the spatial resolution.
Luminal morphology and ulcerations
The morphology of the luminal surface was one of the first features identified in the NASCET trial, and its alteration, in particular ulceration, was associated with an increased risk of cerebrovascular events.54 The luminal surface of carotid plaques can be classified as smooth, irregular, or ulcerated.55 A smooth surface is identified as plain luminal morphology without any sign of ulceration or irregularity. An irregular surface indicates the presence of small alterations of the luminal surface on the luminal profile of the plaque. The third type of morphology is the ulceration. Plaque ulceration has been defined as “an intimal defect larger than 1 mm in width, exposing the necrotic core of the atheromatous plaque.”56 The diagnostic sensitivity of US is lower compared with CT angiography57 even if it has recently been shown that 3D US could be effective in the detection of ulceration in carotid artery plaques.58,59 MR time-of-flight (TOF) shows suboptimal performance in the detection of ulcerations because it is prone to saturation of slowly flowing or recirculating blood protons that can affect the signal within an ulcer crater,60 whereas contrast-enhanced magnetic resonance angiography has been demonstrated to be a sensitive techniques for their identifications.61
Intraplaque hemorrhage
IPH is a common feature of atherosclerotic plaques, and currently it is considered one of the most important features of carotid artery plaque vulnerability. Several studies have found a statistically significant association between the presence of IPH and stroke, and more recently two important meta-analyses47,62 have demonstrated that patients with IPH have a greater risk of stroke even in asymptomatic patients.47 Different hypotheses have been formulated regarding IPH, and most of the authors suggest that it is due to the rupture of neovessels and that some conditions such as inflammation, metabolic disease, or diabetes may trigger this event.63 Currently, it is possible to detect IPH with MRI and CT. Because of the sensitivity of MRI in detecting blood products, some authors suggest that MRI is the best modality for the detection of IPH,55,64,65 and a recent statement published by the Carotid Imaging Consensus Group has strengthened this position.48 The appearance of IPH on MRI depends on the oxidative state of the hemoglobin.66 Detection of IPH using CT is more complex than MRI, and there is no consensus about the use of the CT for the detection of this feature. The authors found that the low attenuation values in the carotid plaque (<30 or 25 HU) are associated with the presence of IPH.67, 68, 69
Fissured FC
The FC is a layer of connective tissue that separates the plaque from vessel lumen. The authors found that vulnerable plaques are characterized by the presence of a thin FC or ruptured FC.70, 71, 72 The fissuring or rupture of the FC exposes the LRNC to luminal blood activating the thromboembolic cascade.
Using US, the detection of the FC and its status (thin-ruptured) showed suboptimal results.73, 74, 75 A potential approach to improve the US performance could be the use of intravascular US, as suggested in some recently published papers76,77
Currently, MRI is the reference standard, and a normal FC is characterized by the presence of a juxtaluminal band of low signal on TOF MR images and/or a hyperintense juxtaluminal region on contrast enhanced T1w images. A thin FC is present when this band of low signal on TOF or the hyperintense region on CE-T1w is not visible or when the juxtaluminal hyperintense region on CE-T1w MRI is interrupted. Using MRI the ruptured FC is identified by: (1) the absence of the juxtaluminal band of low signal and (2) the presence of a bright gray region adjacent to the lumen, corresponding to plaque hemorrhage and/or mural thrombus. It is important to underline that the MRI visualization of the FC is not easy, and its detection requires dedicated carotid surface coils. In 2009, Kerwin et al78 showed that contrast-enhanced MRI has a better sensitivity in detecting the status of the FC, and nowadays the conventional MRI should be used only for those patients with contraindications for contrast injection.
CT is not considered optimal for the evaluation of FC because of the artifacts related to edge-blur and halo effects. However, some authors suggested that, with some advanced approaches, it is also possible to detect the FC also using CT in particular to identify rupture79,80 and that the ruptured FC correlates with the presence of plaque enhancement in CT angiography analysis.81
Lipid LRNC
An LRNC is a collection of heterogeneous materials within the atherosclerotic plaque that consists of cholesterol crystals and necrotic debris of apoptotic cells.72 Previous studies have suggested that the presence of an LRNC is statistically associated with the occurrence of cerebrovascular events.64,82 Moreover, a larger LRNC size correlates with future ipsilateral carotid symptoms. The LRNC can be visualized using CT and MRI, but the limitation of CT is that the LRNC cannot be distinguished from intraplaque hemorrhage because both have overlapping values of attenuation.
MRI is considered the gold standard for the detection and visualization of an LRNC. Prospective evidence for the role of LRNCs in the risk of recurrent ischemic stroke comes from a recent meta-analysis on this topic, demonstrating a pooled hazard ratio of 5.7.83 An LRNC could be detected as a focal hypointense region on T2-W images.84,85 Nowadays, contrast-enhanced MRI is the preferred method to identify an LRNC rather than T2w MRI.11
US grayscale median (GSM) is commonly used to quantify the US appearance of carotid plaques, and several studies have found that it may be valuable for predicting the risk of cerebrovascular events.86 The GSM value of the plaque is a measure of overall echogenicity. It is determined by the median grayscale value (shade) within the segmented plaque.87 In particular, the GSM analyses are demonstrated to have good correlation with the histopathologic comparison in the LRNC detection, and it was found that a necrotic core located in a juxtalumenal position was associated with significantly lower GSM values (P = .009).88 However, one of the limits of the US is that it is very difficult to distinguish the IPH89 from the LRNC.
