Abstract
Objective
Abdominal aortic aneurysm (AAA) is a chronic inflammatory disease, which frequently results in fatal rupture; however, no pharmacologic treatment exists to inhibit AAA growth and prevent rupture. In this study, we investigated whether K-134, a novel phosphodiesterase 3 inhibitor, could limit the progression and rupture of AAA using multiple experimental models.
Methods
A hypoperfusion-induced AAA rat model was developed by inserting of a small catheter and via tight ligation of the infrarenal aorta. Rats were fed with a 0.15% K-134-containing diet (K-134(+) group) or a normal diet (K-134(-) group) from 7 days before the experiment to 28 days after model creation (pretreatment protocol). After the administration period, elastin fragmentation, macrophage infiltration, reactive oxygen species expression, matrix metalloproteinase levels, aneurysmal tissue hypoxia, and adventitial vasa vasorum (VV) stenosis were assessed. In the delayed treatment protocol, rats with AAA >3 mm were randomly divided to K-134(+) or K-134(-) group 7 days after model creation, and the effect of K-134 on suppressing preexisting AAA was examined. Further, elastase-induced rat model and angiotensin II-infused ApoE-/- mouse model were also used to examine the ability of K-134 to prevent rupture.
Results
K-134 prevented AAA rupture and significantly improved survival in the pretreatment protocol (P < .01). In the K-134(+) group, elastin degeneration was prevented; macrophage infiltration and reactive oxygen species production were significantly decreased. At 14 days, the enzymatic activity of matrix metalloproteinase-9 was significantly decreased. Further, K-134 inhibited intimal hyperplasia and VV stenosis. Expressions of hypoxic markers, hypoxia-inducible factor-1α, and pimonidazole, in the aneurysmal wall were also attenuated. In the delayed treatment protocol, K-134 also improved survival of rats with preexisting AAA. Similarly, in the elastase-induced rat model and angiotensin II-infused ApoE-/- mouse model, K-134 inhibited rupture and significantly improved survival (P < .01).
Conclusions
K-134 prevented the rupture of AAA and improved survival through suppressing inflammatory reaction. The inhibition of intimal hyperplasia in the adventitial VV may be associated with reduced hypoxia in the aneurysmal tissue. (JVS–Vascular Science 2020;1:219-32.)
Clinical Relevance
This study shows that K-134, a novel phosphodiesterase 3 inhibitor, suppressed abdominal aortic aneurysm (AAA) rupture. Considering that K-134 had already undergone a phase Ⅱ study in the United States for claudication in peripheral artery occlusive disease patients with good tolerance, K-134 may become a promising new therapeutic option for AAAs and could undergo clinical trials for patients with small AAA.
Keywords: Abdominal aortic aneurysm, Phosphodiesterase 3 inhibitor, Hypoxia, Atherosclerosis, K-134
Article Highlights.
-
•
Type of Research: Rodent model
-
•
Key Findings: A novel phosphodiesterase 3 inhibitor K-134 suppressed aneurysm growth and inhibited the rupture of multiple rodent aneurysm models. K-134 was associated with decreased inflammatory macrophage infiltration, production of reactive oxygen species, and tissue hypoxia.
-
•
Take Home Message: K-134 acts as an antiplatelet, anti-inflammatory, and antiatherosclerosis agent. K-134 is a promising agent for abdominal aortic aneurysm therapy and should be evaluated in future clinical trials.
Abdominal aortic aneurysm (AAA) is a common disease among elderly people with a 4.8% prevalence in the general population.1 The majority of patients remain asymptomatic until AAA rupture occurs. Surgical treatments, such as open repair with prosthetic graft replacement and endovascular treatment with a stent graft, are recommended for patients whose AAA are >55 mm in diameter. Without surgery, AAA progresses to rupture, leading to a high mortality (30%-50%). However, there exists no medical treatment for AAA patients for suppressing AAA growth or inhibiting its rupture.2 Particularly for patients whose AAA are <50 mm in diameter or for patients whose AAA are too large and considered unsuitable for surgical intervention, there is no other treatment option except for the monitoring its growth. Therefore, anti-AAA drugs without adverse effects even after prolonged use are warranted. To develop such therapy, the pathologic target of AAA should be identified.
AAA is a complex, multifactorial disease characterized by the chronic inflammation of the aortic wall, along with the accumulation of macrophages and the degeneration of the extracellular matrix. The excessive expression of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, is reported to be responsible for extracellular matrix degeneration.3 Experimental studies using animal models of aortic aneurysms have obtained promising results, and several drugs have been tested in clinical trials. These drugs are categorized as antihypertensive (eg, calcium channel blockers,4 propranolol,5 angiotensin-converting enzyme inhibitor [perindopril6], angiotensin receptor blocker [telmisartan7], antibiotics [doxycycline8] antiplatelet agent [ticagrelor9], aldosterone antagonist [ephlerenone10], antilipidemic agent [fenofibrate11,12], and anti-inflammatory agents, including anti-IL-1β antibody agent [canakinumab13], mast cell inhibitor [pemirolast14], and immunosuppressant [cyclosporine15]).16 Unfortunately, favorable results have not been obtained to date.
K-134, (-)-6-(3-{3-Cyclopropyl-3-[(1R,2R)-2-hydroxycyclo-hexyl]ureido}-propoxy)-2(1H) -quinolinone, synthesized as a selective phosphodiesterase 3 (PDE3) inhibitor (Fig 1, A), has been shown to exhibit more potent antiplatelet activity than the classical PDE3 inhibitors, including cilostazol, milrinone, and amrinone.17,18 Cilostazol has been demonstrated to inhibit aneurysmal growth in experimental aneurysm models, such as an elastase-induced model or angiotensin II-infused ApoE-/- mouse model.19,20 Similarly, K-134 has shown antithrombotic and antiatherosclerotic effects. According to a phase II dose-ranging study involving patients with intermittent claudication owing to peripheral artery occlusive disease (PAD), K-134 improved walking performance in the per protocol population with good tolerance.21
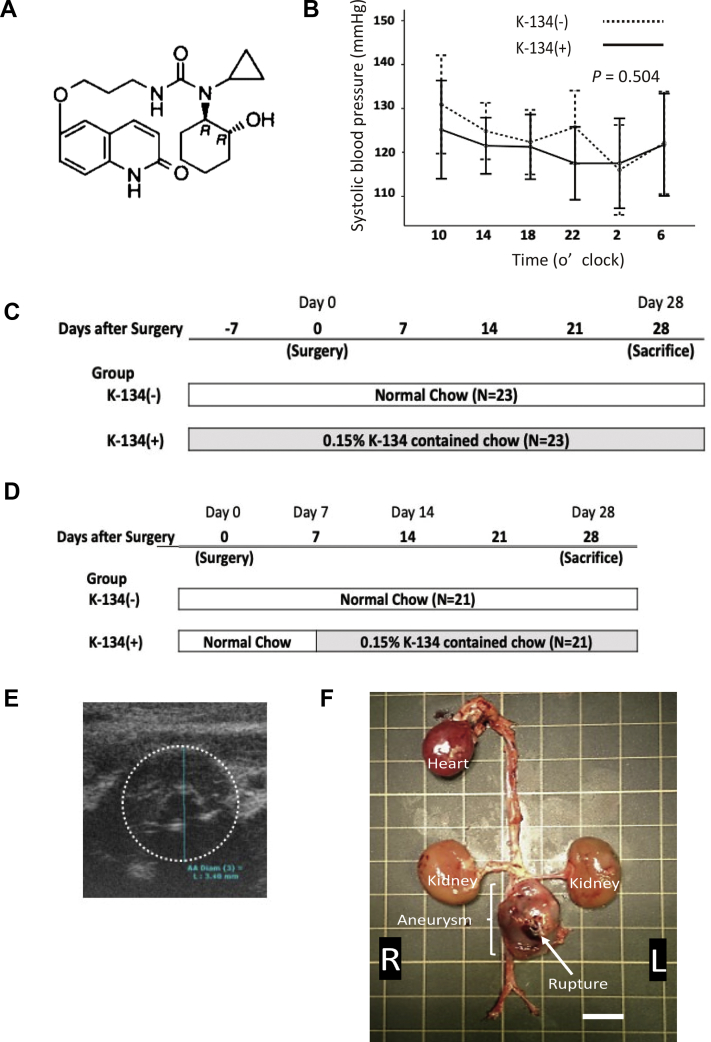
Fig 1.
