Abstract
Objective
In the past five decades, many small caliber vascular grafts have been developed as bypasses for infrapopliteal or coronary arteries. However, reliable grafts have not been obtained owing to poor patency, mainly caused by early thrombosis or neointimal hyperplasia in the intermediate period after implantation. We developed a novel small caliber heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft and evaluated the feasibility to overcome those main causes of graft failure in canine carotid artery implantation.
Methods
The HL-PET graft with a diameter of 3 mm and length of 30 mm was made with combination of three key technologies: (1) weaving with PET ultrafine microfiber with a high biological porosity allowing for cell ingrowth, (2) heparin loading on microfiber surfaces, and (3) an outer coating with a flexible bioabsorbable polymer for prevention of blood leakage and graft kinking. Kink resistance, water permeability, and loaded heparin were assessed. One HL-PET graft each was implanted into a carotid artery of six animals. Graft patency rate and healing were assessed 24 weeks after implantation.
Results
Among the six grafts, five were deemed patent (patency rate of >83%), with one occluded 20 weeks after implantation. Histopathology of the patent grafts showed neointima formation with confluent endothelial cell lining (estimated mean endothelial cell coverage area, 89 ± 18%). Intimal hyperplasia at the anastomotic sites and severe chronic inflammatory responses were not observed. Immunohistochemistry with antibodies to endothelial nitric oxide synthase, alpha 2 smooth muscle actin and calponin 1 revealed luminal surface endothelial cell layer with expression of endothelial nitric oxide synthase and vascular smooth muscle cells with contractile phenotype in the subintimal layer.
Conclusions
The HL-PET graft showed no early postoperative thrombosis and was able to demonstrate a high patency rate with no severe biological response observed after 24 weeks. These results strongly suggest the potential of the HL-PET graft to be used for distal bypasses.
Keywords: Small diameter vascular graft, PET ultrafine microfiber, Heparin-loaded, Bioabsorbable polymer, Bypass graft
Clinical Relevance
The HL-PET graft achieved a high patency rate without early postoperative thrombosis or intimal hyperplasia in a small caliber artery implantation study. The histopathologic analysis demonstrated favorable healing property with a high antithrombogenicity. These results strongly suggested the possible use of the HL-PET graft as a bypass for infrapopliteal or coronary arteries.
Article Highlights.
-
•
Type of Research: Basic research
-
•
Key Findings: The heparin-loaded polyethylene terephthalate ultrafine microfiber woven graft with a diameter of 3 mm exhibited 83% patency 6 months after implantation in a canine carotid artery interposition model. Eighty-nine percent of the luminal surface was covered with endothelial cells and no inflammatory reaction was observed around the graft.
-
•
Take Home Message: The heparin-loaded polyethylene terephthalate ultrafine microfiber woven graft has the potential to be used for distal bypass.
Despite the continuous efforts to develop a small caliber arterial synthetic graft, a reliable graft applicable to bypass for infrapopliteal or coronary artery has not been obtained. Presently, the heparin-bonded expanded polytetrafluoroethylene (HB-ePTFE) graft (Propaten, W. L. Gore & Associates, Inc, Newark, Del) has demonstrated superior 1-year patency rates in popliteal artery bypasses over conventional ePTFE grafts.1,2 It is considered that the results may be achieved by the antithrombogenic modification, and it is suggested that heparin-bonded flow surface is effective for preventing early postoperative thrombotic occlusion after implantation. However, their long-term patency rates for infragenicular artery bypass were not perfect,2 with intimal hyperplasia at anastomotic sites as a cause of late postoperative graft failure. Consequently, it is thought that, to achieve acceptable long-term patency in small caliber prosthetic grafts, not only antithrombogenicity but also excellent healing property are also necessary. It was reported previously that polyethylene terephthalate (PET) ultrafine microfiber grafts accelerated fibroblast and endothelial cell migration, which resulted in early neointima formation without anastomotic intimal thickening.3 Based on those studies, we developed a novel small caliber heparin-loaded PET ultrafine microfiber (HL-PET) graft, and investigated the feasibility of using the HL-PET graft as a small diameter vascular graft and confirmed the proof of concept based on three technologies in assessing patency in a preliminary canine artery implantation study.