Neovascularization
Intraplaque neovascularization is a relatively new feature of carotid artery plaque vulnerability that is nowadays possible to identify with the evolution of plaque imaging.90 From a histologic point of view, it arises from newly formed microvessels that grow into the intima through breaks in the medial wall and are characterized by leaky capillaries with an endothelial lining that is immature. Histopathologic studies have demonstrated that neovessels can be found within carotid artery plaques, and the degree of neovascularization is associated with the inflammatory “activity” of the plaque. It is possible to categorize the neovascularization in (1) adventitial neovascularization (it serves as the main source of ingrowth of new vessels) and (2) intraplaque neovascularization (hallmark of advanced atherosclerotic lesions).
Most papers indicate that MRI represents the best imaging technique to detect and quantify the neovascularization91 using gadolinium perfusion methods correlated with adventitial perfusion as measured by its transfer constant.92 The authors also suggest to grade the adventitial (circumferential) enhancement because it is directly associated with cerebrovascular events. Also US with microbubble injection can detect and quantify the neovascularization, but the reproducibility and utility of this modality for clinical care is not well established.93,94 Recently, Saba et al90,95 suggested that CT can assess the presence and amount of neovascularization through the contrast-plaque enhancement quantification.
Inflammation
Another recently explored feature is the inflammation of the carotid artery plaque. This condition was firstly described and identified in plaques occurring in the coronary arteries. Inflammatory cells are typically found in the plaque shoulder, in the cap, or both, and it is possible to identify different types of inflammatory cells. Macrophages have been found to be predominant cell types96,97 and are significantly associated with the risk of plaque rupture. Thus, imaging methods employed to detect plaque inflammation should target imaging of macrophages.98,99 Currently, the detection and quantification of the inflammation in the carotid plaque remains in the research domain only, and most of the applications are performed with MR using new contrast agents using iron particles (ultrasmall superparamagnetic iron oxide or P947) or 18F-fluorodeoxyglucose (18F-FDG) on positron emission tomography (PET).100,101
Several recent studies have used PET imaging to identify culprit plaques after stroke or transient ischemic attacks and to predict future cerebral ischemic events due to the demonstration that there is an atherosclerotic plaque uptake on FDG-PET, and this parameter is strongly correlated with markers of inflammation such as CD68 macrophages, matrix metaloproteinase-9, and IL-18 on histology as well as plaque angiogenesis and hypoxia.102,103 It is possible to use also 18F-sodium fluoride (18F-NaF) to identify vessel microcalcifications implicated in actively inflamed atherosclerotic lesions.104 Chaker et al105 performed a systematic review and meta-analysis evaluating the association between carotid artery 18F-FDG or 18F-NaF uptake and recent or future cerebral ischemic events, and they found that recent ipsilateral cerebral ischemia may be associated with increased carotid 18F-FDG uptake on PET imaging regardless of degree of carotid stenosis.
Plaque thickness and volume
The maximum thickness of the carotid artery plaque is a simple linear measurement of the maximum plaque thickness and is thought to be associated with other imaging features of plaque vulnerability.106 The maximum plaque thickness seems to increase the risk of stroke, beyond the degree of stenosis. The plaque volume could be a better parameter in stroke risk assessment than thickness but is more difficult to visualize and quantify than maximum thickness, especially with US. However, recent studies have demonstrated that the volume of the carotid artery plaque could play a role in determining plaque “vulnerability” and risk of cardiovascular events.107,108 Plaque composition is known to change with increasing plaque volume. More specifically, there is an increase in the proportion of lipid and calcification with increasing plaque volume. With MR the plaque volume compared across three different MRI vendors showed an interclass correlation of 0.75 for intraplatform reproducibility as well as intrareader (0.83) and interreader (0.99) reproducibility for the lumen, wall, and total vessel areas, indicating strong agreement for repeated measurements.109 Measuring cap thickness remains challenging because of limitations in spatial resolution for resolving thinning FCs.
Initial phantom-based quantification of carotid plaque volume using US demonstrated a mean accuracy of 3.1% ± 0.9% with variability in plaque volume measurements of 4.0% ± 1.0% and 5.1% ± 1.4% cubic mm for intraobserver and interobserver measurements.110,111
CT-based tissue quantification demonstrated a high correlation and low bias with ex vivo histopathologic quantitative measures of atherosclerotic plaque tissue characteristics112; the intrareader variability was low with the repeatability coefficient ranging from 1.50 to 1.83 mm2. Interreader variability was also low with the repeatability coefficients ranging from 2.09 to 4.43 mm2.
