Abstract
Objective
We have previously demonstrated that human abdominal aortic aneurysm (AAA) rupture occurs in zones of low wall shear stress where flow recirculation and intraluminal thrombus (ILT) deposition are increased. Matrix metalloproteinase-9 (MMP-9) is involved in the pathogenesis of AAA via its lytic effect on collagen and elastin. We hypothesize that flow-mediated ILT deposition promotes increased local inflammatory and MMP-9 activity that leads to AAA wall degeneration. The purpose of this study was to examine the correlation between predicted pulsatile flow dynamics and regional differences in MMP-9, elastin, collagen, and ILT deposition in human AAA.
Methods
Full-thickness aortic tissue samples were collected from 24 patients undergoing open AAA repair. Control infrarenal aortic tissue was obtained from 6 patients undergoing aortobifemoral bypass. Full-thickness aortic tissue and ILT were assessed for MMP-9 levels using a cytokine array assay. Histologic and immunohistochemical assessment of inflammation, collagen and elastin content, and MMP-9 levels were also measured. Three-dimensional AAA geometry was generated from computed tomography angiogram (CTA) images using Mimics software and computational fluid dynamics was used to predict pulsatile aortic blood flow.
Results
The majority of AAA showed eccentric ILT deposition which was correlated with predicted recirculation blood flow (R2 = –0.17; P < .05). The regions of high ILT were associated with significant increases in inflammation and loss of elastin and collagen compared with regions of low ILT, or with control tissue. MMP-9 was significantly higher in areas of high ILT deposition compared with areas devoid of ILT. Tissue MMP-9 was correlated with the thickness of ILT deposition (R2 = 0.46; P < .05), and was also present in high levels in thick compared with thin ILT.
Conclusions
We have shown a correlation between flow-mediated ILT deposition with increased tissue levels of MMP-9 activity, increased inflammatory infiltrate, and decreased elastin and collagen content in stereotactically sampled human AAA, suggesting that ILT deposition is associated with local increases in proteolytic activity that may preferentially weaken and promote rupture at selected regions.
Keywords: Abdominal aortic aneurysm, Intraluminal thrombus, MMP-9, Computational fluid dynamics
Article Highlights.
-
•
Type of Research: Basic science research
-
•
Key Findings: Human abdominal aortic aneurysm (AAA) tissue was harvested (n = 24) in a stereotactic manner and compared with control (n = 6) infrarenal aorta to assess the effect of predicted regional hemodynamics on local matrix metalloproteinase (MMP)-9, elastin, and collagen levels. The location of intraluminal thrombus (ILT) was positively correlated with predicted recirculation flow in human AAA. Tissue MMP-9 was significantly higher in areas of high ILT deposition (45.1 ± 5.0 ng/mg protein) compared with areas devoid of ILT (31.2 ± 4.00 ng/mg protein) (P < .05) and was strongly positively correlated with the thickness of ILT deposition (R2 = 0.46; P < .05). Elastin and collagen degradation were also significantly correlated with increased MMP-9 tissue levels.
-
•
Take Home Message: There are significant regional increases in aortic tissue MMP-9 levels in human AAA that are correlated with recirculation blood flow, high ILT deposition, and elastin and collagen degradation.
Rupture of an abdominal aortic aneurysm (AAA) has high morbidity and mortality. Although the decision to repair an AAA is based on size, this single measure fails to address AAA that rupture below size thresholds or that reach extreme sizes without rupture; nor does it explain why AAA in females are more likely to rupture at smaller relative sizes. Furthermore, this single static measure of aortic diameter does not take into consideration the dynamic interactions between physical forces of pulsatile blood flow and biomatrix of the aortic wall.
Pulsatile blood flow may result in unsteady turbulent stresses that produce regions of high and low wall shear stress (WSS) that may contribute to AAA formation.1, 2, 3 Typically, AAA exhibit a dominant flow channel with an associated zone of recirculation flow similar to that of any flow channel that has a sudden expansion. We have previously demonstrated that AAA wall expansion occurs predominantly at the location of maximal flow impingement,4 whereas AAA rupture occurs in zones of low WSS where the flow recirculation and intraluminal thrombus (ILT) deposition were increased.5 Increased thrombus ILT content has been associated with more rapid AAA expansion.6,7 Autopsy specimens from humans with ruptured AAA showed that rupture tends to occur in the posterior-inferior region and not typically at the site of maximal aortic diameter.8 In addition, AAA tended to rupture in regions of higher ILT burden in 80% of cases.8 Because the primary source of oxygen for the aortic wall is luminal blood flow, it has been suggested that increased ILT deposition disrupts oxygen diffusion to the adventitial layer9 and may subsequently lead to aortic wall degeneration, expansion, and rupture.