Treatment protocol for hypoperfusion-induced abdominal aortic aneurysm (AAA) rat model. A, Structural formula of K-134 (-)-6-(3-{3-Cyclopropyl-3-[(1R,2R)-2-hydroxycyclohexyl]ureido}-propoxy)-2(1H)-quinolinone. B, Blood pressure measurement. Transition of systolic blood pressure (SBP) throughout the day with/without K-134 treatment (n = 5 per group). C, K-134 pretreatment protocol. The K-134(+) group (n = 23) was fed with a 0.15% K-134-containing diet, whereas the K-134(-) group (n = 23) was fed with a normal diet. The administration of K-134 started 7 days before the surgery (day 0), and both groups were observed for 28 days after day 0. D, Delayed K-134 treatment protocol. Rats were fed with a normal diet for 7 days after surgery. At day 7, rats with AAA >3.0 mm in diameter identified using ultrasound examination (E) were chosen and randomly divided into two groups: the K-134(+) group, which was fed with a 0.15% K-134-containing diet (n = 21), and the K-134(-) group, which was fed with a normal diet (n = 21). Both groups were observed for 28 days after day 0. F, Representative images of ruptured aneurysm, with the arrow indicating the rupture site, from rats without K-134 treatment. Scale bar = 10 mm.
Therefore, in this study, we hypothesized that K-134 can inhibit the development and prevent the rupture of AAA. Using a hypoperfusion-induced rat AAA model, we aimed to determine the effect of K-134 on the inhibition of AAA rupture through pretreatment before its initiation and delayed treatment for preexisting AAA, which is more clinically relevant for patients with small AAA. Our findings may lead to future clinical trials for AAA treatment.
Methods
Ethics statement
All study protocols were reviewed and approved by the Animal Care and Use Committee of Hamamatsu University School of Medicine and were performed in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996). All surgeries were performed through the intraperitoneal administration of an anesthetic mixture (medetomidine 0.15 mg/kg, midazolam 2 mg/kg, butorphanol 2.5 mg/kg body weight/rat) and all efforts were made to minimize suffering.
Blood pressure monitoring
Preliminary, systolic blood pressure was measured using a sphygmomanometer (BP-98A, Softron, Tokyo, Japan) with a tail cuff system to examine the effect of K-134 on blood pressure. Ten rats were fed with either a 0.15% K-134-containing diet (K-134(+) group) or a normal diet (K-134(-) group) for 7 days before the experiment. Afterward, five rats in each group were placed in a small holder mounted on a thermostatically controlled warming plate and maintained at 37°C. From 10 am until 6 pm the next day, systolic blood pressure was monitored (Fig 1, B).
Drugs and experimental schedules in hypoperfusion-induced rat AAA models
K-134 (molecular weight = 399.48 g/mol) was obtained from Kowa Company, Ltd (Tokyo, Japan) as a drug substance. Sprague-Dawley male rats (300-350 g; Japan SLC, Inc, Shizuoka, Japan) were used. The rats were reared at room temperature (25 ± 1°C), with free access to feed and water.
For the pretreatment protocol (Fig 1, C), administration of K-134 was started 7 days before surgery for the hypoperfusion-induced AAA model (day 0) (n = 23). Both groups fed with or without K-134 and were observed for 28 days after day 0.
For the delayed treatment protocol (Fig 1, D), rats were fed with a normal diet for 7 days after surgery. At day 7, rats with an AAA of >3.0 mm in diameter, identified using ultrasound examination (Fig 1, E), were chosen and randomly divided into two groups: the K-134(+) group was fed with a 0.15% K-134 containing diet (n = 21) and a K-134(-) group fed a normal diet (n = 21). Both groups were observed until day 28, and surviving rats were humanely killed using anesthesia.
K-134 plasma concentrations
Five rats were fed with a 0.15% K-134-containing diet 7 days before surgery. After the surgery, the 0.15% K-134 diet was administered for 14 days. Blood from these rats was taken during the daytime without fasting, and plasma obtained. K-134 concentrations were measured using high-performance liquid chromatography on day 0 (Kowa Company, Ltd). Plasma K-134 concentrations at day 0 (7-day pretreatment with K-134) were 1894.3 ± 1987.8 ng/mL, which was almost equivalent to the pharmacokinetics when K-134 30 mg/kg was orally administered to rats under nonfasting conditions in a single dose per day.22 These K-134 plasma levels were enough to improve the hindlimb circulation and gait disturbance in the rats.22,23 Based on the data of the pharmacokinetics of K-134 mentioned above, a phase II trial was performed in the Unites States to examine the effect of K-134 on improving the peak walking time in patients with peripheral artery disease.21
Creation of the hypoperfusion-induced AAA model
The surgical procedure to induce the hypoperfusion of the aortic wall was performed as previously described.24,25 Briefly, after laparotomy via a ventral midline abdominal incision, the aorta was separated from the periaortic tissue by peeling off the retroperitoneal fat from the aortic adventitia, which blocks blood flow from the perivascular tissue. Aortotomy involving a small incision adjacent to the renal artery branches was performed, and a polyurethane catheter was inserted into the infrarenal aorta. After repairing the incision, a tight ligation of the aorta over the catheter blocked the vasa vasorum (VV) blood flow from the proximal direction through the aortic wall without disturbing the aortic blood flow. This technique can help to develop an infrarenal AAA, which has morphologic and pathologic characteristics similar to those of a human AAA.24 Subsequently, 20% to 40% of the rats died of AAA rupture within 28 days after the surgery. Fig 1, F, shows a representative image of a ruptured AAA in the K-134(-) group, which underwent the pretreatment protocol.
Creation of other established aneurysm models
Elastase-induced rat model
We created an elastase-induced model using Sprague-Dawley male rats (300-350 g; Japan SLC, Inc). After laparotomy and aorta exposure, a polyethylene catheter was threaded into the aorta until its tip was resting in the isolated segment. Temporary ligatures were placed proximally and distally, and the aorta was perfused with 20 μL (30 U) porcine pancreatic elastase (1.5 U/ mL in saline; Sigma-Aldrich, St. Louis, Mo) through the catheter without any aortic expansion for 10 minutes. Afterward, the residual infusate was aspirated; the tubing was withdrawn; and the aortotomy was closed using a 5-0 nylon suture. Then, the rats were placed in warming cages to recover and were similarly pretreated with or without K-134. They were fed either an 0.15% K-134-containing food or normal food 7 days before the model creation and continuously fed for 28 days (n = 11 in each group).
Angiotensin II-infused ApoE-/- mouse model
Spontaneously hyperlipidemic apolipoprotein E-deficient (ApoE-/-) 18- to 20-week-old male mice (C. KOR/Stm slc-Apoe shl, Japan SLC, BALB/c background) were used to establish a model of suprarenal aortic dissection and aneurysm.26,27 Mice fed a standard rodent chow were randomly treated with angiotensin II (Ang II; Sigma-Aldrich) in saline (1000 ng/kg/min) using miniosmotic pumps (Alzet Model 2004, Durect Corp., Cupertino, Calif) implanted beneath the dorsal skin. They were anesthetized with 2% isoflurane inhalation. Mice were similarly pretreated. They were fed with either 0.15% K-134-containing food or normal food for 7 days before the model creation and continuously fed for 28 days (n = 20 in each group).
