Abstract
Background
Peripheral arterial disease (PAD) affects more than 150 million people worldwide and is associated with high rates of lower extremity amputation, myocardial infarction, stroke and death. Fatty acid binding protein 3 (FABP3) is released into circulation in patients with skeletal muscle injury. In this pilot study, we investigated a possible association between PAD and blood levels of FABP3.
Methods
Blood samples were collected from patients with clinical symptoms and diagnostic findings indicative of PAD (PAD group; ankle-brachial index [ABI] <0.9; n = 75) and in those without clinical or diagnostic features of PAD (non-PAD group; ABI >0.9; n = 75) presenting to vascular surgery ambulatory clinics at St. Michael's Hospital. Plasma samples were analyzed by protein multiplex to quantify FABP3 levels.
Results
PAD patients were found to have higher blood levels of FABP3 compared to patients without PAD (mean 3.90 ± 1.69 vs 2.03 ± 0.78; P < .001). A subgroup analysis demonstrated that the FABP3 levels were increased by almost two-fold in patients with PAD, independent of coronary artery disease (P < .001) or diabetes mellitus status (P < .001). Moreover, a significant negative correlation between FABP3 and the ABI was observed in PAD and patients without PAD matched groups (r = –0.51; P = .001). Last, immunohistochemistry demonstrated elevated expressions of FABP3 within skeletal muscle obtained from patients with the most severe form of PAD, chronic limb-threatening ischemia, when compared with patients without PAD.
Conclusions
Patients with PAD have elevated plasma levels of FABP3. An increasing severity of PAD is associated with higher FABP3 levels.
Keywords: Peripheral arterial disease, Biomarker, Fatty acid binding protein, Association
Clinical Relevance
There is a pressing need for a simple, readily accessible, blood-based biomarker for PAD. In this study, we found elevated levels of FABP3 in patients with PAD. This increase in FABP3 was irrespective of history of coronary artery disease or diabetes. Furthermore, our data suggest that an increasing severity of PAD is associated with higher FABP3 levels. Subsequently, FABP3 may be a potential diagnostic biomarker for PAD. However, further studies are needed to confirm the capability of FABP3 to serve as a valid and reliable biomarker for PAD.
Article Highlights.
-
•
Type of Research: Single-center, prospective case-control study
-
•
Key Findings: Patients with peripheral arterial disease (PAD) were found to have higher blood levels of fatty acid binding protein 3 (FABP3) compared with patients without PAD (3.90 ± 1.69 vs 2.03 ± 0.78). A significant negative correlation between FABP3 and ankle-brachial index was also observed in patients with PAD and patients without PAD matched groups (r = –0.51)
-
•
Take Home Message: Patients with PAD have elevated plasma levels of FABP3. Increasing severity of PAD is associated with higher FABP3 levels.
Lower extremity peripheral arterial disease (PAD) is the third most common presentation of atherosclerosis.1,2 More than 50% of patients with PAD are asymptomatic, and the remaining patients typically present with symptoms ranging from calf claudication to tissue loss.3 The wide spectrum of clinical presentations pose a challenge for PAD diagnosis, with community studies suggesting that physicians fail to diagnose PAD in about one-half of patients.4,5 This lack of awareness of the presence of PAD results in the failure of medical therapy initiation and atherosclerosis risk factor optimization, as well as deferred referral to specialists.6 It may also lead to a delay in surgical intervention, which puts patients at significant risk of morbidity, including major limb amputation, and mortality.7
Physicians rely on clinical examination and diagnostic tools such as the ankle-brachial index (ABI) to diagnose patients with PAD. Although the ABI serves as a sensitive and cost-effective screening tool for PAD,8,9 primary health care providers report facing several barriers with using the ABI.10,11 Some of these barriers include time constraints, insufficient staff, limited equipment availability, lack of adequate training or availability of skilled personals, and, in some cases, reimbursement.12 Furthermore, ABI values of patients may sometimes be falsely elevated owing to incompressible calcified vessels in diseases such as with diabetes with medial calcification of tibial vessels, systemic sclerosis, or rheumatic diseases.13
Fatty acid binding protein 3 (FABP3) belongs to a family of multigene fatty acid binding proteins that is primarily expressed in skeletal and cardiac muscle.14, 15, 16 A small intracellular protein involved in fatty acid transfer and metabolism, FABP3 is released into the circulation when there is muscle injury.14, 15, 16, 17, 18, 19, 20 Furthermore, we previously tested the expression of several cardiac markers in patients with PAD and found a positive correlation between FABP3 and patients with symptomatic PAD.21 Therefore, we hypothesized that FABP3 levels are elevated in patients with PAD and might therefore be used as an aid to early diagnosis.
Methods
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the research ethics board at St. Michael's Hospital–University of Toronto in Ontario, Canada. Informed consent (in written) was obtained from all participants.
