Abstract
Objective
The goal of the present study was to test the safety and efficacy of chemical stabilization of the arterial extracellular matrix as a novel nonoperative treatment of abdominal aortic aneurysms (AAAs) in a clinically relevant large animal model.
Methods
To achieve matrix stabilization, we used 1,2,3,4,6-pentagalloylglucose (PGG), a noncytotoxic polyphenolic agent capable of binding to and stabilizing elastin and collagen against the action of degrading enzymes. We first optimized the therapeutic PGG formulation and time of exposure by in vitro testing on porcine aortas using phenol histologic staining with iron chloride, elastic recoil assays, and PGG quantification as a function of tissue thickness. We then induced AAAs in 16 swine using sequential balloon angioplasty and elastase/collagenase and calcium chloride treatment of the infrarenal segment. We monitored AAA induction and development using digital subtraction angiography. At 2 weeks after induction, after the AAAs had reached ∼66% arterial expansion, the swine were randomly assigned to 2 groups. In the treatment group, we delivered PGG to the aneurysmal aorta endoluminally using a weeping balloon and evaluated the AAA diameters using digital subtraction angiography for another 10 weeks. The control swine did not receive any treatment. For the safety evaluation, we collected blood and performed comprehensive metabolic panels and complete blood counts every 2 to 3 weeks for all the animals. The swine were routinely monitored for neurologic and physical attributes such as behavior, inactivity, alertness, appetite, discomfort, and weight gain. After euthanasia and full necropsy, we analyzed the AAA tissue samples for PGG content, elastic recoil, and histologic features.
Results
In vitro, a single 2.5-minute intraluminal delivery of 0.3% PGG to the swine aorta was sufficient for PGG to diffuse through the entire thickness of the porcine arterial tissues and to bind with high affinity to the elastic lamellae, as seen by positive iron chloride staining, a reduction of elastic recoil, and an increase in PGG content. In vivo, the control swine AAA tissues were thickened and showed the typical aspects of AAA, including chronic inflammation, adventitial reactivity, smooth muscle cell proliferation, elastic lamellae degradation, and medial and adventitial calcification. Similar aspects were noted in the PGG-treated arteries, except for the lack of calcification and an apparent diminished hyperplasia. PGG treatment was effective in reducing AAA expansion and reversing the process of AAA dilation by reducing the aortic diameters to ≤30% by week 12 (P < .05). PGG was specifically localized to the aneurysmal segments as seen by histologic examination, the reduction of elastic recoil, and an increase in PGG content. PGG treatment did not affect the swine's neurologic or physical attributes, weight, blood chemistry, blood cells, or functionality of remote organs. The control, untreated swine exhibited progressive increases in AAA diameters up to a mean value of 104%.
Conclusions
Localized delivery of PGG to the aneurysmal aorta attenuated AAA growth and reversed the course of the disease in the swine AAA model. Such specificity for diseased tissue is unprecedented in nonoperative AAA treatment. This novel paradigm-shifting approach has the potential to revolutionize AAA management and save thousands of lives.
Keywords: AAA animal model, Nonoperative management of AAA, Pentagalloylglucose, Polyphenols
Clinical Relevance
Abdominal aorta aneurysm (AAA) is an asymptomatic chronic degenerative disease characterized by localized dilatation of the arterial wall caused by elastin and collagen degradation by proteases. We found that local delivery of 1,2,3,4,6-pentagalloylglucose (PGG) to a developing AAA in a swine animal model safely and effectively stabilized the vascular matrix, reduced AAA expansion, and promoted healing and AAA diameter reduction by limiting tissue degeneration. PGG is a noncytotoxic agent capable of rapid diffusion through arterial tissue and irreversible binding to elastin and collagen. PGG-mediated chemical stabilization of the aortic matrix could be used for safe nonoperative AAA management.
Article Highlights.
-
•
Type of Research: Basic science
-
•
Key Findings: We developed a swine abdominal aortic aneurysm (AAA) model, which reached an aneurysmal expansion of 66% within 2 weeks and 104% within 12 weeks. Localized delivery of 1,2,3,4,6-pentagalloylglucose (PGG) to the aneurysmal aorta 2 weeks after induction safely and effectively attenuated AAA growth and reversed the course by reducing the diameter to ≤30%.
-
•
Take Home Message: Chemical stabilization of the aortic extracellular matrix in medium-size AAAs is a safe and effective nonoperative option for AAA management.
Abdominal aorta aneurysm (AAA) is an asymptomatic chronic degenerative disease characterized by localized dilatation caused by weakening of the arterial wall. The disease affects ∼10% of the population aged >65 years and accounts for 15,000 deaths by sudden rupture annually in the United States.1 Although initially, AAAs were diagnosed incidentally, more recently, active targeted screening (now covered in part by Medicare) demonstrated a 40% reduction in mortality and AAA rupture.2 The pathogenesis of the disease is associated with chronic inflammation, elastin and collagen degradation by matrix metalloproteinases (MMPs), and smooth muscle cell death. Chronic matrix degradation by inflammatory cells and the diminished repair capacity accounts for the chronic weakening of the arterial wall.3 In addition, AAAs exhibit signs of medial calcification, intraluminal thrombus, adventitial thickening, and proliferation of the vasa vasorum. The disease is characterized by slow progression, with the potential for ultimate catastrophic rupture. The risk of rupture increases with increases in diameter, especially when the diameter is >40 mm (ie, expansion of ∼100%). After the initial diagnosis, changes in the AAA diameters are monitored by surveillance imaging every 6 to 12 months, and patients are advised to reduce the main risk factors (ie, smoking, hypertension). Management of uncomplicated AAAs >55 mm in men or >50 mm in women is currently limited to open repair and the less-invasive endovascular grafting.3 Pharmacologic attempts to slow the disease have been targeted at reducing the effects of risk factors, controlling hypertension, limiting oxidative stress, and delivering stem cells, anti-inflammatory drugs, statins, or MMP inhibitors. However, no clinical study has revealed any beneficial effects of these drugs on clinical AAA progression or their propensity to rupture.4 To date, all approved solutions have ignored the chronically inflamed aneurysmal aorta by bypassing flow and reducing pressure with an implant.
We hypothesized that a promising approach would be to implement chemical stabilization of the arterial collagen and elastin such that their degradation by MMPs would be greatly reduced. If that were possible, the smaller aneurysms would not grow, reducing the need for surgery, and the larger aneurysms would remain stable, reducing the risk of late rupture or endoleak. We further hypothesized that matrix stabilization should target small to medium AAAs (ie, 40%-60%), such that therapy could be implemented much earlier and increase the benefits for patients.