Measurement of aortic diameter using ultrasound imaging and specimen preparation
After surgery, the maximum diameter of the infrarenal abdominal aorta of each rat was measured using ultrasound imaging (Vevo770, Visual Sonics, Toronto, Ontario, Canada) under anesthesia with a mixture of medetomide, midazolam, and butorphanol. This examination was performed 7, 14, 21, and 28 days after the surgery. All diameter measurements were performed by a single investigator blinded to study group assignment. At day 28, surviving rats were humanely killed via isoflurane overdose. The aorta from the distal arch to the terminal aorta was harvested, and the aortic samples were frozen in isopentane and stored at −80°C until use. Twenty-three and 21 rats in each group were used for the pretreatment experiment and delayed treatment, respectively.
Elastic fragmentation score
Destruction of the medial elastin was graded as I (mild), II (moderate), III (high), and IV (severe).28 Grade I samples showed mild disruption of the medial layer elastic network with only the external elastic lamina (EEL) disrupted, whereas grade II samples showed moderate disruption, with both the EEL and outer medial layer broken or degraded. Grade III was characterized by high disruption, with the EEL and both medial elastic layers showing breakage and/or degradation. Grade IV was characterized by severe disruption, with all four elastic layers showing signs of breakage and/or degradation. The aortic diameter, which is the maximum distance from one EEL end to another, was determined by tracing contours on digitized images. Specimens from the surviving 23 rats in each group from the pretreatment protocol were used for the assessment. Elastic fragmentation score was assessed by a single investigator blinded to the study group assignment.
Immunohistochemistry
The abdominal aortae of the 23 surviving rats in each group from the pretreatment protocol were harvested and cut using a cryostat (CM1950; Leica) into 8-μm-thick sections. The sections were fixed with 4% paraformaldehyde in phosphate-buffered saline (; pH 7.4) for 10 minutes at room temperature. After rinsing with phosphate-buffered saline, the sections were preincubated with 10% normal goat serum (Nichirei Biosciences, Tokyo, Japan) and incubated overnight at 4°C with mouse anti-CD 68 (Abcam, Cambridge, UK), rabbit anti-MMPs (Abnova, Taipei, Taiwan), rabbit anti-hypoxia-inducible factor (HIF)-1α (Novus Biologicals, Littleton, Colo), and antipimonidazole mouse IgG1 monoclonal antibody (Hypoxyprobe, Inc, Burlington, Mass). Immunoreactivity was visualized using Alexa Fluor 594-conjugated anti-mouse immunoglobulin G and Alexa Fluor 488-conjugated anti-rabbit immunoglobulin G (Molecular Probes, Invitrogen, Carlsbad, Calif). All Alexa-fluoroconjugated secondary antibodies were diluted 200-fold. The slides were mounted in a glycerol-based Vectashield medium (Vector Laboratories, Burlingame, Calif).
Detection of reactive oxygen species
Specimens from the 23 surviving rats in each group from the pretreatment protocol were used for the assessment. Abdominal aortae were harvested and cut using a cryostat (CM1950; Leica) into 8-μm-thick sections. The sections were incubated at room temperature with Oxidative Stress Reagents (CellROX, Life Technologies Japan Ltd, Tokyo, Japan), which are fluorogenic probes designed to reliably measure the content of reactive oxygen species (ROS). The slides were then counterstained with 4′, 6-diamidino-2-phenylindole, and histologic images were analyzed using an inverted microscope (BZX-700, KEYENCE, Osaka, Japan). Fluorescence intensity was quantified using the Hybrid cell count BZ-H2C software (KEYENCE).
Gelatin zymography
AAA samples were harvested at day 14 of the pretreatment protocol in the K-134(+) group and K-134(-) group (n = 4 each). The aortic tissues were homogenized using RIPA buffer (Nacalai Tesque, Kyoto, Japan), and the protein concentrations of each lysate was measured using a TAKARA BCA Protein Assay kit (TAKARA, Shiga, Japan) and H1MF Synergy H1 (BIOTEC, Tokyo, Japan). An equal concentration of protein (3 mg/mL) was applied to the samples as a substrate for MMP activity. The proteolytic activities of MMP-2 and -9 were examined via gelatin zymography using a gelatin-zymography kit (Cosmo-Bio, Tokyo, Japan) according to the manufacturer's instructions. For quantitative analysis, densitometric analysis was performed using Fiji (an open-source platform for biological image analysis) with ChemiDoc Touch Imaging system (BIO RAD, Buckinghamshire, Berkeley, Calif). MMP activity levels were compared with MMP markers and then expressed as a fold difference. Comparisons among the sham (n = 3), K-134(-), and K-134(+) groups were performed.
VV patency
Elastica van Gieson staining was performed to stain elastin. The luminal border and EEL in each adventitial VV were traced at the same magnification. The size of the VV was measured as the total area bound by the EEL, and the lumen patency was measured as the ratio of the luminal area to the total area bound by the EEL in each VV as previously reported.29
Histologic morphometry
Histologic images were analyzed using an inverted microscope (KEYENCE), and quantification was performed using the Hybrid cell count BZ-H2C software (KEYENCE). The number of macrophages (CD68+ cells) was determined by counting all immune-stained cell counts per aortic cross section. The levels of HIF-1α, MMPs, and pimonidazole were evaluated using immunohistochemical staining, and staining images were analyzed using the Image J software (National Institutes of Health, Bethesda, Md). We evaluated three cross-sections from each tissue sample taken at 100-μm intervals and calculated the mean. We set a threshold to compute for the area positive for each protein and then calculated the ratio of the positive area to the total aortic area.
Statistical analysis
Data analyses were performed using the SPSS software (v23; IBM, Armonk, NY). All continuous variables are presented as the mean ± standard deviation. One-way or two-way analysis of variance and nonparametric Mann-Whitney tests were utilized to determine the statistical differences for normally and non-normally distributed data, respectively, in the sham, K-134(-), K-134(+) groups. Post hoc comparisons were performed using Tukey's test. Kaplan-Meier analysis and statistical comparisons were performed using the log-rank test to test the difference in aneurysm mortality between two groups. A P of <.05 was considered significant.
Results
K-134 did not affect blood pressure
The systolic blood pressure of rats showed no significant changes between the K-134(+) and K-134(-) groups. The 0.15% K-134-containing diet had no effect on rat blood pressure (Fig 1, B). Plasma K-134 concentrations at day 0 (7 days of K-134 pretreatment) and day 14 (7 days after surgery with K-134 containing diet) were 1894.3 ± 1987.8 ng/mL and 371.0 ± 112.4 ng/mL, respectively.
