Abstract
Introduction
Current agents for the intravascular embolization of traumatic hemorrhage are used off-label and have been minimally studied with respect to their performance under differing coagulation conditions. We studied the hemorrhage control efficacy of a novel, liquid, polyethylene glycol-based hydrogel delivered as two liquid precursors that polymerize within the target vessel in a unique animal model of severe solid organ injury with and without dilutional coagulopathy.
Methods
Anesthetized swine (n = 36, 45 ± 3 kg) had laparotomy and splenic externalization. Half underwent 50% isovolemic hemodilution with 6% hetastarch and cooling to 33°C-35°C (coagulopathic group). All animals had controlled 20 mL/kg hemorrhage and endovascular proximal splenic artery access with a 4F catheter via a right femoral sheath. Splenic transection and 5-minute free bleeding were followed by treatment (n = 5/group) with 5 mL of gelfoam slurry, three 6-mm coils, up to 6 mL of hydrogel, or no treatment (n = 3, control). Animals received 15 mL/kg plasma and were monitored for 6 hours with continuous blood loss measurement.
Results
Coagulopathy was successfully established, with coagulopathic animals having greater pretreatment blood loss and earlier mean time to death regardless of the treatment group. All control animals died within 100 minutes. Overall survival without coagulopathy was 5/5 for hydrogel, 4/5 for coil, and 3/5 for gelfoam. With coagulopathy, one hydrogel animal survived to the end of the experiment, with 2/4 hydrogel deaths occurring in the final hour of observation. In noncoagulopathic animals, hydrogel demonstrated improved survival time (P < .01) and post-treatment blood loss (1.46 ± 0.8 mL/kg) over controls (18.8 ± 0.7, P = .001), gelfoam (4.7 ± 1.3, P > .05), and coils (4.6 ± 1.5, P > .05). In coagulopathic animals, hydrogel had improved survival time (P = .003) and decreased blood loss (4.2 ± 0.8 mL/kg) compared with control (20.4 ± 4.2, P = .003).
Conclusions
The hydrogel demonstrated equivalent hemorrhage control performance to standard treatments under noncoagulopathic conditions and improved performance in the face of dilutional coagulopathy. This agent should be explored as a potential preferable treatment for the embolization of traumatic solid organ and other injuries. (JVS–Vascular Science 2021;2:43-51.)
Clinical Relevance
In a translational model of severe solid organ injury hemorrhage with and without coagulopathy, a novel hydrogel transarterial embolization agent demonstrated equivalent hemorrhage control performance to standard agents under noncoagulopathic conditions and improved performance in the face of dilutional coagulopathy. This agent represents a promising future treatment for the embolization of traumatic solid organ and other injuries.
Keywords: Angioembolization, Solid organ injury, Hydrogel, Gelfoam, Embolization coils, Endovascular, Uncontrolled hemorrhage, Coagulopathy
Article Highlights.
-
•
Type of Research: Translational large animal model
-
•
Key Findings: In treating experimental uncontrolled splenic hemorrhage, a novel hydrogel transarterial embolization agent performed as well as coils and gelfoam slurry in standard coagulation conditions and resulted in decreased blood loss and improved survival in the face of induced coagulopathy.
-
•
Take Home Message: This novel hydrogel embolization system is a promising new agent for the treatment of hemorrhage.
