Abstract
Objectives
During the COVID-19 pandemic, numerous cases of chilblains have been reported. However, in most cases, RT-PCR or serology did not confirm SARS-CoV-2 infection. Hypotheses have been raised about an interferon-mediated immunological response to SARS-CoV-2, leading to effective clearance of the SARS-CoV-2 without the involvement of humoral immunity. Our objective was to explore the association between chilblains and exposure to SARS-CoV-2.
Methods
In this multicentre case–control study, cases were the 102 individuals referred to five referral hospitals for chilblains occurring during the first lockdown (March to May 2020). Controls were recruited from healthy volunteers' files held by the same hospitals. All members of their households were included, resulting in 77 case households (262 individuals) and 74 control households (230 individuals). Household exposure to SARS-CoV-2 during the first lockdown was categorized as high, intermediate or low, using a pre-established algorithm based on individual data on symptoms, high-risk contacts, activities outside the home and RT-PCR testing. Participants were offered a SARS-CoV-2 serological test.
Results
After adjustment for age, the association between chilblains and viral exposure was estimated at OR 3.3, 95% CI (1.4–7.3) for an intermediate household exposure, and 6.9 (2.5–19.5) for a high household exposure to SARS-CoV-2. Out of 57 case households tested, six (11%) had positive serology for SARS-CoV-2, whereas all control households tested (n = 50) were seronegative (p = 0.03). The effect of potential misclassification on exposure has been assessed in a bias analysis.
Discussion
This case–control study demonstrates the association between chilblains occurring during the lockdown and household exposure to SARS-CoV-2.
Keywords: Acral manifestations, Chilblains, COVID-19, Pernio, Perniosis, SARS-CoV-2
Introduction
In the early months of the COVID-19 pandemic, the emergence of acral lesions, similar to classic chilblains, has been reported worldwide [[1], [2], [3], [4], [5], [6], [7], [8], [9]]. As an outbreak of chilblains is extremely unusual, especially during the spring season and in areas with a mild climate, they were rapidly attributed to COVID-19 under the term ‘COVID toes’ [1,2,4,10]. However, the association between SARS-CoV-2 and chilblains could not be ascertained, due to negative tests for SARS-CoV-2 infection, whether direct (RT-PCR) or indirect (serology), in the large majority of patients with chilblains. Instead, non-viral hypotheses arose: lifestyle changes associated with community lockdown (changes in physical activity, unsuitable footwear, etc.) [3,9,[11], [12], [13], [14], [15]], or a surveillance bias during this anxiogenic pandemic [3].
Several hypotheses have been raised regarding an immunological rationale for SARS-CoV-2 as a trigger for chilblains. Some researchers have suggested that chilblains could result from a strong interferon response, as seen in genetic interferonopathies. The interferon response could lead to rapid clearance of SARS-CoV-2, without the involvement of humoral immunity, explaining the negative SARS-CoV-2 serology in most patients [8,[16], [17], [18]].
To address the controversy, we conducted a multicentre case–control study in western France to estimate the association between chilblains occurring during the first lockdown (17 March to 11 May 2020) and household exposure to SARS-CoV-2 within confined homes. We used an algorithm based on self-reported features related to SARS-CoV-2 exposure rather than serological tests, because the underlying pathophysiological hypothesis implies low sensitivity of serology to document SARS-CoV-2 exposure.
Materials and methods
Overview of the study
This multicentre case–control study took place in western France. Individuals who developed chilblains during lockdown and individuals who had been confined with them (case households) were questioned in June and July 2020 about their exposure to SARS-CoV-2 during the first lockdown (17 March to 11 May 2020). For the control group, individuals from households where no one developed chilblains (control households) were questioned using the same questionnaire covering the same period. Household exposure to SARS-CoV-2, assessed by self-reported information on the questionnaire, was compared between case households and control households. All individuals from these households were offered a COVID-19 serological test.