Methods
Preparation of ultrafine microfiber
The basic cylindrical structure of the HL-PET is PET. The ultrafine microfiber with a single-fiber thickness of 3-5 μm was prepared as follows: PET as an island component and PET copolymerized with 8.0 mol% of 5-sodium sulphthalic acid as a sea component were separately melt and measured. Then they were flown into a spinning pack embedding the composite spinneret to discharge a composite polymer flow though nozzles. The discharged composite polymer flow was cooled to solidify. After adding oil, the solidified fiber was rewound, and drawn between rollers heated to 90°C and 130°C. Using the ultrafine microfiber, a tube graft with an internal diameter of 3 mm was woven. After washing in hot water and heat set at 180°C on a round rod, the graft was incubated in an acid, followed by an alkaline solution to remove the sea component. Finally, additional heating at 180°C was performed (Fig 1).
Fig 1.
Ultrafine microfiber woven polyethylene terephthalate (PET) graft. A, Kink-resistant flexibility. B, Optical endoscopic image of the luminal surface of the graft. C, Macroscopic picture of the outside surface of the graft.
Heparinization in the HL-PET graft
Heparin was loaded to the surface of the ultrafine microfibers of HL-PET graft through ionic interaction. In short, the surface of the ultrafine microfibers of the graft was covalently modified with polyamine by soaking the graft in polyamine aqueous solution (5.0 wt%). After removing excess polyamine by rinsing, heparinization was performed by incubating the modified graft in a heparin aqueous solution (0.75 wt%). The uniformity of the heparin distribution on the luminal surface of HL-PET graft was assessed with toluidine blue staining, and the amount and thickness of the heparin layer were assessed by x-ray photoelectron spectroscopy, which can detect atoms existing in a depth of 10 nm. The intensity of sulfur atoms was also measured by scanning transmission electron microscope-energy dispersive x-ray spectroscope. The surface amount of heparin was also quantified with anti-factor Xa activity, using Testzyme Heparin S (Sekisui Medical Co, Ltd, Tokyo, Japan), according to the instruction for use. The HL-PET graft loaded with heparin of 15-25 IU/cm2 was used in these studies.
To preserve heparin activity on the graft flow surface after heparinization, the outer side of the graft was coated with a bioabsorbable polymer composed of L-lactide, caprolactone, and polyethylene glycol. The polymer was synthesized with a ring-opening polymerization of L-lactide and caprolactone, with subsequent condensation with polyethylene glycol. This bioabsorbable polymer exhibits flexibility with a low tensile modulus of 1.7 MPa, with excellent elongation at a break of 2970%,4 along with resilience. After coating, the graft was dried inside a vacuum oven at room temperature and sterilized in ethylene oxide gas for implantation study.
Physical property of the vascular graft
All tests were performed according to ISO 7198 guidance (Cardiovascular implants and extracorporeal systems–Vascular prostheses–Tubular vascular grafts and vascular patches). The kink resistance of three samples of the HL-PET graft, along with commercially available grafts, was measured via minimum radius around a taper gauge without kink formation. Water permeabilities of an uncoated and polymer-coated graft were measured using the reported method and expressed in milliliters of water per square centimeter per minute of graft wall at a pressure head of 120 mm Hg.5
Measurement of heparin activity after implantation
In vitro testing was performed to detail the time-course of heparin releasing in HL-PET grafts both before implantation, as well as upon retrieved after 24 weeks after implantation. In short, rectangular pieces (1.0 × 0.5 cm) were cut off from the HL-PET graft, weighed and incubated in 5 mL of human plasma at 37°C with gentle shaking. After each incubation time point (15 and 30 minutes, and 1, 3, 6 and 24 hours), 130 μL of human plasma was withdrawn and measured for heparin activity using Testzyme Heparin S. Furthermore, with residual heparin also measured after implantation at 24 weeks. The implanted HL-PET graft retrieved after 24 weeks was rinsed with saline and 3 samples (5 × 5 mm each) cut off from the graft, with residual heparin activity measured quantified using Testzyme Heparin S.