To achieve such stabilization, we developed, patented, and implemented 1,2,3,4,6-pentagalloylglucose (PGG), a unique polyphenolic, noncytotoxic agent capable of diffusing rapidly through arterial tissues, binding to collagen and elastin, and, thus, stabilize the extracellular matrix proteins against the action of degrading enzymes, including MMPs.5 Using a calcium chloride (CaCl2) rodent model of AAA, we showed that localized periadventitial infusion of PGG to the aneurysmal aorta safely and effectively stabilized the collagen and elastin matrix and reduced AAA expansion.5 We also showed that this effect had not resulted from direct interference with MMP activity but, rather, from substrate stabilization (ie, crosslinking of elastin and collagen). A novel finding from the rodent studies was that after PGG treatment, most of AAA segments had started to shrink, reducing their diameter significantly, indicating augmented healing. To the best of our knowledge, this is the first time that PGG-mediated chemical stabilization of the aortic matrix has been used for nonoperative AAA management without surgery or endovascular grafts.
Our team, and many others, has used PGG in numerous other applications, including tissue engineering and stabilization of tissue-derived biologic scaffolds used for regenerative medicine.6, 7, 8 Recently, we also showed that treatment of pure collagen and elastin scaffolds with PGG rendered them resistant to diabetes when implanted in diabetic rodents.9 This has opened new avenues for the generation of “diabetes-resistant” cardiovascular grafts.9 Overall, PGG has been documented as very safe and effective in numerous applications.10,11
In the present study, we performed in vitro mechanistic studies for optimization of the PGG formulation and then tested PGG safety and efficacy in a large animal model of AAA. The animal model generated AAA dilation of ∼66% at 2 weeks after induction, with clinical and histopathologic characteristics very similar to the human pathologic findings. We found that after localized delivery of PGG to the aneurysmal aorta, PGG migrates rapidly through the aortic tissues, binds to aortic elastin and collagen, prevents the expansion of developing aneurysms, and reverses the process of AAA dilation.
Methods
Porcine pancreatic elastase, PGG, 10% ferric chloride (FeCl3) solution, and the Folin-Ciocalteu phenol reagent were all obtained from Sigma-Aldrich (St. Louis, Mo). Collagenase type 3 was obtained from Worthington Biochemical Corporation (Lakewood, NJ).
In vitro mechanistic studies
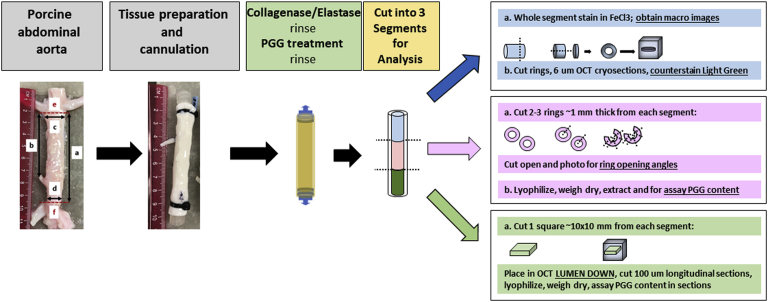
Fresh native porcine abdominal aortas from 6-month-old swine were obtained from Midwest Research Swine LLC (Glencoe, Minn). The tissues were cleaned of fat, and the aorta was cut below the renal arteries and above the trifurcation of the iliac arteries. Rough measurements were performed, and the aortas were cannulated using barbed Luer adapters secured with cable ties (Fig 1). Collaterals (lumbar and mesenteric arteries) were ligated shut, and saline was infused through the Luer adapters to check for leakage. To mimic AAAs, we treated the cannulated porcine aortas with a mixture of elastase (10 U/mL) and collagenase (50 U/mL) in 100 mM Tris buffer and 1 mM CaCl2 in saline (pH 8) by infusion through the lumen, followed by capping the 2 Luer adapters. After incubation for 15 minutes, the enzymes were rinsed with saline. The aortas were then infused endoluminally with PGG via the Luer adapters. The PGG treatment solution consisted of 0.3% ultra-pure PGG (>98% purity), 33% contrast media, saline, and a physiologic buffer to maintain the pH at its optimal binding value of 5.4.5 The concentration of 0.3% PGG was selected in accordance with the results from in vitro efficacy studies. It was the highest concentration that elicited minimal cytotoxicity (<20%) when tested according to International Organization for Standardization 10993-5 standards (data not shown). The aortas were infused with PGG for 2.5 and 5 minutes, followed by rinsing in saline. After treatment, the aortas were cut into 3 equal segments (2.5 cm) for analysis (Fig 1) as follows. First, a 1-mm ring was cut from each segment and fixed in formalin for histologic examination and staining with elastica-Masson,12 a technique that combines Masson's trichrome staining with Verhoeff–van Gieson stain for collagen and elastin, respectively. A first 2.5-cm segment was stained with phenol en bloc with 10% FeCl3 for 5 minutes and rinsed in saline. Next, 1- to 2-mm-wide rings were cut from the FeCl3-stained segment and embedded in optimal cutting temperature compound (OCT) for cryosectioning. Sections of 6 μm were counterstained with Light Green stain (Sigma-Aldrich, St. Louis, Mo), cover slipped with a water-based hard mounting medium, and imaged on a Zeiss microscope (Carl Zeiss AG, Oberkochen, Germany). The remaining FeCl3-stained tubular segment was cut open and photographed. A second 2.5-cm segment was cut into rings ∼1 mm wide. The rings were cut open and allowed to recoil for 10 minutes in saline on a shaker. Next, they were photographed for digital measurement of the opening angles using ImageJ software (National Institutes of Health [NIH], Bethesda, Md). The ring opening assay has been shown to reflect the natural ability of elastin to recoil and a method to assess PGG interactions with vascular elastin.5 The cut rings were further minced into small segments and lyophilized, and the dry weight was recorded. PGG was extracted with methanol, the PGG content was measured using a phenol reagent (Folin-Ciocalteu), and a standard curve was constructed with pure PGG, as described previously.5 Data are expressed as 1 μg of PGG per 1 mg of dry tissue. A third 2.5-cm segment was cut open and processed as follows. Samples of ∼10 × 10 mm from each group were frozen flat on top of a frozen half-full OCT block, lumen side down, OCT was added to fill the block, and the block was frozen at −20°C. Next, we cut 100-μm-thick cross-sections on a cryotome and collected them separately in groups of 2 in individual 15-mL conical tubes with 10 mL of water. Then, the tissue sections were rinsed with 10 mL of water two times by centrifugation at 1000 rpm to completely remove the OCT compound, lyophilized, and weighed dry, and the PGG content was extracted and analyzed as described. PGG is expressed as 1 μg/mg dry tissue as a function of tissue depth.