Transcatheter arterial embolization (TAE) has become an accepted alternative to open surgical management of severe hemorrhage from abdominal solid organ injuries.1, 2, 3, 4 Despite the growing use of TAE in the treatment of traumatic hemorrhage, there are no catheter-delivered agents specifically approved for this indication and a clinical study of existing agents has proved inconclusive in determining best practices.5 We have developed a translational animal model of fatal solid organ injury that has proven suitable for the comparative examination of embolization techniques and agents.6 The addition of a coagulation defect to the hemorrhage model provides a unique opportunity to rigorously study the effectiveness of embolization agents in a clinically relevant translational manner.7,8
The hydrogel embolic evaluated (Instylla Hydrogel Embolic System; Instylla Inc, Bedford, Mass) consists of two aqueous liquid precursors injected through a coaxial dual lumen catheter system. Although the exact composition of the precursors is proprietary, the principal component of the hydrogel produced is polyethylene glycol (PEG) and the polymerized product is composed primarily of water (approximately 95%) and PEG. Contrast media may be added to the precursors to permit fluoroscopic visibility, though the hydrogel itself is radiolucent and does not create the imaging artifacts seen with metal coils or tantalum-based embolic agents. When simultaneously injected, the liquid precursors mix and solidify in the target vessel as they travel with blood flow to form a soft, vessel filling hydrogel embolic. Polymerization occurs with contact between the precursors and no interaction between the precursors, and blood is required for polymerization and formation of the hydrogel plug. In vitro experiments have demonstrated that the polymerization process begins within 2 seconds and the rate of polymerization is dependent on the concentration of PEG in the precursor and the blood flow, with mixing characteristics varying by the size of the treated vessel and the speed of flow within it (unpublished results, Patrick Campbell—Instylla, Inc). During the time immediately after injection, the hydrogel swells slightly, resulting in occlusion of the target vessel and hemostasis.
The hydrogel embolic system evaluated here has had extensive in vitro and in vivo biocompatibility testing and is currently being used in a Food and Drug Administration investigational device exemption human clinical trial for tumor embolization (Instylla HES Hypervascular Tumor Pivotal Study, ClinicalTrials.gov Identifier: NCT04523350). Human trials for hemorrhage have been approved outside of the United States. Herein we present a series of experiments examining the effectiveness of the Instylla Hydrogel Embolic System (hydrogel) for hemorrhage control in a translational solid organ injury model both in the presence and absence of coagulopathy.
Methods
Aspects of the animal model used in this experiment have been previously described.6 The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The study was approved by the United States Army Institute of Surgical Research Institutional Animal Care and Use Committee and was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council.
Animal preparation
Female Yorkshire crossbred swine (n = 36) weighing 45 ± 3 kg underwent the experimental procedures outlined in Fig 1. Female animals alone were used because of institutional experience with this gender in the basic experimental protocol and for uniformity of physiologic response. After induction, animals were ventilated via an endotracheal tube and maintained under inhaled anesthesia with 21%-30% oxygen and 1%-3% isoflurane for the duration of the experiment. Ventilation was titrated to maintain an end tidal PCO2 of 40 mm Hg. Pigs were positioned supine on a radiolucent veterinary surgical table such that their thorax and abdomen could be visualized fluoroscopically. A transurethral Foley catheter was placed for measurement of urine output and core temperature. Vascular access was achieved through open surgical cut-down as follows:
-
•
Right femoral artery—7F introducer sheath (Pinnacle R/O II; Terumo, Elkton, Md) for endovascular procedures
-
•
Left femoral vein—8F introducer (Argon Medical Devices, Argon Medical Devices, Frisco, Tex) for resuscitation fluid administration
-
•
Right carotid artery—Systemic blood pressure monitoring (Mikro-Tip; Millar Instruments, Inc, Houston, Tex)
-
•
Left carotid artery—8F introducer (Argon Medical Devices) for blood sampling.
Fig 1.
Experimental protocol. EBV, Estimated blood volume.