Of the factors involved in AAA growth and rupture, matrix dissolution is an essential pathophysiologic mechanism. The critical role of metalloproteinase-9 (MMP-9) activity in the pathogenesis of AAA has long been recognized.10 MMP-9 is thought to be the most important MMP involved in pathogenesis of AAA via its lytic effect on collagen and elastin.11 Plasma MMP-9 levels are increased in AAA,12 and more MMP-9 mRNA is present in aneurysmal than in normal aortic tissue.13 In addition, MMP-9 levels are significantly elevated in ruptured AAA, particularly at the site of rupture compared with other sites in the aortic wall.14,15
Khan et al examined the correlation between ILT thickness and the levels MMP-9 and tissue inhibitor of MMP-9 in 35 human AAA.16 They reported a statistically significant correlation between ILT thickness and the concentration of tissue inhibitor of MMP-9 and MMP-9, but this finding was based on a single sample from each AAA and only at the area of maximal ILT deposition, because they did not sample other regions of the AAA.
We hypothesize that flow recirculation promotes increased ILT deposition, which in turn promotes increased local inflammatory MMP-9 activity leading to increased elastin and collagen degradation in the AAA wall. The purpose of this study was to examine the correlation between predicted pulsatile blood flow dynamics, using computational fluid dynamics (CFD), and observed ILT deposition patterns in stereotactically sampled human AAA with regional differences in aortic wall MMP-9, elastin, and collagen. Understanding the correlation between aortic blood flow and enzymatic wall decomposition would vastly improve our understanding the mechanisms involved in the development, growth and rupture of AAA.
Methods
Patient population
Twenty-four consecutive patients (18 men, 6 women) undergoing open AAA repair for an AAA 5.0 cm or larger were enrolled in this study; one patient was a planned pilot case used to assess the safety of multisite aortic tissue harvest and those data are not included. All patients were consented to open AAA repair, either owing to a lack of endovascular aneurysm repair suitability or based on patient preference; full-thickness aortic biopsies were taken. Control aortic tissue was collected from nonaneurysmal infrarenal aorta of patients undergoing aortobifemoral (ABF) bypass for nonaneurysmal, aortoiliac occlusive disease (n = 6). Patient demographics were collected, including age, sex, cardiovascular risk factors, and smoking history. All except one AAA patient underwent preoperative computed tomography angiogram (CTA) imaging. The CTA images were used to assess AAA morphology and for CFD analysis of infrarenal aortic blood flow. Control patients undergoing ABF were used for tissue analysis only and did not undergo CFD flow assessment, as most had near distal aortic occlusion.
Tissue harvest
At the completion of AAA repair, but before sac closure, six full-thickness AAA tissue samples of approximately 1 × 1 cm, were biopsied by the vascular surgeon in stereotactic manner covering all regions of the AAA wall; with the exception of the posterior midline (Fig 1). ILT was harvested first at the same stereotactic locations and processed separately from AAA tissue. Control tissues were harvested at the time of ABF bypass from the anterior wall of the normal appearing, nonaneurysmal, infrarenal aorta within 1 cm of the renal arteries (Fig 1). We attempted to harvest closest to the renal arteries and any specimen with associated ILT was rejected. All specimens were divided into two sections immediately after removal; one was placed in liquid nitrogen and the second in 4% buffered formalin. All AAA harvest sites were closed with absorbable suture and the aortic sac and retroperitoneum were closed in the standard fashion. The ABF harvest site was incorporated into the aortic bypass. The location and thickness of thrombus were recorded for all stereotactic sites in each patient from CTA images. All tissue samples were collected with express written consent under study protocol REB B2013:130, University of Manitoba.
Fig 1.
Schematic showing location of full-thickness abdominal aortic tissue sampling (sites 1-6 for abdominal aortic aneurysm [AAA] tissue). C denotes location of control tissue sampling in patients undergoing aortobifemoral (ABF) bypass for aortoiliac occlusive disease. Aortic tissues and associated ILT were collected at ¼ (samples 1 and 3), ½ (samples 5 and 6), and ¾ (samples 2 and 4) of the distance from the renal arteries.