Fig 1.
Experimental design for in vitro studies. To simulate abdominal aortic aneurysms (AAA), we cannulated fresh porcine infrarenal abdominal aortas and treated them with collagenase and elastase. We then treated them with 1,2,3,4,6-pentagalloylglucose (PGG). Assays included phenol staining with ferric chloride (FeCl3) for localization, measurement of ring opening angles as a measure of elastic recoil, and PGG extraction and quantification in arterial tissues as a whole and in arterial longitudinal sections to evaluate PGG diffusion through the tissue. OCT, Optimal cutting temperature compound.
In vivo studies
AAA large animal model
All animal studies were performed at Gateway Medical Innovation Center (Shanghai, China). The procedures were performed in accordance with the Guide for Care and Use of Laboratory Animals, reported by the NIH, under an animal use protocol approved by the Center's animal ethics committee (approval no. SH2018-10003).
Female Yorkshire domestic farm swine obtained from certified breeding facilities and weighing 50 to 55 kg were acclimated at the animal facility for ≥48 hours. Each individual pig was assigned a unique number and a medical record that included medications, operating procedures, anesthesia records, and postoperative observations (Fig 2).
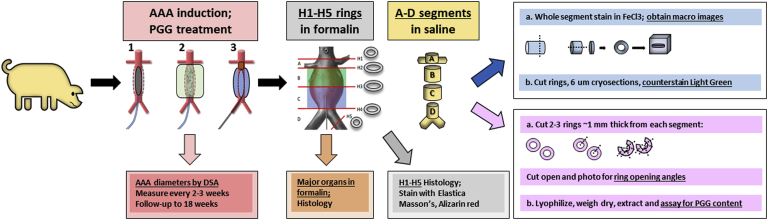
Fig 2.
Experimental design for in vivo studies. At time 0, arterial diameters were measured by digital subtraction angiography (DSA). Next, abdominal aortic aneurysms (AAAs) were induced using balloon dilation (1) and endoluminal collagenase, elastase, and periadventitial calcium chloride (CaCl2) treatment (2). When the AAA diameters had expanded to >40%, 1,2,3,4,6-pentagalloylglucose (PGG) was delivered endoluminally using a weeping balloon catheter (3), and the pigs were followed up for another 10 weeks, with DSA performed every 2 to 3 weeks to assess AAA development. The control pigs did not receive any PGG treatment. Blood was collected at every visit for full biochemistry analysis. On scheduled euthanasia, the aneurysmal aorta was excised and cut into 4 segments (A-D); 1- to 2-mm rings were then cut from each segment and fixed in formalin for histologic examination (H1-H5). Assays included phenol staining with ferric chloride (FeCl3) for localization, measurement of ring opening angles as a measure of elastic recoil, and PGG extraction and quantification in arterial tissues. To evaluate remote organ toxicity, samples were also collected from the major organs and fixed in formalin for histologic analysis.
In brief, the swine underwent the following protocol. At time 0, we obtained baseline aortic diameters using DSA and induced AAA with enzymes and CaCl2. The swine were allowed to recover and underwent DSA at 2 to 3 weeks after AAA induction. If the AAA diameter was >40%, the pigs were treated with PGG during the same session and allowed to recover. The swine underwent DSA again 2 to 3 weeks after PGG treatment and were euthanized after another 2 to 3 weeks, for a total of 10 weeks of follow-up after PGG treatment.
Using reported procedures as a guide,13, 14, 15, 16 AAAs were induced using balloon angioplasty, elastase, collagenase, and CaCl2. The pigs were sedated with a mixture of intramuscular tiletamine hydrochloride with zolazepam hydrochloride (Zoletil; Virbac, Carros, France) and xylazine, washed, and prepared in an aseptic manner for a midline incision. After intubation, anesthesia was maintained with 1% to 5% isoflurane in 100% oxygen, supported by mechanical ventilation. A standard anticoagulation protocol with heparin was observed, with an activated clotting time >250 seconds, measured every 30 to 45 minutes.
The right femoral artery was accessed by inserting the Seldinger needle percutaneously through a skin incision. Baseline digital angiographic measurements were conducted via access through the right femoral artery as described below (PGG treatment section). The viscera were displaced laterally, and the abdominal aorta was fully exposed. A 2-mL topical application of papaverine hydrochloride was delivered to the exposed aorta to reduce vasospasm. After 5 minutes, the 2 lumbar arteries between the renal arteries and the trifurcation were temporarily occluded by string loops. The abdominal aorta was mechanically disrupted by performing balloon angioplasty in the region between 1 cm below the lower renal artery and 1 cm above the inferior mesenteric artery (IMA) for 10 minutes. The targeted oversizing during angioplasty was 30% to 50%. At the segment just above the iliac trifurcation and below the IMA, a purse-string suture was created with a 4-0 polypropylene suture. Inside the suture, a 6F or 7F sheath was placed into the aorta, ensuring that the tip of the sheath was inside the lumen of the aorta and above the IMA. The aorta segments just below the lower renal artery and above the IMA were isolated and occluded with rumel tourniquets.
The blood inside the occluded segment was removed from the sheath and flushed with normal saline. A mixture of sterile 6000 U of collagenase and 500 U of elastase in 10 mL of 100 mM Tris buffer at pH 8.0 was prepared and infused within the isolated region and applied by pressure perfusion. Concurrently, a 5-cm × 5-cm sterile gauze was soaked in 10 mL of sterile 0.5 M CaCl2 solution and placed topically (periadventitially) on the isolated abdominal aorta. The enzyme and CaCl2 treatments lasted for 20 minutes before removal and evacuation. The isolated region was then flushed thoroughly with normal saline. All the ties were removed, and the surgical access was closed. The mean occlusion time was ∼35 minutes, and all animals were monitored for lower limb temperature and blood flow. A single dose of antibiotic (cephalosporin) and analgesic (flunixin meglumine) were administered preoperatively. Postoperatively, the swine received an analgesic (tolfenamic acid or buprenorphine) for 3 days and antibiotics (ceftiofur) for 1 week.
