Abstract
Background
Patients with coronavirus disease 2019 (COVID-19) exhibit high thrombotic risk. The evidence on a potential independent prognostic role of antiplatelet treatment in those patients is limited. The aim of the study was to evaluate the prognostic impact of pre-admission low-dose acetylsalicylic acid (ASA) in a wide series of hospitalized patients with COVID-19.
Methods
This cohort study included 984 COVID-19 patients stratified according to ASA intake before hospitalization: ASA+ (n = 253) and ASA− (n = 731). Patients were included in ASA+ group if they received it daily in the 7 days before admission. 213 (83%) were on ASA 100 mg daily. Primary endpoint was a composite of in-hospital death and/or need for respiratory support upgrade, secondary endpoints were in-hospital death and need for respiratory support upgrade.
Results
Mean age was 72 [62; 81] with 69% of male patients. ASA+ patients were significantly older, with higher prevalence of comorbidities. No significant differences regarding the degree of respiratory dysfunction were observed. At 30-day Kaplan-Meier analysis, ASA+ patients had higher survival free from the primary endpoint and need for respiratory support upgrade, conversely in-hospital death did not significantly differ between groups. At multivariate analysis ASA intake was independently associated with a lower probability of reaching primary endpoint (HR 0.697, 95% C.I. 0.525–0.924; p = 0.012).
Conclusions
In COVID-19 patients undergoing hospitalization, pre-admission treatment with ASA is associated with better in-hospital outcome, mainly driven by less respiratory support upgrade.
Keywords: Acetylsalicylic acid, Platelet aggregation inhibitors, COVID-19, SARS-CoV-2, Thrombosis
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], is responsible for the global pandemic outbreak. At the time of this writing, there have been approximately over 180 million cases reported and more than 3.9 million (~2%) deaths due to COVID-19 across more than 200 countries worldwide [2]. Patients with cardiovascular diseases have been reported to have the highest case fatality [3], [4]. Although most of COVID-19-related physiopathological pathways remain unclear, some evidences suggest that SARS-CoV-2 infection may predispose patients to thrombosis [5], both in the arterial and venous circulations [6], due to inflammation, endothelial dysfunction and, finally, pathological platelet hyperactivation [7], [8]. In fact, as Zhang et al. demonstred [9], SARS-CoV-2 is able to create a spike protein-mediated platelet-ACE2 binding, directly stimulating platelets release of coagulation factors, secretion of inflammatory factors, and formation of leukocyte–platelet aggregates. Furthermore, endothelial cell infection, as evidenced in some autopsy studies [10], [11], or the virus-induced inflammatory response, may contribute to systemic microcirculatory function impairment. The resulting COVID-19-associated endotheliopathy may affect especially, but not only, pulmonary circulation [12] and elicit platelet hyperactivation. For these reasons, antiplatelet therapy, whose impact on outcomes is still under investigation in this subset of patients, may represent an effective therapeutic option [13], [14]. Acetylsalicylic acid (ASA) exerts antithrombotic and anti-inflammatory effects, and it had been demonstrated to play some antiviral activity against deoxyribonucleic and ribonucleic acid viruses [15]. The aim of this study was to evaluate the potential protective effect of chronic ASA-based single antiplatelet therapy in a large cohort of patients undergoing hospitalization because of COVID-19.
2. Methods
This is a multi-center, retrospective, observational study performed at Policlinico San Donato in San Donato Milanese and Ospedale Guglielmo da Saliceto in Piacenza between February 21 and April 22, 2020. The inclusion criteria were: a) patients aged at least 18 years, b) admitted to hospital, c) who were diagnosed COVID-19 according to the interim guidance of the World Health Organization [16]. Clinical information including demographics, comorbidities, medical history, laboratory examinations, baseline and in-hospital treatment measures (including respiratory support) and outcomes was collected after discharge by attending physicians (A.S. and E.P. in San Donato Milanese and A.M. in Piacenza). Each patient underwent admission arterial blood gas analysis, complete blood routine test, including hematologic, biochemical and coagulation function, and chest imaging (X-rays and/or computed tomography) evaluation.
