Abstract
Objective
Novel therapeutic angiogenic concepts for critical limb ischemia are still needed for limb salvage. E-selectin, a cell-adhesion molecule, is vital for recruitment of the stem/progenitor cells necessary for neovascularization in ischemic tissues. We hypothesized that priming ischemic limb tissue with E-selectin/adeno-associated virus (AAV) gene therapy, in a murine hindlimb ischemia and gangrene model, would increase therapeutic angiogenesis and improve gangrene.
Methods
FVB/NJ mice were given intramuscular hindlimb injections of either E-selectin/AAV or LacZ/AAV and then underwent induction of gangrene via femoral artery ligation and concomitant systemic injections of the nitric oxide synthesis inhibitor L-NAME (L-NG-Nitro arginine methyl ester; 40 mg/kg). Gangrene was evaluated via the Faber hindlimb appearance score. The rate of ischemic limb reperfusion and ischemic tissue angiogenesis were evaluated using laser Doppler perfusion imaging and DiI perfusion with confocal laser scanning microscopy of the ischemic footpads, respectively. The treadmill exhaustion test was performed on postoperative day (POD) 8 to determine hindlimb functionality.
Results
The E-selectin/AAV–treated mice (n = 10) had decreased Faber ischemia scores compared with those of the LacZ/AAV–treated mice (n = 7) at both PODs 7 and 14 (P < .05 and P < .01, respectively), improved laser Doppler perfusion imaging reperfusion indexes by POD 14 (P < .01), and greater gangrene footpad capillary density (P < .001). E-selectin/AAV–treated mice also had improved exercise tolerance (P < .05) and lower relative muscular atrophy (P < .01).
Conclusion
We surmised that E-selectin/AAV gene therapy would significantly promote hindlimb angiogenesis, reperfusion, and limb functionality in mice with hindlimb ischemia and gangrene. Our findings highlight the reported novel gene therapy approach to critical limb ischemia as a potential therapeutic option for future clinical studies.
Keywords: Critical limb ischemia; E-selectin, Gangrene; Therapeutic angiogenesis
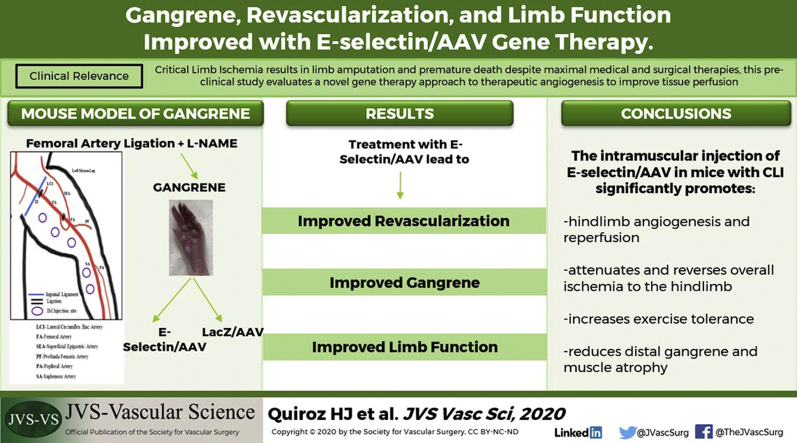
Clinical Relevance
In the United States, >150,000 limb amputations are performed annually for critical limb ischemia (CLI) despite the use of medical and surgical therapy. Thus, novel therapeutic angiogenic concepts for CLI are still needed for limb salvage. We used a novel gene therapy approach in a mouse model of gangrene, the most severe form of CLI, to demonstrate the efficacy of our gene therapy with E-selectin. Preclinical studies such as ours are a vital step in the development of new therapies for CLI patients with threatened limbs and no further medical or surgical options.
Visual abstract
Article Highlights.
-
•
Type of Research: A mouse model of tissue gangrene
-
•
Key Findings: Gene therapy using an E-selectin/adeno-associated virus vector improves gangrene, tissue perfusion, and limb functionality.
-
•
Take Home Message: The results of our preclinical study have demonstrated the novel usage of an adeno-associated virus vector coupled with E-selectin as an effective and biosafe therapeutic angiogenic and regenerative concept.
Peripheral artery disease (PAD) affects ≤10 million people in the United States. It is often incurable and can progress to the end-stage condition, critical limb ischemia (CLI), which develops in ∼20% of all PAD patients.1 Patients with CLI have an overall mortality approaching 50% at 5 years and 70% at 10 years.2,3 Patients with diabetes mellitus have an increased risk of PAD4, 5, 6 and CLI, with the development of lower extremity ischemic ulcers or gangrene (tissue death due to lack of blood supply), which leads to greater rates of lower extremity amputation.7, 8, 9 Our laboratory first described a deficit in stromal cell-derived factor-1-alpha (SDF-1α; an important angiogenic factor) in the diabetic phenotype, which accounts for the more severe impairment in vascularization seen in patients with diabetes mellitus.10 Current therapies, such as combined medical and surgical approaches, are often ineffectual, and >150,000 lower limb amputations occur annually in the United States owing to the failure currently available therapeutic options.11 Thus, novel therapeutic angiogenic concepts are needed to attempt limb salvage and preservation of limb function in this vulnerable patient population.
Therapeutic angiogenesis, which induces postnatal neovascularization and the promotion of collateral vessel formation, is a promising modality that can lead to improved clinical outcomes in patients with CLI. This concept has been shown feasible via gene- and cell-based therapies, which include recombinant proteins, genes, and stem cells.1,12, 13, 14, 15, 16, 17 However, adhesion molecules have not been tested as a therapeutic proangiogenic and proregenerative gene therapy modality. Thus, we sought to test alternative gene targets that do not encode soluble factors yet are critical elements for therapeutic angiogenesis and tissue healing and regeneration. E-selectin, an inducible cell-adhesion molecule, is involved in the regulation of angiogenesis and inflammatory responses. It was originally known to mediate adhesive interactions of the activated vascular endothelium with circulating leukocytes/monocytes in response to inflammatory processes such as rheumatoid arthritis and atherosclerosis18,19 and plays a role in the homing of hematopoietic stem cells20,21 and endothelial progenitor cells (EPCs).22,23 We have recently demonstrated the critical role of cell-bound E-selectin expression in ischemic tissue, regulated by SDF-1α, for the recruitment of EPCs and EPC-dependent angiogenesis24 and have demonstrated improved wound healing after gene therapy with an adeno-associated virus (AAV) vector, which encodes E-selectin.25 Therefore, we sought to test the effect of priming hindlimb tissue with an E-selectin/AAV vector to induce overexpression of cell surface E-selectin and modify the ischemic limb tissue microenvironment in a mouse model of gangrene, the most severe form of CLI. We hypothesized that E-selectin–primed tissue would ultimately promote angiogenesis and regeneration in ischemic, gangrenous tissue and could improve clinically relevant signs and symptoms such as muscular atrophy and functionality.
