Abstract
Background
Quantitative methods for evaluating microstructure of arterial specimens typically rely on histologic techniques that involve random sampling, which cannot account for the unique spatial distribution of features in three dimensions.
Methods
To overcome this limitation, we demonstrate a nondestructive method for three-dimensional imaging of intact human blood vessels using microcomputed tomography (microCT). Human artery segments were dehydrated and stained in an iodine solution then imaged with a standard laboratory microCT scanner. Image visualization and segmentation was performed using commercially available and open source software.
Results
Staining of cadaveric vessels with iodine enabled clear visualization of the arterial wall with microCT, preserved tissue morphology, and generated high-resolution images with a voxel size of 5.4 μm. Various components of the arterial wall were segmented using a combination of manual and automatic thresholding algorithms.
Conclusions
Our approach allows for spatial mapping of human artery tissue samples that can guide targeted histologic analysis of smaller tissue segments, provide geometric data to inform finite element models, quantify degree of atherosclerosis, and help to evaluate the foreign body response to intravascular medical implants. (JVS–Vascular Science 2020;2:13-19.)
Clinical Relevance
In this article, we describe a powerful technique for whole artery analysis of pathologic human tissue specimens that provides high-resolution spatial detail regarding composition of the blood vessel wall. The protocol described here is a valuable adjunct that can be used as a research tool to inform finite element modeling of arteries, quantify pathologic response (ie, neointimal hyperplasia and vascular calcification), and evaluate the tissue/device interface of implanted medical devices.
Keywords: Virtual histology, MicroCT, Soft tissue imaging, Arterial histology
High-resolution cross-sectional imaging of human tissue specimens is essential for analyzing the spatial distribution of pathologies throughout organ systems. However, many techniques currently used lack the scale and spatial resolution to identify regional variations within tissues. For example, high-resolution microscopy typically results planar data and limited depth of analysis in the z-axis. Microcomputed tomography (microCT) is an alternative technique used for three-dimensional (3D) analysis of radiodense tissues such as bone,1 but it has not been routinely used for analysis of soft tissues. Although vascular casting techniques can be combined with microCT to analyze luminal geometry,2 this strategy provides limited information about vessel wall morphology. Therefore, alternative methods are needed to enable high-resolution volumetric imaging of blood vessel segments such that localized disease patterns can be evaluated, regions of interest can be targeted for histologic analysis, and models and simulations of diseased arteries can be adequately informed.
Whole artery imaging has been previously performed with advanced imaging modalities such as magnetic resonance imaging3 and synchrotron radiation microCT,4 which can be used to obtain high-resolution 3D images of nonmineralized biological tissues. Unfortunately, these approaches require costly, specialized equipment and expertise and are unavailable to most laboratories. We sought to develop an imaging strategy for whole artery specimens, using resources that are readily available in most research institutions.
Combining ex vivo whole tissue staining techniques with traditional microCT allows for enhanced visualization of otherwise radiopaque soft tissues, and is an approach that has been used selectively in the field of developmental biology.5 Staining agents such as phosphomolybdic acid (PMA) and phosphotungstic acid are commonly used as a blocking agent in trichrome stains where they are known to bind collagen and fibrin, but may also be used to stain fixed tissue to allow for enhanced visualization with microCT.6 However, the large molecular size of both PMA and phosphotungstic acid leads to slow penetration into large samples, necessitating long staining times, and leading to nonuniform distribution of the stain. Additionally, PMA staining can leave tissue with a blue discoloration, which can interfere with further histology, immunohistochemistry, or immunofluorescence. Iodine, in contrast, is a nontoxic alternative staining agent that rapidly penetrates tissues while enhancing radiodensity7 and is also ubiquitously found in research laboratories worldwide.
Here we describe a simple technique for high-resolution imaging of whole human arterial segments that allows for 3D visualization of the arterial wall using a standard microCT imaging system. Tissue samples are prepared through dehydration in an ethanol gradient then stained with an iodine solution, such that soft tissue architecture can be clearly visualized. This technique can be performed quickly (sample preparation completed in <24 hours) and images are acquired with a standard benchtop or cabinet microCT system.
