Abstract
Background and aims
During the COVID-19 vaccination program in India, the healthcare workers were given the first priority. There are concerns regarding the occurrence of breakthrough infections after vaccination. We aimed to investigate the effictiveness of COVID-19 vaccines in preventing and reducing the severity of post-vaccination infections.
Methods
This retrospective test-negative case-control study examined 28342 vaccinated healthcare workers for symptomatic SARS-CoV-2 infections between January 16 to June 15, 2021. They worked at 43 Apollo Group hospitals in 24 Indian cities. These cohorts received either ChAdOx nCOV-19 (Recombinant) or the whole virion inactivated Vero cell vaccines. Various demographic, vaccination related and clinical parameters were evaluated.
Results
Symptomatic symptomatic post-vaccination infections occurred in a small number of vaccinated cohorts (5.07%, p < 0.001), and these were predominantly mild and did not result in hospitalization (p < 0.0001), or death. Both vaccines provided similar protection, with symptomatic infections in 5.11% and 4.58%, following ChAdOx nCOV-19 (Recombinant) and the whole virion inactivated Vero cell vaccines, respectively (p < 0.001). Nursing and Clinical staff and cohorts >50 years contracted more infections (p < 0.001). Two-dose vaccination has significantly lower odds of developing symptomatic infection (0.83, 95%CI – 0.72 to 0.97). Maximum infections occurred during the peak of the second COVID-19 wave from mid-April to May 2021 (p < 0.001). No significant difference existed in the infection between sex, vaccine type, and the number of vaccine doses received (p ≥ 0.05).
Conclusion
Symptomatic infections occurred in a small percentage of healthcare workers after COVID vaccination. Vaccination protected them from not only infection but also severe disease.
Kewords: SARS-CoV-2, Break through infections, Vaccine, Healthcare workers, COVID-19
1. Introduction
The coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that is highly contagious, has spread globally since it was first detected in December 2019. India has experienced a significant resurgence of the virus during the second wave of the pandemic, which had its peak in April and May 2021 [1]. The only hope for effective control and mitigation of this pandemic are effective and large-scale vaccination efforts. Several vaccines for preventing SARS-CoV-2 infection have been developed in many countries, including India, at a phenomenal speed [2]. The Government of India (GOI) first permitted the clinical use of two types of vaccines, namely ChAdOx nCOV-19 (Recombinant) and a whole virion inactivated Vero cell vaccine [[3], [4], [5]]. A mass vaccination program was implemented in India in multiple phases beginning January 16, 2021. The first phase of vaccinations included healthcare workers (HCWs), people aged over 60 years, and people aged over 45 years with significant medical comorbidities. They were administered one of the two approved vaccines at vaccination centers, with a recommendation to get the 2nd vaccine dose in 4–8 weeks of the 1st vaccination [6].
The ChAdOx nCOV-19 (Recombinant) vaccine [4] is currently the most commonly used in India. It is a viral vector vaccine manufactured by modifying the chimpanzee adenovirus ChAdOx, and was developed by AstraZeneca and Oxford University (UK). The vaccine is manufactured and sold in India by the Serum Institute, Pune. Its reported effectiveness for preventing symptomatic COVID-19 is 76.0% at three weeks after the first and 81.3% after the second. The other approved vaccine in India is a whole virion inactivated Vero cell vaccine [5], developed by Bharat Biotech (Hyderabad, India) in collaboration with ICMR and the National Institute of Virology. It was approved by the GOI for emergency use in the prevention of COVID-19 after a successful phase-2 trial. It has demonstrated 78% effectiveness in preventing mild, moderate, and severe COVID-19 cases and has demonstrated effectiveness in preventing infection by mutant and heterogeneous viral strains of SARS-CoV-2.
HCWs have been on the frontline of efforts to combat the pandemic and were thus prioritized for vaccination when COVID-19 vaccines became available. It was essential to prioritize vaccination of HCWs because they have a greater occupational risk of becoming infected. Additionally, infected HCWs reduce the number of healthy HCWs available at a given time, thereby reducing the availability of their much-needed services during a critical time. Furthermore, HCWs present a risk of transmitting SARS-CoV-2 infection to their family members and the general public [7].
Although it is now widely known that the available COVID-19 vaccines are safe and effective, there have still been concerns about their effectiveness and possible side effects [8]. Recently, cases of post-vaccination infections (PVIs) have been reported [9]. The Centre for Disease Prevention and Control (CDC) has defined the breakthrough infections (BTIs) when the PVI occurs two weeks after the second dose of COVID-19 vaccination [10].