Participants
Individuals with suspicion of chilblains were referred by dermatologists and general practitioners from western France to the Dermatology departments of five referral hospitals (Rennes, Brest, Nantes, Angers and Tours) covering three French regions (Bretagne, Pays de la Loire and Centre Val de Loire). The diagnosis of chilblains was established by a dermatologist in the presence of localized erythema and swelling involving acral sites, persistent for more than 24 hours [19]. All individuals who had chilblains during the first COVID-19 wave in France (March to May 2020) were eligible to participate as cases, whether or not they had had similar lesions in the past. French referral hospitals hold healthy volunteers' files to recruit individuals for clinical research. The above-mentioned hospitals used these files to recruit the controls, so that the individuals would have consulted in the same settings as the cases if they had had similar skin lesions. All controls were asked to check a series of images of chilblains, in order to exclude them if any reported they might have had such lesions during the lockdown. Each individual enrolled (whether case or control) was to get in touch with each person he/she was confined with to complete the questionnaire. Individuals confined together during the lockdown defined the households. Individuals living alone were included in a sensitivity analysis.
Exposure to SARS-CoV-2
Household exposure to SARS-CoV-2 was assessed by processing information from each household member, using an algorithm established before data acquisition by consensus between investigators (including dermatologists, a virologist (V.T.), an infectious disease specialist (P.T.) and an epidemiologist (E.O.)). As a first step, the algorithm determined the individual level of risk for each household member of promoting SARS-CoV-2 circulation at home. Individual risk was classified as high if the household member had RT-PCR-proven COVID-19, specific symptoms (i.e. anosmia or ageusia), or had had unprotected and prolonged contact with a person diagnosed with COVID-19. Individual risk was classified as intermediate in case of other symptoms (fever, asthenia, rhinitis, sore throat, cough, dyspnoea), other types of contact (protected or short-lived) with a person diagnosed with COVID-19, or regular out-of-home activity during lockdown. Individual risk was classified as low otherwise (asymptomatic household member who had no contact with a COVID-19-infected individual and no regular out-of-home activity). As a second step, the algorithm classified household exposure to SARS-CoV-2 into three ordered categories, according to the individual risk obtained for each household member: a household with at least one high-risk household member was considered as having a high level of household exposure to SARS-CoV-2; a household composed exclusively of low-risk members was considered as having a low level of household exposure to SARS-CoV-2. Household exposure to SARS-CoV-2 was considered as intermediate otherwise (i.e., at least one intermediate-risk member, and no one at high risk). The algorithm is presented in Table S1.
SARS-CoV-2 serological testing
A SARS-CoV-2 serological test was offered to all the members of each household, and performed between 21 July 2020 and 19 October 2020. Laboratory methods are detailed in the Supplementary data 1.
Statistical analyses
The association between chilblains occurring during lockdown and household exposure to SARS-CoV-2 was based on OR and 95% CI estimation. The analysis was first stratified on age, then adjusted for age. Age was divided into quartiles in the main analysis, and modelled using a spline function in a sensitivity analysis. The analysis was also stratified and adjusted for the number of individuals confined together. Two additional sensitivity analyses were conducted, one including single-individual households, the other excluding households with a past history of chilblains. The statistical methods are detailed in the Supplementary data 2.
Research ethics
The study was approved on 25 June 2020 by the Comité de Protection des Personnes Ile de France III for the authorization to perform the serological tests (ref. CNRIPH 20.06.17.34600), and by the CHU Rennes's Ethics Committee on June 24th for the questionnaire (ref. 20.79).