Methods
AAV production
Full-length murine genes of E-selectin and LacZ were inserted into multiple cloning sites in the pZac vector and confirmed by gene sequencing. E-selectin/pZac and LacZ/pZac plasmids were then sent to University of North Carolina Gene Therapy Vector Core, where AAV serotype 2/9 (AAV2/9) was packaged. The preparations were performed per standard protocol using the three-plasmid transfection into HEK293 cells.26 Quality assurance and control testing included quantitative polymerase chain reaction quantification of AAV genomes, determination of the infectivity titer, and tests for replication competent AAV.26
Gene therapy priming
FVB/NJ male mice (catalog no. 001800; Jackson Laboratory, Bar Harbor, Me) were given in vivo intramuscular injections of 5 × 1012 viral genome of either E-selectin/AAV2/9 or LacZ/AAV2/9 suspended in phosphate-buffered saline (PBS) at five sites into the left medial thigh and calf tissue (20 μL per injection site using a 31-gauge needle). The local injection was performed preoperatively (4 and 2 days before surgery) and again immediately after femoral artery and vein ligation (FAL). Gene therapy was administered before surgery to compensate for the lag time from vector injection to E-selectin gene expression in vivo.27
Creation of a mouse CLI gangrene model
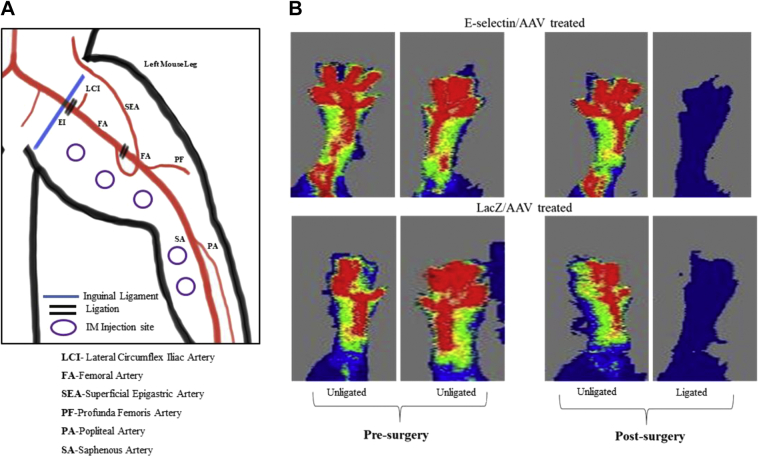
Creation of the hindlimb ischemia and foot gangrene mouse model was performed as previously described.28 The model uses a combination of FAL and intraperitoneal injections of L-NAME (L-NG-Nitro arginine methyl ester), which causes systemic vascular constriction and, overall, results in a reliable murine model of hindlimb gangrene without causing autoamputation of the hindlimb. This model allows for investigators to test treatment strategies to determine whether tissue salvage is feasible with gangrene. In brief, 8- to 10-week-old male FVB/NJ mice (Jackson Laboratory) were cared for and treated in accordance with the Policy on Humane Care and Use of Laboratory Animals and received institutional animal care and use committee approval at the University of Miami Miller School of Medicine (approval no. 16-188). Male mice were used to decreased the potential confounding effects of ovarian steroid hormones on angiogenesis.29 Before surgery, L-NAME (40 mg/kg), a nitric oxide synthesis inhibitor, was given by intraperitoneal injection 2 hours before surgery and, thereafter, on postoperative days (PODs) 1 to 3 after FAL. For FAL, the mice were anesthetized with ketamine 100 mg/kg and xylazine 10 mg/kg intraperitoneally. The hair was sheared from the hindlimbs, and an incision was made to expose the femoral sheath. The femoral artery and vein were dissected and separated from the femoral nerve distally at the saphenopopliteal bifurcation and proximally below the inguinal ligament. The artery and vein were both ligated at these two locations using 7-0 silk sutures, and the intervening segment between sutures was severed with a single incision (Supplementary Fig 1, A). Finally, the skin incision was closed using 7-0 silk suture in a continuous fashion. A single dose of sustained-release buprenorphine (1.0 mg/kg; ZooPharm, Wedgewood Pharmacy, Swedesboro, NJ) was subcutaneously administered for postoperative analgesia.
Supplementary Fig 1.
A, Peripheral vascular anatomy in mice. Femoral artery ligation (FAL) was performed via proximal and distal ligation (double lines) located below the inguinal ligament and immediately above the saphenopopliteal bifurcation. The injection sites were in 5 areas in the left (ligated) medial thigh and calf muscles. B, Preoperative laser Doppler images showing normal perfusion distribution in bilateral limbs of the mice and postoperative laser Doppler images showing successful cessation of limb perfusion in the FAL limb (E-selectin/adeno-associated virus [AAV], n = 10; LacZ/AAV, n = 7).
Evaluating hindlimb ischemia
All FVB mice were evaluated daily for 2 weeks postoperatively for signs of distal hindlimb ischemia. The Faber hindlimb ischemia score was used (0, normal; 1-5, cyanosis or loss of nails, with the score reflecting the number of nails affected; 6-10, partial or complete atrophy of digits, with the score reflecting the number of affected digits; and 11-12, partial atrophy or gangrene of forefoot).30,31 The ischemia scores were compared between the E-selectin/AAV– and LacZ/AAV–treated mice in a blinded manner.
Laser Doppler imaging
Laser Doppler imaging (LDI) was performed using the LDI2-HR System (Moor Instruments, Wilmington, Del). LDI was performed in a blinded manner on the ligated and nonligated legs preoperatively, immediately after FAL, and on PODs 7 and 14. As previously reported, the mice were lightly anesthetized28,30,31 and placed on a heating pad to maintain their core body temperature at 37°C. Images of the plantar foot were of particular interest because this area most effectively demonstrates reperfusion on LDI. The hindlimb reperfusion index was defined as a ratio of the ligated to nonligated leg for each individual mouse.
Live animal DiI perfusion and confocal laser scanning microscopy
The lipophilic carbocyanine dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) was used for vascular perfusion and staining, as previously described.28,32,33 In brief, intracardiac perfusion of filtered PBS, followed by 10 mL of DiI, and, finally, 10% neutral formalin for tissue fixation was performed on POD 14. The area of interest was the footpad, given our interest in distal hindlimb reperfusion. The skinned murine footpad was compressed between two glass microscopic slides using binder clips to allow for adequate imaging using the Zeiss LSM 510 confocal laser scanning microscope (Zeiss, Oberkochen, Germany). The Z-series obtained from confocal imaging were reconstructed into three-dimensional images and analyzed using ImageJ, an image processing program developed at the National Institutes of Health (Bethesda, Md), and the Laboratory for Optical and Computational Instrumentation (University of Wisconsin, Madison, Wis). The intricate network of vessels regenerated in the foot was captured, quantified, and represented by the mean capillary density (volume of total DiI-stained vessels) for each mouse.