Histologic analysis
A total of six full-thickness specimens for each of 24 AAA patients and all six of the control patients were processed for routine histology in the standard fashion. Control and AAA tissues for each tissue site were stained with hematoxylin and eosin to assess for inflammatory infiltrate, or with Verhoeff-Van Geison for assessment of collagen and elastin content. Slides were examined at a magnification of ×100 to ×200. Multiple pictures7, 8, 9, 10 were taken of the slides to represent the tissue sample. For all hematoxylin and eosin-stained tissues, a quantitative analysis was performed to estimate the amount of nucleated cells within the samples. Nucleated cells where assumed to be neutrophils, monocytes, lymphocytes, and fibroblasts, and represent inflammation within the tissue. A Zen Pro Image Analyzer (Zeiss, North York, Ontario, Canada) was used to assess the degree of inflammation and the collagen and elastin content by an examiner blinded to the study group. The designation of minimal (0%-20%), moderate (20%-70%), or abundant (>70%) inflammation, collagen, or elastin were assigned to the sample by taking the average of all the pictures analyzed for each sample. A number value of 1 to 3 was then assigned as minimal (1), moderate (2), or abundant (3) to allow comparison of regional differences.
MMP-9 immunohistochemistry
MMP-9-total, MMP-9-active, and anti-MMP-9 monoclonal antibodies were assessed in paraffin-blocked histologic specimens of AAA and control tissues according to the protocol of Novus Biologicals (MMP-9, 4A3, NBP2-13173). Blocking of the endogenous enzyme was performed in the dark with a horseradish peroxidase/biotin protocol with 3% hydrogen peroxide in methanol mixture. Antigen retrieval with sodium citrated buffer was carried out before immune-staining. Applying a primary antibody and control IgG was preceded by determining the correct concentration and dilutions of each MMP-9-total, MMP-9-active, and IgG that were needed to obtained appropriate analysis of aortic tissue MMP-9. Control IgG dilution was carried out according to the product data sheet of 1:1000 (Novus Biologicals, Littleton, Colo; NB720-B).
MMP-9 content was also assessed using a Zen Image Capture Processor to quantitatively assess the amount MMP-9 total and active staining on each tissue sample at a magnification of ×100. Each slide had one tissue stained with MMP-9 (total or active) and one tissue sample stained with IgG and analyzed to ensure the staining process was accurate and did not pickup background antigens. Multiple pictures (3-5) were taken of the slides to represent the tissue sample as a whole. An analysis was performed for each sample to estimate the amount of MMP-9 that was present within the samples by a blinded examiner. The designation of minimal (0%-20% total area), moderate (20%-70% total area), or abundant (>70% total area) amounts of MMP-9 were assigned to the sample by taking the average of all the pictures analyzed for each sample. A number value of 1 to 3 was then assigned—minimal (1), moderate (2), or abundant (3)—to the samples to allow further analysis.
Measurement of tissue MMP-9
AAA tissues that had been stored at –80°C were weighed and then placed in a mortar. Liquid nitrogen was poured onto the tissue and it was ground into a fine powder using a pestle. Then 1200 μL (30 μL/mg of tissue) of 0.1% Triton-X-100 50 mmol/L Tris-HCl lysis buffer was added to the ground tissue and allowed to sit for 15 minutes. The protein extract was then transferred into 1.5 mL microfuge tubes and spun for 20 minutes at 13,000 rpm. The supernatant protein was pipetted into a new 1.5 mL microfuge tube and sonicated for 10 seconds. The protein was then stored in aliquots at –80°C until analyzed. Protein analysis was performed in the standard fashion using a bovine serum albumin protein assay.17
Aortic tissue and ILT were separately assessed for MMP-9 levels in triplicates using a multiplex immunoassay analyzed with a BioPlex 200 Magnetic Luminex Performance Assay (Eve Technologies, Calgary, Canada). Specimen MMP-9 levels were quantified via extrapolation from a curve of purified standards. All samples were centrifuged before aliquoting. The aliquot (200 μL) was drawn from the middle of the sample. The sensitivity of the assay was 5.7 pg/mL. MMP-9 levels in tissues and plasma are expressed in nanograms per milligram of protein.
CFD
CFD was used to predict pulsatile aortic blood flow. Thin-slice (2.5 mm) CTA images of AAA were obtained. Three-dimensional AAA geometry was generated from CTA images using Mimics software (version 14.0; Materialise, Plymouth, Mich). The open source unstructured finite-volume code OpenFOAM-2.2.2 (OpenCFD Ltd, Bracknell, U) was used to directly solve the governing equations for flow. To simplify the geometry, visceral arterial branches were excluded. At the inlet of the aortic neck an approximation of a physiologic blood flow was implemented using the procedure outlined in Les et al.18 The flow rate was fixed at Q = 15.7 mL s–1, which is equivalent to a resting cardiac output of 5 L/min. A uniform velocity based on the typical arterial pulse profile was implemented at the upstream inlet boundary and at the outlet boundaries, downstream in the iliac arteries, a reference pressure of 0 was imposed. Blood was modeled as an incompressible Newtonian fluid. The arterial walls were assumed to be rigid with a no-slip condition. The initial flow field is set to zero and statistics were collected after five pulses simulated to remove the effect of initial flow conditions.