Laparotomy and splenic preparation
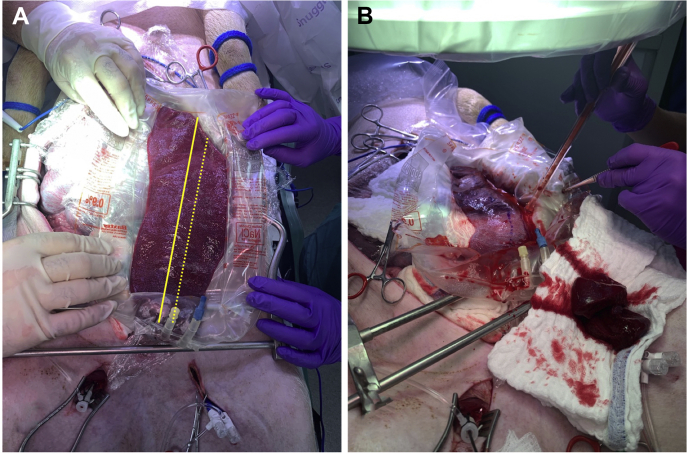
A midline laparotomy was performed, and the spleen externalized. The large left gastroepiploic continuation branch of the porcine splenic artery was ligated and divided. The organ was medialized and placed longitudinally on the open abdomen within a canoe-shaped plastic sheet surrounded by preweighed gauze pads to ensure accurate collection of shed blood from the forthcoming splenic injury (Fig 2).
Fig 2.
A, Exteriorized spleen within the hemorrhage collection bag; the solid yellow line represents splenic midline, and the dashed line represents planned transection. B, Spleen after transection, note midline marking (dashed blue ink line on the spleen), resected portion (on the laparotomy sponge in the foreground), and active shed blood collection. Reprinted with permission from original publication.6
Induction of coagulopathy and hemorrhage
Half (n = 18) of the animals underwent a 50% isovolemic exchange at 50 mL/min with room temperature 6% hetastarch (Hextend; Hospira, Inc, Lake Forest, Ill) and were cooled with an external blanket to a core temperature of 33°C-34°C to induce hypothermic dilutional coagulopathy, as described by Kheirabadi et al.9 All animals then underwent a volume-controlled hemorrhage of 20 mL/kg (approximately 30% of the total blood volume) at 100 mL/min, as described by Sondeen et al.10
Endovascular preparation
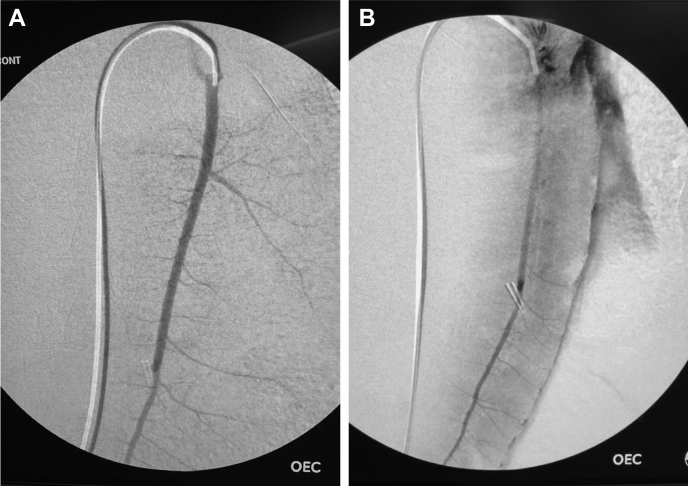
After completion of controlled hemorrhage, a flexible, angled, hydrophilic 0.035″ guidewire (Glidewire; Terumo, Elkton, Md) and a 4F angled hydrophilic catheter (Glidecath; Terumo) were used to select the splenic artery under fluoroscopic guidance (OEC 9900 Elite; GE, Chicago, Ill). Baseline digital subtraction angiography of the spleen was performed using a hand injection of contrast, and a flexible wire was left in the splenic artery while the catheter was withdrawn into the aorta to permit free antegrade splenic flow (Fig 3).
Fig 3.