Histologic examination
Immunofluorescent staining and hematoxylin-eosin staining were performed in accordance with a previously reported protocol.24,34 Tissue section slides were deparaffinized, hydrated, washed, and blocked with protein-blocking serum (Dako, Carpinteria, Calif) for 1 hour at 25°C. To verify E-selectin transgene expression, the slides were incubated with anti–E-selectin/CD62E (Alexa Fluor 488; Novus Biologicals, Centennial, Colo) 5 μg/mL and endothelial cell marker CD31/PECAM-1 (Alexa Fluor 647; Novus Biologicals) 15 μg/mL in protein blocking serum for 12 hours at 4°C. The slides were then washed with 0.1% Tris-buffered saline plus Tween 20 before DAPI (4′,6-diamidino-2-phenylindole) staining (Vector Laboratories, Inc, Burlingame, Calif) for nuclei visualization. A confocal laser scanning microscope with ×20 to ×40 magnification was used to image immunofluorescence. To detect EPCs (defined in the present study as CD34+ endothelial cells), we co-stained hindlimb tissue sections with CD34 (Alexa Fluor 488; Santa Cruz Biotechnology, Dallas, Tex) and endothelial cell marker CD31/PECAM-1 (Alexa Fluor 647; Novus Biologicals). To evaluate the potentially proinflammatory nature of E-selectin, we stained control and treatment hindlimb tissue sections with Mac-2/galectin 3 (Alexa Fluor 594; BioLegend, San Diego, Calif), an inflammatory marker for monocytes/macrophages. For quantification of E-selectin, CD31, CD34, and Mac-2, at least five random ×10 or ×20 magnification fields for each section were blindly scored and/or measured using ImageJ (National Institutes of Health) or Adobe Photoshop CC (Adobe, San Jose, Calif). To determine the amount of muscular atrophy in the ligated limbs of the mice after FAL, hematoxylin-eosin staining was performed on paraffin-embedded transverse muscle tissue sections of both treatment groups, as previously described.35 Images were then acquired at ×10 magnification, and the relative size of the E-selectin/AAV–treated myocytes were set and compared with the myocyte size of the LacZ/AAV–treated group.
Treadmill exhaustion test
Exercise treadmill testing was performed using a rodent treadmill, as previously described36 (model, Exer 3/6; Columbus Instruments, Columbus, Ohio), 8 days after FAL to determine the degree of hindlimb functionality and recovery after gene therapy. In the present study, our protocol started with a 10° slope at a speed of 10 m/min for 5 minutes. The mice were placed on a horizontal treadmill, and the speed was increased by 5 m/min every 5 minutes until a maximum speed of 30 m/min had been reached. The mice were allowed to run for a total of 60 minutes, at which point the test was concluded. The total distance the mice had run during the 60 minutes was recorded and compared between the treatment and control groups. If the mouse remained on the stimulation plate for 10 seconds without any attempt to reengage the treadmill,36 the time to exhaustion (expressed in minutes) and total distance traversed was recorded, as this was defined as exhaustion. Thus, use of the treadmill test allowed us to investigate the running capability of the mice in both treatment and control groups postoperatively.
Evaluating potential prothrombotic effects of E-selectin gene therapy delivered intramuscularly to ischemic limb
To evaluate the potentially prothrombotic effects of E-selectin limb intramuscular gene therapy in vivo, the D-dimer levels were measured in serum. Blood sampling of the E-selectin/AAV– and LacZ/AAV–treated mice was performed on POD 5 to determine the D-dimer levels in serum.
Statistical analysis
The data were parametric and are presented as the mean ± standard deviation. Two-tailed Student's t tests were performed to compare the mean values of two groups. Probability values of P < .05 were interpreted to denote statistical significance.
Results
Effective method of gene delivery after in vivo injection of E-selectin/AAV and LacZ/AAV viral particles
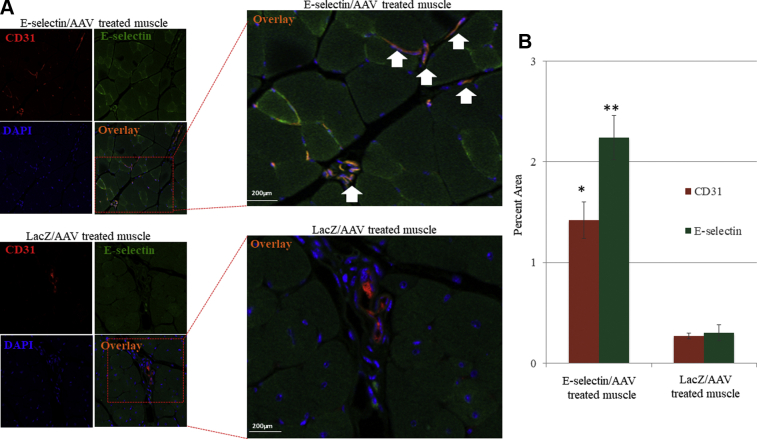
We observed that the E-selectin/AAV–treated mice manifested a greater confluence of the E-selectin (green) and endothelial cell CD31 (red) co-staining, shown by orange, compared with that of the LacZ/AAV–treated mice (Fig 1, A). Quantitatively, the percent area of E-selectin and CD31 co-staining was significantly greater statistically in the E-selectin/AAV–treated muscle vs the LacZ/AAV–treated muscle (Fig 1, B). These results confirmed that AAV2/9-mediated gene delivery effectively increased E-selectin expression of the microvasculature within the ischemic limb tissues of the mice.
Fig 1.
Expression of transgene E-selectin in ischemic tissue. A, Representative image of immunofluorescence of CD31 (red) and E-selectin (green). Overlay images demonstrate higher expression of E-selectin in endothelial cells in ischemic tissue of E-selectin/adeno-associated virus (AAV)–treated muscle vs LacZ/AAV–treated muscle. B, Quantitatively, a two-tailed Student's t test was used to compare the mean values of each treatment group. E-selectin/AAV–treated ischemic tissue had a significantly greater amount of E-selectin (∗∗P < .01) and CD31 (∗P = .01) expression. LacZ/AAV treatment, n = 7; E-selectin/AAV treatment, n = 10.