Patient selection
For the purposes of this pilot study, the first encountered 75 patients with PAD and 75 patients without PAD presenting to ambulatory vascular surgery clinics at St. Michael's Hospital between October 2017 and February 2018 were recruited. The diagnosis of PAD was established by an independent expert using clinical history, physical examination results, and lower limb arterial imaging as per the Rutherford Classification Criteria of chronic limb ischemia. The PAD cohort included patients of all severity levels ranging from claudication to chronic limb-threatening ischemia (CLTI). The non-PAD control cohort did not have a clinical history of claudication, had palpable distal pulses, and a normal arterial ultrasound examination of the lower limbs. We excluded patients (1) on anticoagulants, chemotherapy, or biological anti-inflammatory agents, (2) diagnosed with acute stroke or transient ischemic attack within 3 months, (3) with renal disease (stages 3, 4, and 5) according to the Kidney Disease Outcomes Quality Initiative clinical guidelines, and (4) with acute or a 3-month history of acute coronary syndrome as defined by American College of Cardiology or elevated troponins.
Baseline measurements
Recorded medical history included details of cardiovascular risk factors; prior coronary, cerebral, or peripheral artery disease; congestive heart failure; or cardiac arrhythmia as defined by American College of Cardiology. Hyperlipidemia was defined as total cholesterol of more than 5.2 mmol/L or triglyceride of greater than 1.7 mmol/L or the use of antihyperlipidemic medication. Hypertension was defined as a systolic blood pressure of 130 mm Hg or greater or a diastolic pressure of 80 mm Hg or greater, or the use of an antihypertensive medication. Diabetes mellitus was defined as a glycosylated hemoglobin A1c of 6.5% or greater or the use of antidiabetic medication. Smoking status was recorded for each patient.
Each patient underwent lower limb arterial imaging with ultrasound examination, ABI, or toe-brachial index (TBI). Blood samples were drawn into vacutainer tubes containing EDTA. After centrifugation at 3000 rpm for 10 minutes (4°C), plasma was aliquoted and stored at −80°C.
FABP3 multiplex assay
Plasma samples were analyzed in duplicate using MILLIPLEX MAP Human Cardiovascular Disease (CVD) Magnetic Bead Panel 1 (EMD-Millipore; Billerica, Mass) to determine the concentrations of FABP3. All sample analyses were completed on the same day to eliminate inter-assay variability. Sample intra-assay and inter-assay coefficient of variation were both less than 10%. The MagPix analyzer (Luminex Corp; Austin, Tex) was calibrated prior to analysis using Fluidics Verification and Calibration bead kits (Luminex Corp). A minimum of 50 beads for each targeted biomarker were acquired using Luminex xPonent software and analyzed using Milliplex Analyst software (v.5.1; EMD-Millipore).
Sample processing
Muscle biopsies collected from patients were processed immediately. Each specimen was divided into two pieces. The first muscle piece was fixed in formalin and further processed into paraffin-embedded blocks for immunohistochemistry purposes, whereas the second muscle piece was frozen immediately in liquid nitrogen for Western blot studies.
Immunohistochemistry of FABP3 biomarkers
Three sections of 5 μm thickness were cut from each paraffin block. Medial head gastrocnemius muscle biopsies were obtained from three patients with CLTI undergoing major limb amputation (experimental group) and three patients without PAD undergoing elective surgery to manage varicose veins (negative control). As a positive control, left atrial appendages were obtained from three patients undergoing open heart surgery for acute coronary syndrome. Thereafter, all slides were evaluated with standard protocols for staining with hematoxylin and eosin (Sigma, St. Louis, Mo). For detection of FABP3, sections were stained using antihuman mouse polyclonal FABP3 antibody (Thermo Fisher Scientific, Waltham, Mass) and then incubated again with horseradish peroxidase-conjugated secondary antibody according to the standard immunostaining procedure.
Western blot
All snap frozen muscle samples were homogenized in a lysis buffer (Cell Signaling Technology, Beverly, Mass) using a ultrasonic homogenizer (Biologics Inc, Manassas, Va). Protein concentration was measured in duplicate by the bicinchoninic acid assay (Pierce, Rockford, Ill). In these studies, aliquots equivalent of approximately 40 μg of protein were separated on gradient (10%-15%) polyacrylamide sodium dodecyl sulphate gels. After electrophoresis, proteins were electrotransferred to a nitrocellulose membrane. The membrane was blocked with skimmed milk powder in TBST (0.05% Tween 20, 100 mmol/L NaCl, 10 mmol/L Tris-HCl pH 7.8) for 30 minutes and then incubated with the primary antibody FABP3 (Abcam, Toronto, ON, Canada) overnight at 4°C. Secondary antibody polyclonal goat antimouse horseradish peroxidase conjugated (Cedarlane, Burlington, ON, Canada) was added. Blots were developed in ECL detection reagents (Amersham [Little Chalfont, UK], ECL Western Blotting Detection Reagents; GE Healthcare [Boston, Mass]) and the chemiluminescence emitted from immune complexes was visualized with a ChemiDoc image system (Bio-Rad, Mississauga, ON, Canada). Images were quantified using Image J software.