Patients were included in ASA group if they were on treatment and they received it daily at least 7 days before admission [17], [18]. ASA treatment was continued during the hospitalization on the same dose as before hospitalization. Patients undergoing orotracheal intubation received ASA by nasogastric tube. Chronic kidney disease (CKD) was defined as Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation-derived estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 [19]. The most intense level of oxygen support during hospitalization (nasal cannula, Venturi mask, nonrebreather mask, noninvasive mechanical ventilation [NMV], invasive mechanical ventilation [IMV]) was recorded. According to study institutions' protocols, patients were considered suitable for NMV in the presence of a) moderate-to-high oxygen requirement (partial pressure of oxygen to fractional inspired oxygen ratio [PaO2/FiO2] <200 or PaO2 < 60 mmHg or peripheral oxygen saturation < 94% or 88% in patients with acute or acute on chronic type II respiratory failure, despite 15 L/min oxygen administration via nonrebreather mask), b) in the absence of contraindication to using NMV. IMV was considered after unsuccessful NMV, defined as PaO2/FiO2 tending to decrease and PaO2 < 60 mmHg or if NMV was not advisable, if patient clinical status allowed. Patients requiring IMV at the time of admission were not included in the study because a) only in-hospital death, but not primary endpoint, could have been evaluated since IMV represent the most intense level of respiratory support, and b) those patients belong to critically-ill category, in which the underlying thrombotic and inflammatory damage may have been too advanced to have been influenced by ASA intake. Finally, because of different mechanism of action, P2Y12 inhibitors-assuming patients (i.e. clopidogrel) were not considered in our analysis.
The primary study endpoint was a composite of 30-day in-hospital death or need for respiratory support upgrade to NMV, including Continuous Positive Airway Pressure (CPAP) and Bilevel positive airway pressure (BiPAP) or IMV. The secondary clinical endpoints were in-hospital death and need for respiratory support upgrade up to 30-day, analyzed individually.
This study complied with the principles outlined in the 1975 Declaration of Helsinki. The study was approved by the Local Ethics Committee.
Given the retrospective nature of our study, no statistical sample size calculation was performed a priori, and sample size was equal to the number of eligible patients hospitalized during the study period. Distribution of continuous data was tested with the Shapiro-Wilk test. Non-normally distributed variables were presented as median and interquartile range. Categorical variables were reported as absolute numbers and percentages. Continuous variables were then compared using Mann-Whitney U test; categorical variables were compared with Chi square test. Event-free survival up to 30-day were evaluated according to the unadjusted Kaplan-Meier method and survivals among groups were compared using log-rank test (Cox-Mantel test). Cox proportional hazards regression analysis was used to determine significant predictors of primary and secondary endpoints. Variables with a univariate statistical significance of <0.05 were selected for inclusion into the multivariable model. Multivariate analysis, using stepwise forward selection, was finally performed to analyze the association of baseline characteristics with study endpoints, expressed as hazard ratio (HR) with 95% confidence interval (CI) and p values. All statistical tests were 2-sided, and p values <0.05 were considered statistically significant. The statistical analyses were performed using SPSS software version 25.0.0 (SPSS Inc., Chicago, IL) and GraphPad Prism software version 6 (GraphPad, Inc., San Diego, CA).
3. Results
Nine hundred and eighty-four patients (200 in San Donato Milanese and 784 in Piacenza) with COVID-19 (median age 72 [62; 81] years; 69% male) were included in the study, Fig. S1, Supplementary material. According to baseline pre-admission ASA intake we identified two groups, 253 (26%) patients were receiving ASA (ASA+) and 731 were not (74%) (ASA−). Concerning ASA+ patients, 213 (83%) were on ASA 100 mg daily, meanwhile the remaining were assuming it a daily dose of 75 mg.
Compared to ASA− patients, the group ASA+ was significantly older and suffered more from cardiovascular comorbidities, such as hypertension, diabetes mellitus and dyslipidemia, resulting in higher incidence of coronary artery disease, peripheral artery disease and previous ischemic stroke or transient ischemic attack. Heart failure, chronic kidney disease and chronic obstructive pulmonary disease were less frequent in ASA− group. ASA+ patients were more often on angiotensin-converting enzyme inhibitors or angiotensin type 1 receptor blocker and statin therapy. Of note, there were no differences either on pre-hospitalization infection-related symptoms, except for lower incidence of fever in ASA+ group, or on time between symptoms onset and hospitalization. Arterial blood gas analysis at admission showed similar degree of respiratory impairment. Notably, 32 patients required NMV at the time of admission, without significant differences between groups (p = 0.158). Besides, ASA− patients presented with significantly higher neutrophils to lymphocytes ratio (N/L, 5 [3; 9] vs. 4 [2; 7], p = 0.013) and hemoglobin levels (14 [12; 15] g/dL vs. 13 [12; 15] g/dL, p = 0.016). ASA+ patients showed a worse baseline renal function, as assessed by lower median estimated glomerular filtration rate (eGFR, 59 [43; 80] mL/min/1.73 m2 vs. 70 [51; 89] mL/min/1.73 m2, p = 0.001) and increased high-sensitivity troponin T values (24 [14; 65] ng/L vs. 12 [7; 24] ng/L, p < 0.001), whereas liver function indexes did not differ between study groups. Serum D-dimer level was similar among two groups (2.01 [0.90; 3.53] μg/mL vs. 1.50 [0.69; 3.0] μg/mL, p = 0.276). Chest imaging revealed bilateral interstitial infiltrates in 90% of entire study cohort, without significant difference between study groups (p = 0.774). Admission risk scores assessing in-hospital mortality did not differ significantly between groups. During hospitalization empirical anti-SARS-CoV-2 therapy, including tocilizumab, antibiotic, glucocorticoid and low-molecular weight heparin (LMWH), was administered more often to ASA− patients, as well as oxygen therapy, given that ASA+ patients underwent less NMV or IMV treatments (p < 0.001), Table 1 .