E-selectin/AAV gene therapy increased limb function performance, enhanced exercise tolerance (running distance), and decreased limb skeletal muscle atrophy
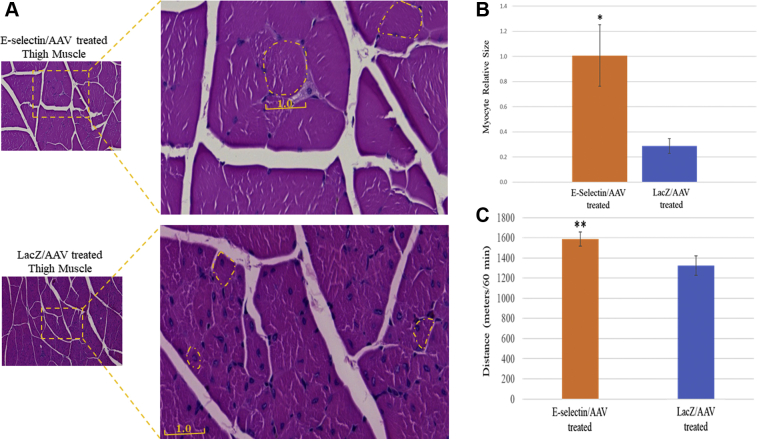
We observed less muscle atrophy in the ischemic limbs treated with E-selectin/AAV gene therapy compared with that in those treated by LacZ/AAV. The myocytes in the E-selectin/AAV–treated mice appeared healthier with a larger relative size (1 ± 0.24) compared with those in the control mice (0.3 ± 0.6; Fig 2, A and B). The E-selectin/AAV–treated mice were found to traverse a statistically significantly greater distance in 60 minutes compared with the LacZ/AAV–treated mice (Fig 2, C). Specifically, the mice in the treatment group were able to run a mean of 1590 m within 60 minutes and the mice in the control group were able to run a mean of 1325 m within 60 minutes (P < .05). Overall, our data have demonstrated that gene therapy with E-selectin/AAV improves limb function recovery and mitigates skeletal muscle atrophy in this murine model of induced CLI and gangrene.
Fig 2.
Increased muscular stamina and decreased atrophy in mice treated with E-selectin/ adeno-associated virus (AAV) gene therapy. A, Representative images of transverse thigh muscle section with hematoxylin-eosin staining. B, Cross-sectional size of individual myocytes, E-selectin/AAV–treated myocytes size set at 1.0. LacZ/AAV–treated myocytes were 30% less than the size of E-selectin/AAV–treated myocytes; the size was significantly larger with E-selectin/AAV treatment (∗∗P < .01). C, Treadmill exhaustion function test in both E-selectin/AAV–treated mice and LacZ/AAV–treated mice on postoperative day (POD) 8. The distance traveled in meters within 60 minutes was measured and compared between groups. The E-selectin/AAV–treated mice had run a significantly greater distance in 60 minutes compared with the LacZ/AAV–treated mice (∗P < .05). Statistical analysis was performed via the two-tailed Student t test. E-selectin/AAV–treated mice, n = 10; LacZ/AAV–treated mice, n = 7.
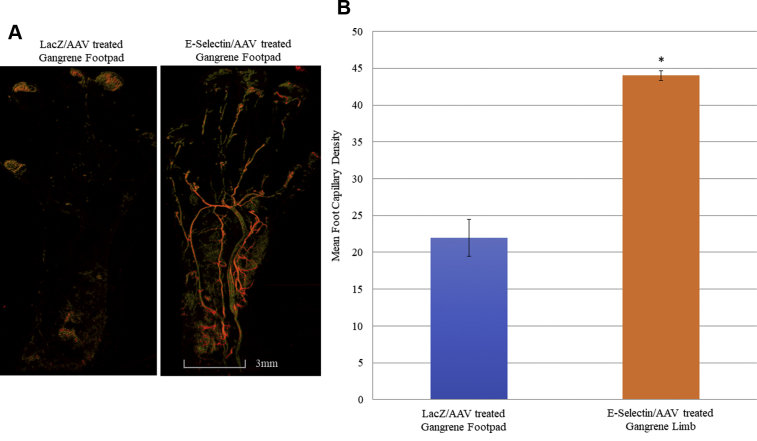
E-selectin/AAV gene therapy increased vessel density in the murine gangrene footpad
Based on confocal microscopy images of DiI-stained blood vessels, the unligated limbs of both E-selectin/AAV– and LacZ/AAV–treated mice showed comparable vessel densities in the normal footpad postoperatively (Supplementary Fig 2). However, a statistically significant difference was found in the number of capillaries in the gangrene footpads of the ligated hindlimbs of the E-selectin/AAV–treated mice compared with the LacZ/AAV–treated mice (Fig 3, A and B). The LacZ/AAV–treated mice showed a scarcity in capillary density postoperatively in the ischemic and gangrenous footpad of the ligated hindlimb (Fig 3, A). Quantitatively, the capillary density within the gangrene footpad of the E-selectin/AAV– and LacZ/AAV–treated mice was 44 and 22 capillaries per myofibril cross-sectional area, respectively (Fig 3, B). Taken together, these data have demonstrated that gene therapy with E-selectin/AAV induces increased vessel density in the footpad in mice with surgically induced hindlimb ischemia and distal gangrene of the digits.
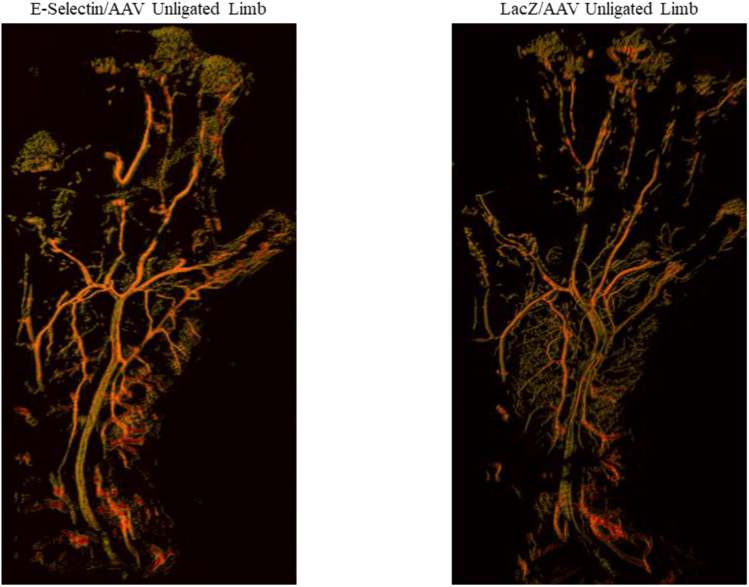
Supplementary Fig 2.
Representative images via confocal laser scanning microscopy of the normal, unligated footpads of mice treated with E-selectin/adeno-associated virus (AAV) and LacZ/AAV. Analysis using two-tailed Student’s t test showed no statistically significant difference in the capillary density between the two groups (E-selectin/AAV treatment, n = 5; LacZ/AAV treatment, n = 5).