Statistical methods
Continuous variables were summarized using means and standard deviation. Categorical variables were summarized as number and proportion. Baseline characteristics were compared between the groups with and without PAD using independent t-tests or the Mann-Whitney U test for continuous variables. Fisher's exact test or χ2 test was used for categorical variables. The Kruskal-Wallis test was used to determine whether there were any significant differences between more than two independent groups. Furthermore, using propensity score adjustment method, patients with and without PAD were matched based on age, coronary artery disease, stroke, smoking history, and diabetes. Using the matched groups, correlation analysis between FABP3 and ABI was performed. SPSS software, version 23 (SPSS Inc., Chicago, Ill) was used for data entry and analysis. All analyses were carried out at a 5% two-sided significance level.
Results
Cohort description
The PAD cohort (n = 75) was mainly composed of males who had a higher prevalence of cardiovascular risk factors compared with the non-PAD patients (n = 75; Table I). Thus, patients with PAD were older; more often had hypertension, hypercholesterolemia, diabetes, smoking, and a history of coronary arterial disease (CAD); and had lower ABI values.
Table I.
Patient demographics and clinical characteristics
| No PAD (n = 75) | PAD (n = 75) | P valuea | |
|---|---|---|---|
| ABIb | 1.1 ± 0.1 | 0.6 ± 0.2 | <.001 |
| Ageb | 52.0 ± 17.2 | 70.1 ± 9.9 | <.001 |
| Sex (male)c | 44 (59) | 53 (71) | .22 |
| Hypertensionc | 28 (38) | 60 (83) | <.001 |
| Hypercholesterolemiac | 26 (35) | 58 (80) | <.001 |
| Diabetesc | 17 (23) | 41 (55) | <.001 |
| Smoking historyc | 33 (45) | 58 (80) | <.001 |
| Coronary artery diseasec | 18 (25) | 33 (45) | .01 |
| Stroke/TIAc | 2 (3) | 11 (16) | .01 |
| Medicationc | |||
| Statin | 23 (32) | 66 (90) | <.001 |
| ACEi/ARB | 18 (25) | 48 (68) | <.001 |
| Beta-blocker | 12 (17) | 26 (37) | .23 |
| CCB | 14 (20) | 24 (34) | .07 |
| Insulin | 1 (1) | 13 (18) | .01 |
| Oral hypoglycemia | 10 (14) | 34 (46) | <.001 |
| ASA | 18 (24) | 43 (57) | <.001 |
ABI, Ankle-brachial index; ACEi, angiotensin-converting-enzyme inhibitors; ARB, angiotensin II receptor blocker; ASA, aspirin; CCB, calcium channel blockers; PAD, peripheral arterial disease;bTIA, transient ischemic attack.
Values are means ± standard deviations (calculated for continuous variables) or number (%). All numbers were rounded to one decimal place.
The significance of the difference between groups with or without PAD.
Differences between groups were compared using the Mann-Whitney U test.
Differences between groups were compared using the χ2 test.
FABP3 levels increase in patients with PAD
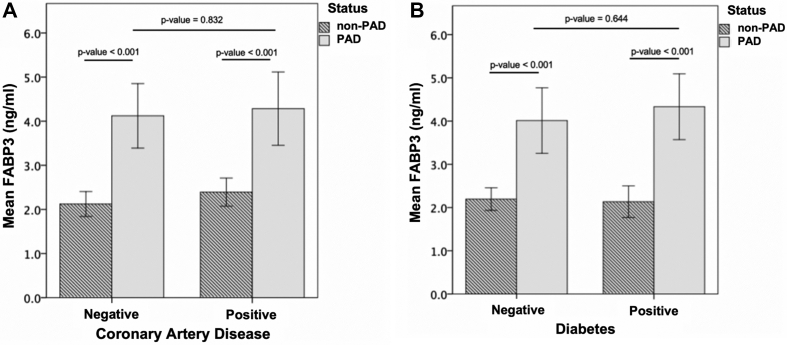
Patients with PAD had significantly higher blood levels of FABP3 compared with patients without PAD (mean 3.90 ± 1.67 vs mean 2.27 ± 0.32, respectively; P < .0001). Furthermore, since FABP3 has been previously suspected to be associated with diabetes mellitus and CAD, we investigated the association between FABP3 levels in PAD and patients without PAD with and without these cardiovascular risk factors.22 As shown in Fig 1, A, patients with PAD had higher levels of FABP3 than those without PAD irrespective of whether they had a history of CAD (mean, 4.15 ng/mL ± 1.83 vs mean, 2.40 ng/mL ± 0.61) or no history of CAD (mean, 4.08 ng/mL ± 1.77 vs mean, 2.11 ng/mL ± 0.72) (P for interaction = .832). A similar pattern was evident for PAD compared with patients without PAD who had diabetes (mean, 4.22 ng/mL ± 1.12 vs mean, 2.28 ng/mL ± 0.66) and in those with no diabetes (mean, 4.00 ng/mL ± 0.98 vs mean, 2.15 ng/mL ± 0.72) (P for interaction = .644; Fig 1, B).