Results
Participants
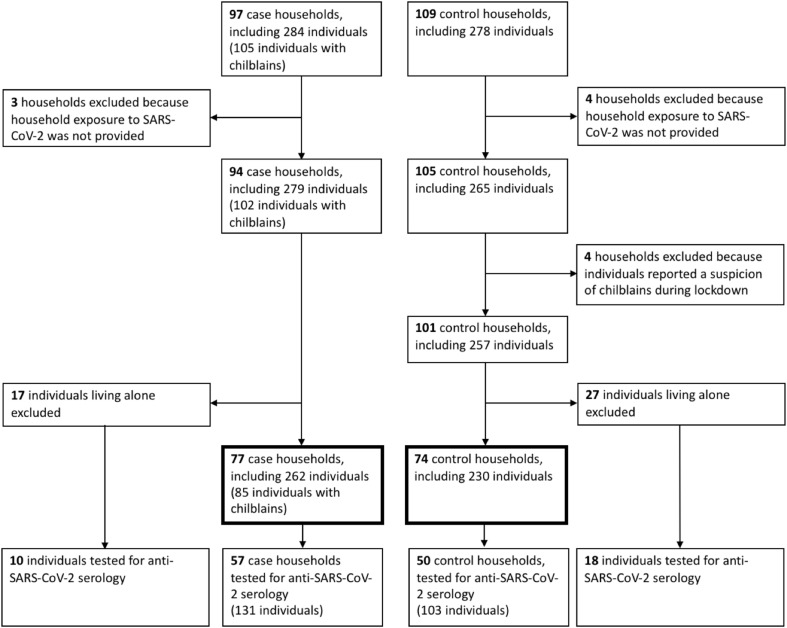
All individuals referred for chilblains answered the questionnaire. Out of 677 individuals included in the healthy volunteer files of the above-mentioned hospitals, 109 (16.1%) answered the questionnaire. Three case and four control households were excluded because of incomplete questionnaires. Four additional control households were excluded because they reported having had lesions resembling chilblains during the lockdown. A total of 94 case households and 101 validated control households were thus included. Single-person households (17 cases and 27 controls) were excluded for the main analysis, leaving 77 case households (262 individuals) and 74 control households (230 individuals) (Fig. 1 ).
Fig. 1.
Study flowchart.
Characteristics of individuals with chilblains
Eighty-five individuals had chilblains, eight case households including several affected individuals. Fifty-three (62.4%) were women, the median age was 24 (range 12–70). Chilblains were located on the feet (75.3%), hands (11.8%) or both (12.9%). The median duration of lesions was 33 days (range 4–143). Eighteen patients (21.2%) reported a past history of chilblains, with less severe previous episodes in all these patients. Ten (11.8%) reported a previous Raynaud phenomenon. Only three patients reported exposure to cold before the occurrence of the lesions.
Characteristics of households
The composition of the households and the conditions of lockdown are described in Table 1 . Case and control households did not differ for their main characteristics, except that they were not evenly distributed across the three regions (all in western France).
Table 1.
Characteristics of case and control households
| Case households n = 77 | Control households n = 74 | p | |
|---|---|---|---|
| Age of the youngest individual in the household in years, median (range) | 17 (1–69) | 25 (2–72) | 0.10 |
| Age of the oldest individual in the household in years, median (range) | 49 (23–70) | 47 (25–94) | 0.33 |
| Proportion of men, median (range) | 50 (0–100) | 50 (0–100) | 0.38 |
| Geographical area, n (%) | <10−5 | ||
| Bretagne | 50 (64.9) | 18 (24.3) | |
| Centre Val de Loire | 7 (9.1) | 26 (35.1) | |
| Pays de la Loire | 18 (23.4) | 23 (31.1) | |
| Not specified | 2 (2.6) | 7 (9.5) | |
| House (vs. apartment), n (%) | 57 (74.0) | 47 (63.5) | 0.16 |
| Home surface area in m2, n (%) | 0.21 | ||
| <50 | 5 (6.5) | 7 (9.5) | |
| 50–100 | 26 (33.8) | 30 (40.5) | |
| 100–150 | 26 (33.8) | 22 (29.7) | |
| >150 | 20 (26.0) | 15 (20.3) | |
| Number of individuals confined, n (%) | 0.16 | ||
| 2 | 25 (32.5) | 34 (45.9) | |
| 3–4 | 41 (53.2) | 30 (40.5) | |
| >4 | 11 (14.3) | 10 (13.5) |
SARS-CoV-2 household exposure
Across the case households, 23 (29.9%), 39 (50.6%) and 15 (19.5%) respectively were classified as having high, intermediate and low level of household exposure to SARS-CoV-2. Across the control households, 9 (12.2%), 33 (44.6%) and 32 (43.2%) respectively were classified as having high, intermediate and low level of household exposure to SARS-CoV-2. Symptoms (fever, asthenia, rhinitis, sore throat, cough, dyspnoea), and a prolonged unprotected contact with an individual diagnosed with COVID-19 were significantly more often reported in case households than in control households (Table 2 ).