Representative angiograms illustrating pretransection (A) and post-transection (B) appearance. Note extravasation along the splenic border and loss of arboreal appearance after transection. Reprinted with permission from original publication.6
Splenic injury and embolization
After marking of the longitudinal midline of the spleen, the organ sharply and completely transected 1 cm lateral to the midline, as described by Sondeen et al11 (Fig 2). Free splenic hemorrhage was allowed for 5 minutes; then the catheter was advanced into the proximal splenic artery over the wire for angiography and treatment (Fig 3). Shed blood from the splenic injury was continuously suctioned and measured. In control animals (n = 3 noncoagulopathic, n = 3 coagulopathic), no treatment was performed. In the treatment groups (n = 5 each), one of three endovascular embolization treatments was performed through the catheter. Smaller control groups were used to minimize the use of animals while ensuring a predictable response to hemorrhage and coagulopathy given previous experimental experience.6
-
•
Gelatin foam slurry comprised 5 mL loosely packed noncompressed gelatin sponge (gelfoam) fragments of 2–5 mm (Surgifoam; Johnson & Johnson, New Brunswick, NJ) agitated into a slurry solution with 5 mL of dilute contrast (n = 5 each coagulopathic and noncoagulopathic animals) injected into the splenic artery. Volume was selected based on the amount required to fill the splenic artery as discovered during model development.
-
•
Three, 0.035″ 6 mm (diameter) by 7 cm (extended length) embolization coils (Nester; Cook Medical, Bloomington, Ind) were placed successively in the proximal splenic artery (n = 5 each). Size and number were selected based on angiographic measurements of the splenic artery during model development.
-
•
Up to 6 mL of hydrogel precursors were injected into the proximal splenic artery until reflux of contrast was noted proximal to the catheter tip (n = 5 each). Volume selected based on volume available in each vial as delivered. In consultation with product engineers, expected performance in the vasculature felt unlikely to require more volume than the intended gelfoam slurry amount (5 mL).
Resuscitation and monitoring
After immediate post-treatment angiography, the treatment catheter was withdrawn into the aorta and coagulopathic animals were warmed with forced-air warming blankets. Resuscitation with swine plasma infused at a rate of 50 mL/min to a total volume of 15 mL/kg was initiated. At the time of the study, forms of plasma were being fielded as resuscitation agents for use in battlefield hemorrhage. Plasma resuscitation was used to expand circulating volume and support blood pressure while providing some coagulation correction. Angiography was repeated 1 hour after splenic injury, and animals were monitored for 6 hours. Any ongoing blood loss from the spleen was collected and measured. Death was defined and animals were euthanized if the mean arterial pressure (MAP) remained less than 20 mm Hg and end-tidal CO2 remained less than 10 mm Hg for 5 minutes. At the completion of the 6-hour observation period, surviving animals were euthanized using a veterinary euthanasia solution. A single animal underwent splenectomy immediately before euthanasia and serial transverse sectioning of the spleen for gross inspection of the splenic artery and its contents (Fig 4).
Fig 4.
Top panel: gross photograph demonstrating hydrogel-thrombus (black asterisk) within the splenic artery (white arrow). Bottom panel: hydrogel-thrombus segments removed from the course of the splenic artery.
Data analysis
Data were analyzed with SPSS version 26 (International Business Machines, Armonk, NY). Because of the difference in experimental conditions resulting from the induced coagulopathy, statistical testing of survival and blood loss data from embolization treatments was compared within and not between coagulation states. The study was designed to test the treatment responses vs the appropriate control group. Mean differences in survival time and other relevant measures between treatment groups and corresponding control groups were determined with analysis of variance with multiple comparisons using appropriate post hoc tests. Data are presented as mean ± standard error of mean. Significance was determined at a P value of ≤.05. The primary outcome of the study was determined a priori to be post-treatment splenic blood loss.
Results
Hemodilution and experimental conditions
Hemodilution with hetastarch and external cooling resulted in significant physiologic derangement with coagulopathy, establishing an independent experimental condition in which to examine the embolization treatments. After hemodilution and controlled hemorrhage and persisting until the time of embolization treatment, significant decreases in core temperature, hematocrit, and platelet count were noted along with increases in prothrombin time and activated partial thromboplastin time among hemodiluted animals. These derangements resulted in increased pretreatment blood loss from the spleen (Table). The mean amount of hydrogel precursors injected per animal was 3.7 ± 0.48 mL (no coagulopathy 3.0 ± 0.54, coagulopathy 4.4 ± 0.68, P = .14).