Thus, this study aimed to investigate the effectiveness of two COVID-19 vaccines in preventing and reducing the severity of symptomatic PVIs in a large cohort of HCWs working at Apollo Group hospitals, the largest chain of hospitals in India [11], after they received the vaccines.
2. Materials and methods
This retrospective, observational cohort study was conducted among HCWs were working at various Apollo Group hospitals across India [11]. We adopted the test-negative study design because it controls for differences in health-seeking behavior of the cohort and the non-symptomatic group acted as the controls. This study was approved by an Ethical Institutional Committee (EIC), and a consent waiver was given by the EIC. The data of the participants of this study were collected from a centralized Human Resource office of Apollo Hospital group, located in Chennai, which collects the data from all the hospitals of the group. We collated and analyzed the data of HCWs obtained. The 5-month study period was from January 16, 2021, to June 15, 2021.
We assessed records of all HCWs who received COVID-19 vaccination and developed symptomatic PVIs. These HCWs were doctors, nurses, paramedics, administrative staff, and support staff. These cohorts administered two types of vaccines: ChAdOx nCOV-19 Recombinant (Covishield™, Serum Institute, India) and a whole virion inactivated Vero cell vaccine (Covaxin™, Bharat Biotech, India) depending on the Government supply of the vaccine at different vaccination centers.
We divided symptomatic PVIs into two groups: (A) PVIs following full vaccination (FV; two doses) and (B) PVIs following partial vaccination (PV; one dose).In addition, the Breakthrough Infections (BTI) were considered when the PVI occurred after two weeks of the two vaccine doses. The day to infection was calculated from the last vaccination date to the positive RT-PCR test date in the symptomatic HCWs.
The inclusion criteria of this study were the participants working at an Apollo Group hospital, receipt of COVID-19 vaccination, and later acquisition of SARS-CoV-2 PVI. Only symptomatic COVID-19 cases with positive reverse transcription-polymerase chain reaction (RT-PCR) test results were included. Our hospital group policy mandates that all the HCWs report any adverse event or symptoms following the vaccination to the respective group hospital where the vaccination was done and do not self-treat or go to another facility for their treatment. We excluded all participants who might have asymptomatic PVIs (due to resource constraints and logistic reasons), non-vaccinated HCWs, and those with negative RT-PCR test results.
The data was collected, analyzed, and compared for various parameters such as age, sex, time to PVI, type of vaccine, PV or FV status, monthly and regional case distribution, clinical severity of PVI, hospitalization requirements, intensive care unit (ICU) need, and death.Statistical analyses were performed using IBM-SPSS version 20™ (IBM, USA). A chi-squared test was performed to determine the association among various categorical variables. The Z-test for differences between proportions was used as appropriate, and relative risks and 95% confidence intervals (CIs) were calculated. Statistical significance was defined by p-values <0.05.
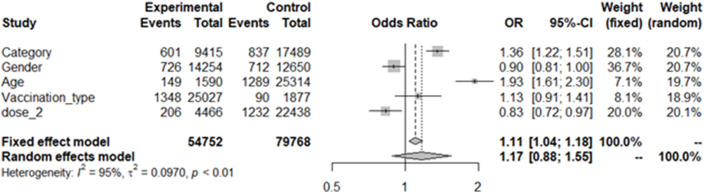
We looked at different variables for the multivariate odds ratio analysis and analyzed the dependent variable of Post Vaccination Infection. In Staff Category, we divided the Doctors and Nurses (as Clinical Staff) as Experimental while Other Non-Clinical Staff as Control. Similarly, the male gender as Experimental and the females as Control. In the age, HCW above 50 years were considered as Experimental and 50 or below as Controls. Vaccination with ChAdOx nCOV-19 (Recombinant) vaccine was taken as Experimental, and the whole virion inactivated Vero cell vaccine as Controls. Lastly, the individuals who received two doses as Experimental and one dose as Control. We ran the multivariate odds ratio on the R package and evaluated the Odds ratios of staff category, gender, age >50 years, type of vaccination, completed two doses as variables, and provided the forest plot (Fig. 1 ).
Fig. 1.
A Forest Plot showing the results of multivariate analysis of the study data.