Table 2.
Features used to define exposure to SARS-CoV-2 in cases and controls, at both household and individual levels
| Cases |
Controls |
Comparison of households, p | |||
|---|---|---|---|---|---|
| Householdsan = 77 | Individuals n = 262 | Householdsan = 74 | Individuals n = 230 | ||
| Symptoms | |||||
| Anosmia, ageusia | 10 (13.0) | 16 (6.1) | 4 (5.4) | 4 (1.7) | 0.16 |
| Other symptomsb | 41 (53.2) | 59 (22.5) | 15 (20.3) | 22 (9.6) | <10−3 |
| Contact with a person diagnosed with COVID-19 | |||||
| Any contact | 30 (39.0) | 40 (15.3) | 22 (29.7) | 29 (12.6) | 0.30 |
| Prolonged contact without protection | 18 (23.4) | 21 (8.0) | 8 (10.8) | 13 (5.7) | 0.05 |
| Activities outside the home | |||||
| Healthcare workers | 19 (24.7) | 25 (9.5) | 12 (16.2) | 14 (6.1) | 0.23 |
| Other regular activities outside the home | 31 (40.3) | 43 (16.4) | 25 (33.8) | 36 (15.7) | 0.50 |
| RT-PCR testc | |||||
| Positive | 2 (2.6) | 2 (0.8) | 0 | 0 | — |
| Tested | 6 (7.8) | 6 (2.3) | 2 (2.7) | 2 (0.9) | — |
Features of at least one individual in the household.
Among fever, asthenia, rhinitis, sore throat, cough, dyspnoea.
Period when RT-PCR testing was restricted to hospitalized patients or health care workers.
Association between chilblains and level of household exposure to SARS-CoV-2
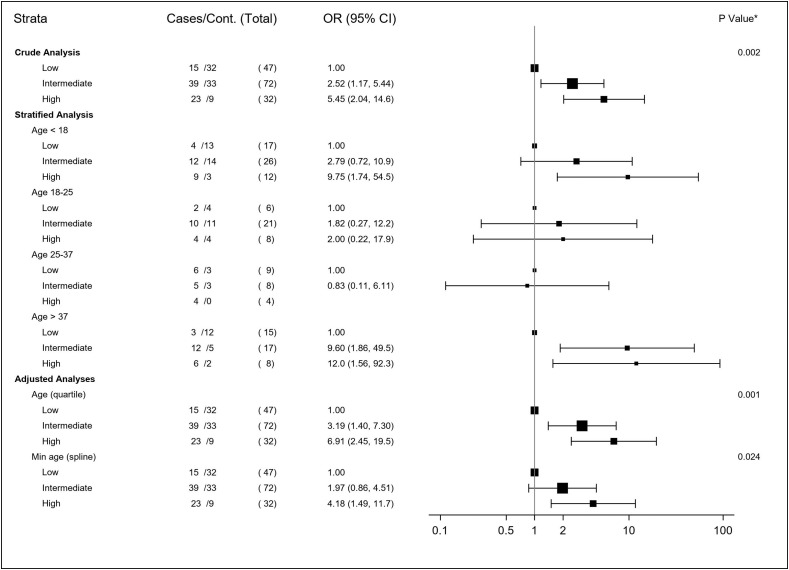
The univariate analysis showed a significant association between chilblains and the level of household exposure to SARS-CoV-2, with a crude OR (95% CI) of 2.5 (1.2–5.4) for an intermediate level of exposure, and 5.5 (2.0–14.6) for a high level of exposure. After adjustment for age, the association was estimated at 3.3 (1.4–7.3) for an intermediate level of household exposure and 6.9 (2.5–19.5) for a high level of household exposure (Fig. 2 ). The homogeneity of the effect of viral exposure across the four age subgroups was not rejected (p for interaction 0.54). The results of the stratified analysis on the four age subgroups and the sensitivity analysis selecting the minimum age within households are presented on the forest plot (Fig. 2). When individuals living alone were included, the results were similar, with an adjusted OR of 2.4 (1.2–4.6) for an intermediate level of exposure, and 5.8 (2.4–14.5) for a high level of exposure. When households with a past history of chilblains were excluded, the adjusted OR was 3.4 (1.3–8.8) for an intermediate level of exposure, and 7.7 (2.3–25.7) for a high level of exposure.