Statistical methods
An independent t test was used to compare regional differences in MMP-9 levels between regions of high and low ILT deposition. Pearson correlation coefficients were calculated to determine the relationship between ILT deposition and MMP-9 levels, and between flow velocity and ILT deposition. Values were considered statistically significant at a P value of .05 or less.
Results
Study population
Patient demographics for AAA and control patients are displayed in the Table. Of the original 24 patients, 23 patients were included in final analysis. There were no adverse results from harvesting multiple full-thickness aortic specimens for the pilot patient or for any of the study patients. One patient's tissues were harvested but were excluded when blood flow calculations could not be made owing to severe renal insufficiency and the lack of contrast imaging. Of the remaining 23 AAA patients, 17 were male and 6 were female. The average AAA size was significantly greater in males at 6.8 ± 1.2 cm compared with females at 5.6 ± 0.5 cm (P < .05).
Table.
Demographics of abdominal aortic aneurysm (AAA) and control patients
| Variables | AAA (n = 24) | Control (n = 6) | P value |
|---|---|---|---|
| AAA size, cm | 6.21 (±0.24) | – | – |
| Age, years | 71 (±7) | 63 (±6) | .43 |
| Male sex | 14 (67) | 3 (38) | .22 |
| Ever smoked | 19 (90) | 7 (88) | >.99 |
| Current smoker | 9 (43) | 3 (38) | >.99 |
| Previous smoker | 10 (48) | 4 (50) | >.99 |
| Hypertension | 13 (62) | 4 (50) | .68 |
| Diabetes mellitus | 9 (43) | 2 (25) | .67 |
| Statin use | 16 (76) | 4 (50) | .21 |
| Peripheral vascular disease | 3 (14) | 8 (100) | <.001a |
| Chronic obstructive pulmonary disease | 4 (19) | 1 (13) | >.99 |
| Cerebrovascular accident | 2 (10) | 1 (13) | >.99 |
| Coronary artery disease | 9 (43) | 1 (13) | .02a |
| Chronic kidney disease | 1 (5) | 0 (0) | .48 |
P ≤ .05.
The aortic tissue from six patients (three men and three women) undergoing ABF bypass grafting for aortoiliac occlusive disease served as control infrarenal aortic tissue. The control patients tended to be younger at 63 ± 6 compared with 71 ± 7 years in AAA patients. All controls had peripheral arterial disease, which was the indication for intervention, and none had an associated AAA. Smoking was a significant factor in 96% with AAA, and in 100% of control patients. There was significantly more coronary artery disease in AAA patients compared with control but there were no other significant differences between AAA and control patients (Table).
ILT deposition
Of the 23 patients included in the final analysis, 21 of the AAA had associated ILT and 2 AAA were devoid of ILT. Of the 21 with ILT, 4 had dense circumferential ILT and 17 had eccentric ILT deposition. Fig 2 shows an axial CTA of an AAA with eccentric ILT deposition with a cross-section of the predicted flow velocity profile. The velocity profile shows recirculating flow adjacent to ILT deposition, with the dominant flow channel impacting on the contralateral side of the AAA. In the 17 patients with eccentric ILT, a correlation between ILT deposition and predicted blood flow velocity is shown in Fig 3. This shows a weak but negative correlation (R2 = –0.17) between ILT thickness and flow velocity in AAA with eccentric ILT.
Fig 2.
A, Axial computed tomography angiography (CTA) of an abdominal aortic aneurysm (AAA) showing eccentric intraluminal thrombus (ILT) deposition. White arrow indicates ILT location. B, A cross-section of the flow velocity profile. Black arrow indicates location of ILT. Red indicates higher velocity flow (m/sec). C, Silhouette of AAA showing location of CTA cross section (black line). D, Silhouette of AAA with blue showing flow lumen and grey indicating ILT deposition. E, Velocity streamlines for AAA. ILT deposition in white. Note recirculation flow (blue end of spectrum) is adjacent to ILT deposition.
Fig 3.
Negative correlation between predicted blood flow velocity and intraluminal thrombus (ILT) deposition (R = –0.42; P < .05).
In AAA with dense circumferential ILT (n = 4), flow was characterized by more laminar flow with a small flow channel through the ILT (Fig 4, A). In AAA without ILT (n = 2), AAA blood flow was characterized by strong recirculation flow involving the entire aortic lumen (Fig 4, B), unlike those with eccentric ILT where there was a dominant flow channel with an adjacent zone of recirculation flow.
Fig 4.