Implantation study in a canine model
In the first study of 4 weeks, the patency of three HL-PET grafts along with three unmodified PET (UM-PET) grafts was compared to confirm the effect of heparin loading. In the second 24-week study, the patency and histopathology of six HL-PET grafts and the residual heparin activity of one HL-PET graft were assessed. The patency of 16 HB-ePTFE grafts at 140-170 days after implantation was between 50% and 60%.6 Six HL-PET grafts were considered to suffice to detect patency at 24 weeks, along with comparison with HB-ePTFE grafts. The grafts were implanted in the carotid artery of quarantined, male Narc Beagle dogs (Kitayama Labs Co Ltd, Nagano, Japan) weighing 10-12 kg. All study procedures were approved by the research facility chairman via an ethics review by the Animal Experiment Committee based on the “Rules for Animal Experiments at Kamakura Techno-Science, Inc, Kanagawa, Japan.” The animal care and use complied with the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Washington: National Academy Press, 1996.
All dogs were intubated and anesthetized using 1%-5% isoflurane, and heparin (100 IU/kg) was administrated intravenously immediately before implantation. The activated clotting time level was controlled in the range of 150-250 seconds, followed by continuous infusion of heparin of 30 IU/kg during the implantation procedure. The graft (inner diameter, 3 mm; length, 30 mm) was interposed in the carotid artery in end-to-end anastomoses with the use of the suture Nyron 6-0. An antibiotic (ampicillin sodium) and analgesic (buprenorphine hydrochloride) were administered immediately after surgery, and aspirin (100 mg/d) and dipyridamole (50 mg/d) were daily administered orally until graft retrieval. For the first study of 4 weeks, the graft patency was measured by duplex ultrasound examination at 1 hour, as well as 1, 2, 3, and 4 weeks. For the second study of 24 weeks, the graft patency was measured by duplex ultrasound examination at 1 hour, as well as 1, 2, 3, 4, 8, 12, 16, and 20 weeks. The animals were humanely killed 24 weeks after implantation, and the grafts including anastomotic sites were retrieved. Six were studied for histopathologic examination and one for heparin activity measurement (as described in previous part).
Morphologic and immunohistochemical analysis
Six HL-PET grafts retrieved at 24 weeks after implantation were longitudinally opened for inspection of the luminal surface, and placed in a 10% buffered formalin solution for 24 hours at 4°C, with the experiment specimens embedded in paraffin, sectioned, and stained with the hematoxylin and eosin. Endothelial cell lining was confirmed by hematoxylin and eosin staining and scanning electron microscopy (SEM). The graft section selected for both histopathologic images as well as a picture by SEM was located at 10 mm from the proximal anastomosis sites.
Immunohistochemistry was used to identify cell types appearing in the graft wall using the avidin-biotin technique, with anti-dog alpha 2 smooth muscle actin monoclonal antibody (#NBP2-32808, Novus Biologicals), anti-dog calponin 1 polyclonal antibody (#NBP1-52399, Novus Biologicals), and anti-dog endothelial nitric oxide synthase (eNOS) polyclonal antibody (#NB300-500SS, Novus Biologicals) as the primary antibodies. After preparation using standard immunohistochemistry procedure, the sections were made visual by means of the avidin-biotin-peroxidase labeling system. Positive staining with diaminobenzidine tetrahydrochloride solution (0.2% H2O2, 0.05 mol/L tris buffer, pH 7.2) appeared brown and the sections were counterstained with hematoxylin.
Estimation of endothelialization
Total length of the endothelialized area (EA) was obtained by light microscopy through a continuous longitudinal scan between both anastomotic site, and % of EA was calculated as:
where LE is the total length of EA and LG is the length between anastomotic sites in retrieved grafts.
Results
HL-PET graft properties
The kink resistance was 8.1 mm, which was within the range of commercially available vascular grafts (Table). The water permeabilities in coated and uncoated HL-PET grafts were 0 and 188 mL/min/cm2 at 120 mm Hg, respectively.