3. Results
-
A)
Demographic characteristics:
31621 HCWs were vaccinated from January 16, 2021, to June 15, 2021, at 43 Apollo Hospitals in 24 cities of India. We excluded 3279 HCWs because of the non-availability of the full data required for this study due to any incomplete or missing information. Thus, the final study cohort comprised 28342 HCWs. The age range of the study population was 18–80 years, with an average age of 33.04 years. Most vaccinated HCWs (76.85%) in the study cohort were younger than 40 years. The average age of HCWs who developed symptomatic PVIs was 35.73 years (Table 1 ). ChAdOx nCOV-19 vaccine (Recombinant) was administered to most HCWs (26375, 93.05%), and the whole virion inactivated Vero cell vaccine was administered to 1967 HCWs (6.95%). Most HCWs (81.92%) received both vaccine doses (Table 1). The sex distribution of the cohort was almost similar (14980 male and 13362 female).
-
B)
Outcomes:
Table 1.
Demographic details and the statistical analysis of the cases included in this study.
| Number of vaccinated Healthcare workers | Symptomatic Post-vaccination infections | Percentage (95% confidence interval) | Statistical significance (p-value) | |
|---|---|---|---|---|
| Total | 28342 | 1438 | 5.07 | – |
| (4.82–5.34) | ||||
| Type of vaccine administered (difference) | 0.53 | |||
| (-0.52–1.41) | ||||
|
|
1348 | 5.11 | |
| (4.85–5.38) | 0.3015 | |||
|
|
90 | 4.58 | |
| (6.95%) | (3.69–5.59) | |||
| Post-vaccination symptomatic infection after one dose | 5125 | 269 | 5.25 | <0.001, Difference- (64.5–67.4%) |
| (18.08%) | (4.65–5.89), p < 0.001 | |||
| - Within 2weeks |
|
46 | 17.0 | |
| - After 2 weeks |
|
223 | 83.0 | |
| Post-vaccination symptomatic infection after two doses | 23217 | 1169 | 5.04 | <0.001, Difference (87.8–88.6%) |
| (81.92%) | (4.76–5.32), P < 0.001 | |||
| - Within 2 weeks | 69 | 5.9 | ||
| - After 2 weeks | 1100 | 94.1 | ||
| Post-vaccination symptomatic infection after two vs. one dose (difference) | 0.21 | 0.5354 | ||
| (-0.44 to 0.91) | ||||
| Sex (difference) | 0.48 | |||
| (-0.32 to 0.99), RR = 1.0995 | ||||
| (0.9941–1.2160) | ||||
|
13362 | 712 | 5.33 | |
| (47.14%) | (4.95–5.72) | 0.0661 | ||
|
14980 | 726 | 4.85 | |
| (52.86%) | (4.51–5.20) | |||
| Average age (in years) | 33.04 | 35.73 | – | – |
| Age distribution (in years) | ||||
|
12767 | 544 | 4.26 | |
|
9014 | 487 | 5.40 | |
|
4893 | 267 | 5.46 | <0.001 |
|
1446 | 111 | 7.68 | |
|
188 | 26 | 13.83 | |
|
34 | 3 | 8.82 | |
| Age groups (in years) | ||||
|
21781 (76.85%) | 1031 | 4.73 | |
|
6339 | 378 | 5.96 | <0.001 |
| (22.37%) | ||||
|
222 | 29 | 13.06 | |
| (0.78%) | ||||
| Regional distribution | <0.001 | |||
|
15777 | 899 | 5.70 | |
|
589 | 31 | 5.26 | |
|
6331 | 210 | 3.31 | |
|
2694 | 157 | 5.82 | |
|
2951 | 141 | 4.78 | |
| Category of Staff | ||||
|
7760 | 448 | 5.77 |  |
|
2256 | 153 | 6.78 | |
|
5172 | 264 | 5.10 | <0.001 
|
|
3000 | 140 | 4.67 | |
|
10154 | 433 | 4.26 | |
| Hospital ward admission | 28342 | 80 | 0.28 | – |
| ICU admission | 28342 | 3 | 0.01 | – |
| Deaths | 28342 | Nil | – | – |
Among the HCWs, 1438 (5.07%; 95% CI = 4.82–5.34%, p < 0.0001) had symptomatic PVIs (Table 1). Among those, who received the ChAdOx nCOV-19 (Recombinant) vaccine, 1348 (5.11%) reported symptomatic PVIs. Whereas those who received the whole virion inactivated Vero cell vaccine, 90 (4.58%) reported symptomatic PVIs with no significant difference between the two types of vaccine (p = 0.3015).