Conclusions
Advanced imaging has significantly evolved in the last 20 years such that several newer features of the highly dynamic and vulnerable carotid artery plaque can be explored with finer detail. This provides unprecedented opportunity to clinicians and vascular surgeons to gather valuable information regarding the morphology and composition of the plaque and offer patient-oriented tailored therapies.
The wide array of features of vulnerability offered by MRI, CT and US need not only to be implemented in routine diagnostic algorithms but also to feed them into deep learning algorithms17 and computer-based decision modeling that will offer optimal strategies for the implementation of these data in an efficient diagnostic process.
Currently, there is a growing debate regarding the use of the imaging biomarker of the plaque for the stroke risk stratification, and some prospective longitudinal studies have demonstrated that the diagnostic algorithms that incorporate the information deriving from the plaque quantification are better associated with the prediction of occurrence of cerebrovascular events.
From the summary we have performed in this review, it is evident that there is a group of histopathologic features (IPH, status of the FC, size of LRNC, inflammation, neovascularization) that are predictive of an increased plaque vulnerability and that nowadays imaging techniques have reached the level of diagnostic sensitivity that allows us to detect these features outside the research domain and moving toward the clinical activity.
In particular, MR have most of the evidence in terms of longitudinal analysis, in particular for the IPH detection, but CT is offering new important evidence thanks to its potentiality to perform the volume quantification of the subcomponents of the plaque. Therefore, MR and CT seem to play a key role in this scenario. Nonetheless, also US and PET could offer significant information as shown in the previous section.
In conclusion, the area of the biomarkers in the carotid artery plaque is an open scenario where in the near future it will be important to define what feature is to be targeted and which are the best imaging modalities to detect it.
Author contributions
Conception and design: LS, CG, RS, GC, YQ, AB, CP, GF
Analysis and interpretation: LS, NA, MP, RM, PL, JS
Data collection: LS, RC
Writing the article: LS, NA, CG, RS, MP, RM, YQ, AB, PL, CP, GF, JS
Critical revision of the article: LS, RC, GC
Final approval of the article: LS, NA, RC, CG, RS, MP, RM, GC, YQ, AB, PL, CP, GF, JS
Statistical analysis: LS, NA, RC, CG, RS, RM, YQ, AB, CP, GF
Obtained funding: LS, NA, RC, CG, MP, GC, YQ, PL, CP, JS
Overall responsibility: LS
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Katan M., Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson C.O., Nguyen M., Roth G.A., Nichols E., Alam T., Abate D. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumamaru H., Jalbert J.J., Nguyen L.L., Gerhard-Herman M.D., Williams L.A., Chen C.-Y. Surgeon case volume and 30-day mortality after carotid endarterectomy among contemporary Medicare beneficiaries: before and after national coverage determination for carotid artery stenting. Stroke. 2015;46:1288–1294. doi: 10.1161/STROKEAHA.114.006276. [DOI] [PubMed] [Google Scholar]
- 5.Abbott A.L., Adelman M.A., Alexandrov A.V., Barber P.A., Barnett H.J.M., Beard J. Why calls for more routine carotid stenting are currently inappropriate: an international, multispecialty, expert review and position statement. Stroke. 2013;44:1186–1190. doi: 10.1161/STROKEAHA.111.000261. [DOI] [PubMed] [Google Scholar]
- 6.Munster A.B., Franchini A.J., Qureshi M.I., Thapar A., Davies A.H. Temporal trends in safety of carotid endarterectomy in asymptomatic patients. Neurology. 2015;85:365–372. doi: 10.1212/WNL.0000000000001781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 8.Abbott A.L. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573–e583. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 9.Naylor A.R., Gaines P.A., Rothwell P.M. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg. 2009;37:625–632. doi: 10.1016/j.ejvs.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Saba L., Saam T., Jäger H.R., Yuan C., Hatsukami T.S., Saloner D. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;4422:1–14. doi: 10.1016/S1474-4422(19)30035-3. [DOI] [PubMed] [Google Scholar]