Methods
The protocol for this study was approved by the NUI Galway Research Ethic Committee. All cadaveric material was bequeathed to the Medical School, National University of Ireland Galway, for further advancement of medical knowledge. This is covered by legislation governing the practice of Anatomy in the Republic of Ireland (Medical Practitioners Act 2007). Whole human cadavers were fixed with embalming fluid containing 21% methanol, 21% glycerin, 5.6% phenol, and 3.1% formaldehyde. Tissue samples were dissected from whole donor cadavers by the study authors and stored in 70% ethanol. Artery segments were divided into 6- to 8-cm lengths and dehydrated in an alcohol gradient for 1 hour each at 70%, 80%, 90%, 95%, and 100% ethanol. Samples were then stained in 1% w/v iodine solution in absolute ethanol for 24 hours, washed in 100% ethanol for 5 minutes to remove excess iodine, then returned to absolute ethanol for imaging. Samples were positioned in microCT sample holders using polyethylene packing foam to hold the specimen in position. Samples were covered completely with 100% ethanol, then the sample holder was capped and sealed with parafilm. MicroCT images were captured with a Scanco uCT 100 at 90 kVp and 116 μA with a 0.5 mm Al filter and voxel size of 5.4 to 36.0 μm. Images and videos were generated using ImageJ and CT Vox (Bruker, Billerica, Mass), and segmentation and modeling was performed using Mimics (Materialize, Leuven, Belgium) and Slicer 3D. After image acquisition, samples were divided into 1-cm lengths, returned to 70% ethanol, processed for histology using an automated tissue processor (Leica, Wetzlar, Germany), and embedded in paraffin. Masson's trichrome stain was then performed for histologic analysis of the vessel wall.
Results
Optimization of staining protocol
We sought to establish a simple and rapid ex vivo sample preparation protocol for soft tissue imaging of large cadaveric arterial segments. Staining of an isolated carotid artery with 2% PMA for 48 hours did not allow for complete penetration of the dye and resulted in an unstained portion of the media (Fig 1, A). Staining of a femoral artery segment with a 2% iodine solution, in contrast, allowed for complete penetration of the stain throughout the vessel wall after 24 hours (Fig 1, B). For this protocol, tissue samples are dehydrated in serial ethanol dilutions and stained in absolute ethanol containing 2% iodine. A schematic of the protocol is highlighted in Fig 1, C. Unlike standard histology, the volumetric reconstructions with microCT enable visualization of not only the axial view of the artery, but also the sagittal and coronal reconstructions along the entire sample length as well as a volumetric 3D rendering (Fig 1, D-G).
Fig 1.
Optimization of whole tissue staining protocol. A, Staining of a common carotid artery with phosphomolybdic acid (PMA) shows a region of poor dye penetration in the center of the arterial wall (∗unstained area), while iodine staining of a common femoral artery (B) penetrates the arterial wall completely. C, The iodine staining protocol involves dehydration of tissue, staining overnight in iodine solution, then scanning with microcomputed tomography (microCT). Reconstructed images of an iodine stained superficial femoral artery (SFA) enable visualization of axial (D), coronal (E), and sagittal (F) views, as well as a three-dimensional (3D) reconstruction (G). Scale bars = 5 mm. ETOH, Ethanol.
The voxel size of acquired images can be varied based on user needs. Because higher resolution scans require longer scanning and image processing times, a low-resolution scan can first be performed to identify regions of interest that are then subsequently reinterrogated with a higher resolution scan. Fig 2, A and B, show microCT slice of a superficial femoral artery (SFA) obtained with a voxel size of 36 μm, with a magnified view in Fig 2, B, showing pixilation at this resolution. When the same section is rescanned with a voxel size of 5.4 μm, the pixilation is improved significantly (Fig 2, C and D). Upon completing the microCT scan, the specimens can be processed for histology or immunolabeling. Fig 2, E-F, shows the same SFA segment after tissue processing, embedding in paraffin, sectioning, and staining with Masson's trichrome. Preparation of tissue for optical microscopy (eg, tissue processing, paraffin embedding, issue sectioning) can result in artifact from tissue orientation and distortion within prepared tissue blocks. MicroCT is nondestructive and enables interrogation of microstructure without disturbing tissue planes. This is evident in Fig 2, where the tissue slice in Fig 2, E and F, seems to be distorted compared with that seen in Fig 2, A-D.