A (a), Axial computed tomography angiography (CTA) of an abdominal aortic aneurysm (AAA) showing circumferential intraluminal thrombus (ILT) deposition. White arrow indicates flow lumen. b, A cross section of the flow velocity profile. Black arrow indicates location of ILT. Red indicates higher velocity flow (m/sec). c, Silhouette of AAA showing location of horizontal transection (black line). d. Silhouette of AAA from (a) with blue showing flow lumen and grey indicating ILT deposition. e, Velocity streamlines for an AAA. ILT deposition in white. Dotted line indicates regions of ILT deposition. Flow is uniform without recirculation. B (a), Axial CTA of AAA showing no ILT deposition. (b) A cross-section of the flow velocity profile. Red indicates higher velocity flow (m/sec). (c) Silhouette of an AAA showing the location of horizontal transection (black line). (d) Silhouette of an AAA with blue showing the flow lumen. (e) Velocity streamlines show vortex flow involving entire AAA lumen.
Histology and MMP-9 immunohistochemistry
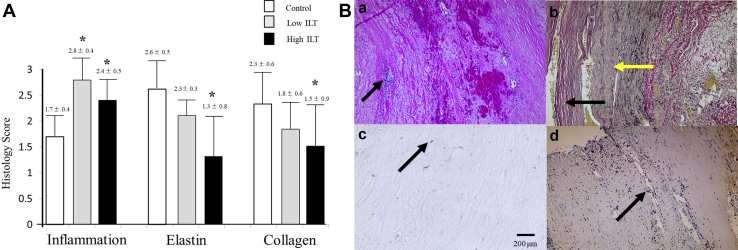
There were significantly higher inflammatory cell scores in histologic specimens of aortic wall in aneurysmal tissue from either ILT-rich (2.8 ± 0.4) or ILT-deplete (2.4 ± 0.5) regions compared with control tissue scores (1.7 ± 0.4) (P ≤ .05; Fig 5, A). There was a non-significant trend toward higher inflammation in ILT-rich compared with the ILT-deplete regions. Elastin and collagen was staining was more prominent in control tissue at (2.6 ± 0.5) and (2.3 ± 0.6), respectively, compared with ILT-rich (1.3 ± 0.8) and (1.5 ± 0.9) tissues (P ≤ .05), but not with ILT-deplete (2.1 ± 0.3) and (1.8 ± 0.6) regions (Fig 5, A). Fig 5, B, shows typical aortic histology showing inflammatory cells, elastin and collagen staining, and immunohistochemistry for MMP-9. In aneurysmal tissue, regions of high active MMP-9 were negatively correlated with lower levels of elastin and collagen staining (Fig 6).
Fig 5.
A, Inflammation, elastin, and collagen scores for control, low intraluminal thrombus (ILT), and high ILT abdominal aortic aneurysm (AAA) regions (∗P < .05). B (a), Routine hematoxylin and eosin staining of AAA tissue. Black arrow shows nucleated inflammatory cells. (b) Van Geisson staining shows with yellow arrow showing collagen and black arrow showing elastin fibers. Immunohistochemistry for matrix metalloproteinase (MMP)-9-total in control (c) and AAA tissue (d). Black arrow indicates with brown areas staining positive for MMP-9.
Fig 6.
Negative correlation between high, moderate, and low total and active matrix metalloproteinase (MMP)-9 vs high, medium, and low (a) elastin content and (b) collagen content in abdominal aortic aneurysm (AAA) tissue. (Elastin: R = –0.43 [total] and R = –0.47 [active]; P < .05). (Collagen R = –0.23 [total] and r = –0.37 [active]; P < .05).
MMP-9 tissue activity
MMP-9 was significantly higher in areas of high ILT deposition (45.1 ± 5 ng/mg protein) compared with areas devoid of ILT (31.2 ± 4 ng/mg protein) (P < .05). Aortic tissue MMP-9 levels in control aorta were significantly lower than in AAA tissue (13.2 ± 7 ng/mg protein) (Fig 7). Tissue MMP-9 was strongly correlated with the thickness of ILT deposition (R2 = 0.46; P < .05; Fig 8). In cases where there was no ILT, MMP-9 levels were uniform throughout the aneurysm with a mean of 18.6 ± 9 ng/mg protein. In those with dense circumferential ILT, MMP-9 was 39.2 ± 11 ng/mg protein, uniform throughout the AAA, and not statistically different when compared with areas of high ILT in patients with eccentric ILT deposition. MMP-9 was also present in high levels in ILT (50 ± 9 ng/mg protein), particularly in areas of greatest ILT deposition; those areas with typically 1 cm or more of ILT. In regions where ILT was present, but very scant (≤0.5 cm), MMP-9 levels were significantly lower at 13 ± 4 ng/mg protein.
Fig 7.