Fig 1.
Fatty acid binding protein 3 (FABP3) levels were higher in patients with peripheral arterial disease (PAD) regardless of prior history of coronary arterial disease (CAD) or diabetes mellitus. A, FABP3 levels in patients with and without PAD with and without a prior medical history of CAD. B, FABP3 levels in patients with and without PAD with and without a prior medical history of diabetes. Significant increase in FABP3 levels was noted in patients with PAD regardless of previous history of CAD or diabetes (P < .001). Significant differences were calculated using Mann-Whitney U test.
FABP3 levels in increased severity of PAD disease and its association with ABI
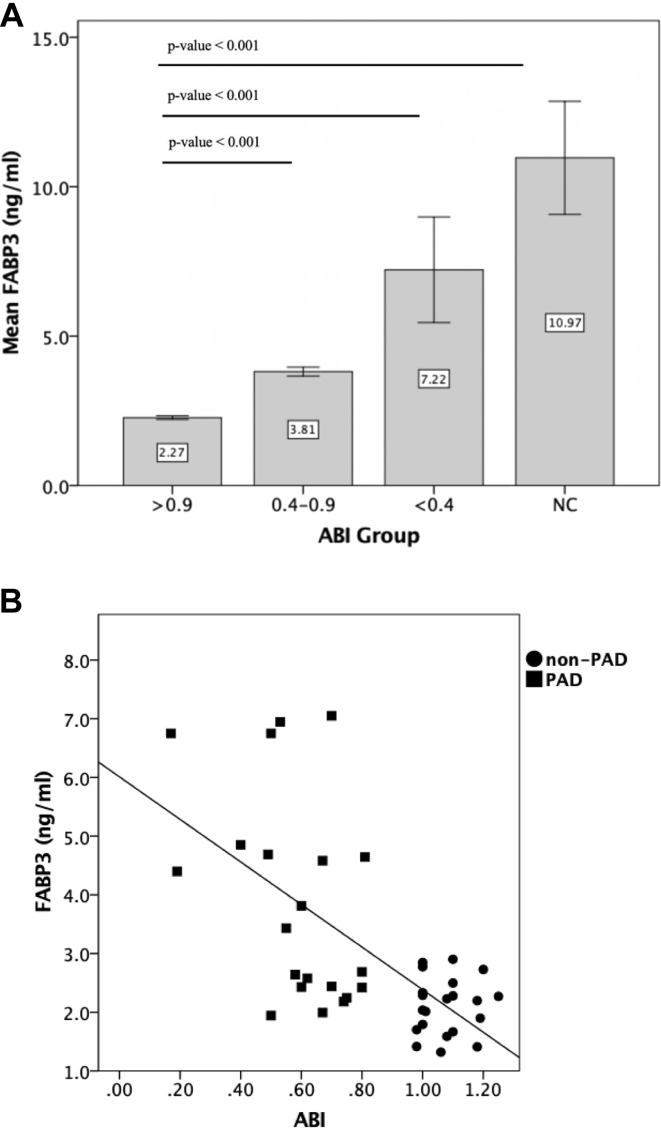
The relationship between worsening PAD severity and FABP3 was also investigated. PAD severity was stratified based on the ABI values into (1) control patients without PAD (1.3 ≤ ABI ≤ 0.9), (2) mild to moderate PAD (0.9 < ABI < 0.4), (3) severe PAD (ABI ≤ 0.40), and (4) patients with noncompressible (NC) tibial vessels (ABI > 1.3). In the NC group, the PAD severity was based on the TBI (n = 5). A TBI value of less than 0.67 was considered abnormal. All of patients in the NC group had a TBI of less than 0.67 with a mean TBI of 0.3. When compared with control patients without PAD, FABP3 levels were higher with increasing severity of PAD (Fig 2, A). FABP3 levels were highest in the NC group, followed by severe PAD, mild to moderate PAD, and control patients without PAD (P for trend < .001).
Fig 2.
A, Fatty acid binding protein 3 (FABP3) levels positively correlated with the severity of peripheral arterial disease (PAD). A bar chart representing mean values with confidence intervals for FABP3 levels in control patients without PAD (n = 75; 1.3 ≤ ankle-brachial index [ABI] ≤ 0.9), patients with PAD (n = 70; ABI <0.9), and non-compressible tibial vessels (n = 5; noncompressible; ABI ≥ 1.3). Levels of FABP3 demonstrated an increase in FABP3 levels as severity of PAD worsen. B, The relationship between values for ABI and FABP3. Levels of FABP3 were compared between patients with PAD (n = 21; squares; ABI < 0.9) and patients without PAD (n = 21; circles; 1.3 ≤ ABI ≤ 0.9) after matching for age, sex, and risk factors (age, sex, coronary artery disease, hypercholesteremia, hypertension, smoking history, and diabetes). FABP3 values were inversely correlated to the ABI using spearmen correlation (r = –0.51; P = .001).