The FV and PV groups included 23217 and 5125 HCWs, respectively. The incidence of PVIs was 5.25% and 5.04% after a single dose (PV) and two doses (FV). The symptomatic PVI incidence following FV was not significantly different from that following PV (95% CI = 0.21% [−0.44 to 0.91], p = 0.5354). However, both single and dual-dose recipients were protected from infection by vaccination (Table 1).
The incidence of symptomatic PVI among female HCWs was slightly higher than the male HCWs (5.33%, vs. 4.85%), but this difference was not significant (p = 0.0661). The relative risk (RR) of acquiring symptomatic PVIs in females was increased compared to males but not reached statistical significance, at RR = 1.0995 (95% CI = 0.9941–1.2160) (Table 1). The maximum incidence of symptomatic PVIs occurred in clinical (6.78%) and nursing staff (5.77%), followed by administrative, paramedical, and support staff (p < 0.001).
Although the total number of symptomatic PVIs in HCWs aged <50 years was higher, the percentage of PVIs in HCWs aged ≥50 years were much greater than those in the younger age (95% CI = 4.59–2.44%). There was a significant influence of age on the incidence of symptomatic PVI, with a substantial number of infections observed in the higher age groups [13.06% in HCWs aged 61–80 years vs. 5.96% in HCWs aged 41–60 years and 4.73% in HCWs aged 18–40 years], with a p-value <0.001.
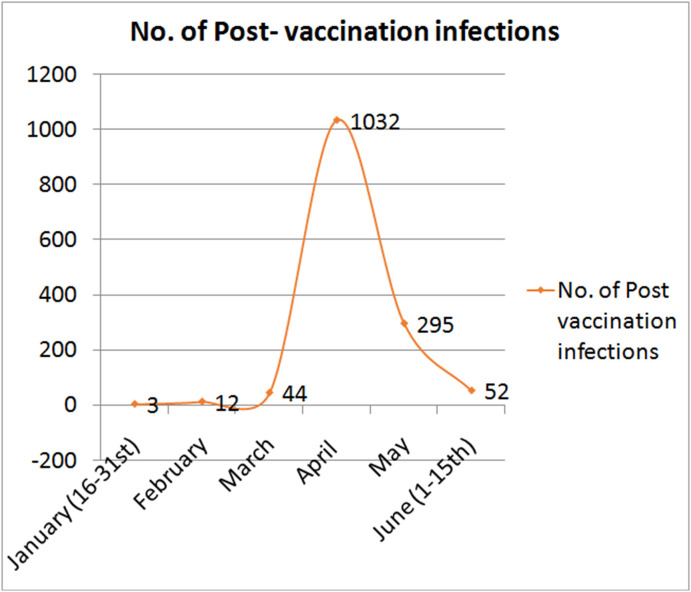
PVI incidence increased during the second COVID-19 wave in April (74.84%) and was the lowest in January (3.31%) (Fig. 2 ). The monthly distribution of symptomatic PVIs was statistically significant (p < 0.001). This higher incidence is likely due to an emergence of a variant of concern (VOC), as the Alpha and Delta variants. In addition, the incidence of symptomatic PVI was significantly different in various regions of India, with the highest incidence (5.82%) in the western region and the lowest incidence (3.31%) in the eastern region (p < 0.001).
Fig. 2.
Monthly distribution of post-vaccination infections in the study.
Among the entire cohort of vaccinated HCWs of 28432, only 80 required hospital admissions (0.28%), and three required ICU admission (0.01%). In addition, there were no deaths among this cohort during the study period (Table 1, Table 2 ). The average duration of developing symptomatic PVI in subjects who were admitted to the non-ICU wards was 54.6 days, compared to an average of 21 days in those who were admitted to the ICU (Table 2).
Table 2.