Table 1.
Baseline clinical features, in-hospital instrumental evaluation and empirical therapy in entire study cohort and the two subgroups identified according to baseline acetylsalicylic acid intake.
| Entire study cohort (n = 984) |
ASA+ (n = 253) |
ASA− (n = 731) |
p value | |
|---|---|---|---|---|
| Clinical features on admission | ||||
| Age (years) | 72 [62; 81] | 76 [67; 82] | 71 [61; 80] | <0.001 |
| Male gender, n (%) | 678 (69) | 171 (68) | 507 (69) | 0.601 |
| Initial common symptomsa | ||||
| Fever, n (%)c | 644 (91) | 165 (86) | 479 (93) | 0.006 |
| Dry cough, n (%) | 369 (56) | 91 (50) | 278 (58) | 0.112 |
| Dyspnea, n (%) | 449 (67) | 129 (69) | 320 (66) | 0.401 |
| Diarrhea, n (%) | 60 (10) | 11 (6) | 49 (11) | 0.094 |
| Symptoms onset to admission (days) | 7 [4; 10] | 7 [3; 9] | 7 [4; 10] | 0.242 |
| Comorbidities, n (%)b | ||||
| Hypertension | 604 (62) | 215 (85) | 389 (54) | <0.001 |
| Diabetes mellitus | 188 (19) | 85 (34) | 103 (14) | <0.001 |
| Dyslipidemia | 237 (24) | 112 (44) | 125 (17) | <0.001 |
| Coronary artery disease | 86 (9) | 81 (33) | 21 (3) | <0.001 |
| Heart failure | 95 (10) | 58 (23) | 37 (5) | <0.001 |
| Atrial fibrillation | 124 (13) | 31 (12) | 93 (13) | 0.774 |
| Peripheral artery disease | 30 (3) | 15 (6) | 15 (2) | 0.002 |
| Previous ischemic stroke/TIA | 31 (3) | 13 (5) | 18 (3) | 0.041 |
| Chronic kidney disease | 98 (10.1) | 42 (17) | 56 (8) | <0.001 |
| Chronic obstructive pulmonary disease | 140 (14) | 53 (21) | 87 (12) | 0.001 |
| History of neoplasia | 60 (6) | 19 (8) | 41 (6) | 0.307 |
| Drugs, n (%)b | ||||
| Anticoagulant | 0.402 | |||
| OAT | 68 (7) | 19 (8) | 49 (7) | |
| DOAC | 45 (5) | 7 (3) | 38 (5) | |
| ACE-I/ARBd | <0.001 | |||
| ACE-I | 250 (26) | 90 (36) | 160 (23) | |
| ARB | 180 (19) | 73 (29) | 107 (15) | |
| Statin | 208 (27) | 100 (48) | 108 (19) | <0.001 |
| Vital signs | ||||
| Systolic blood pressure (mmHg) | 130 [117; 145] | 130 [115; 145] | 130 [120; 145] | 0.888 |
| Diastolic blood pressure (mmHg) | 75 [70; 80] | 70 [65; 80] | 77 [70; 83] | 0.017 |
| Heart rate (bpm) | 90 [80; 100] | 87 [76; 100] | 90 [80; 100] | 0.097 |
| Respiratory rate (min−1) | 22 [18; 25] | 22 [18; 25] | 22 [18; 25] | 0.498 |
| Body temperature (°C) | 38 [37; 38] | 37.7 [37; 38] | 38 [37; 38.5] | 0.027 |
| Peripheral oxygen saturation (%) | 91 [87; 94] | 91 [87; 94] | 91 [87.5; 94] | 0.995 |
| Arterial blood gas | ||||
| pH | 7.47 [7.43; 7.50] | 7.47 [7.43; 7.51] | 7.47 [7.43; 7.50] | 0.964 |
| PaO2 (mmHg) | 60 [50; 70] | 59 [50; 67] | 60 [50; 71] | 0.815 |
| PaO2/FiO2 (mmHg/%) | 2.81 [2.33; 3.20] | 2.79 [2.29; 3.13] | 2.81 [2.34; 3.28] | 0.662 |
| PaCO2 (mmHg) | 33 [30; 37] | 33 [29; 37] | 30 [30; 36] | 0.212 |
| HCO3− (mmol/L) | 24 [22; 27] | 25 [21; 28] | 24 [22; 26] | 0.518 |