Fig. 2.
Forest plot of the analyses estimating the association between chilblains and exposure to SARS-CoV-2 within households.
The stratified, crude and adjusted analyses taking into account the number of individuals confined together did not show substantially different results (Fig. S1).
SARS-CoV-2 serological tests
Out of 57 tested case households, six (10.5%) had at least one SARS-CoV-2 seropositive household member, whereas out of 50 tested control households, none (0%) had a seropositive member (p = 0.03) (Table 3 ). Among the six seropositive case households (17 seropositive individuals), three individuals with chilblains had SARS-CoV-2 antibodies, while in the other three households, all members were seropositive except the individual with chilblains. All seropositive households were categorized at a high level of exposure to SARS-CoV-2 following our algorithm. Among the individuals with serology but excluded because they were living alone (10 individuals with chilblains and 18 without), only one individual, without chilblains, was positive (Table 3).
Table 3.
Anti-SARS-CoV-2 serology in case and control households
| Case households |
Control households |
pa | |||
|---|---|---|---|---|---|
| n = 57 | n = 67b | n = 50 | n = 68 b | ||
| Positive serology | 6 (10.5%) | 6 (9.0%) | 0 (0%) | 1 (1.5%) | 0.03 |
Comparison of households included in the main analysis and who were tested for serology (57 case and 50 control households), using Fisher's exact test.
Including individuals living alone.
Discussion
In this case–control study comparing 77 case households with 74 control households, we observed a strong, significant and proportional association between chilblains occurring during the lockdown and household exposure to SARS-CoV-2. This result is consistent with another case–control study based on serological tests [20].
The association between chilblains during the lockdown and exposure to SARS-CoV-2 within households is an important finding. While numerous reports of chilblain-like cases emerged throughout Europe and the United States during the first lockdown, the proportion of virologically confirmed SARS-CoV-2 infections was generally low, and discordant across studies [[2], [3], [4],[6], [7], [8], [9]], while the absence of a reference group without chilblains hampered the interpretation.
The main strength of our study is the comparison with a control group. Although we identified high exposure to SARS-CoV-2 in only 30% of the case households, the case–control design highlighted the fact that high exposure was about seven times more frequent among case than among control households. In addition, the consistency of the results across age subgroups, with both statistical methods taking age into account, the evidence for a dose effect for chilblains according to the level of viral exposure, and the more frequent seropositivity among case households than control households (10.5 vs. 0%) are additional arguments to support the validity and robustness of our results.
All individuals with chilblains had a diagnostic validation by a dermatologist. The multicentre recruitment ensured the diversity and representativeness of the cases. These cases were similar to those reported in several case series [[2], [3], [4], [5], [6], [7], [8], [9]].