- 11.Saba L., Yuan C., Hatsukami T.S., Balu N., Qiao Y., DeMarco J.K. Carotid artery wall imaging: perspective and guidelines from the ASNR Vessel Wall Imaging Study Group and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. 2018;39:E9–E31. doi: 10.3174/ajnr.A5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett H.J.M., Taylor D.W., Eliasziw M., Fox A.J., Ferguson G.G., Haynes R.B. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 13.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 14.Danese M.D., Pemberton-Ross P., Catterick D., Villa G. Estimation of the increased risk associated with recurrent events or polyvascular atherosclerotic cardiovascular disease in the United Kingdom. Eur J Prev Cardiol. 2021;28:335–343. doi: 10.1177/2047487319899212. [DOI] [PubMed] [Google Scholar]
- 15.Naylor A.R., Ricco J.-B., de Borst G.J., Debus S., de Haro J., Halliday A. Editor’s choice—management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Aboyans V., Ricco J.-B., Bartelink M.-L.E.L., Björck M., Brodmann M., Cohnert T. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1016/j.rec.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Saba L., Biswas M., Kuppili V., Cuadrado Godia E., Suri H.S., Edla D.R. The present and future of deep learning in radiology. Eur J Radiol. 2019;114:14–24. doi: 10.1016/j.ejrad.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Tsakanikas V.D., Siogkas P.K., Mantzaris M.D., Potsika V.T., Kigka V.I., Exarchos T.P. A deep learning oriented method for automated 3D reconstruction of carotid arterial trees from MR imaging. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:2408–2411. doi: 10.1109/EMBC44109.2020.9176532. [DOI] [PubMed] [Google Scholar]
- 19.Biswas M, Saba L, Chakrabartty S, Khanna NN, Song H, Suri HS, et al. Two-stage artificial intelligence model for jointly measurement of atherosclerotic wall thickness and plaque burden in carotid ultrasound: a screening tool for cardiovascular/stroke risk assessment [e-pub ahead of print]. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2020.103847. [DOI] [PubMed]
- 20.Saba L., Pascalis L., Montisci R., Sanfilippo R., Mallarini G., Sulcis R. Stroke risk stratification and its validation using ultrasonic echolucent carotid wall plaque morphology: a machine learning paradigm. Eur J Radiol. 2017;26:1–8. doi: 10.1016/j.compbiomed.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Müller D., Kramer F. MIScnn: a framework for medical image segmentation with convolutional neural networks and deep learning. BMC Med Imaging. 2021;21:12. doi: 10.1186/s12880-020-00543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopczak A., Schindler A., Bayer-Karpinska A., Koch M.L., Sepp D., Zeller J. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol. 2020;76:2212–2222. doi: 10.1016/j.jacc.2020.09.532. [DOI] [PubMed] [Google Scholar]
- 23.Avril G., Batt M., Guidoin R., Marois M., Hassen-Khodja R., Daune B. Carotid endarterectomy plaques: correlations of clinical and anatomic findings. Ann Vasc Surg. 1991;5:50–54. doi: 10.1007/BF02021778. [DOI] [PubMed] [Google Scholar]
- 24.Van Damme H., Vivario M., Boniver J., Limet R. Histologic characterization of carotid plaques. Cardiovasc Pathol. 1994;3:9–17. doi: 10.1016/1054-8807(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 25.Lusby R.J., Ferrell L.D., Ehrenfeld W.K., Stoney R.J., Wylie E.J. Carotid plaque hemorrhage. Arch Surg. 1982;117:1479. doi: 10.1001/archsurg.1982.01380350069010. [DOI] [PubMed] [Google Scholar]
- 26.Imparato A.M., Riles T.S., Mintzer R., Baumann F.G. The importance of hemorrhage in the relationship between gross morphologic characteristics and cerebral symptoms in 376 carotid artery plaques. Ann Surg. 1983;197:195–203. doi: 10.1097/00000658-198302000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammar A.D., Wilson R.L., Travers H., Lin J.J., Farha S.J., Chang F.C. Intraplaque hemorrhage: its significance in cerebrovascular disease. Am J Surg. 1984;148:840–843. doi: 10.1016/0002-9610(84)90450-1. [DOI] [PubMed] [Google Scholar]
- 28.Ricotta J.J., Schenk E.A., Ekholm S.E., DeWeese J.A. Angiographic and pathologic correlates in carotid artery disease. Surgery. 1986;99:284–292. [PubMed] [Google Scholar]
- 29.Bassiouny H.S., Davis H., Massawa N., Gewertz B.L., Glagov S., Zarins C.K. Critical carotid stenoses: morphologic and chemical similarity between symptomatic and asymptomatic plaques. J Vasc Surg. 1989;9:202–212. [PubMed] [Google Scholar]
- 30.Lennihan L, Kupsky WJ, Mohr JP, Hauser WA, Correll JW, Quest DO. Lack of association between carotid plaque hematoma and ischemic cerebral symptoms. Stroke. 18:879-881. [DOI] [PubMed]
- 31.Bassiouny H.S., Sakaguchi Y., Mikucki S.A., McKinsey J.F., Piano G., Gewertz B.L. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J Vasc Surg. 1997;26:585–594. doi: 10.1016/s0741-5214(97)70056-9. [DOI] [PubMed] [Google Scholar]
- 32.Fisher C.M., Ojemann R.G. A clinico-pathologic study of carotid endarterectomy plaques. Rev Neurol (Paris) 1986;142:573–589. [PubMed] [Google Scholar]