Table.
Kink resistance comparison of the uncoated heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft and commercially available synthetic vascular grafts
| Graft | Material | Diameter, mm | Kink radius, mm |
|---|---|---|---|
| Uncoated HL-PET graft | PET | 3 | 8.1 ± 1.2 |
| Gelsoft | PET | 6 | 4.7 ± 0.1 |
| Interguard K | PET | 6 | 3.9 ± 0.1 |
| Interguard W | PET | 6 | 3.1 ± 0.5 |
| J Graft | PET | 7 | 5.6 ± 0.6 |
| Fusion | ePTFE/PET | 6 | 8.1 ± 0.5 |
| Impra | ePTFE | 6 | 4.2 ± 10.1 |
| Intering | ePTFE | 6 | 4.5 ± 0.0 |
| SESL PTFE | ePTFE | 5 | 3.9 ± 0.4 |
ePTFE, Expanded polytetrafluoroethylene; PET, polyethylene terephthalate; PTFE, polytetrafluoroethylene.
Three repetitions were performed for all grafts. Kink radius are described as mean ± standard deviation (SD).
Impra: C.R. Bard, Inc; Gelsoft; SEAL PTFE: Terumo Corporation; J Graft: Japan Lifeline Co, Ltd; Fusion, Interguard: Getinge; Intering: W. L. Gore & Associates, Inc.
Regarding heparin loading, the luminal surface of the HL-PET graft was uniformly stained with toluidine blue. The scanning transmission electron microscope-energy dispersive x-ray spectroscope in a cross-section showed an accumulation layer of sulfur atom on the luminal surface with a thickness of approximately 55 nm (Fig 2, A), and x-ray photoelectron spectroscopy revealed high counts of sulfur ion, proving that the accumulation was a heparin layer (Fig 2, B).
Fig 2.
Distribution and thickness of sulfur atoms on the luminal surface of the heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft showing heparin localization with a thickness of approximately 55 nm. A, High-angle annular dark field scanning transmission electron microscope (HAADF-STEM) image showing the heparin layer between (a) and (b), with a thickness of about 55 nm. The high intensity of sulfur atoms between (a) and (b) shows a heparin layer. Bar = 50 nm. B, Energy dispersive x-ray spectroscope (EDX) line analysis.