Details of the subjects who were admitted to the hospitals for symptomatic post-vaccination infections.
|
Hospital Admissions |
Hospital Admissions |
|
|---|---|---|
| (in wards) | (in ICU) | |
| Number of Post vaccination infections | N = 80 | N = 03 |
| Percentage out of total vaccinated population (n = 28432) | 0.28 | 0.01 |
| Percentage out of total post vaccination infections (n = 1438) | 5.56 | 0.21 |
| Age (in years) | Average: 36.1 (Range: 22–70) | Average: 33.67 (Range: 25–48) |
| Sex | ||
| Female | N = 37 | N = 01 |
| Male | N = 43 | N = 02 |
| Category of Staff | ||
| Administrative | 20 | 01 |
| Clinical | 11 | 02 |
| Nursing | 30 | – |
| Paramedical and Supportive | 19 | – |
| Vaccine administered | All (N = 80) | All (N = 03) |
| Type of Vaccine used | ||
| ChAdOx nCOV-19 Recombinant | N = 72 | N = 03 |
| Whole virion inactivated Vero cell | N = 08 | Nil |
| Number of vaccination doses taken, prior to infection | ||
| Single dose | N = 11 | N = 1 |
| Double dose | N = 69 | N = 2 |
| Days to symptomatic post vaccination infections | Average: 54.6 (Range: 02 to 109) | Average: 21.0 (Range: 06–38) |
| Outcome | Good in all | Good in all |
| Mortality | Nil | Nil |
On the multivariate analysis, the adjusted odds ratios for Clinical and Nursing staff who were directly in the care of patients was 1.36 (95%CI – 1.22 to 1.51) and significant.The Odds for the female HCWs were slightly higher at 1.11. The individuals above 50 years had higher odds for PVI at 1.93 (95%CI – 1.61 to 2.30). The participants who received ChAdOx nCOV-19 (Recombinant) vaccinehad a slightly higher odds of PVI at 1.13 than thewhole virion inactivated Vero cell vaccine. The subjects who completed their two-dose vaccination hada significantly lower odds of developing symptomatic PVI (0.83, 95%CI – 0.72 to 0.97).
4. Discussion
With HCWs being the frontline workers for combating the COVID-19 pandemic, this population has an increased risk of acquiring SARS-CoV-2 infections and thus requires protection. Therefore, most governments worldwide, including GOI, have prioritized the vaccination of HCWs during vaccination rollouts. Unvaccinated HCWs have a higher risk of infections of approximately 7.3% compared with 5% in the general population [7,12,13]. It makes a strong case for prioritizing HCWs for vaccination against COVID-19. HCWs who acquire infection cannot provide critical services and care to patients during increased need. Furthermore, vaccination of HCWs may contribute to preventing SARS-CoV-2 spread and the development of further mutations. Italy became the first European country to make COVID-19 vaccination mandatory for HCWs on April 1, 2021, to contain a third wave of the disease [14].
Symptomatic PVIs may occur before and even after full immunization; however, the reported incidence of PVI is low [9,14,15]. We found incidence of PVIs of 5.01% after two doses and 5.07% after a single dose of vaccination. Vaccination prevented symptomatic PVI in both groups (p < 0.001), however, there was no significant difference found in our study between the single and dual-dose vaccination groups (p = 0.5354). A study from California reported a rapid and sustained decline in symptomatic and asymptomatic infections following vaccination of their HCWs and concluded that the vaccination reduced overall infections rates, viral shedding, and asymptomatic and symptomatic transmission [16].
Most of our symptomatic PVIs did not result in ICU admissions, and there were no deaths during the study period. Although the period of this study coincided with the second wave of COVID-19 in India, the symptomatic PVI rates were low. It reflects on the dual effect of vaccination in preventing COVID-19 and reducing the severity of SARS-CoV-2 infection. In this study, most BTIs (81.92%) occurred after an average of 46 days. It could be related to relaxation in personal safety measures or the emergence of viral mutants or variants of concern (VOCs). The VOCs are highly transmissibleand can evade or bypass existing immunity in the host, leading to severe disease [17,18]. Various VOCs such as B.1.617.2 (delta variant) and B.1.1.7 (alpha variant) are detected in multiple parts of India, as well as in 85 countries worldwide [17].