Fig 2.
Comparison of acquisition parameters using microcomputed tomography (microCT) histology. Axial section of a low-resolution microCT scan (36.0-μm voxel) of a superficial femoral artery (SFA) segment (A and B) compared with a high-resolution (5.4-μm voxel) scan of the same iodine stained section (C and D). Masson's trichrome stain was performed on the same arterial segment (E and F). The dashed boxes identify the region of interest for the magnified views.
Whole organ imaging
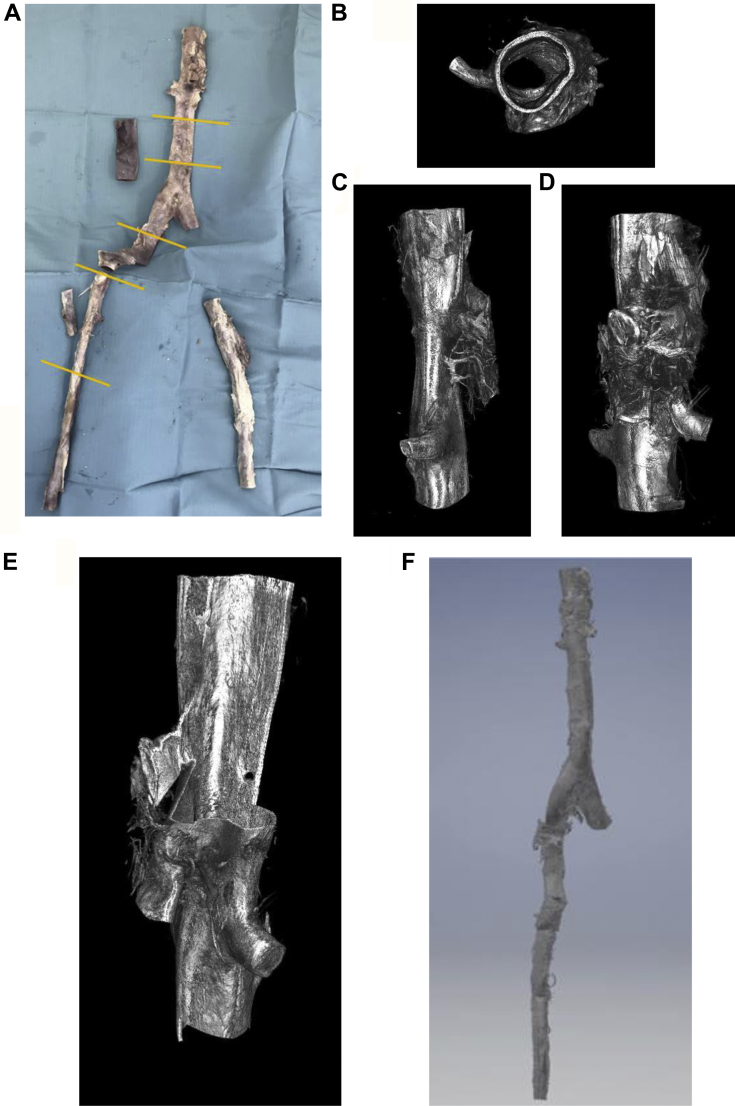
Our staining protocol is easily adapted for imaging of large segments of tissue, such that continuous portions of the arterial tree can be assessed. To demonstrate this process, we dissected an entire cadaveric aorta from the visceral segment to the aortic bifurcation, and the iliofemoral arterial system distally to the right proximal SFA (Fig 3, A). The arteries were divided into shorter segments of approximately 8 cm so that they could be housed in the microCT sample containers in a single piece. Samples were then stained with iodine solution and imaged with microCT. The virtual reconstructions of each individual segment were manipulated for viewing at different angles (Fig 3, B-D), and the reconstructed volume was cropped to visualize internal structures (Fig 3, E). A 3D object was also generated from each imaged segment, and the entire aorta reconstructed by linking the segments (Fig 3, G). Thus, the entire arterial system is spatially mapped and regions of interest can be identified for further study.