The in vitro heparin-releasing test demonstrated that almost 80% of the initially loaded heparin was released within 24 hours; however, approximately 5% of the loaded heparin remained even at 24 weeks after implantation.
Implantation study in a canine model
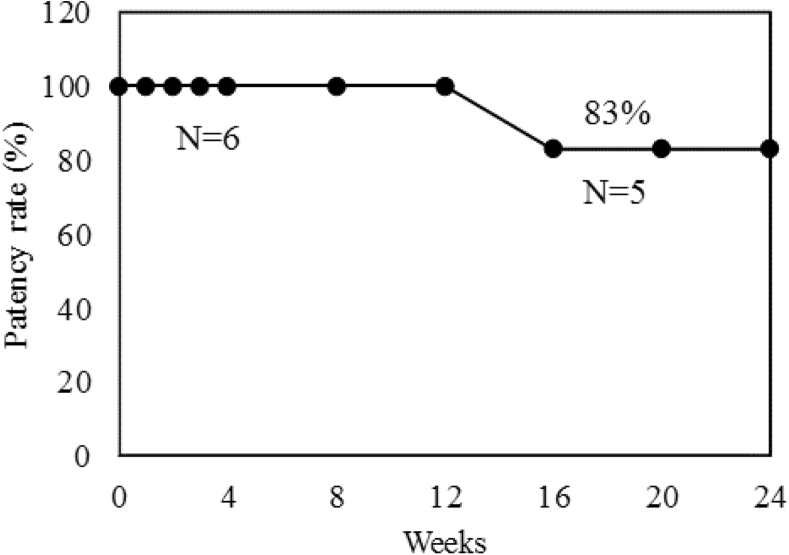
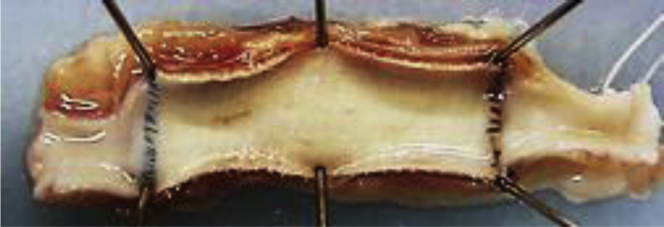
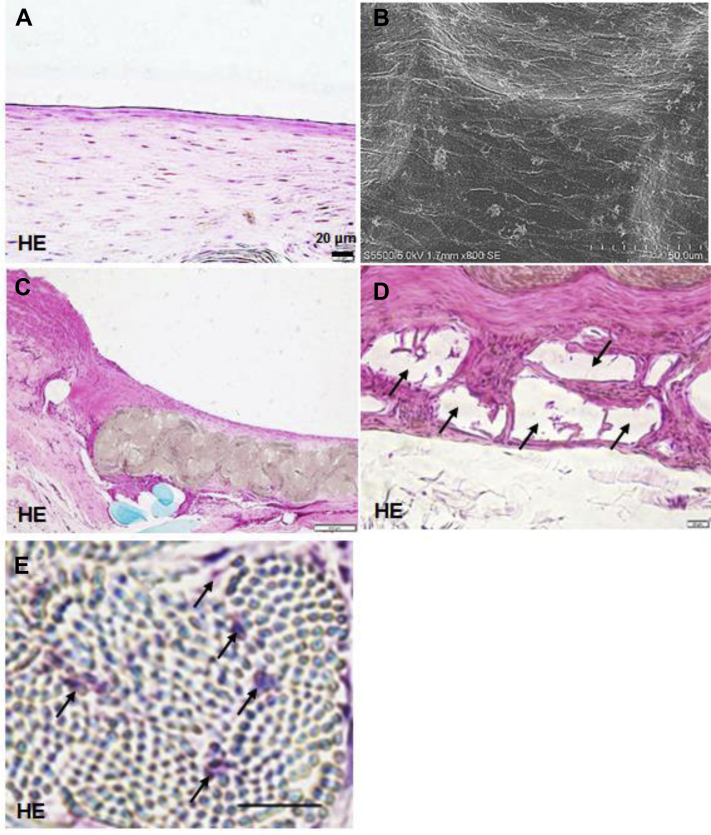
In the first study, all three HL-PET grafts and two of UM-PET grafts were patent at 4 weeks after implantation. No flow was detected in one UM-PET graft at 3 weeks after implantation. In the second study, five of six grafts were patent and the patency rate was 84% at 24 weeks (Fig 3). The five patent grafts exhibited a glossy flow surface without thrombus formation (Fig 4), and light microscopy and SEM showed a confluent endothelial cell lining on the flow surface (Fig 5, A and B), and the mean percent of EA was 89 ± 18% (n = 5). No intimal hyperplasia was observed on the graft, including both anastomotic sites (Fig 5, C). Infiltration of fibroblast-like cells, macrophages, and some foreign body giant cells were observed in the interstices of ultrafine microfibers (Fig 5, D), showing the ongoing graft healing process. The bioabsorbable material in the outer layer showed fragmentation accompanied by macrophages, suggesting there was no inhibitive effect on the graft healing (Fig 5, E). Immunohistochemical analysis detected contractile-phenotype vascular smooth muscle cells and luminal surface cell lining with eNOS expression (Fig 6). Similar immunohistochemical results were observed among all five grafts.
Fig 3.
The cumulative patency rate of heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) grafts in a canine carotid artery implantation using the Kaplan-Meier method.
Fig 4.
Flow surface of the heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft 24 weeks after implantation, showing a smooth glossy appearance without thrombi or stenosis.
Fig 5.