Matrix metalloproteinase (MMP)-9 levels in control aortic tissue and in abdominal aortic aneurysm (AAA) with regions of high and no intraluminal thrombus (ILT) deposition (∗P < .05 compared with control tissue; +P < .05 compared with high ILT).
Fig 8.
Matrix metalloproteinase (MMP)-9: levels (ng/mg protein) in abdominal aortic aneurysm (AAA) in areas of absent and high intraluminal thrombus (ILT) deposition in 17 patients with eccentric ILT deposition. Right upper insert, Correlation between MMP-9 level and ILT deposition. R = 0.68 (P < .05).
Discussion
Until recently, ILT was thought to passively accumulate in AAA, and possibly provide protection against rupture by reducing peak wall stress.19 There is increasing evidence that ILT is biologically active and may promote aortic wall degeneration and rupture, either by stimulating inflammatory and proteolytic mechanisms,10,11 and/or by providing a barrier to aortic wall oxygenation increasing local oxidative stress.9 Using CFD, we have previously shown that AAA tend to rupture at areas of flow recirculation, where there is higher ILT deposition.5 Autopsy studies have also shown that AAA rupture occurs through regions of maximal ILT deposition in 80% of cases.8 In the present study, we have demonstrated regional differences in MMP-9 activity in human AAA that are associated with different blood flow and ILT deposition patterns. In particular, we have shown that areas of flow recirculation are associated with greater ILT deposition, increased inflammatory activity, increased elastin and collagen degradation, and increased MMP-9 activity.
Unlike other studies, we used a stereotactic AAA tissue sampling method that sampled aortic tissue and ILT from the entire AAA in the same manner in all subjects and allowed an accurate correlation between computational hemodynamics, the degree of ILT deposition, and AAA wall degenerative changes. In addition, unlike studies that have used thoracic aorta from autopsy of healthy, non-age-matched controls, we used age-matched, nonaneurysmal infrarenal aortic tissue as control. The control aorta was taken from a visually normal section of the infrarenal aorta in those undergoing ABF bypass for aortoiliac occlusive disease. Even in aortic occlusion, the aorta nearest the renal arteries tends to be spared significant inflammation. Although it could be argued that infrarenal aortic tissue in patients with severe aortoiliac disease is not entirely normal, the infrarenal aorta serves as a better control than that of non-aged-matched thoracic aorta; as the thoracic aorta is comprised of different embryonic cells, has different responses to various cytokines and growth factors,20 and has a different elastin and collagen ratio.21,22 More importantly, the infrarenal aorta is more prone to aneurysmal degeneration and is completely dependent on luminal to abluminal diffusion of nutrients and oxygen.9 In control infrarenal aorta, we found very little inflammation and no ILT deposition despite the fact that the distal aorta and iliac arteries were significantly atherosclerotic. Elastin and collagen were also well-preserved in our control aortic tissue.
This study was limited by a small sample size; however, considering that the bulk of AAA repair and treatment for aortoiliac occlusive disease is predominantly endovascular, we had a relatively high number of open cases to provide adequate aortic tissue, especially considering that majority of AAA had eccentric ILT deposition, allowing a direct stereotactic comparison of MMP-9 levels within AAA.
This study was also limited by our computational assumptions. We used CFD to predict luminal flow and did not take into consideration the effect of flow on wall motion or wall stress; however, we have previously demonstrated that low WSS and low velocity recirculation flow are typical of high ILT locations and of the site of AAA rupture.5
Ninety-one percent of AAAs in this study had associated ILT. The majority of our cohort of patients exhibited an eccentric pattern of aortic ILT deposition. CFD assessment of flow in AAA with eccentric ILT deposition showed that ILT was significantly associated with zones of recirculation; whereas, the dominant flow channel and location of flow impingement were rarely associated with ILT deposition. In AAA that were completely devoid of ILT, flow typically did not separate into a dominant flow channel with a zones of recirculation. We found that AAA with no ILT showed higher velocity vortex flow within the entire lumen, suggesting that ILT deposition required slower recirculation flow for deposition of ILT to occur. Turbulent or recirculating blood flow has also been associated with platelet activation and increases ILT deposition.23,24
In cases with dense circumferential ILT, flow was more laminar and similar to that in the nonaneurysmal aorta. We have previously examined ILT deposition over time in human AAA25 and showed that ILT deposition is a dynamic process building increasing ILT in varying regions over time. It is not known why some AAA have relatively greater amounts of ILT but it may reflect a slower AAA growth with longer deposition times.
ILT was a common feature in more than 90% of cases in this study. In addition, ILT itself had higher levels of MMP-9 in regions where there was a greater thickness of deposition. Regions with absent ILT showed significantly lower levels of MMP-9, less inflammation, and a better preservation of elastin and collagen; however, they still showed higher levels of MMP-9 compared to control aorta.