Table.
Pretreatment physiologic measures between animals in the noncoagulopathic and coagulopathic states
| No coagulopathy | Coagulopathy | P | |
|---|---|---|---|
| Temperature (°C) | 38.2 ± 0.09 | 34.5 ± 0.06 | <.001 |
| Hematocrit (%) | 30.1 ± 0.6 | 14.9 ± 0.35 | <.001 |
| Platelets (×109/L) | 334.6 ± 14.8 | 144.3 ± 5.5 | <.001 |
| Prothrombin time (seconds) | 11.0 ± 0.29 | 13.4 ± 1.1 | <.001 |
| Partial thromboplastin time (seconds) | 15.4 ± 0.6 | 21.1 ± 4.7 | <.001 |
| Fibrinogen (mg/dL) | 245.5 ± 5 | 171.4 ± 13.6 | <.001 |
| Pretreatment blood loss (mL/kg) | 7.3 ± 0.69 | 11.6 ± 1.3 | .006 |
Blood pressure
All groups experienced an expected decrease in systemic MAP with the combination of controlled and splenic hemorrhage. Plasma resuscitation resulted in a modest MAP increase in all groups, but this was not continued or sustained in the absence of endovascular embolization treatment. Among the noncoagulopathic groups, all three treatments resulted in a significantly higher MAP than the control group by 30 minutes after treatment. In coagulopathic animals, only the hydrogel group experienced a MAP significantly greater than the control group by the 30-minute timepoint. No intergroup blood pressure differences were noted between individual treatments (Fig 5).
Fig 5.
Average group mean ± standard error of mean arterial blood pressure (MAP) at experimental timepoints in noncoagulopathic (left panel) and coagulopathic (right panel) study arms. N = 5 for each treatment group, and N = 3 for control groups. ∗P < .05 for each treatment group over the control group. ∗∗P < .05 for the hydrogel treatment group over the control group.
Survival
In the absence of splenic artery treatment, the model produced the anticipated 100% mortality in both control groups. All noncoagulopathic hydrogel-treated animals survived the entire 6-hour observation period, with one coil-treated and two gelfoam-treated animals dying before 180 minutes. In the coagulopathic groups, a single hydrogel-treated animal survived for 6 hours. Three coil-treated and all gelfoam-treated animals died within 3 hours (Fig 6). All three noncoagulopathic treatment groups experienced significantly longer mean survival times than the control group, and no survival time differences were noted between the treatments themselves. Among the coagulopathic treatment groups, only the hydrogel group had a significantly longer mean survival time than did the control group. The hydrogel group had longer mean survival than gelfoam, but not coils (Fig 7).
Fig 6.
Survival curves for the noncoagulopathic (left panel) and coagulopathic (right panel) study arms. N = 5 for each treatment group, and N = 3 for control groups. No coagulopathy P < .05 for all treatments vs control; coagulopathy P < .05 for hydrogel vs control and gelfoam.
Fig 7.
Mean group survival times (minutes) in the noncoagulopathic (left) and coagulopathic (right) study arms. N = 5 for each treatment group, and N = 3 for control groups. ∗P < .05 vs the corresponding control group. ∗∗P < .05 vs the hydrogel group.
Blood loss
Immediate post-treatment angiograms in all treatment groups revealed complete cessation of flow within the splenic artery and no extravasation (Fig 8). Similar results were observed when analyzing postembolization treatment blood loss. In noncoagulopathic conditions, all three treatments resulted in significantly reduced splenic bleeding than was seen in the control group. With coagulopathy, however, only hydrogel treatment produced a significant reduction in bleeding over controls. No significant intergroup differences were observed between individual treatments (Fig 9).
Fig 8.