| SO2 (%) | 94 [91; 96] | 93 [91; 94] | 94 [91; 96] | 0.320 |
| Lactate (mmol/L) | 1.3 [0.9; 1.8] | 1.3 [0.7; 1.8] | 1.3 [0.9; 1.7] | 0.689 |
| Laboratory indices | ||||
| White blood cells (109/L) | 6.9 [5.2; 9.5] | 6.5 [4.8; 8.6] | 7.1 [5.3; 9.8] | 0.061 |
| Neutrophils (109/L) | 5.0 [3.1; 7.9] | 4.6 [2.8; 7.1] | 5.2 [3.2; 8.1] | 0.101 |
| Lymphocytes (109/L) | 1.2 [0.8; 1.6] | 1.2 [0.8; 1.8] | 1.1 [0.8; 1.6] | 0.043 |
| N/L | 5 [2; 9] | 4 [2; 7] | 5 [3; 9] | 0.013 |
| Hemoglobin (g/dL) | 14 [12; 15] | 13 [12; 15] | 14 [12; 15] | 0.016 |
| Hematocrit (%) | 41 [37; 44] | 40 [36; 44] | 41 [37; 45] | 0.104 |
| Platelets, (109/L) | 200 [150; 263] | 206 [140; 282] | 195 [153; 260] | 0.125 |
| Creatinine (mg/dL) | 1.0 [0.9; 1.3] | 1.1 [0.9; 1.43] | 1.0 [0.8; 1.3] | 0.135 |
| eGFR (mL/min/1.73 m2) | 68 [48; 87] | 59 [43; 80] | 70 [51; 89] | 0.001 |
| Urea (mg/dL) | 45 [32; 64] | 48 [35; 68] | 44 [31; 63] | 0.097 |
| Sodium (mEq/L) | 137 [134; 139] | 137 [134; 140] | 137 [134; 139] | 0.806 |
| Potassium (mEq/L) | 4.14 [3.80; 4.53] | 4.27 [3.80; 4.70] | 4.10 [3.80; 4.50] | 0.172 |
| Lactate dehydrogenase (UI/L) | 451 [344; 588] | 443 [323; 580] | 455 [351; 592] | 0.484 |
| Creatinine kinase (UI/L) | 119 [64; 259] | 126 [68; 257] | 118 [63; 26] | 0.579 |
| Total bilirubin (mg/dL) | 0.69 [0.51; 0.93] | 0.70 [0.51; 0.96] | 0.69 [0.52; 0.91] | 0.654 |
| Glutamic pyruvic transaminase (UI/L) | 30 [20; 49] | 29 [18; 47] | 30 [21; 50] | 0.857 |
| Glutamic oxaloacetic transaminase (UI/L) | 42 [30; 66] | 42 [30; 67] | 42 [31; 65] | 0.298 |
| High-sensitivity troponin T (ng/L)e | 16 [8; 30] | 24 [14; 65] | 12 [7; 24] | <0.001 |
| C-reactive protein (mg/dL) | 10 [5; 16] | 9 [5; 15] | 10 [5; 17] | 0.103 |
| Serum ferritin (ng/mL) | 876 [510; 1460] | 715 [446; 1601] | 940 [529; 1460] | 0.120 |
| D-dimer (μg/mL) | 1.53 [0.77; 3.18] | 2.01 [0.90; 3.53] | 1.50 [0.69; 3.0] | 0.276 |
| Fibrinogen (mg/dL) | 602 [486; 734] | 633 [485; 718] | 595 [482; 739] | 0.811 |
| Chest imaging, n (%) | ||||
| Bilateral infiltrates | 886 (90) | 228 (90) | 658 (90) | 0.774 |
| Pleuric effusion | 177 (18) | 66 (26) | 110 (15) | 0.004 |
| Risk scores | ||||
| qSOFA | 1 [0; 1] | 1 [0; 1] | 1 [0; 1] | 0.226 |
| CURB-65 | 1 [0; 2] | 1 [0; 1.75] | 1 [0; 1] | 0.394 |
| Drugs, n (%)a | ||||
| Hydroxychloroquine | 494 (78) | 127 (76) | 367 (74) | 0.323 |
| Tocilizumab | 57 (16) | 4 (4) | 53 (20) | <0.001 |
| Antibioticf | 439 (69) | 102 (60) | 337 (73) | 0.001 |
| Glucocorticoid | 207 (34) | 43 (27) | 164 (37) | 0.019 |
| Low-molecular weight heparin | 0.015 | |||
| None | 246 (40) | 79 (47) | 167 (37) | |
| Prophylactic dose | 39 (6) | 14 (9) | 25 (6) | |
| Therapeutic dose | 331 (54) | 74 (44) | 257 (57) | |
| Oxygen therapy, n (%)a | <0.001 | |||
| None | 193 (20) | 35 (14) | 158 (22) | |