Histopathologic findings of the heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft 24 weeks after implantation. A, C-E, Hematoxylin and eosin (HE)-stained image. B, Scanning electron microscopy (SEM) image. A, Thin neointima formation on the flow surface (longitudinal section). B, SEM, showing a confluent endothelial lining. C, Proximal anastomotic site, showing smooth surface without intimal hyperplasia (longitudinal section). D, Tissue ingrowth between the ultrafine microfibers (black arrows) (longitudinal section). E, Outer layer of the graft, showing a well-organized outer capsula involving fragmentation of the bioabsorbable material (black arrows) (longitudinal section). Scale bar in A, D, E = 20 μm; bar in B = 50 μm; bar in C = 200 μm.
Fig 6.
Immunohistochemistry of the heparin-loaded polyethylene terephthalate ultrafine microfiber (HL-PET) graft retrieved 24 weeks after implantation. A, Endothelial nitric oxide synthase (eNOS) positive cell lining on the flow surface. B, Alpha 2 smooth muscle (SM) actin positive cells in the medial layer of the graft wall, suggesting migration and infiltration of smooth muscle cells. C, Calponin 1-positive cells in the medial layer of the graft wall, suggesting smooth muscle cells with contractile phenotype. Scale bar = 20 μm.
Discussion
We developed a novel small caliber graft, the HL-PET ultrafine microfiber woven graft. We were able to confirm an acceptable graft patency rate 24 weeks after implantation in a canine carotid artery. The retrieved grafts showed smooth flow surface without anastomotic intimal thickening, with both microscopy and SEM revealing thin neointima with low inflammatory reactions, as well as no intimal hyperplasia at the anastomotic sites. These results suggest that the HL-PET graft may have potential for the clinical application as a new small caliber vascular prosthesis for infrapopliteal or coronary artery bypass use.
The HL-PET graft is designed to prevent both early postoperative thrombosis and neointimal hyperplasia based on three key technologies: (1) an ionically heparin loaded flow surface, (2) smooth surface of microfiber woven for obtaining a thin fibrin flow surface with subsequent neointimal formation, and (3) a biological high porosity fabric with ultrafine microfibers for enhancing early graft healing.
The heparin-loading process is based on chemical reaction which is different from the heparin-containing polymer coating reported previously.7 Heparin is loaded on each of the ultrafine microfiber surfaces at a thickness of 55 nm. Therefore, the biological high porosity is preserved, and the graft retains not only antithrombogenicity, but also cell affinity which enhances early graft healing. The loaded heparin is released, with approximately 80% of the initially loaded heparin released within 24 hours, with the rest gradually released resulting in 5% of the loaded heparin remaining on the flow surface at the end. In the first study of 4 weeks, all three HL-PET graft were patent at 4 weeks; one UM-PET graft occluded within 3 weeks. These results suggest that the graft retains sufficient heparin activity in the early phase during which antithrombogenicity is required. The HB-ePTFE graft used covalent end point attachment of heparin to a biomaterial surface, referred to as Carmeda Bioactive Surface technology,6,8,9 with heparin bioactivity decreasing after implantation (24.7 ± 7.9 pmol/cm2 at 2 weeks to 15.3 ± 3.7 pmol/cm2 at 12 weeks) in a canine model.10 The heparin activity 24 weeks after implantation has not been reported, but a decrease activity may be speculated. Graft bleeding owing to the heparin coating is a possible side effect and could occur immediately after implantation, regardless of the low water porosity woven of the HL-PET graft. To prevent bleeding, graft sealing using a bioabsorbable polymer was necessary. However, conventional bioabsorbable polymers have a high young's modulus and hard enough to hinder graft flexibility (PLLA, 0.12-2.30 GPa; PDLLA, 0.04-0.05 GPa; PGA, 0.08-1.00 GPa).11 Therefore, we created a flexible, stretchable bioabsorbable natural rubber-like polymer, and then applied to the outer side of the graft. Histopathology of the 24-week grafts revealed that the coating polymer degraded into fragments, with inflammatory cells infiltrating into the interstitial spaces and between the fragments, suggesting that the coating material did not interrupt cell migration and tissue ingrowth.