- 33.AbuRahma A.F., Wulu J.T., Crotty B. Carotid plaque ultrasonic heterogeneity and severity of stenosis. Stroke. 2002;33:1772–1775. doi: 10.1161/01.str.0000019127.11189.b5. [DOI] [PubMed] [Google Scholar]
- 34.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunning A.J., Pickering G.W., Robb-Smith A.H., Russell R.R. Mural thrombosis of the internal carotid artery and subsequent embolism. Q J Med. 1964;33:155–195. [PubMed] [Google Scholar]
- 36.Eikelboom B.C., Riles T.R., Mintzer R., Baumann F.G., DeFillip G., Lin J. Inaccuracy of angiography in the diagnosis of carotid ulceration. Stroke. 1983;14:882–885. doi: 10.1161/01.str.14.6.882. [DOI] [PubMed] [Google Scholar]
- 37.Svindland A., Torvik A. Atherosclerotic carotid disease in asymptomatic individuals: an histological study of 53 cases. Acta Neurol Scand. 1988;78:506–517. doi: 10.1111/j.1600-0404.1988.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 38.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 39.Golledge J., Greenhalgh R.M., Davies A.H. The symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 40.Davies M.J., Thomas A.C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas A.C., Davies M.J. Post-mortem investigation and quantification of coronary artery disease. Histopathology. 1985;9:959–976. doi: 10.1111/j.1365-2559.1985.tb02880.x. [DOI] [PubMed] [Google Scholar]
- 42.Falk E. Morphologic features of unstable atherothrombotic plaques underlying acute coronary syndromes. Am J Cardiol. 1989;63:E114–E120. doi: 10.1016/0002-9149(89)90242-7. [DOI] [PubMed] [Google Scholar]
- 43.Carr S., Farb A., Pearce W.H., Virmani R., Yao J.S. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23:755–765. doi: 10.1016/s0741-5214(96)70237-9. discussion: 765-6. [DOI] [PubMed] [Google Scholar]
- 44.Shindo S., Fujii K., Shirakawa M., Uchida K., Enomoto Y., Iwama T. Morphologic features of carotid plaque rupture assessed by optical coherence tomography. Am J Neuroradiol. 2015;36:2140–2146. doi: 10.3174/ajnr.A4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien M., Chandra A. Carotid revascularization: risks and benefits. Vasc Health Risk Manag. 2014;10:403–416. doi: 10.2147/VHRM.S48923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karlsson L., Kangefjärd E., Hermansson S., Strömberg S., Österberg K., Nordanstig A. Risk of recurrent stroke in patients with symptomatic mild (20–49% NASCET) carotid artery stenosis. Eur J Vasc Endovasc Surg. 2016;52:287–294. doi: 10.1016/j.ejvs.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Schindler A., Schinner R., Altaf N., Hosseini A.A., Simpson R.J., Esposito-Bauer L. Prediction of stroke risk by detection of hemorrhage in carotid plaques: meta-analysis of individual patient data. JACC Cardiovasc Imaging. 2020;13:395–406. doi: 10.1016/j.jcmg.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 48.Saba L., Moody A.R., Saam T., Kooi M.E., Wasserman B.A., Staub D. Vessel wall–imaging biomarkers of carotid plaque vulnerability in stroke prevention trials: a viewpoint from the Carotid Imaging Consensus Group. JACC Cardiovasc Imaging. 2020;13:2445–2456. doi: 10.1016/j.jcmg.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 49.Lu M., Peng P., Cui Y., Qiao H., Li D., Cai J. Association of progression of carotid artery wall volume and recurrent transient ischemic attack or stroke. Stroke. 2018;49:614–620. doi: 10.1161/STROKEAHA.117.019422. [DOI] [PubMed] [Google Scholar]
- 50.Saam T., Hetterich H., Hoffmann V., Yuan C., Dichgans M., Poppert H. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Mughal M.M., Khan M.K., DeMarco J.K., Majid A., Shamoun F., Abela G.S. Symptomatic and asymptomatic carotid artery plaque. Expert Rev Cardiovasc Ther. 2011;9:1315–1330. doi: 10.1586/erc.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corti R., Fayad Z.A., Fuster V., Worthley S.G., Helft G., Chesebro J. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 53.Underhill H.R., Yuan C. Carotid MRI: a tool for monitoring individual response to cardiovascular therapy? Expert Rev Cardiovasc Ther. 2011;9:63–80. doi: 10.1586/erc.10.172. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson G.G., Eliasziw M., Barr H.W.K., Clagett G.P., Barnes R.W., Wallace M.C. Surgical results in 1415 patients. Stroke. 2015;30:1751–1758. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 55.Saba L., Anzidei M., Marincola B.C., Piga M., Raz E., Bassareo P.P. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol. 2014;37:572–585. doi: 10.1007/s00270-013-0711-2. [DOI] [PubMed] [Google Scholar]
- 56.Sitzer M., Muller W., Siebler M., Hort W., Kniemeyer H.W., Jancke L. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke. 1995;26:1231–1233. doi: 10.1161/01.str.26.7.1231. [DOI] [PubMed] [Google Scholar]