We know that ILT develops from the deposition of blood-borne factors, such as platelets, red blood cells, white blood cells, and fibrin in the lumen of AAA26; however, it is not known why some AAA regions are spared ILT, but the process seems to be flow-mediated, suggesting that a longer resident time of blood-borne particles may favor deposition.27 Flow-mediated ILT deposition is not the only factor. This multifactorial process of ILT development is demonstrated in the inhomogeneous make-up of ILT, with luminal layers showing higher inflammatory cell content compared with abluminal layers, which are virtually devoid of cells.26 This heterogenous make-up of the ILT likely reflects the fact that luminal layers of ILT have been most recently deposited. In addition, Siennicka et al28 have shown inhomogeneity of coagulation and fibrinolytic activity in human AAA depending on ILT thickness. There is also evidence that abluminal layers of ILT have higher levels of MMP-9,29 suggesting that MMP-9 in the deeper ILT layers is not due to circulating factors, but rather to factors in the adjacent degenerating aortic wall.
Conclusions
This work was the first to correlate computationally-derived hemodynamic changes with increased ILT deposition and tissue MMP9 levels in human AAA. Using CFD we have demonstrated that human AAA typically have blood flow patterns characterized by a dominant flow channel and a flow impingement location, that is relatively devoid of ILT, and a zone of recirculation flow that was significantly associated high ILT deposition and significantly higher MMP-9 tissue levels. Further, these areas of high ILT were associated with local AAA wall degeneration as evidenced by increased elastin and collagen loss. It is possible that ILT deposition results in relative hypoxia, especially if the vasa vasorum is already affected by inflammation, that preferentially weakens the underlying aortic wall ultimately leading to rupture. The initial factors that lead to early aortic dilation and altered flow that may precipitate ILT deposition are still not understood. These results suggest that flow-mediated ILT deposition may promote local increases in proteolytic activity that may preferentially weaken and promote rupture at selected regions. Understanding the interaction between ILT deposition and proteolytic degradation of the aortic wall is an exciting area of investigation that may lead to targeted therapies for AAA and may ultimately lead to a more accurate assessment of AAA rupture risk.
Author contributions
Conception and design: DK, AB
Analysis and interpretation: AD, RL, LB
Data collection: AD, RL, LB
Writing the article: AD, LB, AB
Critical revision of the article: AD, RL, LB, DK, AB
Final approval of the article: AD, RL, LB, DK, AB
Statistical analysis: AD, LB, AB
Obtained funding: Not applicable
Overall responsibility: AB
Footnotes
This study was funded by Thorlakson Foundation Fund, University of Manitoba.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Rutherford R.B., McCroskey B.L. Ruptured abdominal aortic aneurysms. Special considerations. Surg Clin North Am. 1989;69:859–868. doi: 10.1016/s0039-6109(16)44891-7. [DOI] [PubMed] [Google Scholar]
- 2.Panek B., Gacko M., Palka J. Metalloproteinases, insulin-like growth factor-I and its binding proteins in aortic aneurysm. Int J Exp Pathol. 2004;85:159–164. doi: 10.1111/j.0959-9673.2004.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treska V., Kocova J., Boudova L., Neparsova P., Topolcan O., Pecen L. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomology of aneurysm. Cyto Cell Mol Therapy. 2002;7:91–97. doi: 10.1080/13684730310001652. [DOI] [PubMed] [Google Scholar]
- 4.Lozowy R.J., Ducas A.A., Kuhn D.S.C., Boyd A.J. The relationship between pulsatile flow impingement and intraluminal thrombus deposition in abdominal aortic aneurysms. Cardiovasc Eng Technol. 2017;8:57–69. doi: 10.1007/s13239-016-0287-5. [DOI] [PubMed] [Google Scholar]
- 5.Boyd A.J., Kuhn D.C., Lozowy R.J., Kulbisky G.P. Low wall shear stress predominates at sites of abdominal aortic aneurysm rupture. J Vasc Surg. 2016;63:1613–1619. doi: 10.1016/j.jvs.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Satta J., Mennander A., Soini Y. Increased medial TUNEL-positive staining associated with apoptotic bodies is linked to smooth muscle cell diminution during evolution of abdominal aortic aneurysms. Ann Vasc Surg. 2002;16:462–466. doi: 10.1007/s10016-001-0071-2. [DOI] [PubMed] [Google Scholar]