Fig 3.
Evaluation of vessel density within the ischemic, gangrenous footpads of the murine ligated hindlimb with E-selectin/adeno-associated virus (AAV) vs control (LacZ/AAV) gene therapy. Treatment with E-selectin/AAV gene therapy resulted in increased footpad vessel density. A, Representative images showing DiI perfusion capillary density was improved in treatment vs control groups on postoperative day (POD) 14. B, Quantification of capillary density in ischemic, gangrene footpads of both E-selectin/AAV treatment (n = 5) and LacZ/AAV treatment (n = 5); E-selectin/AAV–treated ischemic footpad capillary density was 44 capillaries per myofibril cross-sectional area, and LacZ/AAV–treated footpad capillary density was 22 capillaries per myofibril cross-sectional area (∗P < .001, two-tailed Student's t test).
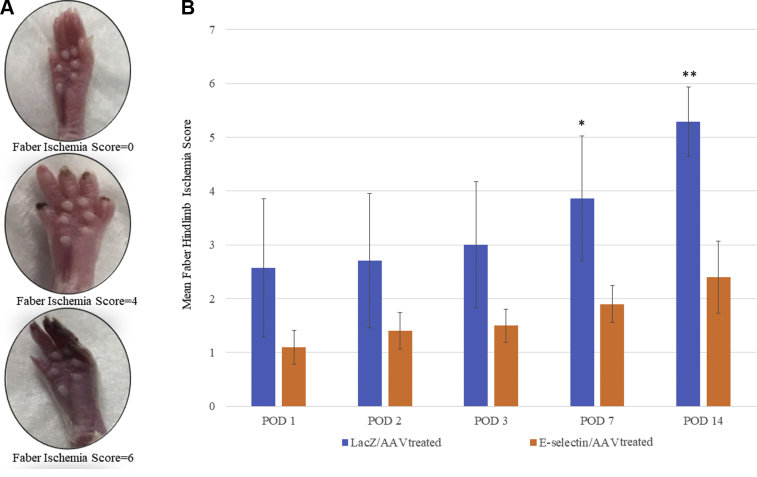
E-selectin/AAV gene therapy improved the hindlimb ischemia score in the murine model of distal gangrene
The E-selectin/AAV–treated mice demonstrated fewer visual signs of ischemia/gangrene to the distal hindlimb and digits compared with the LacZ/AAV–treated mice (Fig 4, A and B) and demonstrated lower Faber hindlimb ischemia scores on each postoperative day compared with the LacZ/AAV–treated mice. The difference in CLI severity between the treatment and control groups reached statistical significance by POD 7 (P < .05) and was maintained through POD 14 (P < .01; Fig 4, B). On POD 14, the E-selectin/AAV mice had a mean Faber ischemia score of ∼2, equating to two ischemic or gangrene nails. In contrast, the LacZ/AAV mice had a mean Faber ischemia score of ∼5, equivalent to five ischemic or gangrene nails (Fig 4, B). These data revealed that E-selectin/AAV gene therapy, not only promoted therapeutic angiogenesis, but also reduced the signs of CLI, such as the extent of gangrene and tissue loss related to severe limb ischemia.
Fig 4.
Postoperative Faber ischemia scores in murine gangrene model improved with E-selectin/adeno-associated virus (AAV) treatment vs LacZ/AAV treatment. A, Representative images of Faber score 0, 4, and 6 (top to bottom). B, E-selectin/AAV treated mice demonstrated a significantly lower ischemia score in their ischemic limbs on postoperative day (POD) 7 (∗P < .05) and POD 14 (∗∗P < .01) compared with the ischemic limbs of LacZ/AAV–treated mice using a two-tailed Student's t test. LacZ/AAV, n = 7; E-selectin/AAV, n = 10.
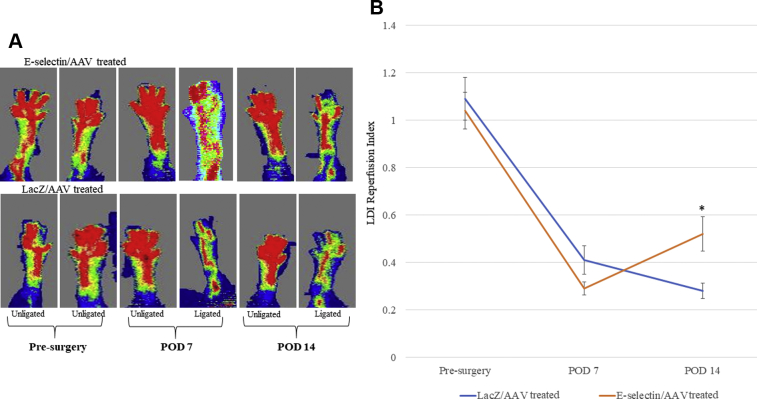
E-selectin/AAV gene therapy increased hindlimb skin reperfusion
The LDI measurements performed preoperatively and immediately postoperatively were comparable between the two groups, with both groups of mice manifesting normal perfusion to the foot before surgery and severely diminished perfusion to the foot immediately after surgery (Supplementary Fig 1, B). However, by POD 14, the mice treated with E-selectin/AAV demonstrated statistically significantly increased distal hindlimb reperfusion indexes on LDI compared with their LacZ/AAV counterparts (Fig 5, A and B; P < .01). Overall, these results revealed an increased level of reperfusion in the ischemic hindlimbs treated with E-selectin/AAV, as evidenced by the increased perfusion seen on LDI and that the increased reperfusion occurred at the skeletal muscle, skin, and subcutaneous levels (Fig 1, Fig 3, and 5).
Fig 5.
Laser Doppler imaging (LDI) demonstrated improved ischemic limb reperfusion in E-selectin/adeno-associated virus (AAV)–treated mice vs LacZ/AAV–treated mice by postoperative day (POD) 14. A, Representative images of LDI at various days between treatment and control groups. B, Quantitative reperfusion index at various days for treatment and control groups (reperfusion index = ligated/unligated hindlimb). A two-tailed Student's t test was used to compare the mean values of each treatment group. By POD 14, the E-selectin/AAV–treated mice demonstrated significantly greater foot reperfusion vs LacZ/AAV–treated mice (∗P < .01). LacZ/AAV, n = 7; E-selectin/AAV, n = 10.