K-134 inhibited AAA progression and improved survival in the hypoperfusion-induced aneurysm rat model (pretreatment protocol)
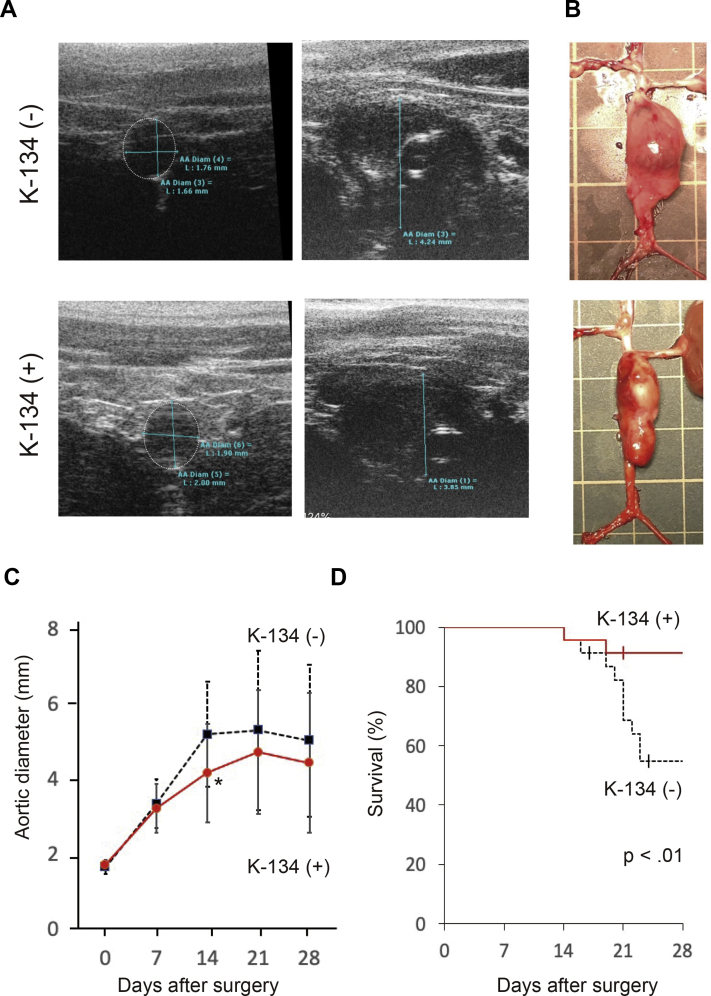
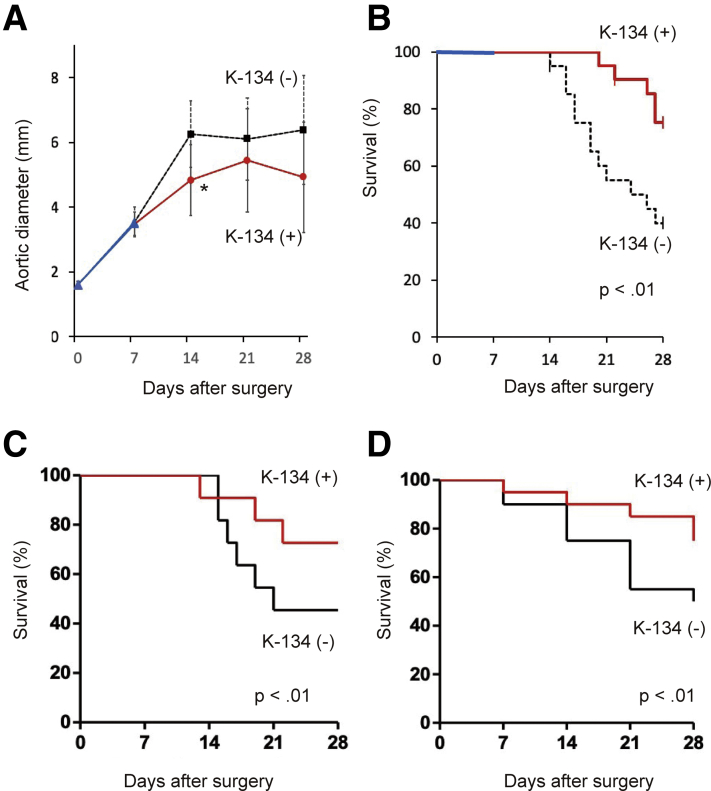
The incidence of AAA in both the K-134(-) and the K-134(+) groups was 100% (Fig 2, A). The AAA samples at day 28 showed infrarenal saccular aneurysm (Fig 2, B). The aortic outer diameter was significantly larger in the K-134(-) group than in the K-134(+) group at day 14 (5.18 ± 1.39 mm and 4.18 ± 1.31 mm, respectively; P < .01) (n = 22 each, because one rat died of rupture in each group) (Fig 2, C). In the K-134(-) group, ruptures occurred on days 14, 16, 19, 20, 21, 21, 21, 22, 23, and 23 after surgery, and two rats died on days 17 and 24 for unknown reasons (survival rate of 56.5%). In contrast, in the K-134(+) group, ruptures occurred on days 14 and 19 after surgery, and one rat died on day 21 for unknown reasons after surgery (survival rate of 87.0%) (Fig 2, D). Overall, K-134 significantly inhibited aneurysm rupture and improved the survival (P < .01).
Fig 2.
A, Representative ultrasound images of the aorta and AAA with/without K-134 treatment at day 0 and day 28. B, Representative photographs of AAA at day 28 (pretreatment) with/without K-134 treatment. Grid size = 10 mm. C, The aortic outer diameter of abdominal aortic aneurysm (AAA). The aortic diameter was significantly suppressed in the K-134(+) group compared with the K-134(-) group at day 14 (5.18 ± 1.39 mm and 4.18 ± 1.31 mm, respectively; P < .01). D, Kaplan-Meier survival curves in rats with/without K-134 treatment showing that K-134 treatment significantly improved survival. Data are presented as the mean ± standard deviation. K-134(+) group (n = 23); K-134(-) group (n = 23).
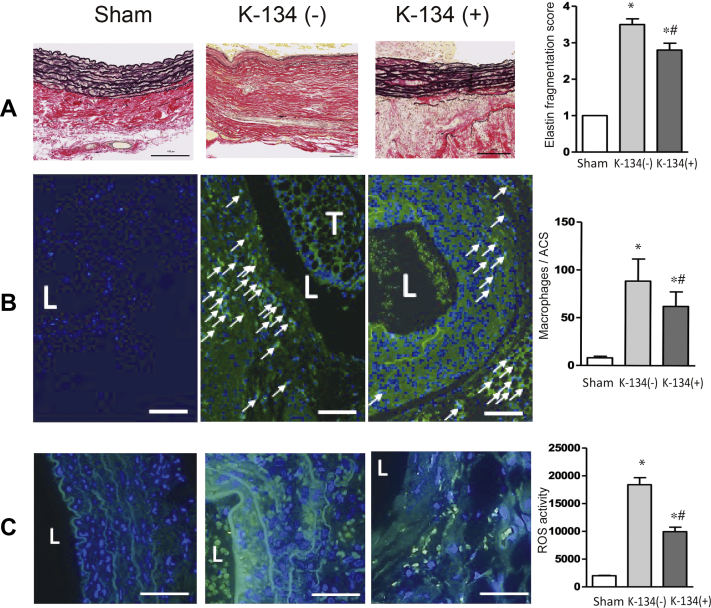
K-134 decreased medial disruption, mural macrophage infiltration, and ROS generation
Medial elastin degradation is a critical histologic feature of AAAs. Histologic assessment revealed that medial elastic lamellae were significantly preserved in AAA on day 28 in the K-134(+) group treated with K-134 (Fig 3, A). Massive accumulation of macrophages was observed in both the media and adventitia of the aneurysmal wall (Fig 3 and B), and K-134 treatment significantly reduced this macrophage accumulation. Similarly, hypoperfusion-induced generation of ROS was decreased in the aneurysmal wall in the K-134(+) group (Fig 3, C).
Fig 3.
Effect of K-134 on abdominal aortic aneurysm (AAA) pathology. Treatment with K-134 suppressed medial elastin degradation, macrophage accumulation, and reactive oxygen species (ROS) production at day 28 (pretreatment protocol). Comparisons among the sham (n = 3), K-134(-) group (n = 13), and K-134 (+) groups (n = 20) are shown. Scale bar = 100 μm. L, lumen. T, thrombus. ∗P < .05 vs sham; #P < .05 vs K-134(-). A, Representative images of elastin. Medial elastic disruption was scored as mild (1), high (3), and severe (4). B, Representative images of immunofluorescence staining for macrophage (indicated by green and arrows) and 4′, 6-diamidino-2-phenylindole (DAPI) (blue). Merged images showed that macrophage was accumulated in the media and adventitia. The number of macrophages (CD68+ cells) was counted on each aortic section. C, Representative images of ROS activity (indicated by green) and DAPI (blue). Merged images showed that K-134 treatment significantly decreased ROS activity in the hypoperfusion-induced AAA rat model.