Representative immediate post-treatment angiogram. Note lack of opacification of the splenic artery and collected, previously extravasated blood.
Fig 9.
Mean group post-treatment splenic blood loss (mL/kg) in the noncoagulopathic (left) and coagulopathic (right) study arms. N = 5 for each treatment group, and N = 3 for control groups. ∗P < .05 vs the corresponding control group.
Discussion
We demonstrated the effectiveness of a novel hydrogel TAE agent in controlling solid organ hemorrhage in a translational model of severe trauma. Under conditions of normal coagulation, the hydrogel's performance was similar to that of the most common agents used clinically, embolization coils, and gelfoam slurry. With hemodilution and cooling resulting in coagulopathy, however, the hydrogel outperformed these treatments with decreased hemorrhage and improved survival vs controls. The coagulopathy generated in this model is based on previous experimental work aiming to model trauma-induced coagulopathy in translational research.7,9,12 We have previously examined the effects of this coagulopathy on the effectiveness of embolization coils and gelfoam slurry and found that the physiologic derangement establishes a rigorous test for TAE agents in controlling hemorrhage.6
In previous work using this model, we found that the introduction of coagulopathy resulted in alterations of the performance characteristics of standard TAE agents. In the current experiment, coagulopathy had less of an effect on the hydrogel treatment than the standard treatments. This is most likely accounted for by the hydrogel's novel mechanism of action within the arterial system. When injected, the hydrogel liquid precursors rapidly polymerize, effectively creating a hydrogel-thrombus “cast” within the lumen of the target vessel as the hydrogel that swells slightly and ceases flow (Fig 8). Unlike coils and gelfoam that require the native coagulation process to generate hemostasis, the hydrogel-thrombus cast creates a zone of stasis independent of coagulation, likely explaining our observed results in the presence of coagulopathy.
Neither placement of coils within the proximal target vessel nor injection of gelfoam slurry into its downstream branches has emerged as the ideal catheter-based treatment for solid organ or pelvic hemorrhage.13,14 Proximal coil treatment has the potential advantage of definitively eliminating inflow, but at the cost of potential continued hemorrhage from collateral vessels. Distal slurry embolization results in tissue-level hemostasis at the potential cost of loss of target organ perfusion and function. The nature of the hydrogel system used in this study is such that the hydrogel polymerizes within the vascular system, resulting in a hybrid of these two techniques—large vessel occlusion with a liquid injection. The initially injected precursors travel with blood flow some distance within the target vessel before polymerizing, resulting in the “building” of an intravascular cast from distal to proximal. Polymerization occurs quickly (<2 seconds) and the hydrogel occupies the target vessel with the potential for sparing of smaller distal vessels and preserved collateral perfusion. The distal and proximal extents of the hydrogel-thrombus cast and the filling of branch vessels can be altered by the operator by modulating the location and rate of injection as well as the injection volume. This flexibility distinguishes the hydrogel embolic system from other existing embolization products and techniques. The liquid hydrogel precursors are delivered via coaxial independent lumens within the injection catheter. The polymerization process takes place gradually as the components mix while traveling downstream with the flow of blood. This mechanism eliminates the potential for hydrogel adherence to the catheter resulting in “plugging” with loss of vascular access.
Hemostasis within the splenic artery generated by the hydrogel-thrombus cast was tolerant of the increase in blood pressure observed with plasma resuscitation. This is in contrast to our previous findings6 in which increases in systemic blood pressure resulted in early rebleeding from the spleen after coil and gelfoam treatment in the presence of improving coagulopathy. The ability of the hydrogel to maintain hemostasis in the setting of normotension and coagulopathy and the precise controllability of the polymerization process with injection modulation have positive implications for the potential broader use of this system in a number of vascular beds.