E-selectin/AAV gene therapy increased recruitment of EPCs
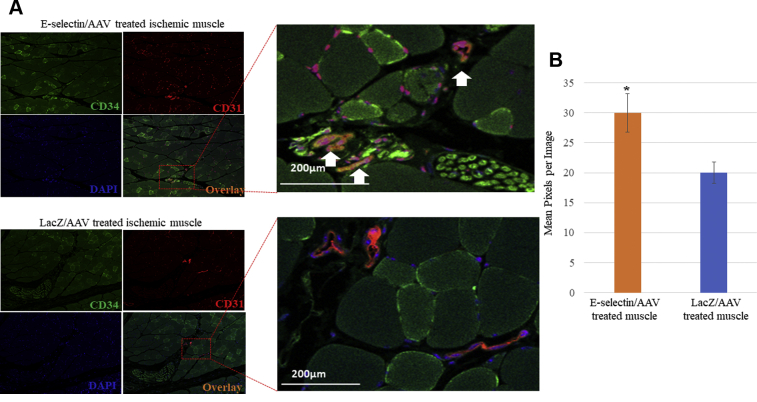
We found greater amounts of CD34+/CD31+ cells, which are surrogate markers of EPCs, in mice treated with E-selectin/AAV compared with those treated with LacZ/AAV (Fig 6, A and B). This suggests that the increased availability of EPCs in the ischemic hindlimb of mice primed with E-selectin/AAV might play a key mechanistic role in postnatal limb neovascularization and skeletal muscle function preservation, which was imputed in previous studies.37, 38, 39
Fig 6.
Gene therapy with E-selectin/adeno-associated virus (AAV) increased recruitment of endothelial progenitor cells (EPCs) in ischemic tissue. A, Representative images of immunofluorescent staining of CD34 (green) and CD31 (red) of ischemic muscle tissues of E-selectin/AAV–treated mice and LacZ/AAV–treated mice. E-selectin/AAV–treated ischemic tissue demonstrates increased expression of CD34 and CD31. B, Quantitative levels of CD34/CD31 per image in mice undergoing E-selectin/AAV treatment vs LacZ/AAV treatment. Student's t test revealed a significant increase in CD34/CD31 levels in E-selectin/AAV–treated ischemic tissue vs LacZ/AAV–treated ischemic tissue (P < .05). E-selectin/AAV sections, n = 15; LacZ/AAV sections, n = 15.
E-selectin/AAV gene therapy did not appear to induce inflammation, thrombosis, or systemic toxicity in the ischemic mouse model
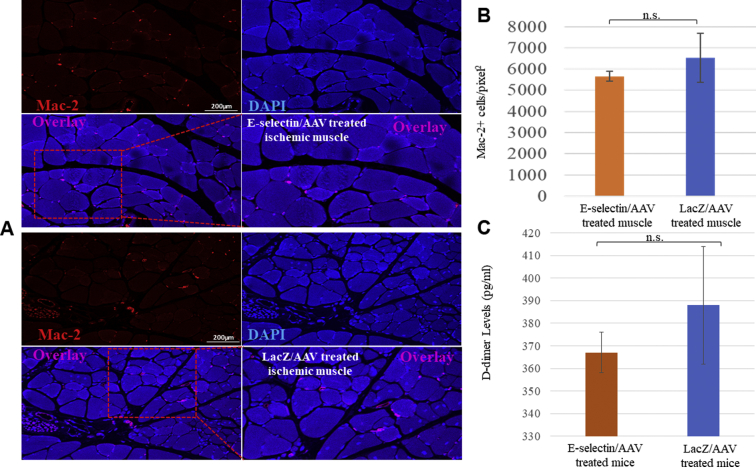
These two experimental groups of mice (E-selectin/AAV and LacZ/AAV) showed comparable expression of the Mac-2+ cell marker (Fig 7, A and B), indicating that E-selectin/AAV gene therapy does not appear to induce a stronger proinflammatory response. Our analysis revealed no statistically significant differences in this marker of thrombosis in vivo between the E-selectin/AAV– and LacZ/AAV–treated mice (Fig 7, C), suggesting that mice receiving E-selectin/AAV gene therapy are not more likely to develop clots than mice treated with the LacZ control vector. We did not find any abnormalities in liver and kidney function test results among the mice treated with E-selectin/AAV, LacZ/AAV, or PBS (control, no virus), with no statistically significant differences among the three groups (Supplementary Table). These data together have demonstrated that E-selectin/AAV gene therapy does not appear to induce inflammation or thrombosis or cause major organ toxicity in the ischemic/gangrene mouse model and suggest that this novel gene therapy is generally nontoxic to these key metabolic organs in the short term.
Fig 7.
Gene therapy via E-selectin/adeno-associated virus (AAV) did not seem to significantly induce inflammation or thrombosis. A, Representative images of immunofluorescence of Mac-2+ cells in E-selectin/AAV–treated ischemic muscle and LacZ/AAV–treated ischemic muscle. B, Quantification of Mac-2+ cells per square pixel in ischemic muscle in E-selectin/AAV–treated mice vs LacZ/AAV–treated mice. Student's t test revealed no significant differences in expression of Mac-2 in ischemic muscle tissues between E-selectin/AAV treatment and LacZ/AAV treatment (P = .602). C, Serum D-dimer levels for E-selectin/AAV–treated mice and LacZ/AAV–treated mice. No significant difference was found in serum D-dimer levels at postoperative day (POD) 5 between treatment and control groups (P = .6). E-selectin/AAV sections, n = 15; LacZ/AAV sections, n = 15. DAPI, 4′,6-Diamidino-2-phenylindole; n.s., not significant.
Discussion
In the present study, we have provided a novel approach for postnatal neovascularization of severely ischemic limb tissues by the usage of gene therapy with E-selectin to prime the ischemic tissue microenvironment via a safe and efficient AAV2/9 vector to augment the body's own neovascularization mechanisms through the enhancement of recruitment of CD34+ progenitor cells.
Under hypoxic conditions, the endothelium in ischemic tissues expresses certain adhesion molecules, including E-selectin owing to stimulation of several hypoxia-inducible factor-1α–induced cytokines and chemokines.23 We previously reported that SDF-1α can upregulate E-selectin expression on endothelial cells in tumor and wound vasculature22 and that E-selectin is involved in EPC-dependent neovascularization via recruitment of circulating leukocytes and hematopoietic stem cells through interaction with counterpart ligands.23,24 In the present study, we have demonstrated, for the first time, to the best of our knowledge, that effective overexpression of the cell-bound E-selectin transgene into ischemic gangrenous hindlimb tissues, not only improves angiogenesis, limb perfusion, and recruitment of CD34+ progenitor cells, but also improves mouse exercise tolerance and ameliorates the atrophy effects of severe ischemia in limb skeletal muscle. Ostensibly, this gene therapy approach primes the microenvironment of the ischemic tissue via overexpression of the cell adhesion molecule E-selectin, which then allows for increased capture of stem/progenitor cells to maximize the neovascularization and healing process.