- 57.Saba L., Caddeo G., Sanfilippo R., Montisci R., Mallarini G. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: potentialities and advantages of multidetector row CT angiography. Am J Neuroradiol. 2007;28:1061–1066. doi: 10.3174/ajnr.A0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rafailidis V., Chryssogonidis I., Tegos T., Kouskouras K., Charitanti-Kouridou A. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging. 2017;8:213–225. doi: 10.1007/s13244-017-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafailidis V., Chryssogonidis I., Xerras C., Nikolaou I., Tegos T., Kouskouras K. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. Eur Radiol. 2019;29:2137–2145. doi: 10.1007/s00330-018-5773-8. [DOI] [PubMed] [Google Scholar]
- 60.Fellner C., Lang W., Janka R., Wutke R., Bautz W., Fellner F.A. Magnetic resonance angiography of the carotid arteries using three different techniques: accuracy compared with intraarterial x-ray angiography and endarterectomy specimens. J Magn Reson Imaging. 2005;21:424–431. doi: 10.1002/jmri.20282. [DOI] [PubMed] [Google Scholar]
- 61.Etesami M., Hoi Y., Steinman D.A., Gujar S.K., Nidecker A.E., Astor B.C. Comparison of carotid plaque ulcer detection using contrast- enhanced and time-of-flight MRA techniques. Am J Neuroradiol. 2013;34:177–184. doi: 10.3174/ajnr.A3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A., Baradaran H., Schweitzer A.D., Kamel H., Pandya A., Delgado D. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–3077. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 63.Pasterkamp G., Van Der Steen A.F.W. Intraplaque hemorrhage: an imaging marker for atherosclerotic plaque destabilization? Arterioscler Thromb Vasc Biol. 2012;32:167–168. doi: 10.1161/ATVBAHA.111.241414. [DOI] [PubMed] [Google Scholar]
- 64.Brinjikji W., Iii J.H., Rabinstein A.A., Kim G., Lerman A., Lanzino G. Plaque vulnerability. J Neurosurg. 2016;124:27–42. doi: 10.3171/2015.1.JNS142452. [DOI] [PubMed] [Google Scholar]
- 65.Tartari S., Rizzati R., Righi R., Deledda A., Capello K., Soverini R. High-resolution MRI of carotid plaque with a neurovascular coil and contrast-enhanced MR angiography: one-stop shopping for the comprehensive assessment of carotid atherosclerosis. Am J Roentgenol. 2011;196:1164–1171. doi: 10.2214/AJR.10.4751. [DOI] [PubMed] [Google Scholar]
- 66.Fuster V., Moreno P.R., Fayad Z.A., Corti R., Badimon J.J. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–954. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 67.Porcu M., Anzidei M., Suri J.S., Wasserman B.A., Anzalone N., Lucatelli P. Carotid artery imaging: the study of intra-plaque vascularization and hemorrhage in the era of the “vulnerable” plaque. J Neuroradiol. 2020;47:464–472. doi: 10.1016/j.neurad.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Saba L., Micheletti G., Brinjikji W., Garofalo P., Montisci R., Balestrieri A. Carotid intraplaque-hemorrhage volume and its association with cerebrovascular events. AJNR Am J Neuroradiol. 2019;40:1731–1737. doi: 10.3174/ajnr.A6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saba L., Francone M., Bassareo P.P., Lai L., Sanfilippo R., Montisci R. CT attenuation analysis of carotid intraplaque hemorrhage. Am J Neuroradiol. 2018;39:131–137. doi: 10.3174/ajnr.A5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hatsukami T.S., Ross R., Polissar N.L., Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 71.Chu B., Kampschulte A., Ferguson M.S., Kerwin W.S., Yarnykh V.L., O’Brien K.D. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004;35:1079–1084. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 72.Cai J., Hatsukami T.S., Ferguson M.S., Kerwin W.S., Saam T., Chu B. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 73.Sztajzel R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med Wkly. 2005;135:635–643. doi: 10.4414/smw.2005.11038. [DOI] [PubMed] [Google Scholar]
- 74.Varetto G., Gibello L., Castagno C., Quaglino S., Ripepi M., Benintende E. Use of contrast-enhanced ultrasound in carotid atherosclerotic disease: limits and perspectives. Biomed Res Int. 2015;2015:293163. doi: 10.1155/2015/293163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Funaki T., Iihara K., Miyamoto S., Nagatsuka K., Hishikawa T., Ishibashi-Ueda H. Histologic characterization of mobile and nonmobile carotid plaques detected with ultrasound imaging. J Vasc Surg. 2011;53:977–983. doi: 10.1016/j.jvs.2010.10.105. [DOI] [PubMed] [Google Scholar]