| Nasal cannula/Venturi mask/nonrebreather mask | 454 (48) | 146 (59) | 308 (44) | |
| Noninvasive ventilation | 184 (19) | 41 (17) | 143 (20) | |
| Invasive mechanical ventilation | 123 (13) | 25 (10) | 98 (14) | |
ACE-I: angiotensin-converting enzyme inhibitors. ARB: angiotensin 1 receptor blocker. ASA: acetylsalicylic acid. DOAC: direct oral anticoagulant. eGFR: estimated glomerular filtration rate. HCO3−: hydrogen carbonate. N/L: neutrophils to lymphocytes ratio. NT-proBNP: N-terminal prohormone of brain natriuretic peptide. OAT: oral anticoagulant therapy. PaO2: partial pressure of oxygen. PaO2/FiO2: partial pressure of oxygen to fractional inspired oxygen ratio. qSOFA: quick sepsis related organ failure assessment. SO2: oxygen saturation. TIA: transient ischemic attack.
Data are presented as n (%), mean ± SD or median [IQR].
Values are avaible for ~ 70% of the entire study cohort.
Values are avaible for ~ 97% of the entire study cohort.
Fever was classified as highest patient temperature 37.3 °C or higher. To minimize interference of treatment, the highest patient temperature was defined using the self-reported highest temperature before taking antipyretic drug.
ACE-I/ARB use was defined as use of these drugs at the time of admission that continued through hospitalization.
Values are avaible for ~ 25% of the entire study cohort.
Including azithromycin 500 mg daily dose p.o. and/or ceftriaxone 2000 mg daily dose i.v.
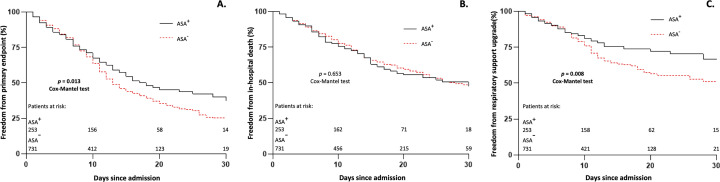
Median length of in-hospital stay was 11 [7; 18] days, similar between the two groups (p = 0.980). Furthermore, no significant differences were observed in patients assuming ASA 100 mg daily compared to those taking 75 mg daily (p = 0.331) concerning duration of hospitalization. At 30-day Kaplan Meier analysis in the entire study cohort, compared to ASA− patients, ASA+ suffered less adverse events in terms of both primary endpoint (63% vs. 75%; HR 0.788, log-rank p = 0.013) and need for respiratory support upgrade (33% vs. 49%; HR 0.640, log-rank p = 0.008), Fig. 1 , panel A and C, respectively, with 19% ASA+ patients vs. 25% ASA− patients needing upgrade to NMV (log-rank p = 0.006), and 15% ASA+ patients vs. 25% ASA− patients needing upgrade to IMV (log-rank p = 0.017). Meanwhile in-hospital death did not differ significantly between two groups (ASA+ 52% vs. ASA− 53%; HR 1.042, log-rank p = 0.653), Fig. 1 , panel B. Primary and secondary endpoints are shown in Table S1, Supplementary material.
Fig. 1.
Entire study cohort 30-day Kaplan-Meier analysis of primary and secondary endpoints.
Entire study cohort 30-day Kaplan-Meier analysis of survival free from primary endpoint (panel A), in-hospital death (panel B) and need for respiratory support upgrade (panel C).
ASA: acetylsalicylic acid.