PGG treatment
All the pigs were monitored for weight on a weekly basis and for AAA expansion by DSA at 2 to 3 weeks after induction. When the aneurysm had reached ≥40% relative to the baseline measure, the pigs were randomly assigned to receive PGG treatment or no treatment at all (controls) at a 2:1 randomization ratio. The sterile PGG treatment solution consisted of 0.3% ultra-pure PGG (>98% purity), 33% contrast media, saline, and a physiologic buffer to maintain pH at its optimal binding value of 5.4. The concentration of PGG was selected in accordance with the results from in vitro efficacy studies and correlated with the highest concentration that elicited minimal cytotoxicity when tested in accordance with the International Organization for Standardization 10993-5 standards (data not shown).
For PGG delivery, we designed a system (patent pending) composed of a central occlusion balloon, a weeping delivery balloon, and a manifold with 3 ports. A central radiopaque marker allowed the central occluding balloon to be placed at the AAA neck, just proximal to the ostium of the aortic aneurysmal sac. The occlusion port allowed for the contrast plus saline solution to be infused in the central balloon until wall apposition was confirmed. Within the infusion port, PGG was delivered under fluoroscopic guidance until the weeping delivery balloon had inflated and had fully apposed the sac wall. Next, at a controlled pressure of <100 mm Hg (using the attached digital gauge), PGG was delivered at ∼10 mL/1 minute for 2.5 minutes. Then, in reverse order, the balloons were deflated, and the delivery system was retrieved over the guidewire. The time, volume, and infusion rate of PGG delivery were determined by the results from the in vitro studies (described previously). Studies were also performed to ensure that PGG binding and activity were not hindered by the presence of the contrast material (data not shown). The pigs were followed up for ≤10 weeks after AAA induction, with the diameters monitored every 2 to 3 weeks by DSA.
DSA was used to assess the arterial diameters in sedated and anesthetized pigs. Percutaneous angiographic measurements were conducted using 4F sheaths and 4F pigtail catheters. The digital measurements were performed at the aorta just below the renal arteries, at a couple of places in the AAA target treatment area, and just above the iliac trifurcation. All the DSA scans before and at PGG treatment were conducted via the right femoral artery, and all the follow-up DSA scans after PGG treatment was conducted via the left femoral artery.
Blood was collected from each pig at baseline, at AAA induction surgery, at PGG treatment, and at every DSA examination. The blood was analyzed in a standard biochemistry analyzer for the comprehensive metabolic panel and complete blood count. The pigs were routinely monitored for neurologic and physical attributes such as behavior, inactivity, alertness, appetite, discomfort, and weight gain.
At the scheduled dates, euthanasia was performed on anesthetized pigs with a lethal dose of veterinary euthanasia solution of saturated potassium chloride, and the aortic tissues were collected. The aortas were divided into 4 anatomical segments (marked A-D). From each segment, a 1-mm-wide ring was cut and placed in formalin for histologic examination (marked H1-H5; details shown in Fig 2). Segments A to D were placed in saline and frozen in liquid nitrogen for transportation to the test laboratory.
A full necropsy was also performed on each pig to collect, weigh, and inspect the heart, kidneys, brain, lungs, and liver. Small segments from each organ were also dissected and fixed in formalin for histologic examination. All paraffin-embedded samples were sectioned at 5 μm and stained with elastica-Masson and Alizarin Red S stains.
Segments A to D were thawed and divided for analysis as follows (details shown in Fig 2). First, a segment was stained with phenol en bloc with 10% FeCl3 for 5 minutes and rinsed in saline. Next, 1-mm rings were cut from the FeCl3-stained segment and embedded in OCT for cryosectioning. Sections at 6 μm were counterstained with Light Green, cover slipped with a water-based hard mounting medium, and imaged on a Zeiss microscope (Carl Zeiss AG). The remaining tubular segment was cut open and photographed. Second, two 1- to 2-mm-wide rings were cut open and allowed to open unaided for 10 minutes in saline on a shaker. Next, they were photographed for digital measurement of the opening angles using ImageJ software (NIH). The cut rings were further minced into small segments, lyophilized, and PGG was extracted and measured as described previously. The data are expressed as 1 μg of PGG/1 mg of dry tissue.
Statistical analysis
The results are presented as the mean ± standard deviation. We used the Shapiro-Wilks test for normality and compared ≤2 sets of data using single-factor analysis of variance with 95% confidence intervals (P < .05).
Results
In vitro studies
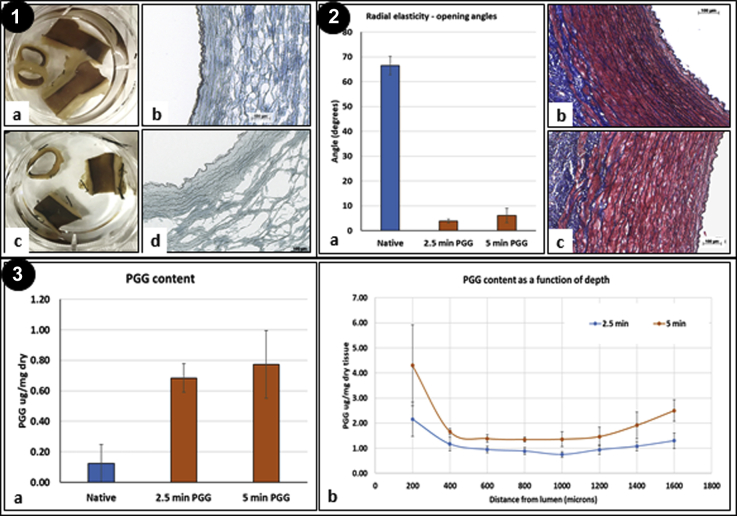
Intraluminal treatment of porcine aortas with PGG resulted in very rapid binding to the tissue (within minutes) as noted after en bloc staining with FeCl3 (Fig 3, Panel 1). PGG had diffused within 2.5 minutes through the arterial tissues, as evidenced by staining of most elastin layers, including the external elastic lamina and adventitial fibers. Binding of PGG to elastin significantly reduced the ability of arteries to recoil, as demonstrated by the ring opening analysis (Fig 3, Panel 2). The phenolic content of the native aorta (Fig 3, Panel 3) was ∼0.125 μg/1 mg dry tissue. The PGG-treated arteries exhibited PGG levels of 0.7 to 0.8 μg/1 mg dry tissue, and the histologic analysis showed excellent preservation of the overall tissue structure. Assessment of PGG content as a function of depth showed a slight decrease after the first 400 μm, after which the PGG content remained relatively stable, indicating rapid diffusion through the arterial tissue. No significant quantitative or qualitative differences were found between aortas treated with PGG for 2.5 minutes and those treated for 5 minutes, indicating that 2.5 minutes might be sufficient for efficient delivery of PGG to the aortic tissues, minimizing the occlusion time and risk of lower limb ischemia.