- 76.Zacharatos H., Hassan A.E., Qureshi A.I. Intravascular ultrasound: principles and cerebrovascular applications. AJNR Am J Neuroradiol. 2010;31:586–597. doi: 10.3174/ajnr.A1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suh W.M., Seto A.H., Ronan M., Margey J.P., Cruz-Gonzalez I., Jang I.-K. Intravascular detection of the vulnerable plaque. Circ Cardiovasc Imaging. 2011;4:169–178. doi: 10.1161/CIRCIMAGING.110.958777. [DOI] [PubMed] [Google Scholar]
- 78.Kerwin W.S., Zhao X., Chun Y., Hatsukami T.S., Maravilla K.R., Underhill H.R. Contrast-enhanced MRI of carotid atherosclerosis: dependence on contrast agent. J Magn Reson Imaging. 2009;30:35–40. doi: 10.1002/jmri.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saba L., Potters F., van der Lugt A., Mallarini G. Imaging of the fibrous cap in atherosclerotic carotid plaque. Cardiovasc Intervent Radiol. 2010;33:681–689. doi: 10.1007/s00270-010-9828-8. [DOI] [PubMed] [Google Scholar]
- 80.Saba L., Mallarini G. Fissured fibrous cap of vulnerable carotid plaques and symptomaticity: Are they correlated? preliminary results by using multi-detector-row CT angiography. Cerebrovasc Dis. 2009;27:322–327. doi: 10.1159/000202008. [DOI] [PubMed] [Google Scholar]
- 81.Saba L., Tamponi E., Raz E., Lai L., Montisci R., Piga M. Correlation between fissured fibrous cap and contrast enhancement: preliminary results with the use of CTA and histologic validation. AJNR Am J Neuroradiol. 2014;35:754–759. doi: 10.3174/ajnr.A3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun J., Zhao X.Q., Balu N., Neradilek M.B., Isquith D.A., Yamada K. Carotid plaque lipid content and fibrous cap status predict systemic CV outcomes: the MRI substudy in AIM-HIGH. JACC Cardiovasc Imaging. 2017;10:241–249. doi: 10.1016/j.jcmg.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng F., Mu C., Yang L., Li H., Xiang X., Li K. Carotid plaque magnetic resonance imaging and recurrent stroke risk: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19377. doi: 10.1097/MD.0000000000019377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trivedi R.A., U-King-Im J.M., Graves M.J., Horsley J., Goddard M., Kirkpatrick P.J. MRI-derived measurements of fibrous-cap and lipid-core thickness: the potential for identifying vulnerable carotid plaques in vivo. Neuroradiology. 2004;46:738–743. doi: 10.1007/s00234-004-1247-6. [DOI] [PubMed] [Google Scholar]
- 85.Toussaint J.F., LaMuraglia G.M., Southern J.F., Fuster V., Kantor H.L. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation. 1996;94:932–938. doi: 10.1161/01.cir.94.5.932. [DOI] [PubMed] [Google Scholar]
- 86.Spence J.D. Uses of ultrasound in stroke prevention. Cardiovasc Diagn Ther. 2020;10:955–964. doi: 10.21037/cdt.2019.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitchell C. Grayscale analysis of carotid plaque: an overview. J Am Soc Echocardiogr. 2019;32:A21–A22. doi: 10.1016/j.echo.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 88.Sztajzel R., Momjian S., Momjian-Mayor I., Murith N., Djebaili K., Boissard G. Stratified gray-scale median analysis and color mapping of the carotid plaque: correlation with endarterectomy specimen histology of 28 patients. Stroke. 2005;36:741–745. doi: 10.1161/01.STR.0000157599.10026.ad. [DOI] [PubMed] [Google Scholar]
- 89.Kume S., Hama S., Yamane K., Wada S., Nishida T., Kurisu K. Vulnerable carotid arterial plaque causing repeated ischemic stroke can be detected with B-mode ultrasonography as a mobile component: jellyfish sign. Neurosurg Rev. 2010;33:419–430. doi: 10.1007/s10143-010-0270-9. [DOI] [PubMed] [Google Scholar]
- 90.Saba L., Lai M.L., Montisci R., Tamponi E., Sanfilippo R., Faa G. Association between carotid plaque enhancement shown by multidetector CT angiography and histologically validated microvessel density. Eur Radiol. 2012;22:2237–2245. doi: 10.1007/s00330-012-2467-5. [DOI] [PubMed] [Google Scholar]
- 91.Millon A., Boussel L., Brevet M., Mathevet J.L., Canet-Soulas E., Mory C. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke. 2012;43:3023–3028. doi: 10.1161/STROKEAHA.112.662692. [DOI] [PubMed] [Google Scholar]
- 92.Kerwin W.S., Oikawa M., Yuan C., Jarvik G.P., Hatsukami T.S. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 93.Jaipersad A.S., Shantsila A., Silverman S., Lip G.Y.H., Shantsila E. Evaluation of carotid plaque neovascularization using contrast ultrasound. Angiology. 2013;64:447–450. doi: 10.1177/0003319712457013. [DOI] [PubMed] [Google Scholar]