We also performed a correlation study between FABP3 levels and ABI (which reflects PAD status), among matched patients with PAD (n = 35; ABI <0.9) and patients without PAD (n = 35; ABI ≥ 0.9; Table II). As expected, there were no significant differences between non-PAD and PAD groups in the measured risk factors (age, sex, coronary artery disease, hypercholesteremia, hypertension, smoking history, and diabetes) except for ABI. Relative to patients without PAD, FABP3 levels were significantly higher in patients with PAD (PAD, 3.9 ± 1.6 ng/mL; non-PAD, 2.2 ± 0.6 ng/mL; P < .001). After applying spearmen's correlation test on this matched cohort, the plasma FABP3 levels were significantly inversely correlated with ABI (r = –0.51; P = .001; Fig 2, B).
Table II.
Patient demographics and clinical characteristics for the matched groups with and without peripheral arterial disease (PAD)
| No PAD (n = 21) | PAD (n = 21) | P value | |
|---|---|---|---|
| ABIa | 1.1 ± 0.1 | 0.6 ± 0.2 | <.001 |
| Agea | 69 ± 8.3 | 70 ± 10.2 | .95 |
| Sex (male)b | 17 (81) | 12 (57) | .09 |
| Hypertensionb | 13 (62) | 16 (76) | .32 |
| Hypercholesterolemiab | 17 (81) | 11 (52) | .05 |
| Diabetesb | 7 (33) | 10 (48) | .35 |
| Smoking historyb | 16 (76) | 14 (67) | .50 |
| Coronary artery diseaseb | 13 (62) | 13 (52) | .53 |
| Stroke/TIAb | 1 (5) | 4 (19) | .15 |
ABI, Ankle-brachial index; TIA, transient ischemic attack.
Values are mean ± standard deviations (calculated for continuous variables) or number (%; calculated for categorical variables); all numbers were rounded to one decimal place.
Differences between groups were compared using the Mann-Whitney U test.
Differences between groups were compared using χ2 test.
Localization of FABP3 within lower limb skeletal muscles
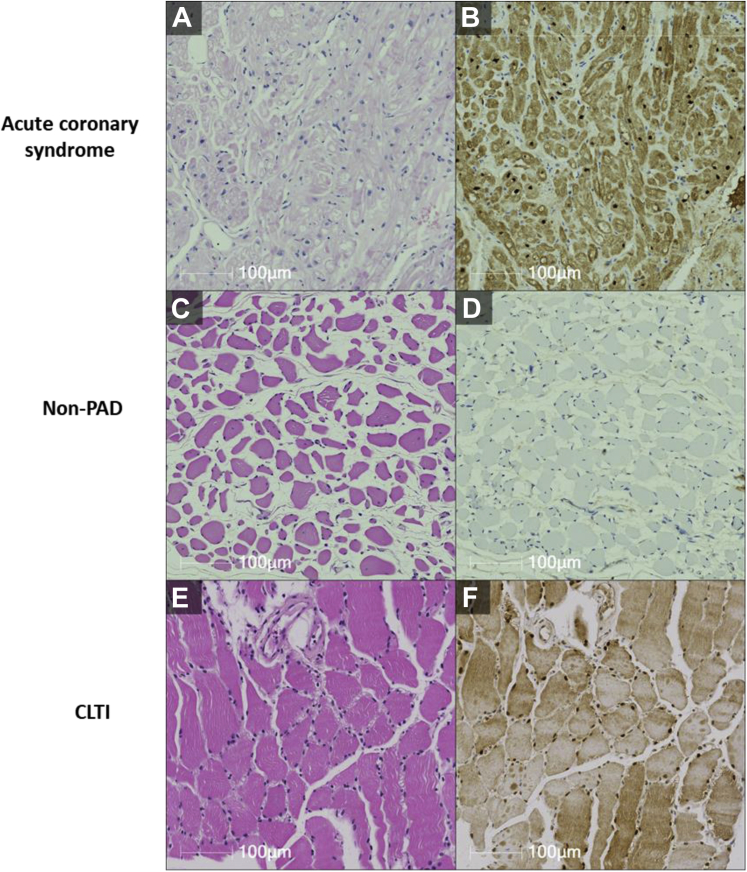
Muscle biopsies were obtained from three patients with CLTI undergoing major limb amputation, three patients without PAD undergoing elective lower limb surgery to manage varicose veins (negative control), and three patients undergoing open heart surgery for acute coronary syndrome (positive control). Our results demonstrated higher levels of FABP3 in patients with CLTI as compared with control patients without PAD (Fig 3). Using Western blot studies, FABP3 expression was investigated in gastrocnemius muscle obtained from the control group without PAD and the experimental CLTI patient group. A quantitative analysis revealed a significant increase in the expression of FABP3 in muscle tissue obtained from patients with CLTI, when compared with control patients without PAD (Fig 4). This finding is very interesting, because this experiment demonstrates an increase in FABP3 protein expression within muscle biopsies obtained from patients with CLTI.