The results of the present study have demonstrated that gene therapy with E-selectin/AAV confers hindlimb tissue salvage, regeneration of hindlimb blood vessels, and reperfusion of ischemic hindlimbs, with an improved running distance and decreased skeletal muscle atrophy. The tests performed in the CLI mouse model were designed to correlate clinically with how human patients with CLI are monitored. Most patients with CLI are monitored via physiologic testing such as the ankle brachial index (ABI)40 and pulse volume recording waveform analysis41 owing to their relative cost-effective, low-risk nature and typical congruence with clinical features. However, these relatively simple diagnostic tests are not feasible in the mouse; therefore, we used LDI as a correlate test because it allows for determination of blood flow to the limb, much like the ABI and pulse volume recording waveform analysis. Morphologic examinations of the human vasculature such as computed tomography angiography, magnetic resonance angiography, or procedural angiography are used in patients with CLI to determine the vascular anatomy and location of arterial blockage, often before an attempt at revascularization. These examinations are also impractical in the mouse model; therefore, we used confocal microscopy after DiI perfusion as a proxy to determine whether the foot had had increased blood vessel density. Overall, the acceleration of tissue regeneration after CLI in the treatment mice can be appreciated by many different modalities that underscore the beneficial biologic and functional effects of E-selectin/AAV gene therapy. These promising preclinical results suggest potential translatable effects in humans. The ABI could serve as a compass to identify patients in need of treatment who are neither surgical nor endovascular candidates. Although further testing is necessary, we believe that no-option patients with a certain ABI could potentially qualify for preemptive treatment with gene therapy in initial clinical studies to deter disease progression and, potentially, reverse tissue ischemia, thus preventing amputation.
In vivo, E-selectin–mediated adhesion and signaling can contribute to adverse pathophysiologic outcomes such as atherosclerosis, arterial and deep vein thrombosis, ischemia–reperfusion injury, and, conceivably, other cardiovascular diseases.42,43 Because of the potential proinflammatory and thrombogenic nature of either increased soluble E-selectin or systemic generalized cell-bound E-selectin expression, determining the safety profile of this locoregional limb intramuscular gene therapy approach is important. Monocyte/macrophage staining demonstrated comparable levels of Mac-2+ cells between the hindlimbs in the E-selectin/AAV– and LacZ/AAV–treated mice. It is important to recognize, however, that macrophages of differing phenotypes exist, including an activated, proinflammatory macrophage (M1) and a proregulatory macrophage (M2). We did not differentiate between these two macrophage phenotypes, which is a potential area of further study to determine whether E-selectin/AAV treatment results in a change in ischemic tissue macrophage differentiation.44 Furthermore, based on our histologic analysis of the tissue samples, no evidence of microthrombi was noted within the blood vessels, and the serum D-dimer levels were normal and similar between the groups. These results have shown that this novel adhesion molecule-based gene therapy approach is effective and appears to be generally safe in preclinical testing.
Conclusion
The present study has demonstrated the novel usage of an AAV vector coupled with E-selectin as an effective and biosafe therapeutic angiogenic and regenerative concept. The intramuscular injection of E-selectin/AAV in mice with CLI significantly promoted hindlimb angiogenesis and reperfusion, attenuating and reversing overall ischemia to the hindlimb, increasing exercise tolerance, and reducing distal gangrene and muscle atrophy. Our study has highlighted this novel gene therapy approach to CLI as a potential therapeutic option to be tested in future clinical studies.
Author contributions
Conception and design: RV, JP, ZL, OV
Analysis and interpretation: HQ, PP, RLS, YL, HS, ZL, OV
Data collection: HQ, PP, RLS, MR, YL, HS
Writing the article: HQ, PP
Critical revision of the article: HQ, RLS, MR, YL, HS, RVP, JP, ZL, OV
Final approval of the article: HQ, PP, RLS, MR, YL, HS, RVP, JP, ZL, OV
Statistical analysis: HQ, PP
Obtained funding: ZL, OV
Overall responsibility: OV
HQ and PP contributed equally to this article and share co-first authorship.
Acknowledgments
We thank the Histology Core and Image Core, Miller School of Medicine, University of Miami, for assistance with tissue processing, sectioning, and confocal microscopy.
Footnotes
The present study was supported by the National Institutes of HealthVascular Interventions/Innovations and Therapeutic Advances program (grants NHLBl-CSB-HV-2017-01-JS, R01DK-071084, and R01GM081570 to O.V. and Z.-J.L.).
Author conflict of interest: O.V. and Z.-J.L., in conjunction with the University of Miami, hold a patent for “Method for Treating Ischemic Tissue,” licensed to Ambulero Inc. H.J.Q., P.P.P., R.L.S., M.M.R., Y.L., H.S., R.V.P., J.P., Z.J.L., and O.C.V. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Appendix
Supplementary Table.
Liver and kidney function blood test resultsa
| Biochemistry test | E-selectin/AAV | LacZ/AAV | PBS (no virus) | P value | Normal range |
|---|---|---|---|---|---|
| AST, U/L | 123 | 118 | 117 | .91 | 50-270 |
| ALT, U/L | 32 | 40 | 67 | .1 | 29-77 |
| ALP, U/L | 29 | 33 | 31 | .84 | 51-285 |
| Bilirubin, mg/dL | |||||
| Total | 0.5 | 0.5 | 0.8 | .21 | 0.1-0.9 |
| Direct | 0.5 | 0.5 | 0.8 | .46 | 0.1-0.9 |
| Albumin, g/dL | 1.6 | 1.9 | 1.6 | .17 | 2.5-2.8 |
| BUN, mg/dL | 23 | 24 | 21 | .24 | 18-29 |
| Creatinine, mg/dL | 0.1 | 0.2 | 0.1 | .27 | 0.1-0.4 |
AAV, Adeno-associated virus; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; PBS, phosphate-buffered saline.
Intracardiac blood samples were taken before animal sacrifice; no differences were found among mice treated with E-selectin/AAV (n = 4), LacZ/AAV (n = 7), or PBS (no virus; n = 5) via analysis of variance statistical analysis.