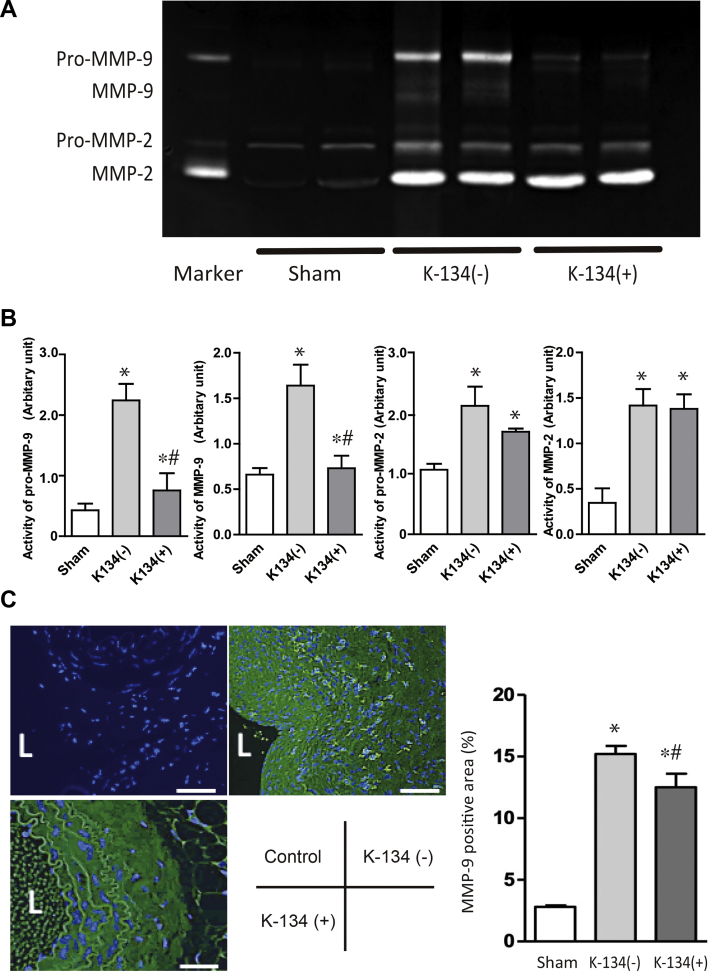
K-134 decreased MMP activity in the aneurysmal wall
The activities of all MMPs (ie, pro-MMP-2, MMP-2, pro-MMP-9 and MMP-9) were increased compared with those in aorta harvested from sham rats at day 28. In contrast, K-134 treatment significantly inhibited the elevation of pro-MMP-9 and MMP-9 activities (Fig 4, A and B). Histologic morphometry revealed that K-134 treatment decreased MMP-9 expression in the aneurysmal tissue (Fig 4, C).
Fig 4.
Gelatin zymography of the activities of matrix metalloproteinase (MMP)-2 and -9 and the immunohistochemical analysis of MMP-9 in the aorta. Comparisons among the sham (n = 3), K-134(-) (n = 4), and K-134(+) (n = 4) groups are shown. Aortic samples were harvested at day 28. (A) Gelatin zymography. B, Quantitation of the activities of pro-MMP-9, MMP-9, pro-MMP-2, and MMP-2 obtained using gelatin zymography. ∗P < .05 vs sham; #P < .05 vs K-134(-). C, Representative images of immunofluorescence staining for MMP-9 (indicated by green) and 4′, 6-diamidino-2-phenylindole (DAPI) (blue). Merged images showed that K-134 treatment significantly decreased the MMP-9 activity in the hypoperfusion-induced abdominal aortic aneurysm (AAA) rat model. Bar scale = 100 μm. L, lumen.
K-134 treatment prevented neointimal formation of adventitial VV in AAA wall
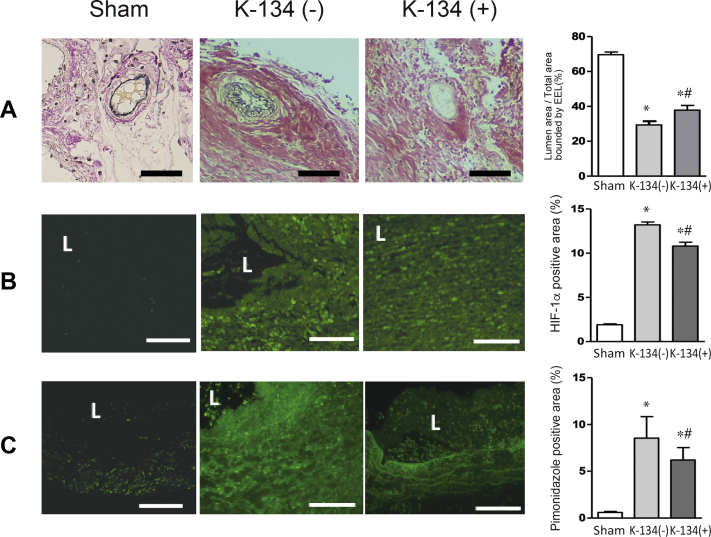
At day 28, the adventitial VV in the hypoperfusion-induced aneurysm showed stenosis with proliferation of smooth muscle cells (SMCs). We found that the adventitial VV in the aneurysmal sac with normal diet (n = 13) was significantly stenotic in comparison with that of sham rats (n = 5). K-134 treatment (n = 20) significantly prevented VV stenosis compared with nontreated rats (Fig 5, A).
Fig 5.
Effect of K-134 treatment on the adventitial vasa vasorum (VV) intimal hyperplasia and tissue hypoxia in abdominal aortic aneurysm (AAA). K-134 ameliorated VV intimal hyperplasia and attenuated hypoxia in the hypoperfusion-induced aneurysmal wall at day 28 (pretreatment protocol). Comparisons among the sham (n = 5), K-134(-) (n = 13), and K-134 (+) (n = 20) groups are shown. L, lumen. ∗P < .05 vs sham; # P < .05 vs K-134(-). A, Representative images of the adventitial VV with Elastica van Gieson staining. Scale bar = 20 μm. Lumen patency of the VV was measured as the ratio of the luminal area to the total area bound by the EEL in each VV. K-134 treatment significantly suppressed VV stenosis owing to intimal hyperplasia in the hypoperfusion-induced AAA rat model. B, Representative images of immunofluorescence staining for HIF-1α (indicated by green). K-134 significantly suppressed HIF-1α expression. Scale bar = 100 μm. C, Representative images of immunofluorescence staining for pimonidazole (indicated by green). K-134 significantly suppressed pimonidazole expression. Scale bar = 100 μm.
K-134 attenuated expression of hypoxia markers HIF-1α, and pimonidazole, in the aneurysmal wall
The expressions of HIF-1α and pimonidazol were both attenuated in the K-134(+) group compared with the K-134(-) group, suggesting that K-134 attenuated hypoxic conditions in the aneurysmal wall (Fig 5, B and C).
K-134 inhibited progression of hypoperfusion-induced aneurysm and improved survival in the hypoperfusion-induced aneurysm rat model (delayed treatment protocol)
We then assessed whether K-134 suppressed aneurysm progression and prevented rupture after AAA formation through a delayed treatment protocol. The aortic diameters of the rats before surgery (day 0) were 1.60 ± 0.11 mm and 1.59 ± 0.12 mm in the K-134(-) group (n = 21) and K-134(+) group (n = 21), respectively, with no significant difference. At day 7, aortic ultrasound images revealed that the incidence of an AAA of >3 mm was 100%. At day 28, the aortic outer diameter was significantly larger in the K-134(-) group than in the K-134(+) group at day 14 (6.25 ± 1.03 mm and 4.83 ± 1.09 mm, respectively; P < .01) (Fig 6, A). In the K-134(-) group, ruptures occurred on days 14, 16, 16, 17, 17, 19, 19, 20, 21, 24, 26, and 27 after surgery and one rat died on day 14 for unknown reasons (survival rate of 38.1%) in K-134(-) group. In contrast, in the K-134(+) group, ruptures occurred on days 20, 22, 26, 27, and 27 after surgery, and one rat died on day 22 for unknown reasons after surgery (survival rate of 71.4%) (Fig 6, B). K-134 significantly inhibited aneurysm rupture and improved survival (P < .01).
Fig 6.