The effectiveness of various vaccines used in India and abroad has been demonstrated in several published studies [9,[19], [20], [21]]. Al –Kuwari et al. have published a randomized, multicenter clinical trial that reported an effectiveness rate of more than 70% for two vaccines assessed and compared with an adjuvant-only control [23]. Keehner et al. [9] analyzed the data of 36659 HCWs who had mRNA vaccine and reported BTI incidence of 1.03%. The higher incidence of PVI in our series was higher. We attribute these infections to the second COVID-19 wave in India, which happened during the last two months of this study when the VOCswere accounted for more than half of the PVIs [[20], [21], [22],24]. Shah et al. reported the effect of vaccination among HCWs and their household in the UK and concluded that the vaccination was associated with a significant reduction in the incidence of COVID-19 among their household contacts [25]. Substantial early symptomatic infections following the first dose of vaccination were found in a study conducted in Israel [26]. We also noted the protective effect of vaccination following a single dose (p < 0.001). A UK surveillance study reported a reduction of SARS-CoV-2 infections by 65% after receiving the first vaccine dose (Oxford-AstraZeneca or Pfizer-BioNTech). These reductions were further increased to 70% after receiving a second dose of the vaccine [27]. Our findings are also corroborated by those of Dogan et al. [17], who demonstrated that vaccination effectively prevents severe outcomes after PVIs, with lower hospitalization rates, severe illness, and death (p < 0.0001) [15,28]. Most studies involving vaccinated HCWs demonstrated a lower incidence of severe complications and mortality following PVIs, as we believe that their cohorts were younger and did not have significantmedical comorbidities, known as high-risk factors. However, the varied incidence of COVID-19 has been reported among HCWs, from different centers in India, including our study, ranging from 1.6% to 11% [15,[20], [21], [22]]. We have identified and suggested probable reasons for these variable incidences of PVI (Table 3 ).
Table 3.
Suggested reasons for the variable incidence of post-vaccination infection in the healthcare workers.
| S. No. | VARIABLES | RELEVANCE |
|---|---|---|
| I) | PATHOGEN RELATED | |
|
Variant of Concern are highly virulent mutants and can bypass the immunity | |
| II) | HOST RELATED | |
|
Increasing age is a risk factor of acquiring infection and more severe | |
|
Medical comorbidities like Respiratory disease, Diabetes, Hypertension, Obesity, Immuno-compromised conditions are identified high risk factors | |
|
Higher immunity is present in individuals with a history of prior COVID-19 disease, and post vaccination (especially after two weeks of the 2nd dose) | |
|
Routine and random testing for SARS-COV-2 infections can pick up asymptomatic infections, which will add to the total number of cases | |
| III) | VACCINE RELATED | |
|
|
|
|
|
|
| IV) | STUDY RELATED | |
|
If the study is done during the period of an ongoing COVID ‘wave’, the incidence would be higher | |
|
If the study location falls in the area of higher SARS-COV-2 infections, there would be higher rate of infection in the healthcare workers also | |
|
A COVID dedicated hospital is likely to have higher incidence of infections in the healthcare workers | |
|
A multi-centre study provides more heterogeneous data and better projection of the incidence and outcomes of the infections | |
|
Larger the sample, better inferences can be derived | |
Little information is available on the effect of vaccination on reducing asymptomatic infection or transmission globally. The SIREN study confirmed that the BNT162b2 mRNA vaccine does not prevent all cases of infection. Hence, vaccinated HCWs should continue to observe COVID-appropriate behavior and continue regular asymptomatic testing until COVID prevalence has been considerably reduced [29,30]. The CDC has also emphasized and supported the continuation of safety measures after complete vaccination [18].
We noted comparable effectiveness of both types of vaccines administered in our cohorts, with symptomatic PVI incidence of 5.11% and 4.58% for the ChAdOx nCOV-19 (Recombinant) vaccine and the whole virion inactivated Vero cell vaccine, respectively.
Information concerning BTIs has started to be reported but remains sparse [15,24,[31], [32], [33], [34], [35]] SARS-CoV-2 mutants (e.g., Delta and Alpha variants) are recognized as important causes of increasing BTIs across the globe. Genome sequencing is valuable in identifying the nature of and is necessary to monitor the evolution and spread of mutant viruses [34]. The CDC has reported the collated data of 10262 BTIs in the US population during the first four months of 2021. One hundred and one million people in the US were vaccinated by April 30, 2021. Hence, the incidence of BTIs is very low, even though there may be under-reporting of such cases. The symptomatic patients accounted for 73% of these. A total of 10% of BTI cases required hospital admission, of which 29% were asymptomatic cases admitted for non-COVID medical reasons. The cumulative fatality was 2%. Only 5% of these cases underwent genome sequencing, which revealed a high incidence (64%) of VOCs, with Alpha variant and B.1.429 being the most common. In these data, no cases related to the Delta variant were reported [34]. Coincidentally, this VOC was predominantly responsible for the massive second wave in India [[20], [21], [22]].