High biological porosity and a smooth flow surface of the HL-PET graft were essential to obtain a thin neointima formation, two important features of the PET ultrafine microfiber. A previous study reported that PET ultrafine microfiber accelerated the migration of fibroblast and neointima formation in a canine implantation model.5 The HL-PET graft had a high-density woven texture, but no crimp modification was applied to retain a smooth interior flow surface. Low-porosity woven grafts commonly showed rigidity and low flexibility, with crimp modification necessary to increase the flexibility and kink resistance seen in commercially available woven grafts. In the HL-PET graft, warp yarns, and weft yarns were arranged to move in response to graft kinking, with sufficient kink resistance created without crimp modification. The kink resistance was within the range of commercially available vascular grafts and we believe it might be acceptable to clinical use.
In the canine carotid artery implantation study, we observed that the HL-PET graft exhibited high patency, the absence of stenosis at anastomotic sites owing to intimal hyperplasia, and the absence of severe chronic inflammatory reaction at 24 weeks. Histopathologic analysis confirmed that approximately 90% of the luminal surface was covered with endothelial cells with eNOS expression, and that subintimal layer consisted of contractile-phenotype smooth muscle cells. These results clearly demonstrate the efficacy of ultrafine microfiber fabric with biological high porosity, which induced the excellent healing property and the stability of patency in intermediate periods. The HB-ePTFE grafts achieved high early patency rates (73% at 1 year) for infrainguinal bypasses for critical limb ischemia.12 The ePTFE graft had a low porosity structure and had low surface energy, which impairs adhesion of serum proteins, cell components and tissues.13,14 These deficiencies inhibited the graft healing process, resulting in poor patency during the intermediate period. In contrast, the HL-PET graft exhibited not only antithrombogenicity owing to heparin bonding, but also has excellent healing properties of biological tissue formation around the graft after implantation. Although one of the HL-PET grafts exhibited no-flow at 20 weeks, suggesting possible thrombosis, we believe that the general superior attributes of the HL-PET graft contributed to the stability of patency in intermediate period.
Limitations
There were three limitations to this study. First, there was no control in the 24-week implantation study. Second, the effectiveness of heparin loading would need to be demonstrated in a sufficient number of grafts compared with nonheparinized grafts. Third, the end-to-end interposition into the carotid artery is a very preliminary model. Future studies should incorporate more practical models such as end-to-side anastomosis, long bypass, and low blood flow rate to better evaluate effectiveness.
Conclusions
The HL-PET graft demonstrated a high patency rate in the 24-week implantation study without severe biological response, the results suggesting that the graft might be applicable for bypass use with infragenicular or coronary arteries.
Author contributions
Conception and design: MF, NT, TK, HT, KT
Analysis and interpretation: MF, YS, YT, TK, TS, KT
Data collection: MF, YS, YT
Writing the article: MF, YS, YT, TK, TS, KT
Critical revision of the article: MF, NT, HT, TS, KT
Final approval of the article: MF, NT, YS, YT, TK, HT, TS, KT
Statistical analysis: Not applicable
Obtained funding: KT
Overall responsibility: KT
Acknowledgments
The authors thank Mr So Kakiyama, Ms Megumi Nakanishi, Mr Yuichi Koyamatsu, Dr Koji Kadowaki, Mr Keishi Miwa, Dr Tetsuya Goto, Dr Yoshiki Makabe, Mr Motoki Takaoka, Mr Kazumasa Mizuno, Mr Masato Shimagami, Mr Satoshi Yamada, Mr Yuki Ninomiya, Mr Atsushi Kuwabara, and Mr Hajimu Kurumatani for assisting development of the graft. The authors also thank Toray Research Center, Inc for XPS and STEM-EDX analysis, and Kamakura Techno-science, Inc for conducting animal experiment.