Fig 3.
Interactions of 1,2,3,4,6-pentagalloylglucose (PGG) with porcine abdominal aortic tissue in vitro. Panel 1, En bloc phenol stain with ferric chloride (dark brown) after 2.5 minutes (a) and 5 minutes (c) of endoluminal PGG treatment. PGG had bound mostly to elastin sheets, as seen in cryosections through the ferric chloride-stained tissue (dark brown; b and d). Panel 2, Radial elasticity of abdominal aorta measured via the ring opening angle assay. a, Digitally measured angles of fresh native artery (blue bar) and arteries treated with PGG for 2.5 and 5 minutes (orange bars). N = 3 for each group. Elastica-Masson histologic staining showed normal structure at 2.5 minutes (b) and 5 minutes (c) of PGG-treated arteries. Blue indicates collagen; dark brown, elastin; and red, cells. The lumen is on the right. Panel 3, a, Quantitative PGG content assayed in whole native fresh aortic tissues (blue bar) and tissues treated with PGG for 2.5 and 5 minutes (orange bars). b, PGG content in longitudinal tissue sections as a function of depth, with the intima (lumen) on the left. N = 3 for each data point and group.
In vivo studies
A total of 44 swine were investigated in the present study. For clinical relevance, we set the target pathologic entity as small to medium AAA, defined as aortic dilatation of >50%, and the follow-up duration as 10 weeks after AAA induction. A first cohort of 14 pigs was used to determine the optimal animal age and weight, the duration of elastase plus collagenase treatment for AAA induction, the adequate imaging modality, the timeline of AAA development, and the parameters for PGG delivery. The data obtained from these 14 pigs were not included in the present report. Once the protocol was established, a second cohort of 30 pigs underwent AAA induction and randomization for PGG treatment or no treatment (controls) at a 2:1 randomization ratio. Of these 30 pigs, 6 died prematurely: 2 during AAA induction, 2 of cardiac arrest, and 2 at 7 to 10 days after surgery of unknown reasons but unrelated to PGG treatment. Another 4 pigs died of a ruptured AAA (the pigs were found dead with the abdominal cavity full of blood) before PGG treatment, and 4 pigs did not develop a large enough AAA (<40%) to be included in the present study. The mean overall survival rate after AAA induction was 76%. In the present study, we report the data obtained from the 6 controls and 10 pigs who had undergone PGG treatment and been followed up for ≤12 weeks.
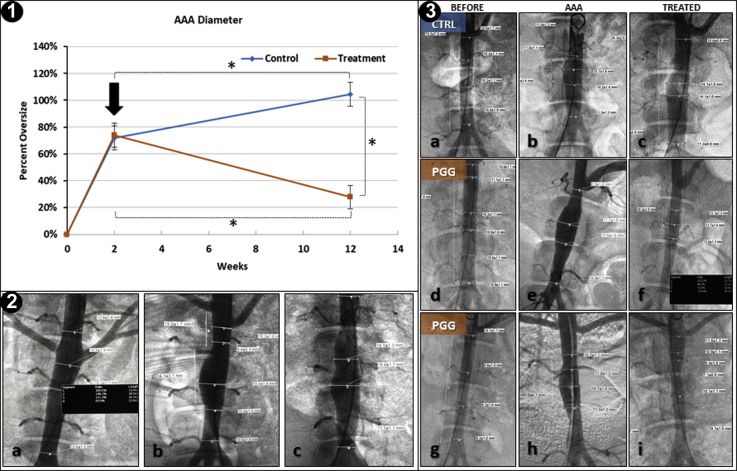
Assessment of the aortic diameter changes (Fig 4, Panel 1) showed that most pigs reached a mean increase in the AAA diameter of 66% at 2 weeks after induction. The diameter of the AAAs in the control pigs continued to increase, reaching 104%. In contrast, the AAAs treated with PGG slowly diminished and had reached significantly lower levels at 12 weeks after induction (mean, 30%; P < .05). A few PGG-treated animals that had been allowed to survive for ≤18 weeks exhibited unchanged AAA diameters (mean, 24%; not significantly different statistically from the 12-week values). A variety of AAA shapes and infrarenal locations were found in our study (Fig 4, Panel 2).
Fig 4.
Experimental results for in vivo studies. Panel 1, Diameters of abdominal aortas in controls and 1,2,3,4,6-pentagalloylglucose (PGG)-treated aneurysmal swine. Two weeks after abdominal aortic aneurysm (AAA) induction, the mean diameters had reached ∼66%, at which time, the pigs were randomly selected for PGG treatment (arrow) or no treatment. The AAA diameters were monitored using digital subtraction angiography (DSA) every 2 to 3 weeks. Data presented as mean of N = 6 for controls and N = 10 for PGG treatment. ∗Statistically significant differences determined by analysis of variance with 95% confidence intervals (P < .05). Panel 2, Representative examples of infrarenal AAA shapes and locations obtained in the present study. Relative to the 2 renal arteries, we found AAAs in proximal segments (a), medial segments (b), and even a “double hump” aspect (c). Panel 3, Representative DSA images of AAA development in controls (CTRL; a-c) and 2 PGG-treated pigs (PGG; d-f and g-i). Images shown at time 0 (BEFORE), after AAA induction, and 6 to 8 weeks after PGG treatment (TREATED).