Effect of K-134 treatment of abdominal aortic aneurysm (AAA) on progression and survival under delayed treatment protocol (treatment for preexisting AAA). A, The aortic outer diameter of AAA. The aortic diameter was significantly suppressed in the K-134(+) group compared with the K-134(-) group at day 14 (6.25 ± 1.03 mm and 4.83 ± 1.09 mm, respectively; ∗P < .01; n = 21 per group). B, Kaplan-Meier survival curves of rats with/without K-134 treatment. K-134 significantly improved survival. Effect of K-134 treatment on survival in elastase-induced AAA rat model and angiotensin II-induced AAA ApoE-/- mouse model. C, Survival free from rupture-induced death in the elastase-induced AAA rat model (n = 11 per group). The animals were similarly pretreated with K-134 and fed with a 0.15% K-134-containing diet 7 days before and after the creation of the model. D, Survival free from rupture--induced death in the angiotensin II-infused ApoE-/- mouse model (n = 20 per group). Mice were similarly pretreated with K-134 and fed with a 0.15% K-134-containing diet 7 days before and after the establishment of the model.
K-134 treatment improves survival in other established aneurysm models
In the elastase-induced rat AAA model, at day 28, the survival rate of the K-134(-) group (n = 11) was 45.5%, whereas that of the K-134(+) group was 72.7% (n = 11) at the same day (Fig 6, C). In the angiotensin II-infused ApoE-/- mouse aneurysm model, at day 28, survival rate of the K-134(-) group (n = 10) was 50%, whereas that of the K-134(+) group was 75.0% (n = 15) at the same day (Fig 6, D).
All cases of fatality were due to the bleeding of the abdominal cavity owing to aneurysmal rupture. These results suggested that K-134 treatment might be effective in inhibiting aneurysm rupture and improving survival on multiple animal models
Discussion
In this study, we demonstrated that K-134 treatment was effective in preventing the progression of aneurysms in not only a hypoperfusion-induced AAA rat model, but also other established models, namely, elastase-induced rat AAA and angiotensin II-infused ApoE-/- mouse aneurysm models. Furthermore, using the delayed treatment protocol in the hypoperfusion-induced rat model, K-134 prevented AAA rupture and improved survival in preexisting AAA (>3 mm in diameter). K-134-induced aneurysm inhibition was associated with the relative preservation of the aortic architecture and reduced inflammation.
K-134 is a novel PDE3 inhibitor that exhibits antiatherosclerosis properties because of its antiplatelet, antithrombus, and vasodilator effects. Its antiplatelet activity has been shown to be more potent than cilostazol,18 a commercial drug available for >30 years.30 However, the cellular mechanisms underlying these have not been fully elucidated. PDE3 inhibitor increases intracellular levels of cycle adenosine monophosphate (cAMP) and cyclic guanosine monophosphate and activate the cAMP-protein kinase A (PKA) pathway. The activation of the cAMP-PKA pathway can upregulate the peptide intermedin (IMD; also known as adrenomodulin-2) in the endothelium.31 IMD can inhibit oxidative stress and reduce neointima formation by the SMCs.32 It also causes vasodilation and inhibits the proliferation of aortic SMCs,33 which is beneficial to inhibit VV stenosis and maintain tissue perfusion. IMD can attenuate AAA development in Ang-II-induced ApoE-/- mouse aneurysm models by inhibiting oxidative stress.34 The activation of the cAMP-PKA pathway may also exert pivotal effects on anti-inflammation. A previous study speculated that the activation of the cAMP-PKA pathway in endothelial cells could enhance the integrity of the endothelial barrier in the aortic wall, and that the PDE3 inhibitor may inhibit macrophage accumulation in the aortic media by enhancing the endothelial barrier integrity through reducing inflammation in Ang-II-induced ApoE-/- mouse aneurysm models.20 Taken together, the antiplatelet, anti-inflammatory, and antiatherosclerosis effects of K-134 may be attributed to the activation of cAMP-PKA pathway. However, most of these mechanisms were suggested based on the results obtained from experimental animal models. Therefore, further studies to confirm these cellular mechanisms using human AAA samples are warranted.
When K-134 was developed, its initial targets were stroke patients and claudicants owing to PAD. Indeed, a 2012 phase II study in the United States, Russia, and Japan performed as a double-blinded, randomized, placebo-controlled trial assessed the safety, tolerability, and efficacy of K-134 in patients with claudication secondary to PAD. The trial demonstrated that K-134 had a safe profile and improved the patients' treadmill-walking performance, similar to the effects of cilostazol.21,35 During the trial, minor side effects, such as palpitation (4.8%) and headache (7.1%), occurred in the trial participants.21
Because the intraluminal thrombus (ILT) of AAA was identified to contain many inflammatory cells, ILT may induce aortic wall inflammation.36 The volume of the thrombus has also been found to be associated with AAA growth.37 Histologic studies revealed multiple layers in the intramural thrombus of AAA,38 which suggests that thrombin generation and fibrin formation were repeated during AAA growth. Focal thrombolysis or bleeding into the organized thrombi may also be associated with layer formation. These repetitive protease activities inherently produce inflammation.39 The presence of thick ILT may prevent the luminal perfusion of oxygen from the aortic blood flow to the aortic wall, thus causing the aortic tissue to be hypoxic. Vorp et al40 reported that thicker ILT was associated with localized tissue hypoxia, neovascularization, and inflammation in the human AAA wall.
Accordingly, antiplatelet therapy has recently been highlighted as a possible pharmacologic treatment to suppress AAA. Several antiplatelet agents such as aspirin, clopidogrel, and cilostazol have been investigated to suppress aneurysms in established rodent models.19,20,41,42 In experimental studies, the antiplatelet agents commonly inhibited the infiltration of macrophages, generation of ROS, upregulation of MMPs, elastin degradation, and aneurysm growth. Further, a large cohort study revealed that the administration of aspirin or P2Y12 inhibitors might slow AAA progression and reduce AAA rupture.42 From these findings, a double-blinded, placebo-controlled clinical trial in Sweden (TicAAA trial) was conducted to evaluate the effect of ticagrelor, a P2Y12 inhibitor, on the growth of small AAA. However, the results reported that ticagrelor did not reduce their growth.9
In this study, we revealed that K-134 suppresses AAA growth and prevents rupture in multiple rodent models. Similar to other antiplatelet agents, as inhibition of macrophage infiltration, ROS generation, MMPs activation, and elastin degradation in a hypoperfusion-induced rat model were obtained. Unlike aspirin and the P2Y12 inhibitors, ticagrelor and clopidogrel, K-134 is a PDE3 inhibitor. As we described elsewhere in this article, PDE3 acts not only as antiplatelet, anti-inflammatory agents, but also as antiatherosclerosis agents that suppresses the intimal thickening by preventing SMCs proliferation and inhibits intimal thickening via the activation of cAMP-PKA pathway.43
In human AAA, we have previously demonstrated that marked intimal hyperplasia owing to SMC proliferation occurred in the adventitial VV, and that the aortic walls were ischemic and hypoxic.29 Next, we created a hypoperfusion-induced AAA model in rats,24 in which tight ligation of the aorta over the inserted catheter induced hypoperfusion of the aortic wall, subsequently developing AAA. Further, the adventitial VV became stenotic owing to intimal hyperplasia. The hypoperfusion-induced AAA model mimics human AAA pathologic features well, such as the existence of ILT, tissue hypoxia, infiltration of the inflammatory cells, medial degeneration, adipogenesis, and spontaneous rupture.44 In the other established rodent models, K-134 prevented aneurysmal rupture and improved survival. Among the different effects of K-134, the inhibition of the adventitial VV intimal thickening and the attenuation of hypoxic conditions in aneurysmal tissue are the most notable. The adventitial VV intimal thickness can perturb oxygen and nutrient diffusion and, thus, result in regional hypoxia in the arterial wall, which may cause ischemic injury of the inner arterial wall and eventually induce VV neovascularization.45 Hypertension increases the aortic wall tensile force and interferes with VV blood flow. Smoking or nicotine inhalation is another known risk factor that perturbs VV blood flow.46 Considering that both hypertension and smoking are the most important risk factors for AAA,47 we hypothesize that VV hypoperfusion and the subsequent hypoxia may be the pivotal mechanisms AAA pathogenesis. Furthermore, the topologic distribution of adventitial VV is densest in the aortic arch and decreases as the aortic segments become more distal, with the lowest amount in the infrarenal abdominal aorta, thus making this region the most susceptible to hypoxia.48 Overall, these results indicate that infrarenal AAA with dense ILT is likely to become most hypoxic owing to VV hypoperfusion and perturbed oxygen diffusion from the aortic blood flow. Hypoxia could further exacerbate macrophage-induced vascular inflammation and remodeling.49 Therefore, we speculated that the initial mechanisms involved in the inhibition of AAA development by K-134 may be its antiatherosclerosis and antiplatelet properties as they inhibit stenosis of aortic adventitial VV and ameliorate tissue hypoperfusion. During aneurysm progression, the common mechanisms in our models were sustained inflammation and oxidant stress,50 although all three rodents models exhibited distinct mechanisms of AAA development. Therefore, the anti-inflammatory and antioxidant properties of K-134 may be more beneficial for inhibiting AAA progression and rupture.