Fig 3.
Fatty acid binding protein 3 (FABP3) is expressed in the skeletal muscles of patients with peripheral arterial disease (PAD). Hematoxylin and eosin staining (A, C, and E) and FABP3 staining (B, D, and F) were used to assess cellular histology and localization of FABP3 in cardiac myocytes obtained from patients with acute coronary syndrome (B) as well as skeletal muscles obtained from patients without PAD (D) and patients with chronic limb-threatening ischemia (CLTI) (F).
Fig 4.
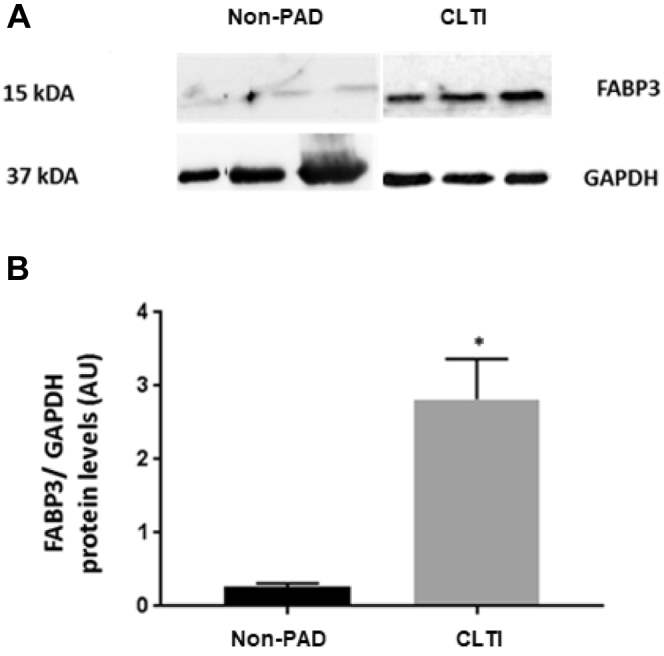
Increased fatty acid binding protein 3 (FABP3) expression levels in the skeletal muscles of patients with chronic limb-threatening ischemia (CLTI) in comparison with control patients without peripheral arterial disease (PAD). A, Western blot demonstrating FABP3 and GAPDH expressions in the skeletal muscle of control patients without PAD (n = 3) and patients with CLTI (n = 3). B, The histogram shows a quantitative representation of the levels of protein obtained from a densitometry analysis. Each value represents the mean ± standard error of the mean. A significant difference of comparison was determined by t-test as indicated by asterisk. P < .05 vs control skeletal muscles. AU, Absolute units.
Discussion
In this pilot study, we found that patients with PAD had significantly higher blood levels of FABP3 than those without PAD. This association was evident irrespective of whether patients had a history of CAD or diabetes. Furthermore, FABP3 levels correlated with disease severity, with higher levels in those with more advanced PAD. We also found that muscle biopsies obtained from patients with CLTI compared with control patients without PAD had higher FABP3 protein expression.
FABP3, also known as heart-type FABP, belongs to a family of multi-gene fatty acid binding proteins.23 FABP3 is primarily expressed in skeletal and cardiac muscle, where it constitutes 4% to 5% of all cellular proteins, but it is also found in the brain.15,16 Because of the abundance of FABP3 in the myocardium and its rapid release into the circulation after the onset of cardiomyocyte damage, FABP3 has been used as a biomarker of acute coronary artery syndrome.14 Recently, FABP3 has also been shown to be potentially linked to the progression of atherosclerosis owing to its contributing role in endothelial dysfunction.24 Within muscle cells, FABP3 is found mainly within the cytosol,25 where it mediates the uptake of intracellular fatty acids as well as their transport to the mitochondrial β-oxidation system.26 During physical exercise, there is an increased demand of fatty acid use,27 and this demand results in increased in FABP3 expression.28 Prior studies have reported the release of FABP3 from skeletal muscle in patients with myopathy, after exercise or skeletal muscle injury.17, 18, 19, 20
Patients with PAD are considered to have an acquired form of myopathy caused by repetitive tissue ischemia and reperfusion29, 30, 31, 32 that is characterized by alterations in the expression of mitochondrial enzymes, deregulation of mitochondrial respiration, increased mitochondrial oxidative stress, and muscle apoptosis.33, 34, 35 This process can potentially result in the release of FABP3 into circulation; however, additional studies are needed to confirm this finding. Our findings of elevated FABP3 blood levels in patients with PAD, and of a correlation between FABP3 levels and severity of PAD disease, are consistent with previously published studies demonstrating FABP3 release from patients with myopathy.36,37 Thus, our data suggest that FABP3 is released into plasma at increased levels with worsening PAD disease status. However, further studies are needed to confirm this hypothesis.