Although not a permanent indwelling occlusive device like an occlusion plug or coils, the hydrogel-thrombus cast and target vessel occlusion do persist long enough to result in ischemia if all inflow to a target organ is treated. Over time, water sensitive linkages between the PEG molecules within the hydrogel are lysed such that the agent liquifies within approximately 6 months, releasing PEG and polyacrylic acid to be absorbed and cleared via renal filtration. The accompaniment of the hydrogel by conventional thrombus may result in permanent fibrotic occlusion of the target vessel, though long-term studies have not been performed to confirm this and the eventual fate of the hydrogel itself and the target vessel.
This study is limited in several important ways. First, the experimental conditions do not mimic the clinical situation perfectly, especially in the extracorporeal placement of the spleen. Such placement does not permit the intra-abdominal tamponade that may help stop splenic hemorrhage after blunt trauma. Extracorporeal placement did permit complete, uninterrupted shed blood collection during the postinjury period and presented a rigorous test of hemostatic agents. In addition, the nature of the resuscitation used here may not mimic that used in the resuscitation of a hemorrhaging patient. The use of plasma as a first-line resuscitation agent is a military casualty care standard, however, which is why it was chosen here. Experimental technique differed slightly between the hydrogel group, where only enough agent was given to occlude the target artery, and the coil and gelfoam treatment groups, in which the number and volume of treatment was standardized. This is a potential source of experimental bias, though it likely biases the results to favor the more aggressive treatment in the coil and gelfoam groups. The clinical applicability of these data may be limited by the use of only female animals, which, while standardizing experimental conditions, may have impacted the range of physiologic responses observed. Finally, we do not have histologic or other specific data on the in vivo or in vitro behavior of the hydrogel to explain its performance in this model. Such data should be included in future translational work using this compound.
Conclusions
Using a translational model of solid organ hemorrhage with and without dilutional coagulopathy, we have demonstrated the effectiveness of the transcatheter arterial injection of a novel hydrogel compound in providing hemostasis. The hydrogel compound performed at least as well as the current standard TAE agents, coils, and gelfoam slurry under standard coagulation conditions, and outperformed them in the face of significant coagulopathy. Further investigation of this promising embolization system in various translational and clinical scenarios is warranted.
Author contributions
Conception and design: DK, IP, MP, BK, MD
Analysis and interpretation: DK. MD
Data collection: DK, IP, RDG, MP, AV
Writing the article: DK
Critical revision of the article: DK, IP, RDG, MP, AV, BK, MD
Final approval of the article: DK, IP, RDG, MP, AV, BK, MD
Statistical analysis: DK
Obtained funding: DK, MD
Overall responsibility: DK
Footnotes
The hydrogel used in this study was provided to the United States Army Institute of Surgical Research by Instylla, Inc under a Cooperative Research and Development Agreement. Instylla, Inc was not involved in the design of the experiments, their execution, or in the analysis of the data. This study was solely funded by the United States Army Medical Research and Development Command.
This publication is dedicated to the memory of Michael A. Dubick, PhD, whose decades of scientific contribution saved lives on the battlefield and beyond.