Accordingly, the PDE3 inhibitor K-134 has shown great potential for pharmacologic therapy of AAA because it acts not only as an antiplatelet and anti-inflammatory agent, but also as an antiatherosclerosis agent that can be administered daily. Its antiatherosclerosis activity may also be beneficial in preventing other atherosclerotic manifestations in AAA patients at a high risk for developing future cardiovascular events. Considering that the phase II trial of K-134 has already been conducted in PAD claudicants without major adverse events, K-134 may be a promising candidate for a randomized, controlled study on the growth of small AAAs.
This study has several limitations. First, our established rodent models have relatively small sample sizes. Second, because rodents are smaller in size than humans, a larger animal AAA model, such as pig, may further elucidate the mechanism of K-134 and confirm its effectiveness before clinical trials in humans. Last, some of authors hold a patent associated with K-134, which might cause potential bias during result evaluation.
Conclusions
K-134 suppressed aneurysm growth and inhibited rupture in multiple rodent aneurysm models. In rat models of hypoperfusion-induced AAA, K-134 improved survival even in rats with preexisting aneurysm. K-134's antiatherosclerotic properties inhibited intimal hyperplasia of the adventitial VV in AAA and attenuated hypoxia, thus proving its potential as pharmacologic treatment for AAA.
Author contributions
Conception and design: NU
Analysis and interpretation: NU, HT, TY, MS, KI, EN, HT
Data collection: NU, HT, TY, TK, YY, HT, MS
Writing the article: NU, HT
Critical revision of the article: NU, HT, TY, TK, YY, HT, MS, KI, EN, HT
Final approval of the article: NU, HT, TY, TK, YY, HT, MS, KI, EN, HT
Statistical analysis: NU, HT, TY, EN
Obtained funding: NU
Overall responsibility: NU
Acknowledgments
We thank the Kowa Company Ltd, for kindly providing us with the K-134 drug substance and measuring the plasma concentration of K-134 in rats. We also thank H. Kugo, and N. Zaima (Kindai University, Nara, Japan) for their technical assistance in gelatin zymography.
Footnotes
Funded in part by a JOINT RESEARCH CONTRACT-grant from Kowa Company, Ltd. The authors received no support or funding for the study. Kowa Company, Ltd had no involvement in the study design, analysis, and interpretation of data. Kowa Company, Ltd was not involved in the decision to submit the manuscript for publication.
Author conflict of interest: NU, TY, and HT have a patent associated with K-134 (WO/2019/054432). The other authors report no conflicts.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Li X., Zhao G., Zhang J., Duan Z., Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population--a meta-analysis. PLoS One. 2013;8:e81260. doi: 10.1371/journal.pone.0081260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura K., Morikage N., Nishino-Fujimoto S., Furutani A., Shirasawa B., Hamano K. Current status and perspectives on pharmacologic therapy for abdominal aortic aneurysm. Curr Drug Targets. 2018;19:1265–1275. doi: 10.2174/1389450119666171227223331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuivaniemi H., Ryer E.J., Elmore J.R., Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13:975–987. doi: 10.1586/14779072.2015.1074861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey M.A., Sohrabi S., Flood K., Griffin K.J., Rashid S.T., Johnson A.B. Calcium channel blockers enhance sac shrinkage after endovascular aneurysm repair. J Vasc Surg. 2012;55:1593–1599. doi: 10.1016/j.jvs.2011.12.075. [DOI] [PubMed] [Google Scholar]
- 5.Propanolol Aneurysm Trial Investigators Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2002;35:72–79. doi: 10.1067/mva.2002.121308. [DOI] [PubMed] [Google Scholar]
- 6.Bicknell C.D., Kiru G., Falaschetti E., Powell J.T., Poulter N.R., AARDVARK Collaborators An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK) Eur Heart J. 2016;37:3213–3221. doi: 10.1093/eurheartj/ehw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowbotham S.E., Krishna S.M., Moran C.S., Golledge J. Fenofibrate and telmisartan in the management of abdominal aortic aneurysm. Curr Drug Targets. 2018;19:1241–1246. doi: 10.2174/1389450119666171227224655. [DOI] [PubMed] [Google Scholar]
- 8.Meijer C.A., Stijnen T., Wasser M.N., Hamming J.F., van Bockel J.H., Lindeman J.H. Doxycycline for stabilization of abdominal aortic aneurysms. Ann Intern Med. 2013;159:815–823. doi: 10.7326/0003-4819-159-12-201312170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Wanhainen A., Mani K., Kullberg J., Svensjo S., Bersztel A., Karlsson L. The effect of ticagrelor on growth of small abdominal aortic aneurysms - a randomized controlled trial. Cardiovasc Res. 2020;116:450–456. doi: 10.1093/cvr/cvz133. [DOI] [PubMed] [Google Scholar]
- 10.Davis F.M., Rateri D.L., Daugherty A. Abdominal aortic aneurysm: novel mechanisms and therapies. Curr Opin Cardiol. 2015;30:566–573. doi: 10.1097/HCO.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowbotham S.E., Cavaye D., Jaeggi R., Jenkins J.S., Moran C.S., Moxon J.V. Fenofibrate in the management of AbdoMinal aortic anEurysm (FAME): study protocol for a randomised controlled trial. Trials. 2017;18:1. doi: 10.1186/s13063-016-1752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinchbeck J.L., Moxon J.V., Rowbotham S.E., Bourke M., Lazzaroni S., Morton S.K. Randomized placebo-controlled trial assessing the effect of 24-week fenofibrate therapy on circulating markers of abdominal aortic aneurysm: outcomes from the FAME -2 trial. J Am Heart Assoc. 2018;7:e009866. doi: 10.1161/JAHA.118.009866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraga-Silva R.A., Trachet B., Stergiopulos N. Emerging pharmacological treatments to prevent abdominal aortic aneurysm growth and rupture. Curr Pharm Des. 2015;21:4000–4006. [PubMed] [Google Scholar]
- 14.Sillesen H., Eldrup N., Hultgren R., Lindeman J., Bredahl K., Thompson M. Randomized clinical trial of mast cell inhibition in patients with a medium-sized abdominal aortic aneurysm. Br J Surg. 2015;102:894–901. doi: 10.1002/bjs.9824. [DOI] [PubMed] [Google Scholar]
- 15.Golledge J., Norman P.E., Murphy M.P., Dalman R.L. Challenges and opportunities in limiting abdominal aortic aneurysm growth. J Vasc Surg. 2017;65:225–233. doi: 10.1016/j.jvs.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Lindeman J.H., Matsumura J.S. Pharmacologic management of aneurysms. Circ Res. 2019;124:631–646. doi: 10.1161/CIRCRESAHA.118.312439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudo T., Tachibana K., Toga K., Tochizawa S., Inoue Y., Kimura Y. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000;59:347–356. doi: 10.1016/s0006-2952(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H., Okamura Y., Watanabe N., Ikeda Y., Handa M. Shear-dependent suppression of platelet thrombus formation by phosphodiesterase 3 inhibition requires low levels of concomitant Gs-coupled receptor stimulation. Thromb Haemost. 2011;105:487–495. doi: 10.1160/TH10-07-0439. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q., Huang J.H., Xia R.P., Duan X.H., Jiang Y.B., Jiang Q. Suppression of experimental abdominal aortic aneurysm in a rat model by the phosphodiesterase 3 inhibitor cilostazol. J Surg Res. 2011;167:e385–e393. doi: 10.1016/j.jss.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Umebayashi R., Uchida H.A., Kakio Y., Subramanian V., Daugherty A., Wada J. Cilostazol attenuates angiotensin II-induced abdominal aortic aneurysms but not atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2018;38:903–912. doi: 10.1161/ATVBAHA.117.309707. [DOI] [PubMed] [Google Scholar]