Our pilot study has some limitations. First, we included only a modest number of patients, which limited the power to adjust for confounders. Second, we measured FABP3 at only one time point and thus we are unaware of whether blood levels change over time. Third, we excluded patients with acute coronary ischemia and those with moderate to severe kidney disease. Fourth, we are uncertain whether FABP3 is associated with PAD after treatment or independent of treatment.
In conclusion, we have demonstrated an association between circulating blood levels of FABP3 and the presence and severity of PAD. Although the findings are robust and the association seems to be independent of other factors that may elevate blood levels of FABP3 within this patient population, they require replication in larger cohorts that include a broader range of patients with PAD and with other comorbidities. If our findings are confirmed, FABP3 may be a valuable biomarker to aid primary care physicians in screening patients for PAD, evaluating disease severity, and in making decisions regarding the initiation of medical therapy, thereby helping to further reduce the burden of morbidity and mortality in this undertreated population.
Author contributions
Conception and design: RA, MQ
Analysis and interpretation: MS, AZ, KS, TF, OR, RA, JE, MQ
Data collection: HK, MQ
Writing the article: MS, RA, MQ
Critical revision of the article: AZ, HK, KS, TF, OR, RA, JE, MQ
Final approval of the article: MS, AZ, HK, KS, TF, OR, RA, JE, MQ
Statistical analysis: AZ, RA, MQ
Obtained funding: MQ
Overall responsibility: MQ
Acknowledgments
The authors thank Dr Michael Conte for generously reviewing this manuscript.
Footnotes
Funded by the Blair Foundation.
Author conflict of interest: J.E. reports consulting fees/honoraria and/or grant support from Astra-Zeneca, Bayer Boehringer-Ingelheim, Bristol-Myer-Squibb/Pfizer, Daiichi-Sankyo, Eli-Lilly, GlaxoSmithKline, Pfizer, Janssen, and sanofi-aventis, Servier. The remaining authors have no competing interests.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Fowkes F.G., Rudan D., Rudan I., Aboyans V., Denenberg J.O., McDermott M.M. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Dhaliwal G., Mukherjee D. Peripheral arterial disease: epidemiology, natural history, diagnosis and treatment. Int J Angiol. 2007;16:36–44. doi: 10.1055/s-0031-1278244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weitz J.I., Byrne J., Clagett G.P., Farkouh M.E., Porter J.M., Sackett D.L. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch A.T., Criqui M.H., Treat-Jacobson D., Regensteiner J.G., Creager M.A., Olin J.W. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 5.Conte S.M., Vale P.R. Peripheral arterial disease. Heart Lung Circ. 2018;27:427–432. doi: 10.1016/j.hlc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Lovell M., Harris K., Forbes T., Twillman G., Abramson B., Criqui M.H. Peripheral arterial disease: lack of awareness in Canada. Can J Cardiol. 2009;25:39–45. doi: 10.1016/s0828-282x(09)70021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker C.M., Bunch F.T., Cavros N.G., Dippel E.J. Multidisciplinary approach to the diagnosis and management of patients with peripheral arterial disease. Clin Interv Aging. 2015;10:1147–1153. doi: 10.2147/CIA.S79355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alahdab F., Wang A.T., Elraiyah T.A., Malgor R.D., Rizvi A.Z., Lane M.A. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg. 2015;61(3 Suppl):42S–53S. doi: 10.1016/j.jvs.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Crawford F., Welch K., Andras A., Chappell F.M. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev. 2016;9:CD010680. doi: 10.1002/14651858.CD010680.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies J.H., Kenkre J., Williams E.M. Current utility of the ankle-brachial index (ABI) in general practice: implications for its use in cardiovascular disease screening. BMC Fam Pract. 2014;15:69. doi: 10.1186/1471-2296-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolai S.P., Kruidenier L.M., Rouwet E.V., Bartelink M.L., Prins M.H., Teijink J.A. Ankle brachial index measurement in primary care: are we doing it right? Br J Gen Pract. 2009;59:422–427. doi: 10.3399/bjgp09X420932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hageman D., Pesser N., Gommans L.N.M., Willigendael E.M., van Sambeek M., Huijbers E. Limited adherence to peripheral arterial disease guidelines and suboptimal ankle brachial index reliability in Dutch primary care. Eur J Vasc Endovasc Surg. 2018;55:867–873. doi: 10.1016/j.ejvs.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 14.O'Donoghue M., de Lemos J.A., Morrow D.A., Murphy S.A., Buros J.L., Cannon C.P. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 15.Smathers R.L., Petersen D.R. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics. 2011;5:170–191. doi: 10.1186/1479-7364-5-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varrone F., Gargano B., Carullo P., Di Silvestre D., De Palma A., Grasso L. The circulating level of FABP3 is an indirect biomarker of microRNA-1. J Am Coll Cardiol. 2013;61:88–95. doi: 10.1016/j.jacc.2012.08.1003. [DOI] [PubMed] [Google Scholar]