At univariate Cox regression analysis, ASA, as well as glucocorticoid therapy, was associated with better outcome in terms of primary endpoint, whereas age, male gender, hypertension, admission N/L > 3 and eGFR <60 mL/min/1.73m2 correlated with a worse one. Multivariate analysis identified ASA use as an independent positive prognostic factor in terms of primary endpoint (HR 0.697, 95% C.I. 0.525–0.924; p = 0.012), Fig. 2 . ASA was also identified as independent protective factor in terms of need for less respiratory support upgrade (HR 0.529, 95% C.I. 0.333–0.839; p = 0.007), meanwhile it was not able to predict in-hospital death, as evidenced in Table 2 .
Fig. 2.
Forest plot showing results from multivariate Cox regression analysis regarding primary endpoint.
ASA: acetylsalicylic acid. eGFR: estimated glomerular filtration rate. N/L: neutrophils to lymphocytes ratio.
Data are presented as hazard ratio (HR) with 95% confidence interval (CI).
Table 2.
Primary endopoint-related univariate and multivariate Cox regression analysis in entire study cohort.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| In-hospital death and/or respiratory support upgrade | ||||||
| Age | 1.024 | 1.016–1.031 | <0.001 | |||
| Male gender | 1.312 | 1.085–1.585 | 0.005 | 1.424 | 1.100–1.842 | 0.007 |
| Hypertension | 1.244 | 1.039–1.490 | 0.018 | |||
| ASA | 0.788 | 0.647–0.960 | 0.018 | 0.697 | 0.525–0.924 | 0.012 |
| N/L > 3 | 1.549 | 1.239–1.938 | <0.001 | 1.483 | 1.145–1.919 | 0.003 |
| eGFR <60 mL/min/1.73m2 | 1.466 | 1.207–1.780 | <0.001 | 1.351 | 1.054–1.731 | 0.018 |
| Glucocorticoid | 0.698 | 0.558–0.872 | 0.002 | 0.782 | 0.622–0.985 | 0.036 |
| In-hospital death | ||||||
| Age | 1.069 | 1.058–1.081 | <0.001 | 1.066 | 1.047–1.085 | <0.001 |
| Hypertension | 1.577 | 1.251–1.987 | <0.001 | |||
| Heart failure | 1.728 | 1.275–2.342 | <0.001 | |||
| Previous ischemic stroke/TIA | 1.619 | 1.008–2.601 | 0.046 | |||
| Chronic obstructive pulmonary disease | 1.411 | 1.069–1.862 | 0.015 | |||
| N/L > 3 | 1.519 | 1.128–2.045 | 0.006 | 1.468 | 1.054–2.045 | 0.023 |
| Hemoglobin | 0.913 | 0.855–0.975 | 0.007 | |||
| eGFR <60 mL/min/1.73m2 | 2.693 | 2.071–3.502 | <0.001 | 1.728 | 1.263–2.365 | 0.001 |
| Low-molecular weight heparin | 0.640 | 0.477–0.858 | 0.003 | 0.660 | 0.487–0.893 | 0.007 |
| Respiratory support upgrade | ||||||
| Male gender | 2.084 | 1.473–2.949 | <0.001 | 1.855 | 1.232–2.794 | 0.003 |
| ASA | 0.640 | 0.458–0.894 | 0.009 | 0.529 | 0.333–0.839 | 0.007 |
| N/L > 3 | 1.706 | 1.211–2.404 | 0.002 | |||
| Low-molecular weight heparin | 1.652 | 1.154–2.364 | 0.006 | |||
| Glucocorticoid | 0.477 | 0.346–0.659 | <0.001 | 0.556 | 0.395–0.782 | 0.001 |
ASA: acetylsalicylic acid. eGFR: estimated glomerular filtration rate. N/L: neutrophils to lymphocytes ratio. TIA: transient ischemic attack.
Data are presented as hazard ratio (HR) with 95% confidence interval (CI) and p values. Only covariates with a univariate statistical significance of <0.05 were reported.
Finally, Cox regression analysis showed no significant impact of different doses of ASA in terms of primary endpoint (HR 0.769, 95% C.I. 0.489–1.209, p = 0.256), in-hospital death (HR 0.864, 95% C.I. 0.525–1.422, p = 0.565) and need for respiratory support upgrade (HR 0.828, 95% C.I. 0.368–1.865, p = 0.649).
4. Discussion
The cardinal finding of this multi-center, observational analysis with the prespecified hypothesis of a protective role of ASA in COVID-19 infection was that pre-admission chronic ASA therapy resulted in a better in-hospital outcome mainly driven by less respiratory support upgrade. This noteworthy finding was associated with no difference concerning in-hospital death among patients with or without ASA.