- 7.Wolf Y.G., Thomas W.S., Brennan F.J., Goff W.G., Sise M.J., Bernstein E.F. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg. 1994;20:529–538. doi: 10.1016/0741-5214(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 8.Simão da Silva E., Rodrigues A.J., Magalhães Castro de Tolosa E., Rodrigues C.J., Villas Boas do Prado G., Nakamoto J.C. Morphology and diameter of infrarenal aortic aneurysms: a prospective autopsy study. Cardiovasc Surg. 2000;8:526–532. doi: 10.1016/s0967-2109(00)00060-0. [DOI] [PubMed] [Google Scholar]
- 9.Vorp D.A., Lee P.C., Wang D.H., Makaroun M.S., Nemoto E.M., Ogawa S. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34:291–299. doi: 10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 10.Thompson R.W., Parks W.C. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 11.Busuttil R., Rinderbriecht H., Flesher A., Carmack C. Elastase activity: the role of elastase in aortic aneurysm formation. J Surg Res. 1982;32:214–217. doi: 10.1016/0022-4804(82)90093-2. [DOI] [PubMed] [Google Scholar]
- 12.Wilson W.R., Anderton M., Schwalbe E.C., Jones J.L., Furness P.N., Bell P.R.F. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113:438–445. doi: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]
- 13.Elmore J.R., Keister B.F., Franklin D.P., Youkey J.R., Carey D.J. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg. 1998;12:221–228. doi: 10.1007/s100169900144. [DOI] [PubMed] [Google Scholar]
- 14.Wilson W.R., Anderton M., Choke E.C., Dawson J., Loftus I.M., Thompson M.M. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:580–584. doi: 10.1016/j.ejvs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Arzani A., Shadden S.C. Characterization of the transport topology in patient-specific abdominal aortic aneurysm models. Phys Fluids. 2012;24:1–16. doi: 10.1063/1.4744984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan J.A., Abdul Rahman M.N.A., Mazari F.A.K., Shahin Y., Smith G., Madden L. Intraluminal thrombus has a selective influence on matrix metalloproteinases and their inhibitors (tissue inhibitors of matrix metalloproteinases) in the wall of abdominal aortic aneurysms. Ann Vasc Surg. 2012;26:322–329. doi: 10.1016/j.avsg.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Les A.S., Yeung J.J., Schultz G.M., Herfkens R.J., Dalman R.L., Taylor C.A. Supraceliac and intrarenal blood flow in patients with abdominal aortic aneurysms: mean flows, waveforms, and allometric scaling relationships. Card Eng Tech. 2010;1:39–51. doi: 10.1007/s13239-010-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mower W.R., Quinones W.J., Gambhir S.S. Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J Vasc Surg. 1997;26:602–608. doi: 10.1016/s0741-5214(97)70058-2. [DOI] [PubMed] [Google Scholar]
- 20.Ruddy J.M., Jones J.A., Ikonomidis J.S. Pathophysiology of thoracic aortic aneurysm (TAA): is it not one uniform aorta? Role of embryologic origin. Prog Cardiovasc Dis. 2013;56:68–73. doi: 10.1016/j.pcad.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruddy J.M., Jones J.A., Spinale F.G., Ikonomidis J.S. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg. 2008;136:1123–1130. doi: 10.1016/j.jtcvs.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolinsky H., Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circ Res. 1969;25:677–686. doi: 10.1161/01.res.25.6.677. [DOI] [PubMed] [Google Scholar]
- 23.Stein P.D., Sabbah H.N. Measured turbulence and its effect on thrombus formation. Circ Res. 1974;35:608–614. doi: 10.1161/01.res.35.4.608. [DOI] [PubMed] [Google Scholar]
- 24.Hathcock J.J. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 25.Launcelott S.L., Lozowy R.J., Kuhn D.S.C., Boyd A.J. Abdominal aortic aneurysm growth is associated with changes in thrombus deposition. J Vasc Surg. 2016;64:1546. [Google Scholar]
- 26.Adolph R., Vorp D.A., Steed D.L., Webster M.W., Kameneva M.V., Watkins S.C. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25:916–926. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- 27.Suh G.Y., Andrea S., Les S., Tenforde A.S., Shadden S.C., Spilker R.L. Quantification of particle residence time in abdominal aortic aneurysms using magnetic resonance imaging and computational fluid dynamics. Ann Biomed Eng. 2011;39:864–883. doi: 10.1007/s10439-010-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siennicka A., Zuchowski M., Kaczmarczyk M., Cnotliwy M., Clark J.S., Jastrzębskae M. Tissue factor levels and the fibrinolytic system in thin and thick intraluminal thrombus and underlying walls of abdominal aortic aneurysms. J Vasc Surg. 2017;68:30S–37S. doi: 10.1016/j.jvs.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Fontaine V., Jacob M.P., Houard X., Rossignol P., Plissonnier D., Angles-Cano Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2012;161:1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]