- 17.Sorichter S., Mair J., Koller A., Pelsers M.M., Puschendorf B., Glatz J.F. Early assessment of exercise induced skeletal muscle injury using plasma fatty acid binding protein. Br J Sports Med. 1998;32:121–124. doi: 10.1136/bjsm.32.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burch P.M., Greg Hall D., Walker E.G., Bracken W., Giovanelli R., Goldstein R. Evaluation of the relative performance of drug-induced skeletal muscle injury biomarkers in rats. Toxicol Sci. 2016;150:247–256. doi: 10.1093/toxsci/kfv328. [DOI] [PubMed] [Google Scholar]
- 19.Zhen E.Y., Berna M.J., Jin Z., Pritt M.L., Watson D.E., Ackermann B.L. Quantification of heart fatty acid binding protein as a biomarker for drug-induced cardiac and musculoskeletal necroses. Proteomics Clin Appl. 2007;1:661–671. doi: 10.1002/prca.200700006. [DOI] [PubMed] [Google Scholar]
- 20.Pritt M.L., Hall D.G., Recknor J., Credille K.M., Brown D.D., Yumibe N.P. Fabp3 as a biomarker of skeletal muscle toxicity in the rat: comparison with conventional biomarkers. Toxicol Sci. 2008;103:382–396. doi: 10.1093/toxsci/kfn042. [DOI] [PubMed] [Google Scholar]
- 21.Jain S., Khan H., Afxentiou S., Abdin R., Harlock J., Narang T. Status of cardiac markers in patients with peripheral arterial disease and critical limb ischemia. J Vasc Surg. 2018;68:e68–e69. [Google Scholar]
- 22.Otaki Y., Watanabe T., Takahashi H., Hirayama A., Narumi T., Kadowaki S. Association of heart-type fatty acid-binding protein with cardiovascular risk factors and all-cause mortality in the general population: the Takahata study. PLoS One. 2014;9:e94834. doi: 10.1371/journal.pone.0094834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertzel A.V., Bernlohr D.A. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y.-W., Chen J.-W. The potential link between fatty acid-binding protein 3 and endothelial dysfunction. J Am Coll Cardiol. 2020;75(11 Suppl 1):185. [Google Scholar]
- 25.Stremmel W., Strohmeyer G., Borchard F., Kochwa S., Berk P.D. Isolation and partial characterization of a fatty acid binding protein in rat liver plasma membranes. Proc Natl Acad Sci U S A. 1985;82:4–8. doi: 10.1073/pnas.82.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tunstall R.J., Mehan K.A., Wadley G.D., Collier G.R., Bonen A., Hargreaves M. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E66–E72. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- 28.Mulya A., Haus J.M., Solomon T.P., Kelly K.R., Malin S.K., Rocco M. Exercise training-induced improvement in skeletal muscle PGC-1alpha-mediated fat metabolism is independent of dietary glycemic index. Obesity (Silver Spring) 2017;25:721–729. doi: 10.1002/oby.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brass E.P., Hiatt W.R. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 30.Koutakis P., Miserlis D., Myers S.A., Kim J.K., Zhu Z., Papoutsi E. Abnormal accumulation of desmin in gastrocnemius myofibers of patients with peripheral artery disease: associations with altered myofiber morphology and density, mitochondrial dysfunction and impaired limb function. J Histochem Cytochem. 2015;63:256–269. doi: 10.1369/0022155415569348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipinos I.I., Judge A.R., Selsby J.T., Zhu Z., Swanson S.A., Nella A.A. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg. 2008;42:101–112. doi: 10.1177/1538574408315995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pipinos I.I., Judge A.R., Selsby J.T., Zhu Z., Swanson S.A., Nella A.A. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg. 2007;41:481–489. doi: 10.1177/1538574407311106. [DOI] [PubMed] [Google Scholar]
- 33.Pipinos I.I., Sharov V.G., Shepard A.D., Anagnostopoulos P.V., Katsamouris A., Todor A. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg. 2003;38:827–832. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 34.Pipinos I.I., Judge A.R., Zhu Z., Selsby J.T., Swanson S.A., Johanning J.M. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell R.G., Duscha B.D., Robbins J.L., Redfern S.I., Chung J., Bensimhon D.R. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc Med. 2007;12:285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 36.Spitali P., Hettne K., Tsonaka R., Charrout M., van den Bergen J., Koeks Z. Tracking disease progression non-invasively in Duchenne and Becker muscular dystrophies. J Cachexia Sarcopenia Muscle. 2018;9:715–726. doi: 10.1002/jcsm.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hathout Y., Brody E., Clemens P.R., Cripe L., DeLisle R.K., Furlong P. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2015;112:7153–7158. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]