Footnotes
Author conflict of interest: T.S. has been paid a consulting fee by Toray Industries, Inc.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Lindholt J.S., Gottschalksen B., Johannesen N., Dueholm D., Ravn H., Christensen E.D. The Scandinavian propaten trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – A randomized clinical controlled multi-centre trial. Eur J Vasc Endovasc Surg. 2011;41:668–673. doi: 10.1016/j.ejvs.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Samson R.H., Morales R., Showalter D.P., Lepre M.R., Jr., Nair D.G. Heparin-bonded expanded polytetrafluoroethylene femoropopliteal bypass grafts outperform expanded polytetrafluoroethylene grafts without heparin in a long-term comparison. J Vasc Surg. 2016;64:638–647. doi: 10.1016/j.jvs.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 3.Noishiki Y., Watanabe K., Okamoto M., Mori Y. Evaluation of a new vascular graft prosthesis fabricated from ultrafine polyester fiber. ASAIO J. 1986;32:309–314. [PubMed] [Google Scholar]
- 4.Kogawa T, Kadowaki K, Fujita M, Sakaguchi Y, Sakaguchi H, Sakaguchi H, et al. Development of highly pliable biodegradable polymer and its application to small-diameter vascular graft. The 99th Chemical Society Japan Annual Meeting, Kobe, Japan, March 16-19, 2019.
- 5.Wesolowski S.A. In: Vascular graft. Sawyer P.N., Kaplitt M.J., editors. Appleton-Century Crofts; New York: 1978. Foundations of modern vascular graft; pp. 27–49. [Google Scholar]
- 6.Sanchez J., Elgue G., Riesenfeld J., Olsson P. Inhibition of the plasma contact activation system of immobilized heparin: relation to surface density of functional antithrombin binding sites. J Biomed Mater Res. 1997;37:37–42. doi: 10.1002/(sici)1097-4636(199710)37:1<37::aid-jbm5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Ikuta T., Fujii H., Shibata T., Hattori K., Hirai H., Kumano H. A new poly-2-methoxyethlacrylate-coated cardiopulmonary bypass circuit possesses superior platelet preservation and inflammatory suppression efficacy. Ann Thorac Surg. 2004;77:1678–1683. doi: 10.1016/j.athoracsur.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 8.Amander C., Dryjski M., Larsson R., Olsson P., Swedenborg J. Thrombin uptake and inhibition on endothelium and surfaces with a stable heparin coating: a comparative in vitro study. J Biomed Mater Res. 1986;20:235–246. doi: 10.1002/jbm.820200212. [DOI] [PubMed] [Google Scholar]
- 9.Pasche B., Elgue G., Olsson P., Riesenfeld J., Rasmuson A. Binding of antithrombin to immobilized heparin under varying flow conditions. Artif Organs. 1991;15:481–491. [PubMed] [Google Scholar]
- 10.Begovac P.C., Thomson R.C., Fosher J.L., Hughson A., Gällhagen A. Improvements in GORE-TEX® vascular graft performance by Carmeda® Bioactive Surface heparin immobilization. Eur J Vasc Endovasc Surg. 2003;25:432–437. doi: 10.1053/ejvs.2002.1909. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji H. Poly(lactide) sterecomplexes; formation, structure, properties, degradation, and applications. Macromol Biosci. 2005;5:569–597. doi: 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- 12.Pulli R., Dorigo W., Castelli P., Dorrucci V., Ferilli F., De Blasis G. Midterm results from a multicenter registry on the treatment of infrainguinal critical limb ischemia using a heparin-bonded ePTFE graft. J Vasc Surg. 2010;51:1167–1177. doi: 10.1016/j.jvs.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Chen M., Zamora P.O., Som P., Pena L.A., Osaki S. Cell attachment and biocompatibility of polytetrafluoroethylene (PTFE) treated with glow-discharge plasma of mixed ammonia and oxygen. J Biomater Sci Polym Ed. 2003;14:917–935. doi: 10.1163/156856203322381410. [DOI] [PubMed] [Google Scholar]
- 14.Dekker A., Reitsma K., Beugeling T., Bantjes A., Feijen J., vsn Aken W.G. Adhesion of endothelial cells and adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials. 1991;12:130–138. doi: 10.1016/0142-9612(91)90191-c. [DOI] [PubMed] [Google Scholar]