- 21.Brass E.P., Cooper L.T., Morgan R.E., Hiatt W.R. A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication. J Vasc Surg. 2012;55 doi: 10.1016/j.jvs.2011.09.004. 9.e1. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida H., Ashikawa Y., Itoh S., Nakagawa T., Asanuma A., Tanabe S. K-134, a phosphodiesterase 3 inhibitor, prevents brain damage by inhibiting thrombus formation in a rat cerebral infarction model. PLoS One. 2012;7:e46432. doi: 10.1371/journal.pone.0046432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki Y., Suzuki H., Itoh S., Yoshida H., Kondo S., Inoue K. K-134, a phosphodiesterase 3 inhibitor, improves gait disturbance and hindlimb blood flow impairment in rat peripheral artery disease models. Eur J Pharmacol. 2012;689:132–138. doi: 10.1016/j.ejphar.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H., Zaima N., Sasaki T., Sano M., Yamamoto N., Saito T. Hypoperfusion of the adventitial vasa vasorum develops an abdominal aortic aneurysm. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka H., Unno N., Yata T., Kugo H., Zaima N., Sasaki T. Creation of a rodent model of abdominal aortic aneurysm by blocking adventitial vasa vasorum perfusion. J Vis Exp. 2017;129:55763. doi: 10.3791/55763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daugherty A., Manning M.W., Cassis L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega R., Collado A., Selles F., Gonzalez-Navarro H., Sanz M.J., Real J.T. SGLT-2 (Sodium-Glucose Cotransporter 2) inhibition reduces Ang II (Angiotensin II)-induced dissecting abdominal aortic aneurysm in ApoE (Apolipoprotein E) knockout mice. Arterioscler Thromb Vasc Biol. 2019;39:1614–1628. doi: 10.1161/ATVBAHA.119.312659. [DOI] [PubMed] [Google Scholar]
- 28.Hamblin M., Chang L., Zhang H., Yang K., Zhang J., Chen Y.E. Vascular smooth muscle cell peroxisome proliferator-activated receptor-gamma deletion promotes abdominal aortic aneurysms. J Vasc Surg. 2010;52:984–993. doi: 10.1016/j.jvs.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka H., Zaima N., Sasaki T., Hayasaka T., Goto-Inoue N., Onoue K. Adventitial vasa vasorum arteriosclerosis in abdominal aortic aneurysm. PLoS One. 2013;8:e57398. doi: 10.1371/journal.pone.0057398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reilly M.P., Mohler E.R., 3rd Cilostazol: treatment of intermittent claudication. Ann Pharmacother. 2001;35:48–56. doi: 10.1345/aph.19408. [DOI] [PubMed] [Google Scholar]
- 31.Aslam M., Pfeil U., Gunduz D., Rafiq A., Kummer W., Piper H.M. Intermedin (adrenomedullin2) stabilizes the endothelial barrier and antagonizes thrombin-induced barrier failure in endothelial cell monolayers. Br J Pharmacol. 2012;165:208–222. doi: 10.1111/j.1476-5381.2011.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Q., Ni X.Q., Lu W.W., Zhang J.S., Ren J.L., Wu D. Intermedin reduces neointima formation by regulating vascular smooth muscle cell phenotype via cAMP/PKA pathway. Atherosclerosis. 2017;266:212–222. doi: 10.1016/j.atherosclerosis.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi S., Oida K., Fujiwara R., Maeda H., Hayashi S., Takai H. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol. 1992;20:900–906. doi: 10.1097/00005344-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Lu W.W., Jia L.X., Ni X.Q., Zhao L., Chang J.R., Zhang J.S. Intermedin1-53 attenuates abdominal aortic aneurysm by inhibiting oxidative stress. Arterioscler Thromb Vasc Biol. 2016;36:2176–2190. doi: 10.1161/ATVBAHA.116.307825. [DOI] [PubMed] [Google Scholar]
- 35.Shigematsu H. The novel phosphodiesterase 3 inhibitor K-134 improves walking performance in Japanese patients with intermittent claudication. Circulation. 2011;124:A14430. [Google Scholar]
- 36.Kazi M., Thyberg J., Religa P., Roy J., Eriksson P., Hedin U. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 37.Parr A., McCann M., Bradshaw B., Shahzad A., Buttner P., Golledge J. Thrombus volume is associated with cardiovascular events and aneurysm growth in patients who have abdominal aortic aneurysms. J Vasc Surg. 2011;53:28–35. doi: 10.1016/j.jvs.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michel J.B., Martin-Ventura J.L., Egido J., Sakalihasan N., Treska V., Lindholt J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foley J.H., Conway E.M. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 40.Vorp D.A., Lee P.C., Wang D.H., Makaroun M.S., Nemoto E.M., Ogawa S. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34:291–299. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 41.Liu O., Jia L., Liu X., Wang Y., Wang X., Qin Y. Clopidogrel, a platelet P2Y12 receptor inhibitor, reduces vascular inflammation and angiotensin II induced-abdominal aortic aneurysm progression. PLoS One. 2012;7:e51707. doi: 10.1371/journal.pone.0051707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owens A.P., 3rd, Edwards T.L., Antoniak S., Geddings J.E., Jahangir E., Wei W.Q. Platelet inhibitors reduce rupture in a mouse model of established abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:2032–2041. doi: 10.1161/ATVBAHA.115.305537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo K., Umemura K., Miyaji M., Nakashima M. Milrinone, a phosphodiesterase inhibitor, suppresses intimal thickening after photochemically induced endothelial injury in the mouse femoral artery. Atherosclerosis. 1999;142:133–138. doi: 10.1016/s0021-9150(98)00203-2. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka H., Unno N., Suzuki Y., Sano H., Yata T., Urano T. Hypoperfusion of the aortic wall secondary to degeneration of adventitial vasa vasorum causes abdominal aortic aneurysms. Curr Drug Targets. 2018;19:1327–1332. doi: 10.2174/1389450119666180122154409. [DOI] [PubMed] [Google Scholar]
- 45.Bjornheden T., Levin M., Evaldsson M., Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol. 1999;19:870–876. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- 46.Xu J., Lu X., Shi G.P. Vasa vasorum in atherosclerosis and clinical significance. Int J Mol Sci. 2015;16:11574–11608. doi: 10.3390/ijms160511574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent K.C., Zwolak R.M., Egorova N.N., Riles T.S., Manganaro A., Moskowitz A.J. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 48.Sano M., Unno N., Sasaki T., Baba S., Sugisawa R., Tanaka H. Topologic distributions of vasa vasorum and lymphatic vasa vasorum in the aortic adventitia-implications for the prevalence of aortic diseases. Atherosclerosis. 2016;247:127–134. doi: 10.1016/j.atherosclerosis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama T., Kurobe H., Sugasawa N., Kinoshita H., Higashida M., Matsuoka Y. Role of macrophage-derived hypoxia-inducible factor (HIF)-1alpha as a mediator of vascular remodelling. Cardiovasc Res. 2013;99:705–715. doi: 10.1093/cvr/cvt146. [DOI] [PubMed] [Google Scholar]
- 50.Kim H.W., Blomkalns A.L., Ogbi M., Thomas M., Gavrila D., Neltner B.S. Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am J Physiol Heart Circ Physiol. 2017;313:H1168–H1179. doi: 10.1152/ajpheart.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]