- 94.Akkus Z., Hoogi A., Renaud G., van den Oord S.C.H., ten Kate G.L., Schinkel A.F.L. New quantification methods for carotid intra-plaque neovascularization using contrast-enhanced ultrasound. Ultrasound Med Biol. 2014;40:25–36. doi: 10.1016/j.ultrasmedbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 95.Saba L., Mallarini G. Carotid plaque enhancement and symptom correlations: an evaluation by using multidetector row CT angiography. Am J Neuroradiol. 2011;32:1919–1925. doi: 10.3174/ajnr.A2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Finn A.V., Nakano M., Narula J., Kolodgie F.D., Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 97.Virmani R., Ladich E.R., Burke A.P., Kolodgie F.D. Histopathology of carotid atherosclerotic disease. Neurosurgery. 2006;59(Suppl 3):219–227. doi: 10.1227/01.NEU.0000239895.00373.E4. [DOI] [PubMed] [Google Scholar]
- 98.Kolodgie F.D., Yahagi K., Mori H., Romero M.E., Trout H.H., Finn A.V. High-risk carotid plaque: lessons learned from histopathology. Semin Vasc Surg. 2017;30:31–43. doi: 10.1053/j.semvascsurg.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 99.Goikuria H., Vandenbroeck K., Alloza I. Inflammation in human carotid atheroma plaques. Cytokine Growth Factor Rev. 2018;39:62–70. doi: 10.1016/j.cytogfr.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Tang T.Y., Moustafa R.R., Howarth S.P., Walsh S.R., Boyle J.R., Li Z.Y. Combined PET-FDG and USPIO-enhanced MR imaging in patients with symptomatic moderate carotid artery stenosis. Eur J Vasc Endovasc Surg. 2008;36:53–55. doi: 10.1016/j.ejvs.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Vöö S., Kwee R.M., Sluimer J.C., Schreuder F.H.B.M., Wierts R., Bauwens M. Imaging intraplaque inflammation in carotid atherosclerosis with 18F-fluorocholine positron emission tomography-computed tomography. Circ Cardiovasc Imaging. 2016;9:e004467. doi: 10.1161/CIRCIMAGING.115.004467. [DOI] [PubMed] [Google Scholar]
- 102.Pedersen S.F., Hag A.M.F., Klausen T.L., Ripa R.S., Bodholdt R.P., Kjær A. Positron emission tomography of the vulnerable atherosclerotic plaque in man—a contemporary review. Clin Physiol Funct Imaging. 2014;34:413–425. doi: 10.1111/cpf.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kerwin W., Alessio A., Ferguson M., Hatsukami T., Caldwell J., Miyaoka R. High-resolution [18]fluorodeoxyglucose-positron emission tomography and coregistered magnetic resonance imaging of atherosclerotic plaque from a patient undergoing carotid endarterectomy. Circ Cardiovasc Imaging. 2012;5:683–684. doi: 10.1161/CIRCIMAGING.112.975144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mechtouff L, Sigovan M, Douek P, Costes N, Le Bars D, Mansuy A, et al. Simultaneous assessment of microcalcifications and morphological criteria of vulnerability in carotid artery plaque using hybrid 18F-NaF PET/MRI [e-pub ahead of print]. J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02400-0. [DOI] [PubMed]
- 105.Chaker S., Al-Dasuqi K., Baradaran H., Demetres M., Delgado D., Nehmeh S. Carotid plaque positron emission tomography imaging and cerebral ischemic disease. Stroke. 2019;50:2072–2079. doi: 10.1161/STROKEAHA.118.023987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saba L., Mannelli L., Balestrieri A., Serra A., Bassareo P., Murgia A. CT and MR imaging of carotid wall and plaque. J Neurosonol Neuroimaging. 2019;11:115–125. [Google Scholar]
- 107.Saba L., Raz E., Grassi R., Di Paolo P.L., Iacomino A., Montisci R. Association between the volume of carotid artery plaque and its subcomponents and the volume of white matter lesions in patients selected for endarterectomy. Am J Roentgenol. 2013;201:W747–W752. doi: 10.2214/AJR.12.10217. [DOI] [PubMed] [Google Scholar]
- 108.Nicolaides A.N., Kakkos S.K., Griffin M., Sabetai M., Dhanjil S., Tegos T. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Eur J Vasc Endovasc Surg. 2005;30:275–284. doi: 10.1016/j.ejvs.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 109.Saam T., Hatsukami T.S., Yarnykh V.L., Hayes C.E., Underhill H., Chu B. Reader and platform reproducibility for quantitative assessment of carotid atherosclerotic plaque using 1.5T Siemens, Philips, and General Electric scanners. J Magn Reson Imaging. 2007;26:344–352. doi: 10.1002/jmri.21004. [DOI] [PubMed] [Google Scholar]
- 110.Landry A., Spence J.D., Fenster A. Measurement of carotid plaque volume by 3-dimensional ultrasound. Stroke. 2004;35:864–869. doi: 10.1161/01.STR.0000121161.61324.ab. [DOI] [PubMed] [Google Scholar]
- 111.Landry A., Fenster A. Theoretical and experimental quantification of carotid plaque volume measurements made by three-dimensional ultrasound using test phantoms. Med Phys. 2002;29:2319–2327. doi: 10.1118/1.1510130. [DOI] [PubMed] [Google Scholar]
- 112.Sheahan M., Ma X., Paik D., Obuchowski N.A., St Pierre S., Newman W.P. Atherosclerotic plaque tissue: noninvasive quantitative assessment of characteristics with software-aided measurements from conventional CT angiography. Radiology. 2018;286:622–631. doi: 10.1148/radiol.2017170127. [DOI] [PMC free article] [PubMed] [Google Scholar]