Fig 3.
Whole organ imaging of a cadaveric human aorta. Microcomputed tomography (microCT) was performed on sections along the length of an explanted cadaveric aorta (A). Volumetric renderings of the visceral aortic segment with the axial (B), sagittal (C), and coronal (D) views. The reconstructed volume can be cropped so internal features can be viewed (E). The individual segments were converted into three-dimensional (3D) models and merged so that the entire arterial segment from supraceliac aorta to superficial femoral artery (SFA) could be rendered (F).
Image segmentation and quantitative analysis
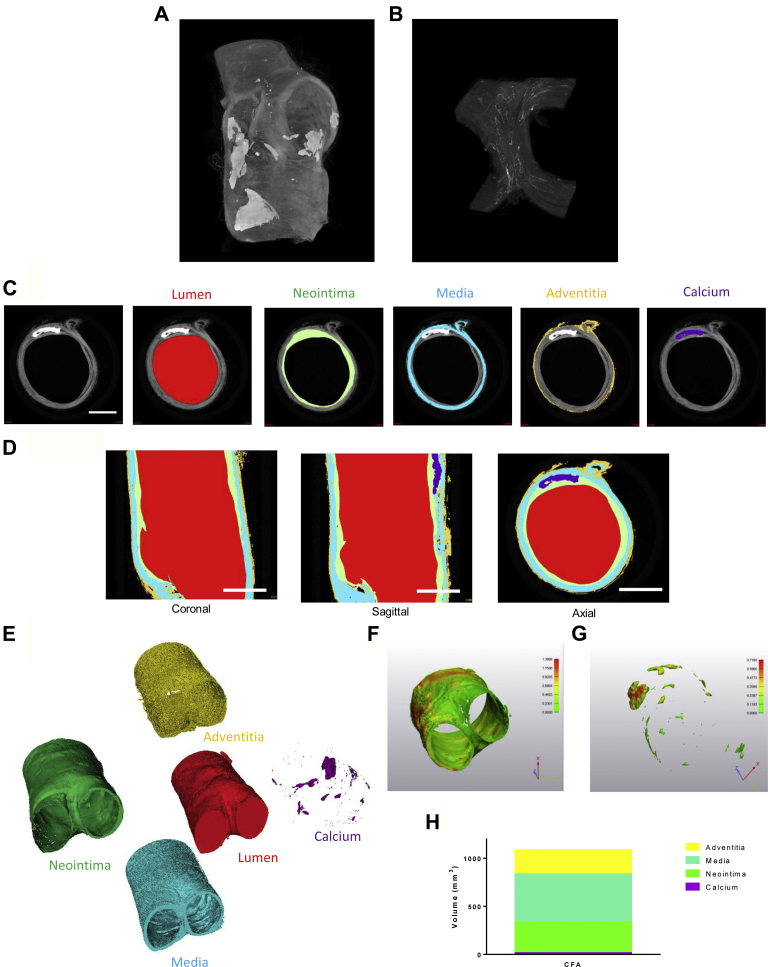
Based on the staining pattern with iodine and the nature of the pathology of atherosclerotic disease, several distinct features of the arterial wall can be visualized for qualitative purposes. Calcium deposits are highlighted in a maximum intensity projection of a 3D volumetric rendering of a carotid artery bifurcation without segmentation (Fig 4, A). The propensity of iodine to bind fibrin also allows for visualization of thrombus retained within arteriole and capillary networks. This is demonstrated by a maximum intensity projection of a magnified portion of the arterial wall at the femoral artery bifurcation that enables visualization of the vasa vasorum (Fig 4, B).