An intriguing question involving the scientific community is the definition of the role played by antithrombotic treatment in COVID-19 patients [7], [20], primarily focusing on anticoagulation and its clinical impact [21], [22]. Considering the lack of a standard of care, dominant related questions are: 1) what is the best antithrombotic strategy (anticoagulant with or without antiplatelet and eventually which specific drug)? and, 2) which kind of clinical benefit to expect from, and primarily which kind of benefit to consider as still useful (freedom from complications and/or survival improvement) for each patient within the broad spectrum of presentation?
The present study is an attempt to provide some potential answers and to make a firm focus on the role of ASA, a therapeutic regimen approved in patients phenotype with multiple cardiovascular comorbidities and more exposed to COVID-19 injury. To the best of our knowledge, this is the largest analysis showing an association between ASA and favourable outcome in COVID-19 patients. The present findings are consistent with a multi-centre study, where ASA was independently associated with decreased risk of mechanical ventilation, intensive care unit admission and finally in-hospital mortality, though in a smaller sample size (412 patients) [23]. Conversely, in our analysis ASA failed to predict overall survival. Apparently divergent results may be associated to either patient selection resulting in different baseline clinical features or different level of adjustment for several prognostic confounders. However, since a sub-analysis of the TARGET-COVID study showed an insufficient pharmacodynamic effect of 81 mg daily ASA in a high percentage of COVID-19 patients, most of whom African Americans [24], it is at least surprising how low-dose (median 81 mg daily) ASA is sufficient to provide such a meaningful clinical effect.
Our results suggest that, although suffering from a similar extent of disease, ASA− patients underwent in-hospital progressive clinical deterioration and were in greater need of empirical anti-SARS-CoV-2 therapy and respiratory support, potentially related to a pathological platelet hyperactivation. Through inhibition of synthesis of cyclooxygenase and activation of nuclear factor-κB [13], ASA exerts a simultaneous antiplatelet and anti-inflammatory effect, potentially able to prevent intravascular coagulation and neutrophil-mediated microvascular thrombosis, as showed in animal model [25]. Since platelets may represent a bridge between immune system and thrombosis, therefore the frontline of COVID-19 pathogenesis [26], antiplatelet therapy may constitute a cost-effective, relatively low risk-associated [27], therapeutic strategy to prevent patients from clinical worsening during SARS-CoV-2 infection in addition to LMWH, especially in non-critically ill patients. Indeed, our analysis identified LMWH as an independent predictor of in-hospital mortality. That is consistent with recently published data deriving from a single multiplatform, randomized, controlled trial suggesting that in the moderately ill patients therapeutic-dose LMWH appeared to increase the probability of survival until hospital discharge [28]. Furthermore, the preprint article reporting the findings of the Therapeutic Anticoagulation versus Standard Care as a Rapid Response to the COVID-19 Pandemic (RAPID) trial showed as therapeutic anticoagulation group had a lower incidence of death at 28 days [29].
Therefore, it is reasonable that, rather than a single medication, combination therapies targeting several pathological pathways (e.g., inflammation, coagulopathy, thrombocytopathy and endotheliopathy) are more likely to be successful.
The identification of single-patient thrombogenic phenotype, based upon not only thrombotic biomarkers such as D-dimer, fibrinogen, prothrombin time and activated partial thromboplastin time, but also whole blood viscoelastic analysis performed by thromboelastography or rotational thromboelastometry, would allow personalizing antithrombotic therapy in COVID-19 patients and possibly improve outcomes [30]. Interestingly, as Gurbel et al. suggested [31], systemic concentrations of oral-administered ASA may not reach the airway and alveolus to effectively reduce the virus load. In this perspective, unconventional routes of administration, including inhaled nanoparticle, should be considered to achieve locally effective concentrations.
To date available data are not sufficient to influence standard of care. Randomized controlled trial, such as the ongoing Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial (NCT04381936), will definitively evaluate whether antiplatelet therapy prevents adverse outcome in patients with COVID-19.
The present study suffers from the following limitations. In view of the observational nature of our analysis, patient selection and ascertainment bias may have influenced event rates. Particularly, identifying study groups according to pre-admission ASA intake represents a selection bias, since patients were on treatment because of the presence of more cardiovascular comorbidities. Furthermore, we did not account for safety endpoints, such as major bleeding.
Accordingly, our results should be considered as hypothesis generating and need confirmation in further larger observational studies or randomized trials.