4.1. Study limitations and strengths
We acknowledge some limitations of this retrospective study. First, there was a lack of a comparative group of unvaccinated population, and we did not analyze asymptomatic cases.We also did not include the cases with a history of previous COVID 19 infections. Second, the genome sequencing and antibody titers were not tested in all infected individuals due to operational and logistical reasons. This study reported clinical outcomes of symptomatic PVIs in the short term, and therefore, more long-term effects of the infection need to be evaluated and reported on these cohorts. Third, the participants in the study were primarily healthy young HCWs. Hence, it did not say the effectiveness of vaccination among those with higher risk, e.g., those with chronic diseases, older adults, and those with previous SARS-CoV-2 infections. Finally, being a retrospective study, it has the potential for type II errors, biases, and confounding.
The main strength of this study is being a multicenter study representing almost all geographical areas of India and had a large cohort to draw meaningful conclusions. This observational study was a real-world study to assess the effectiveness of vaccines outside of a randomized control trial, which we believe is helpful for the scientific community, as it provides a piece of direct evidence. A comparison of the two vaccines administered to this cohort could be made. Furthermore, as literature on the Vero Cell vaccine's effectiveness is scarce, this study could be particularly insightful.
5. Conclusion
There was a very low incidence of symptomatic COVID-19 (5.07%) in vaccinated HCWs and even lower incidence of hospitalization and ICU admissions. Nursing and clinical staff, HCWs aged more than 50 years, and HCWs from western India were significantly more affected by symptomatic PVIs. The two-dose vaccination has lower odds of developing symptomatic PVI.This multicenter study showed no significant influence of sex or type of vaccine received on symptomatic PVI incidence. This study lays out the evidence that vaccination provided prevention from symptomatic infection and protection from severe disease.
Declaration of competing interest
None.
Acknowledgments
We are grateful to Dr. Mohit Kumar Patralekh of Safdarjung Hospital, New Delhi, for helping us out with the statistical analysis of the study data. We are also grateful to the MRD and HR departments of the 43 Apollo group of hospitals, who participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dsx.2021.102306.
Conflict of interest declaration
On behalf of all t he co-authors, I declare that none of the authors of this study titled ‘Symptomatic post-vaccination SARS-CoV-2 infections in healthcare workers– A multicenter cohort study’ had any conflict of interest, and did not receive any financial grants or support.
Funding
None.
Conflicts of interest
None.
Data sharing
The data collected for the study, including individual participant data and a data dictionary defining each field in the set, will be available to the journal whenever required.
Financial disclosure
None.
Ethical approval
Taken.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vaishya R. The havoc caused by the 2nd wave of COVID-19 in India. Apollo Medicine. 2021 doi: 10.4103/am.am_36_21. [DOI] [Google Scholar]
- 2.Coronavirus. World Health Organization (WHO) 2021. https://www.who.int/health-topics/coronavirus#tab=tab_1 Last accessed on 25th April.
- 3.Different COVID-19 vaccines. Centers for disease control and prevention. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html Last accessed on 25th April. [PubMed] [Google Scholar]
- 4.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., eet al Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021 Dec 19;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sapkal G.N., Yadav P.D., Ella R., Deshpande G.R., Sahay R.R., Gupta N., et al. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Trav Med. 2021 Jun 1;28(4) doi: 10.1093/jtm/taab051. taab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidance note for COWIN 2.0. 2021. https://www.mohfw.gov.in/pdf/GuidancedocCOWIN2.pdf Ministry of Health and Family Welfare. Last accessed on 25th April.
- 7.da Silva P.C.M.C. Vaccination against COVID-19 in health care workers. Rev Bras Med Trab. 2021;19(1):1–2. doi: 10.47626/1679-4435-2021-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodd R.H., Pickles K., Nichel B., et al. Concerns and motivations about COVID-19 vaccination. The Lancet Infectious Diseases. Feb. 2021;21(2):161–163. doi: 10.1016/S1473-3099(20)30926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keehner J., Horton L.E., Pfeffer M.A., et al. SARS-CoV-2 infection after vaccination in health care workers in California. New Eng J Med March. 2021 doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 breakthrough case investigations and reporting. https://www.cdc.gov/vaccines/covid-19/health-departments/breakthrough-cases.html Centers for Disease Control and Prevention. Accessed on 12th.
- 11.Indraprastha Apollo Hospital 2021. https://delhi.apollohospitals.com/corporate/indraprastha-apollo-hospitals Last accessed on 25st April.