Fig 4.
Segmentation of individual arterial segments. A, Calcium deposits throughout the arterial wall are visualized with a maximum intensity projection of a carotid artery bifurcation. B, The maximum intensity projection of a stained femoral artery bifurcation highlights the course of the vasa vasorum (B). Segmentation performed on an isolated common femoral artery identifies the lumen, neointima, media, adventitia, and calcium deposits (C). Coronal, sagittal, and axial views (D) can be visualized along the arterial segment, and three-dimensional (3D) models can be generated of each individual component (E). The corresponding 3D models can then be used for quantitative analysis, which we demonstrate by performing a thickness analysis of the neointimal layer (F) and the calcium deposits (G). Volumetric analysis of each component of the vessel wall can be performed to assess the relative volume of each segment. CFA, Common femoral artery.
MicroCT imaging of stained tissues can be used to identify different components of the arterial wall, which can in turn be used to quantify pathology (eg, calcium or neointimal volume). These volumetric data can then inform solid or fluid mechanical modeling of the artery. Here we demonstrate this concept through segmentation of an ex vivo stained common femoral artery bifurcation (Fig 4, C and D). The inner lumen was outlined by intensity thresholding, while the outer boundary of the intima and media were manually segmented. The adventitia and calcium deposits were then also segmented through intensity thresholding, and a 3D model of the artery was generated using these components (Fig 4, E). These data can, in turn, be used to quantify vessel pathology. For example, a thickness analysis of the neointima and calcium deposits (Fig 4, F and G) can be performed to identify the regions of greatest tissue thickness or disease. Additionally, the volumetric data obtained from the segmentation process allows for a comparison of fractional relationships of the arterial wall components. Thus, segment volumes can be calculated and compared (Fig 4, H). These results are highlighted in Supplementary Video 1 (online only), in which 3D models and volumetric reconstructions are manipulated for improved visualization.
Conclusions
We have demonstrated the use of MicroCT histology to interrogate large cadaveric human arterial segments. MicroCT histology offers numerous advantages over optical or fluorescence microscopy. With microCT, the entire specimen can be visualized, whereas standard histology requires targeted or random preparations of small fields of view taken from a larger sample; thus, key features of the specimen may be missed owing to a sampling error. MicroCT is nondestructive, and the iodine solution is easily washed out of specimens, so that the tissue can subsequently be used for additional testing (eg, immunohistochemistry, mechanical testing). MicroCT histology requires minimal tissue preparation and manipulation, such that the tissue architecture is preserved. This factor significantly decreases sectioning artifact that is frequently encountered with optical microscopy. Finally, microCT enables in situ assessment of features that can be extremely challenging to analyze with traditional tissue processing techniques. For example, specimens with calcium deposits must be decalcified for histologic processing and sectioning but require no additional steps with microCT. Tissues with metallic implants, such as stents and grafts, require the implant to be separated from the tissue before histology, or a tedious preparation must be performed methyl methacrylate and sectioning with a diamond blade to preserve the tissue architecture. MicroCT alleviates the need for these additional steps, enabling easy visualization of all specimen components.
Although this study is limited to the use of whole human arterial segments, this approach can be applied to tissue from a variety of sources. MicroCT histology allows whole tissue imaging with voxel size as low as 5.4 μm. Smaller tissue fragments, such as those from rodent studies or pathology specimens, can also be prepared and imaged using this technique. Since spatial resolution with microCT is dependent on distance of the x-ray source from the sample center, even higher resolution images can be obtained with smaller samples because the x-ray source can be positioned closer to the sample center.