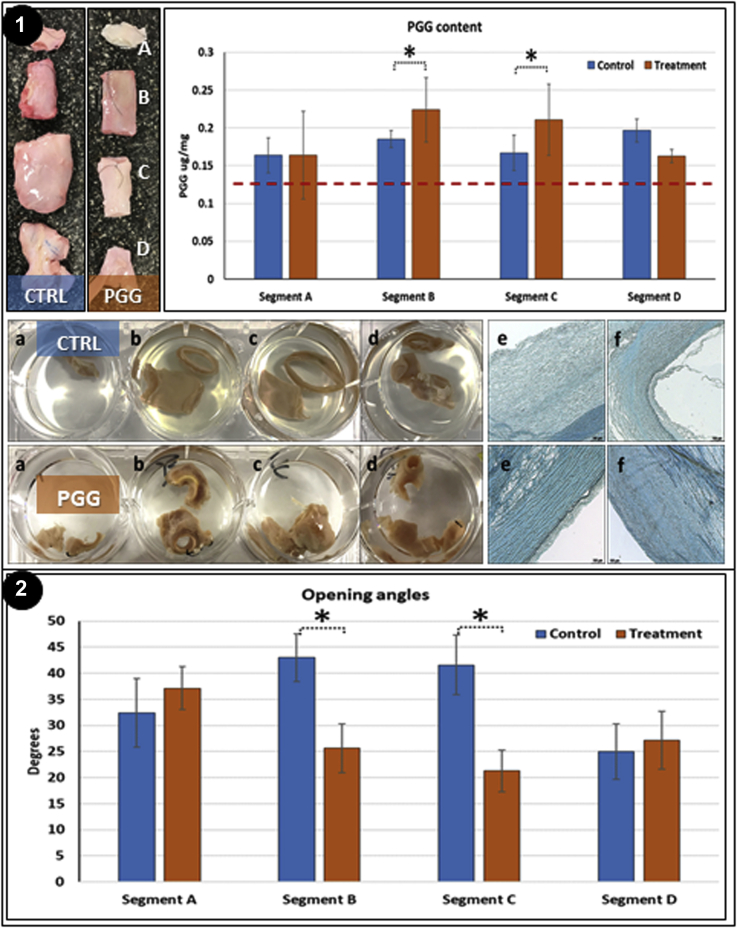
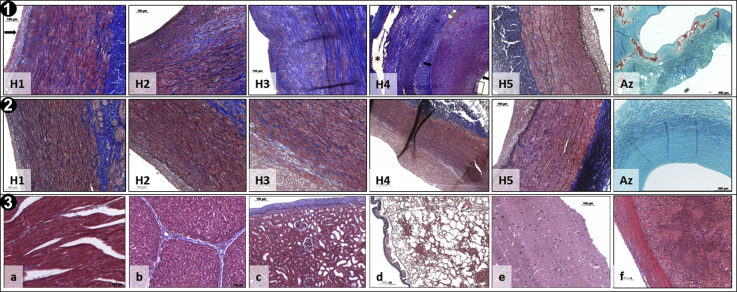
The aortic tissue samples retrieved at the end of the study from the control and treated pigs were cut into 4 segments (A-D) and tested for PGG content (Fig 5, Panel 1). Segments B and C, corresponding to the area where the PGG had been delivered in vivo, exhibited significantly higher levels of PGG statistically compared with segments A and D, indicating that PGG delivery had been localized mainly to the targeted AAA area. Segments B and C also stained positively with FeCl3. The opening angles were significantly smaller in segments B and C compared with controls (Fig 5, Panel 2), indicating that PGG had interacted with the arterial elastin in these segments. The tissue segments collected from the 4 segments (labeled H1-H5) were analyzed by histologic examination (Fig 6). Segments H1, H2, and H5 did not exhibit significant alterations, except for mild neointimal hyperplasia, possibly as a reaction to the balloon angioplasty. Segments H3 and H4 were thickened and showed aspects of chronic inflammation, adventitial reactivity, adventitial neovascularization, smooth muscle cell proliferation, elastin and collagen degradation, and medial and adventitial calcification. Similar aspects were noted in the PGG-treated arteries, except for lack of calcification in all segments analyzed and an apparent diminished hyperplasia in the H3 and H4 segments (Fig 6, Panels 1 and 2).
Fig 5.
Analysis of retrieved abdominal aortic aneurysm (AAA) tissue samples. Panel 1, Top, Left, Representative macroscopic images of whole AAA segments from controls and 1,2,3,4,6-pentagalloylglucose (PGG) treatment groups after sectioning into 4 segments (A-D). Renal arteries shown at top and iliac arteries at bottom. Right, PGG content of tissue samples from each segment in control (blue) and PGG-treated arteries (orange). Bottom, En bloc phenol stain with ferric chloride (PGG-treated; dark brown) of each segment (a-d) in controls (CTRL) and PGG-treated tissues and their corresponding cryosections (e, f). N = 3 for each group. Panel 2, Radial elasticity of abdominal aorta from each segment of controls (blue) and PGG-treated AAA (orange) measured via the ring opening angle assay. N = 3 for each group.
Fig 6.
Histologic analysis. Panel 1, Representative histologic images of H1 to H5 sections collected from different abdominal aortic aneurysm (AAA) areas in control swine showing developing neointimal hyperplasia, inflammation, smooth muscle cell proliferation (H4; arrows), adventitial thickening and neovascularization, and elastin degradation. Az, Representative Alizarin Red S (Az) stain showing extensive calcification (red) of AAA media and adventitia. Panel 2, Representative histologic images of H1 to H5 sections collected from AAA areas in 1,2,3,4,6-pentagalloylglucose (PGG)-treated swine showing apparently diminished hyperplasia and no calcification (Az). Panel 3, Representative histologic images of remote organs from PGG-treated swine showing unaffected normal structures of myocardium (a), liver (b), kidney (c), lung (d), brain (e), and spleen (f). All sections (except for Az) were stained with elastica-Masson, which revealed collagen (blue), elastin (dark brown), and cells (red).
Overall, the health of the pigs was very good throughout the study. The pigs did not exhibit any signs of neurologic or physical distress and the weight of both groups slowly increased, without showing any changes as a result of surgery or repeated imaging. A full chemistry panel was performed on blood collected at every imaging visit for all control and treatment swine. The panel included liver enzymes, creatinine, glucose, ions, and urea. All parameters tested were within established normal ranges, with no changes attributable to the surgical procedures or imaging studies. The complete blood count analysis showed minor changes in the white blood cell counts (10%-20% increase) after the surgical procedures, which had returned to normal levels by 7 to 10 days postoperatively. The full necropsy performed on each individual swine revealed a lack of any visible alterations in organ weights or overall aspects and no histologic signs of structural alterations (Fig 6, Panel 3).
Discussion
In vitro studies
Our in vitro studies showed that intraluminal treatment of porcine aortas with PGG for 2.5 and 5 minutes resulted in very rapid diffusion through the entire thickness of the tissue (1-2 mm) and binding to the elastic lamellae, essentially plateauing after 2.5 minutes. We chose the shorter treatment duration of 2.5 minutes for the in vivo study to minimize the occlusion time and prevent lower limb ischemia. In previous studies, the PGG treatment duration had been tested at 10 minutes for periadventitial delivery in rodents5 and 30 minutes in a swine elastase-induced model of AAA.13 The PGG bound to the arteries at a ratio of ∼0.7 to 0.8 μg PGG/1 mg of dry tissue, in agreement with previous studies performed with purified elastin.17 The rapid diffusion and binding characteristics of PGG prompted us to pursue a large animal study.