Author conflict of interest: none. The opinions and assertions contained herein are solely those of the authors and do not represent those of the U.S. Army or Department of Defense.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Cinquantini F., Simonini E., Di Saverio S., Cecchelli C., Kwan S.H., Ponti F. Non-surgical management of blunt splenic trauma: a comparative analysis of non-operative management and splenic artery embolization—experience from a European trauma center. Cardiovasc Intervent Radiol. 2018;41:1324–1332. doi: 10.1007/s00270-018-1953-9. [DOI] [PubMed] [Google Scholar]
- 2.Cimbanassi S., Chiara O., Leppaniemi A., Henry S., Scalea T.M., Shanmuganathan K. Nonoperative management of abdominal solid-organ injuries following blunt trauma in adults: results from an International Consensus Conference. J Trauma Acute Care Surg. 2018;84:517–531. doi: 10.1097/TA.0000000000001774. [DOI] [PubMed] [Google Scholar]
- 3.Stassen N.A., Bhullar I., Cheng J.D., Crandall M.L., Friese R.S., Guillamondegui O.D. Selective nonoperative management of blunt splenic injury: an eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(Suppl 4):S294–S300. doi: 10.1097/TA.0b013e3182702afc. [DOI] [PubMed] [Google Scholar]
- 4.Chang R., Fox E.E., Greene T.J., Eastridge B.J., Gilani R., Chung K.K. Multicenter retrospective study of noncompressible torso hemorrhage: anatomic locations of bleeding and comparison of endovascular versus open approach. J Trauma Acute Care Surg. 2017;83:11–18. doi: 10.1097/TA.0000000000001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rong J.J., Liu D., Liang M., Wang Q.H., Sun J.Y., Zhang Q.Y. The impacts of different embolization techniques on splenic artery embolization for blunt splenic injury: a systematic review and meta-analysis. Mil Med Res. 2017;4:1–12. doi: 10.1186/s40779-017-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauvar D.S., Schechtman D.W., Thomas S.B., Polykratis I.A., De Guzman R., Prince M.D. Endovascular embolization techniques in a novel swine model of fatal uncontrolled solid organ hemorrhage and coagulopathy. Ann Vasc Surg. 2021;70:143–151. doi: 10.1016/j.avsg.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Parr M.J., Bouillon B., Brohi K., Dutton R.P., Hauser C.J., Hess J.R. Traumatic coagulopathy: Where are the good experimental models? J Trauma. 2008;65:766–771. doi: 10.1097/TA.0b013e31818606d2. [DOI] [PubMed] [Google Scholar]
- 8.Hess J.R., Brohi K., Dutton R.P., Hauser C.J., Holcomb J.B., Kluger Y. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 9.Kheirabadi B.S., Mace J.E., Terrazas I.B., Fedyk C.G., Valdez K.K., MacPhee M.J. Clot-inducing minerals versus plasma protein dressing for topical treatment of external bleeding in the presence of coagulopathy. J Trauma. 2010;69:1062–1072. doi: 10.1097/TA.0b013e3181fa0f21. [DOI] [PubMed] [Google Scholar]
- 10.Sondeen J.L., Dubick M.A., Holcomb J.B., Wade C.E. Uncontrolled hemorrhage differs from volume- or pressure-matched controlled hemorrhage in swine. Shock. 2007;28:426–433. doi: 10.1097/shk.0b013e31804a5791. [DOI] [PubMed] [Google Scholar]
- 11.Sondeen J.L., Prince M.D., Kheirabadi B.S., Wade C.E., Polykratis I.A., De Guzman R. Initial resuscitation with plasma and other blood components reduced bleeding compared to hetastarch in anesthetized swine with uncontrolled splenic hemorrhage. Transfusion. 2011;51:779–792. doi: 10.1111/j.1537-2995.2010.02928.x. [DOI] [PubMed] [Google Scholar]
- 12.Kheirabadi B.S., Crissey J.M., Deguzman R., Perez M.R., Cox A.B., Dubick M.A. Effects of synthetic versus natural colloid resuscitation on inducing dilutional coagulopathy and increasing hemorrhage in rabbits. J Trauma. 2008;64:1218–1228. doi: 10.1097/TA.0b013e31816c5c6c. [DOI] [PubMed] [Google Scholar]
- 13.Hymel A., Asturias S., Zhao F., Bliss R., Moran T., Marshall R.H. Selective versus nonselective embolization versus no embolization in pelvic trauma. J Trauma Acute Care Surg. 2017;83:361–367. doi: 10.1097/TA.0000000000001554. [DOI] [PubMed] [Google Scholar]
- 14.Rasuli P., Moosavi B., French G.J., Petrcich W., Hammond I. Splenic artery embolization in blunt trauma: a single-center retrospective comparison of the use of gelatin sponge versus coils. Am J Roentgenol. 2017;209:W382–W387. doi: 10.2214/AJR.17.18005. [DOI] [PubMed] [Google Scholar]