The controls were unevenly recruited across western France, but the area was overall moderately affected by the COVID-19 pandemic, the highest incidence of COVID-19 in France during spring 2020 being located in Ile-de-France (Paris area) and Grand-Est (northeast of France) [21]. While we were initially concerned about a selection bias in the inclusion of controls towards individuals who might have been more exposed and seeking a confirmation of their infection by serological testing, our results rule out the possibility of an overestimation of seropositivity among the controls as none was positive. We used healthy volunteers' files from the same referral hospitals where cases were recruited to limit selection bias. Regarding a possible underestimation of the seropositivity rate, the prevalence of SARS-CoV-2 infection in this area of France was estimated at 1.7% (weighting the prevalence from the three regions of interest by the number of cases originating from these regions) [21]. Among the 68 control households tested (including individuals living alone), 1.18 positive serology was expected and a value of 1 was observed. Although seropositivity was not the main outcome in this study, these findings suggest that we selected a fairly unbiased control group.
Our study has limitations. First, our main outcome was based on self-reported items. Hence, exposure misclassification may have occurred. Non-differential misclassification, affecting both cases and controls, may have occurred for viral symptoms that are not specific to SARS-CoV-2. However, these were only used to determine the intermediate level of SARS-CoV-2 exposure (Table S1). In addition, protective measures during the lockdown resulted in a low incidence of other respiratory viruses. Also, past exposures may be more readily recalled by cases, possibly because of repeated questioning by physicians, leading to a differential misclassification. However, the OR would remain significant, even with a 20% misclassification rate. False-positive and false-negative rates among case households, control households or both were simulated in the bias analysis in Table S2 [22]. Furthermore, a significant association was also observed for objective criteria not subject to misclassification, such as serology results. Second, asymptomatic cases make analyses of intra-household transmission unreliable, and this is the reason we considered the household as a whole for the entire duration of the lockdown. Third, residual confounding is possible. For example, urban and rural living area was not specified in the questionnaire and could thus not be taken into account in the analysis. Last, seropositivity could have been underestimated in our study, because serologies were performed 2–5 months after the study period, and anti-nucleoprotein antibodies may wane within months [23]. However, antibody waning would have affected case and control households similarly, which would have led to an underestimation of the association [24,25].
Several issues remain regarding the relationship between chilblains and SARS-CoV-2. Among them, one can wonder whether SARS-CoV-2 exposure acts as a stimulus for a flare (a first or a subsequent one) among subjects with an immuno-genetic background predisposing to chilblains, or whether SARS-CoV-2 is an independent cause of chilblains; whether any other clusters of chilblains have occurred following anti-SARS-CoV-2 vaccination, since eight cases have been reported in a registry-based study [26]; and on practical grounds, what is the medium- to long-term evolution of patients with chilblains occurring during the first lockdown, given that persistent symptoms following COVID-19 are increasingly being described [[27], [28], [29], [30], [31], [32], [33]]. Persistent systemic and acral manifestations among the individuals with chilblains included in this study have been described in another article [34].
Conclusion
This comparative multicentre case–control study demonstrates an association between chilblains during lockdown and exposure to SARS-CoV-2 within confined households.
Transparency declaration
Mahtab Samimi has received reimbursement for travel and/or accommodation expenses for attending medical meetings from Bristol Myers Squibb, Lilly, Galderma International, Janssen, AbbVie, MSD, outside the submitted work. Alain Dupuy reports reimbursement for travel and/or accommodation expenses for attending medical meetings from Sanofi, and personal fees from Sanofi and Leo Pharma, outside the submitted work.
Funding: This work was supported by a grant from the Programme Hospitalier de Recherche Clinique Interrégional (Ministry of Health, France) GIRCI Grand-Ouest.
Trial registration
ClinicalTrials.gov Identifier NCT04455308.
Author contributions
Substantial contribution to study concept and design: F.P., E.O., S.B., Y.L.C., M.S., E.B., C.L., L.Ma., L.Mi., A.D. Substantial contribution to the acquisition of data: F.P., S.B., Y.L.C., M.S., E.B., H.A., V.G., C.H., A.T., V.T., A.D. Substantial contribution to the statistical analysis or interpretation of data: F.P., E.O., S.B., Y.L.C., M.S., E.B., E.C., C.D., C.H., C.L., P.T., V.T., A.D. Drafting the manuscript: F.P., A.D. Critical review of the manuscript for important intellectual content and final approval for publication: all authors.