- 12.Dong X.C., Li J.M., Bai J.Y., Liu Z.Q., Zhou P.H., Gao L., et al. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghualiuxingbingxueza. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhanin China. Clin Infect Dis. 2020;71:2218–2221. doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterlini M. Covid-19: Italy makes vaccination mandatory for healthcare workers. BMJ. 2021;373:n905. doi: 10.1136/bmj.n905. [DOI] [PubMed] [Google Scholar]
- 15.Vaishya R, Sibal A, Arpita M, Hariprasad K. SARS-CoV-2 infections after COVID-19 immunization in Health care workers– A retrospective, case cohort study. Indian J Med Res2021; doi: 10.4103/ijmr.ijmr_1485_21.
- 16.Gohil S.K., Olenslager K., Quan K.A., et al. Asymptomatic and symptomatic COVID-19 infections among health care personnel before and after vaccination. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.15980. Published 2021 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. DOI: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed]
- 18.Centers for Disease Control and Prevention . 2021. COVID-19 vaccines.https://www.cdc.gov/vaccines/covid-19/index.html Last accessed 25th April. [PubMed] [Google Scholar]
- 19.SARS-CoV-2 Variant Classifications. Definitions . 17th May 2021. Centers for disease control and prevention.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html#:%7E:text=Viral%20mutations%20and%20variants%20in,Variant%20of%20Interesty [Google Scholar]
- 20.Dhar M.S., Marwal R., Radhakrishnan V.S., et al. 3rd June 2021. Genomic characterization and Epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. medRxiv preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta N, Kaur H, Yadav P, Mukhopadhyay L, Sahay RR, Kumar AK et al. Clinical characterization and Genomic analysis of COVID-19 breakthrough infections during second wave in different states of India. MedRxiv preprint. 10.1101/2021.07.13.21260273. [DOI] [PMC free article] [PubMed]
- 22.Kale P., Gupta E., Bihar C., Patel N., Rooge S., Pandey A., et al. Clinicogenomic analysis of breakthrough infections by SARS CoV2 variants after ChAdOx1 nCoV- 19 vaccination in healthcare workers. medRxiv. 2021:21259546. doi: 10.1101/2021.06.28.21259546. 06.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-KuwariMG, AbdulMalik M.A., Al-Nuaimi A.A., et al. Epidemiology characteristics of COVID-19 infection amongst primary health care workers in Qatar: march-october 2020. Frontiers in Public Health. May 2021;9 doi: 10.3389/fpubh.2021.679254. Article 679254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlcochova P., Kemp S., Dhar M.S., Vaishya R., et al. SARS-CoV-2 B.1.617.2 Delta variant emergence and vaccine breakthrough. Research Square. 2021 doi: 10.21203/rs.3.rs-637724/v1. [DOI] [Google Scholar]
- 25.Shah A.S.V., Gribben C., Bishop J., et al. medRxiv preprint 21st March; 2021. Effect of vaccination on transmission of COVID19: an observational study in healthcare workers and their households. [DOI] [Google Scholar]
- 26.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 6th March 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacobucci G. Covid-19: infections fell by 65% after first dose of AstraZeneca or Pfizer vaccine, data show. BMJ 23rdApril. 2021;373:n1068. doi: 10.1136/bmj.n1068. [DOI] [PubMed] [Google Scholar]
- 28.Vaishya R., Sibal A., Singh S.K., et al. ChAdOx1 n-COV 19 Vaccine protected against severe infection caused by the Variants. Mayo Clin Proc. August 2021 doi: 10.1016/j.mayocp.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall V., Foulkes S., Charlett A., et al. Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? 2020. Large multicentre prospective cohort study (the SIREN study), England: June to November. medRxiv2021; published online 15th January. [DOI]
- 30.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in healthcare workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. The Lancet 23rd April. 2021 doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagi K., Ghosh A., Nair D., Dutta A.K., Singh B.P., Ahmed A.I., Misra A. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India, diabetes & metabolic syndrome. Clin Res Rev. 2021 doi: 10.1016/j.dsx.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacisuleyman E., Hale C., Saito Y., et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021 21st April doi: 10.1056/NEJMoa2105000. Epub ahead of print. PMID: 33882219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021 Jul 28 doi: 10.1056/NEJMoa2109072. Epub ahead of print. PMID: 34320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health. https://www.who.int/publications/i/item/9789240018440 Last accessed on 25th April 2021:
- 35.Centers for Disease Control and Prevention COVID-19 vaccine breakthrough infections reported to CDC — United States, january 1–april 30, 2021. 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7021e3.htm May 28. 70(21); 792-793. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.