In vivo study
AAA development in large animals has been investigated extensively in the past few years.18 Attempts to adapt rodent models, which typically use only 1 type of injury (elastase, CaCl2, angiotensin II, or β-aminopropionitrile) to larger animals have not proved fully successful. Therefore, combinations of ≥1 injuries have been used in reported studies.3,18 We chose to combine an initial mechanical injury induced by balloon angioplasty, with elastase plus collagenase treatment, and periadventitial CaCl2 application, as originally reported by Czerski et al.14 Our accelerated model generated 66% AAAs within 2 weeks, reaching >100% AAA in 12 weeks if left untreated and exhibited good parallels with human pathologic features, including inflammation, adventitial reactivity, elastin degradation, and medial and adventitial calcification. We observed a variety of AAA shapes and locations, similar to the anatomic variability seen in human patients.3 Four of our pigs developed AAAs very rapidly and died prematurely of ruptured AAAs (these were excluded from the present study). Intraluminal, acute PGG delivery to the medium-size AAAs resulted in PGG binding to the targeted area and stabilization and diminution of the AAA diameters, reaching statistical significance at 10 weeks after treatment. Similar results were obtained in the rodent CaCl2 AAA model5 and the elastase-induced model of AAA in swine.13 In the present study, we noted an apparent diminished hyperplasia and reduction in medial and adventitial calcification in the PGG group. The anticalcification effect of PGG has been demonstrated previously.9 Finally, PGG treatment was determined to be safe locally and systemically, which has been supported by others.11,19
The expansion rate and propensity to rupture of AAAs in diabetic patients have been notoriously lower than in nondiabetic patients. This effect has been reported in numerous clinical studies and was explained by invoking the hypothesis that glycoxidation, or glucose-mediated cross-linking of the aortic matrix, reduces its susceptibility to degradation. Thus, diabetes is considered to have a protective effect on AAA.20, 21, 22, 23 Therefore, it is possible that locally induced matrix stabilization such as the PGG treatment we have reported, could effectively slow AAA progression and lower the incidence of AAA rupture.
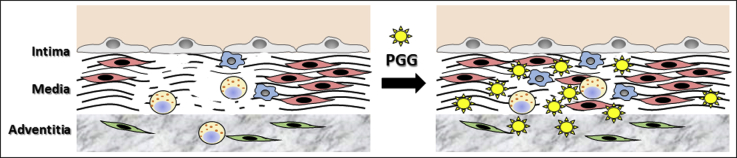
The mechanisms of PGG reduction of AAA progression are not fully understood. However, based on the data obtained thus far, we believe that (1) PGG binds covalently to elastin and collagen and prevents their proteolytic degradation; (2) diminution of matrix degradation reduces the amounts of soluble products, most of which are proinflammatory; (3) stabilization of the matrix fibers directly reduces cell infiltration; and (4) diminishing degeneration and levels of proinflammatory signals promotes more effective healing by resident cells (Fig 7).
Fig 7.
Hypothetical mechanisms of action of 1,2,3,4,6-pentagalloylglucose (PGG). Aneurysmal aorta (Left) is characterized by intact, but activated, endothelium (top layer: gray cells) lining the intima, inflammatory cell infiltration (blue cells, spotted yellow cells), which secrete matrix metalloproteinases and induce extensive degradation of elastin and collagen fibers (black), death of medial smooth muscle cells (red cells), and activation of adventitial fibroblasts (green cells). PGG (yellow star) diffuses rapidly through arterial tissues, binds to, cross-links, and stabilizes elastin and collagen, and reduces their degradation, which, in turn, reduces inflammatory cell infiltration, promotes more effective healing, and reduces aneurysmal expansion.
The present study had several limitations. First, we recognize that the in vitro data obtained from PGG treatment of fresh arteries was not entirely similar to the in vivo study in which the arteries had undergone a more complex pathogenic process. However, we used the in vitro data to establish a safe and effective PGG concentration and time of exposure that would suggest possible therapeutic potential. Second, the present study lacked a saline control (ie, aneurysmal animals subjected to saline delivery via the same weeping balloon delivery system as used for PGG delivery. Third, we recognize that the role of the vascular cells in this model is only speculative; additional immunohistochemical analyses are required for insight into the mechanistic aspects of this animal model. Fourth, some of the AAAs exhibited nonuniform morphologic features (ie, saccular, fusiform, eccentric shapes), which could be construed as a deficiency of the animal model. However, because these anatomic variations are also regularly found in human pathology, these do not detract from the value of the model. Finally, we realize that the usage of only female swine was a limitation. Ours was a pilot study that took advantage of the local availability of female pigs near the animal facility. The choice was not made in relation to gender and the availability at that time would have not allowed using both males and females or only males. Future studies with both genders are warranted.
Conclusions
Degradation of the aortic wall extracellular matrix is the hallmark of AAAs and the main factor responsible for fatal aortic ruptures. Once initiated, the chronic degenerative processes that degrade elastin and collagen cannot be stopped. Thus, the only nonoperative option to treat AAAs is to stabilize, cross-link, and maintain the existing structural proteins intact. We found earlier in our work that PGG is a highly effective agent capable of stabilizing elastin and collagen against the action of proteases. The acute local delivery of PGG to developing AAAs in a clinically relevant swine model attenuated and reversed AAA expansion and aortic dilation. As a result of PGG delivery, the small- to medium-size AAAs did not increase, and the aortic diameters stabilized and even showed signs of diminution and healing. PGG could be delivered to aneurysmal tissue with high precision using a weeping balloon system and only required 2.5 minutes of infusion time to diffuse rapidly through arterial tissues, bind with high affinity to collagen and elastin, and stabilize the arterial extracellular matrix proteins and promote a healing response. No signs of local, systemic, or remote organ toxicity were detected in association with PGG delivery. Targeting chemical stabilization of arterial collagen and elastin appears to be more promising than targeting the inflammatory process or reducing the activity of proteases. The use of PGG was deemed safe and effective in the swine model, allowing for implementation of this revolutionary AAA management solution in patients and saving thousands of lives.
Author contributions
Conception and design: DS
Analysis and interpretation: DS, MC, JT, NR
Data collection: DS, MC, JT, NR, JY, KN
Writing the article: DS, MC, JT, NR, JY
Critical revision of the article: DS, KN
Final approval of the article: DS, MC, JT, NR, JY, KN
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: DS
Footnotes
The present study was funded by Nectero Medical, Inc (to K.N.), the Harriet and Jerry Dempsey Endowment (to D.S.), and a Clemson University Research Foundation Award (to D.S.).