References
- 1.Powell R.J. Update on clinical trials evaluating the effect of biologic therapy in patients with critical limb ischemia. J Vasc Surg. 2012;56:264–266. doi: 10.1016/j.jvs.2012.03.255. [DOI] [PubMed] [Google Scholar]
- 2.Van Belle E., Nikol S., Norgren L., Baumgartner I., Driver V., Hiatt W.R. Insights on the role of diabetes and geographic variation in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg. 2011;42:365–373. doi: 10.1016/j.ejvs.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Varu V.N., Hogg M.E., Kibbe M.R. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 4.Marso S.P., Hiatt W.R. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 5.Jaff M.R., White C.J., Hiatt W.R., Fowkes G.R., Dormandy J., Razavi M. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Vasc Med. 2015;20:465–478. doi: 10.1177/1358863X15597877. [DOI] [PubMed] [Google Scholar]
- 6.Kannel W.B., McGee D.L. Update on some epidemiologic features of intermittent claudication: the Framingham study. J Am Geriatr Soc. 1985;33:13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 7.Jude E.B., Oyibo S.O., Chalmers N., Boulton A.J. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 8.Faglia E., Favales F., Quarantiello A., Calia P., Clelia P., Brambilla G. Angiographic evaluation of peripheral arterial occlusive disease and its role as a prognostic determinant for major amputation in diabetic subjects with foot ulcers. Diabetes Care. 1998;21:625–630. doi: 10.2337/diacare.21.4.625. [DOI] [PubMed] [Google Scholar]
- 9.Bild D.E., Selby J.V., Sinnock P., Browner W.S., Braveman P., Showstack J.A. Lower-extremity amputation in people with diabetes: epidemiology and prevention. Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher K.A., Liu Z.J., Xiao M., Chen H., Goldstein L.J., Buerk D.G. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clair D., Shah S., Weber J. Current state of diagnosis and management of critical limb ischemia. Curr Cardiol Rep. 2012;14:160–170. doi: 10.1007/s11886-012-0251-4. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R., Tongers J., Losordo D.W. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sneider E.B., Nowicki P.T., Messina L.M. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem. 2009;108:753–761. doi: 10.1002/jcb.22315. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopalan S., Mohler E.R., III, Lederman R.J., Mendelsohn F.O., Saucedo J.F., Goldman C.K. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh R., Walsh S.R., Tang T.Y., Noorani A., Hayes P.D. Gene therapy as a novel therapeutic option in the treatment of peripheral vascular disease: systematic review and meta-analysis. Int J Clin Pract. 2008;62:1383–1390. doi: 10.1111/j.1742-1241.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- 16.Powell R.J., Simons M., Mendelsohn F.O., Daniel G., Henry T.D., Koga M. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- 17.Belch J., Hiatt W.R., Baumgartner I., Driver I.V., Nikol S., Norgren L. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. doi: 10.1016/S0140-6736(11)60394-2. [DOI] [PubMed] [Google Scholar]
- 18.Bevilacqua M.P., Pober J.S., Mendrick D.L., Cotran R.S., Gimbrone M.A., Jr. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlos T.M., Clark R.S., Franicola-Higgins D., Schiding J.K., Kochanek P.M. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 20.Mazo I.B., Gutierrez-Ramos J.C., Frenette P.S., Hynes R.O., Wagner D.D., von Andrian U.H. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweitzer K.M., Drager A.M., van der Valk P., Thijsen S.F., Zevenbergen A., Theijsmeijer A.P. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z.J., Tian R., An W., Zhuge Y., Li Y., Shao H. Identification of E-selectin as a novel target for the regulation of postnatal neovascularization: implications for diabetic wound healing. Ann Surg. 2010;252:625–634. doi: 10.1097/SLA.0b013e3181f5a079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh I.Y., Yoon C.H., Hur J., Kim J.H., Kim T.Y., Lee C.S. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891–3899. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z.J., Tian R., Li Y., Zhang L., Shao H., Yang C. SDF-1alpha-induced dual pairs of E-selectin/ligand mediate endothelial progenitor cell homing to critical ischemia. Sci Rep. 2016;6:34416. doi: 10.1038/srep34416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh P.P., Lassance-Soares R.M., Shao H., Regueiro M.M., Li Y., Liu Z.J. Intramuscular E-selectin/adeno-associated virus gene therapy promotes wound healing in an ischemic mouse model. J Surg Res. 2018;228:68–76. doi: 10.1016/j.jss.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 26.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther Methods Clin Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boden J., Lassance-Soares R.M., Wang H., Wei Y., Spiga M.G., Adi J. Vascular regeneration in ischemic hindlimb by adeno-associated virus expressing conditionally silenced vascular endothelial growth factor. J Am Heart Assoc. 2016;5:e001815. doi: 10.1161/JAHA.115.001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh P.P., Castilla D., Lassance-Soares R.M., Shao H., Regueiro M., Li Y. A reliable mouse model of hindlimb gangrene. Ann Vasc Surg. 2018;48:222–232. doi: 10.1016/j.avsg.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Rubanyi G.M., Johns A., Kauser K. Effect of estrogen on endothelial function and angiogenesis. Vascul Pharmacol. 2002;38:89–98. doi: 10.1016/s0306-3623(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 30.Faber J.E., Zhang H., Lassance-Soares R.M., Prabhakar P., Najafi A.H., Burnett M.S. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalothorn D., Faber J.E. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 2010;42:469–479. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Song Y., Zhao L., Gaidosh G., Laties A.M., Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3:1703–1708. doi: 10.1038/nprot.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boden J., Wei J., McNamara G., Layman H., Abdulreda M., Andreopoulos F. Whole-mount imaging of the mouse hindlimb vasculature using the lipophilic carbocyanine dye DiI. Biotechniques. 2012;53 doi: 10.2144/000113907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao H., Cai L., Grichnik J.M., Livingstone A.S., Velazquez O.C., Liu Z.J. Activation of Notch1 signaling in stromal fibroblasts inhibits melanoma growth by upregulating WISP-1. Oncogene. 2011;30:4316–4326. doi: 10.1038/onc.2011.142. [DOI] [PubMed] [Google Scholar]
- 35.Wang C., Yue F., Kuang S. Muscle histology characterization using H&E staining and muscle fiber type classification using immunofluorescence staining. Bioprotocol. 2017;7:e2279. doi: 10.21769/BioProtoc.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bi P., Yue F., Sato Y., Wirbisky S., Liu W., Shan T. Stage-specific effects of Notch activation during skeletal myogenesis. Elife. 2016;5:e17355. doi: 10.7554/eLife.17355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalka C., Masuda H., Takahashi T., Kalka-Moll W.M., Silver M., Kearney M. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majka S.M., Jackson K.A., Kienstra K.A., Majesky M.W., Goodell M.A., Hirschi K.K. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 40.Aboyans V., Criqui M.H., Abraham P., Allison M.A., Creager M.A., Diehm C. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 41.Lewis J.E., Owens D.R. The pulse volume recorder as a measure of peripheral vascular status in people with diabetes mellitus. Diabetes Technol Ther. 2010;12:75–80. doi: 10.1089/dia.2009.0061. [DOI] [PubMed] [Google Scholar]
- 42.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 43.Luster A.D., Alon R., von Andrian U.H. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 44.Porcheray F., Viaud S., Rimaniol A.C., Léone C., Samah B., Dereuddre-Bosquet N. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]