We have performed whole organ imaging on multiple tissue types (skin, heart, abdominal wall, intestine, kidney) from mice, rats, pigs, and humans. Our technique is easily modified for different tissue types, and with minimal changes to sample preparation and imaging acquisition parameters it can be used for analysis animal tissues and pathology specimens (data not shown). Thus, with slight modification to the sample preparation protocol and imaging acquisition parameters, microCT histology can be used for qualitative and quantitative analysis of tissue from animal experiments or intraoperative pathology samples. We do recommend that this technique be used with fixed tissue, because the staining and image acquisition processes can be prolonged, exposing the samples to degradative processes.
We have described a versatile, rapid, and inexpensive technique using common laboratory supplies and equipment that provides a detailed insight into arterial wall architecture. Because microCT systems are now frequently used among engineering and biomedical research facilities, this technology is readily available to many vascular scientists. Combining standard microCT imaging with an iodine staining technique, whole arterial segments can be visualized to allow for spatial discrimination of unique features of the blood vessel wall, helping to guide further histologic analyses or computational modeling.
Author contributions
Conception and design: SR, RL, GD
Analysis and interpretation: SR, RL, RB, ED, NO, PD, PH, GD
Data collection: SR, RL, RB, DC
Writing the article: SR, RL, RB, DC, GD
Critical revision of the article: ED, NO, PD, PH
Final approval of the article: SR, RL, RB, DC, ED, NO, PD, PH, GD
Statistical analysis: Not applicable
Obtained funding: SR, GD
Overall responsibility: SR
Footnotes
S.T.R. and G.P.D. acknowledge funding from Science Foundation Ireland under grant SFI/12/RC/2278, Advanced Materials and Bioengineering Research (AMBER) Centre, National University of Ireland Galway and Trinity College Dublin, Ireland. G.P.D. acknowledge financial support from the National University of Ireland Galway. E.B.D. and G.P.D. acknowledge the DRIVE project which has received funding from the European Union's Horizon 2020 framework program under grant agreement number 645991. S.T.R. has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 713567. The authors acknowledge the facilities and scientific and technical assistance of the Centre for Microscopy & Imaging at the National University of Ireland Galway (www.imaging.nuigalway.ie).
Author conflict of interest: none.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS-Vascular Science policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Appendix (online only).
Combining microcomputed tomography with a whole organ iodine staining technique allows for visualization of three-dimensional models and volumetric reconstructions of human arteries in high resolution.
References
- 1.Holdsworth D.W., Thornton M.M. Vol. 20. Elsevier; New York (NY): 2002. Micro-CT in small animal and specimen imaging; pp. S34–S39. (Trends in Biotechnology). [Google Scholar]
- 2.Duvall C.L., Taylor W.R., Weiss D., Guldberg R.E. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–H310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 3.Edlow B.L., Mareyam A., Horn A., Polimeni J.R., Witzel T., Tisdall M.D. 7 Tesla MRI of the ex vivo human brain at 100 micron resolution. Sci Data. 2019;6:244. doi: 10.1038/s41597-019-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonanno G., Coppo S., Modregger P., Pellegrin M., Stuber A., Stampanoni M. Ultra-high-resolution 3D imaging of atherosclerosis in mice with synchrotron differential phase contrast: a proof of concept study. Sci Rep. 2015;5:11980. doi: 10.1038/srep11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metscher B.D. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn. 2009;238:632–640. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- 6.Faraj K.A., Cuijpers V.M.J.I., Wismans R.G., Walboomers X.F., Jansen J.A., Van Kuppevelt T.H. Micro-computed tomographical imaging of soft biological materials using contrast techniques. Tissue Eng Part C Methods. 2009;15:493–499. doi: 10.1089/ten.tec.2008.0436. [DOI] [PubMed] [Google Scholar]
- 7.Juliana J.M., Zanette I., Noël P.B., Cardoso M.B., Kimm M.A., Pfeiffer F. Three-dimensional non-destructive soft-tissue visualization with X-ray staining micro-tomography. Sci Rep. 2015;5:14088. doi: 10.1038/srep14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combining microcomputed tomography with a whole organ iodine staining technique allows for visualization of three-dimensional models and volumetric reconstructions of human arteries in high resolution.