In conclusion, in this retrospective analysis of patients with COVID-19 undergoing hospitalization, ASA is associated with a better in-hospital outcome in terms of in-hospital death or need for respiratory support upgrade, whose definitive evidence is mainly supported by the latter.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
All authors have participated in the work and have reviewed and agree with the content of the article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.09.058.
Appendix A. Supplementary data
Supplementary material
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asia S., Mediterranean E., Pacific W. 2020. COVID-19 Weekly Epidemiological Update. (December) [Google Scholar]
- 3.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganatra S., Hammond S.P., Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020;2(2):350–355. doi: 10.1016/j.jaccao.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. April 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu S.X., Tyagi T., Jain K. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021;2 doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S., Liu Y., Wang X., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13(1):120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. April 2020 doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. http://europepmc.org/abstract/MED/32294809 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang Y., Wang Y., Liu B., Ma X., Ding R. Effects of antiplatelet therapy on the mortality rate of patients with sepsis: a meta-analysis. J. Crit. Care. 2019;50:162–168. doi: 10.1016/j.jcrc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Panka B.A., de Grooth H.-J., Spoelstra-de Man A.M.E., Looney M.R., Tuinman P.-R. Prevention or treatment of ards with aspirin: a review of preclinical models and meta-analysis of clinical studies. Shock. 2017;47(1):13–21. doi: 10.1097/SHK.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianconi V., Violi F., Fallarino F., Pignatelli P., Sahebkar A., Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID - 19 ? Drugs. 2020;80(14):1383–1396. doi: 10.1007/s40265-020-01365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization WH. Geneva PP - Geneva: World Health Organization; 13 March 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance.https://apps.who.int/iris/handle/10665/331446 [Google Scholar]
- 17.Gurbel P.A., Bliden K.P., Butler K. 2009. Randomized Double-Blind Assessment of the ONSET and OFFSET of the Antiplatelet Effects of Ticagrelor Versus Clopidogrel in Patients With Stable Coronary Artery Disease The ONSET / OFFSET Study. [DOI] [PubMed] [Google Scholar]
- 18.Eikelboom J.W., Hirsh J., Spencer F.A., Baglin T.P., Weitz J.I. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: american College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e89S–e119S. doi: 10.1378/chest.11-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godino C., Scotti A., Maugeri N., et al. Antithrombotic therapy in patients with COVID-19? -Rationale and evidence. Int. J. Cardiol. September 2020;S0167–5273(20):33894–33898. doi: 10.1016/j.ijcard.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ACB Lemos, do Espírito Santo D.A., Salvetti M.C. Therapeutic versus prophylactic anticoagulation for severe COVID-19: arandomized phase II clinical trial (HESACOVID) Thromb. Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paranjpe I., Fuster V., Lala A., et al. Association of treatment dose anticoagulation withwith in-hospitalin-hospital survival amongamong hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow J.H., Khanna A.K., Kethireddy S. Aspirin use is associated with decreased mechanical ventilation, Intensive Care Unit admission, and in-hospital mortality in hospitalized patients with Coronavirus Disease 2019. Anesth Analg. 2020;132(4):930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 24.Gurbel P.A., Bliden K.P., Rout A., et al. Bedside thromboelastography to rapidly assess the pharmacodynamic response of anticoagulants and aspirin in COVID-19: evidence of inadequate therapy in a predominantly minority population. J. Thromb. Thrombolysis. 2021;51(4):902–904. doi: 10.1007/s11239-021-02435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mj D., Hannah S., Karlheinz P. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes Z., Florian P., Isabelle A., et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiarito M., Sanz-Sánchez J., Cannata F., et al. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet. 2020;395(10235):1487–1495. doi: 10.1016/S0140-6736(20)30315-9. [DOI] [PubMed] [Google Scholar]
- 28.The REMAP-CAP, ACTIV-4a, and ATTACC Investigators Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. August 2021 doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sholzberg M., Tang G.H., Rahhal H. Heparin for moderately Ill patients with Covid-19. medRxiv Prepr Serv Heal Sci. July 2021 doi: 10.1101/2021.07.08.21259351. 2021.07.08.21259351. [DOI] [Google Scholar]
- 30.Chaudhary R., Kreutz R.P., Bliden K.P., Gurbel P.A. Personalizing Antithrombotic Therapy in COVID-19 : Role of Thromboelastography and Thromboelastometry. 2020. pp. 1594–1596. [DOI] [PubMed] [Google Scholar]
- 31.Gurbel P.A., Bliden K.P., Schrör K. Can an old ally defeat a new Enemy? Circulation. 2020;142(4):315–317. doi: 10.1161/CIRCULATIONAHA.120.047830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material