Author conflict of interest: Nectero Medical, Inc. funded most of this project; however, all performance data analysis and interpretation were performed by the authors, independent of input or interpretation from Nectero Medical, Inc. Dr Simionescu is a co-inventor of Clemson University technology using 1,2,3,4,6-pentagalloylglucose for the treatment of abdominal aortic aneurysms (U.S. Patent and Trademark Office no. 8,435,553). K.N. is Senior Vice President at Nectero Medical, Inc, which licensed the PGG technology from Clemson University. M.C., J.T., N.R., J.Y. have no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Carino D., Sarac T.P., Ziganshin B.A., Elefteriades J.A. Abdominal aortic aneurysm: evolving controversies and uncertainties. Int J Angiol. 2018;27:58–80. doi: 10.1055/s-0038-1657771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16:225–242. doi: 10.1038/s41569-018-0114-9. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow N.J., Wu W.W., Schermerhorn M.L. Open and endovascular management of aortic aneurysms. Circ Res. 2019;124:647–661. doi: 10.1161/CIRCRESAHA.118.313186. [DOI] [PubMed] [Google Scholar]
- 4.Lindeman J.H., Matsumura J.S. Pharmacologic management of aneurysms. Circ Res. 2019;124:631–646. doi: 10.1161/CIRCRESAHA.118.312439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isenburg J.C., Simionescu D.T., Starcher B.C., Vyavahare N.R. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–1737. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 6.Pennel T., Fercana G., Bezuidenhout D., Simionescu A., Chuang T.H., Zilla P. The performance of cross-linked acellular arterial scaffolds as vascular grafts: pre-clinical testing in direct and isolation loop circulatory models. Biomaterials. 2014;35:6311–6322. doi: 10.1016/j.biomaterials.2014.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sierad L.N., Simionescu A., Albers C., Chen J., Maivelett J., Tedder M.E. Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc Eng Technol. 2010;1:138–153. doi: 10.1007/s13239-010-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedder M.E., Liao J., Weed B., Stabler C., Zhang H., Simionescu A. Stabilized collagen scaffolds for heart valve tissue engineering. Tissue Eng Part A. 2009;15:1257–1268. doi: 10.1089/ten.tea.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow J.P., Simionescu D.T., Warner H., Wang B., Patnaik S.S., Liao J. Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol. Biomaterials. 2013;34:685–695. doi: 10.1016/j.biomaterials.2012.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patnaik S.S., Piskin S., Pillalamarri N.R., Romero G., Escobar G.P., Sprague E. Biomechanical restoration potential of pentagalloyl glucose after arterial extracellular matrix degeneration. Bioengineering (Basel) 2019;6:E58. doi: 10.3390/bioengineering6030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik S.S., Simionescu D.T., Goergen C.J., Hoyt K., Sirsi S., Finol E.A. Pentagalloyl glucose and its functional role in vascular health: biomechanics and drug-delivery characteristics. Ann Biomed Eng. 2019;47:39–59. doi: 10.1007/s10439-018-02145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita H., Umezawa T., Omine Y., Kasahara M., Rodriguez-Vazquez J.F., Murakami G. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol. 2013;46:39–48. doi: 10.5115/acb.2013.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloster B.O., Lund L., Lindholt J.S. Inhibition of early AAA formation by aortic intraluminal pentagalloyl glucose (PGG) infusion in a novel porcine AAA model. Ann Med Surg (Lond) 2016;7:65–70. doi: 10.1016/j.amsu.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czerski A., Bujok J., Gnus J., Hauzer W., Ratajczak K., Nowak M. Experimental methods of abdominal aortic aneurysm creation in swine as a large animal model. J Physiol Pharmacol. 2013;64:185–192. [PubMed] [Google Scholar]
- 15.Cullen J.M., Lu G., Shannon A.H., Su G., Sharma A., Salmon M. A novel swine model of abdominal aortic aneurysm. J Vasc Surg. 2019;70:252–260.e2. doi: 10.1016/j.jvs.2018.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon A.H., Cullen J.M., Dahl J.J., Scott E.J., Tyerman Z., Spinosa M.D. Porcine model of infrarenal abdominal aortic aneurysm. J Vis Exp. 2019 doi: 10.3791/60169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenburg J.C., Simionescu D.T., Vyavahare N.R. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25:3293–3302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Patelis N., Moris D., Schizas D., Damaskos C., Perrea D., Bakoyiannis C. Animal models in the research of abdominal aortic aneurysms development. Physiol Res. 2017;66:899–915. doi: 10.33549/physiolres.933579. [DOI] [PubMed] [Google Scholar]
- 19.Shaikh Q.U., Yang M., Memon K.H., Lateef M., Na D., Wan S. 1,2,3,4,6-Pentakis[-O-(3,4,5-trihydroxybenzoyl)]-alpha,beta-D-glucopyranose (PGG) analogs: design, synthesis, anti-tumor and anti-oxidant activities. Carbohydr Res. 2016;430:72–81. doi: 10.1016/j.carres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Taimour S., Avdic T., Franzen S., Zarrouk M., Acosta S., Nilsson P. Survival, cardiovascular morbidity, and reinterventions after elective endovascular aortic aneurysm repair in patients with and without diabetes: a nationwide propensity-adjusted analysis. Vasc Med. 2019;24:539–546. doi: 10.1177/1358863X19870243. [DOI] [PubMed] [Google Scholar]
- 21.Betancourt-Garcia M.M., Vatcheva K., Thakur A., Gupta P.K., Postevka E., Martinez R. Diabetes and its effect on abdominal aortic aneurysm growth rate in Hispanic patients. Ann Vasc Surg. 2019;61:254–260. doi: 10.1016/j.avsg.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Taimour S., Franzen S., Zarrouk M., Acosta S., Nilsson P., Miftaraj M. Nationwide comparison of long-term survival and cardiovascular morbidity after acute aortic aneurysm repair in patients with and without type 2 diabetes. J Vasc Surg. 2020;71:30–38.e3. doi: 10.1016/j.jvs.2019.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Zarrouk M., Franzen S., Acosta S., Nilsson P., Miftaraj M., Eliasson B. Long-term survival and cardiovascular morbidity after elective open aortic aneurysm repair in patients with and without type 2 diabetes: a nationwide propensity-adjusted analysis. Ann Vasc Surg. 2019;59:110–118. doi: 10.1016/j.avsg.2019.01.011. [DOI] [PubMed] [Google Scholar]