Acknowledgements
Institut Dermatologique du Grand Ouest (IDGO), as the institutional framework for developing and conducting the study.
Additional contributions: Loïc Fin and Valérie Visseiche (CHU Rennes) supervised the administrative part of the study. Sabrina Nekmouche-Messis helped in elaborating the online questionnaire. Marion Jacob (CHU Rennes), Antoine Boulay, Anne Lefebvre (CHU Nantes), Hélène Humeau, Chloé Blondin (CHU Angers), Helene Blansard (CHU Tours), Sonia Haddad (CHU Brest) helped to collect the data. Angela Verdier, MA, and Sarah Leyshon, MA, SARL L'Auracoise, revised the article for the English language. They received compensation for their contribution.
Editor: Luigia Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.09.032.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Recalcati S., Barbagallo T., Frasin L.A., Prestinari F., Cogliardi A., Provero M.C., et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346–e347. doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman E., McMahon D., Lipoff J., Rosenbach M., Kovarik C., Takeshita J., et al. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le Cleach L., Dousset L., Assier H., Fourati S., Barbarot S., Boulard C., et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866–874. doi: 10.1111/bjd.19377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A., Moreno-Arrones O.M., Saceda-Corralo D., Arana-Raja A., et al. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83:e61–e63. doi: 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Masson A., Bouaziz J., Sulimovic L., Cassius C., Jactiez M., Ionescu M., et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández-Nieto D., Rodríguez-Villa Lario A., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccolo V., Neri I., Filippeschi C., Oranges T., Argenziano G., Battarra V.C., et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34:e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubiche T., Cardot-Leccia N., Le Duff F., Seitz-Polski B., Giordana P., Chiaverini C., et al. Clinical, Laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202–206. doi: 10.1001/jamadermatol.2020.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman A., Peeters C., Verroken A., Tromme I., Tennstedt D., Marot L., et al. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156:998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hachem M., Diociaiuti A., Concato C., Carsetti R., Carnevale C., Ciofi Degli Atti M., et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:2620–2629. doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeck M., Herman A. Emerging evidence of the direct association between COVID-19 and chilblains – Reply. JAMA Dermatol. 2021;157:239–240. doi: 10.1001/jamadermatol.2020.4655. [DOI] [PubMed] [Google Scholar]
- 12.Caselli D., Chironna M., Loconsole D., Aricò M. Response to “No evidence of SARS-CoV-2 infection by polymerase chain reaction or serology in children with pseudo-chilblain”. Reply from the authors. Br J Dermatol. 2020;183:1156–1157. doi: 10.1111/bjd.19563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca-Ginés J., Torres-Navarro I., Sánchez-Arráez J., Abril-Pérez C., Sabalza-Baztán O., Pardo-Granell S., et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156:992. doi: 10.1001/jamadermatol.2020.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeck M., Peeters C., Herman A. Chilblains and COVID-19: further evidence against a causal association. J Eur Acad Dermatol Venereol. 2021;35:e2–e3. doi: 10.1111/jdv.16901. [DOI] [PubMed] [Google Scholar]
- 15.McCleskey P.E., Zimmerman B., Lieberman A., Liu L., Chen C., Gorouhi F., et al. Epidemiologic analysis of chilblains cohorts before and during the COVID-19 pandemic. JAMA Dermatol. 2021;157:947–953. doi: 10.1001/jamadermatol.2021.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magro C.M., Mulvey J.J., Laurence J., Sanders S., Crowson A.N., Grossman M., et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184:141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsker D. Paraviral eruptions in the era of COVID-19: do some skin manifestations point to a natural resistance to SARS-CoV-2? Clin Dermatol. 2020;38:757–761. doi: 10.1016/j.clindermatol.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damsky W., Peterson D., King B. When interferon tiptoes through COVID-19: pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83:e269–e270. doi: 10.1016/j.jaad.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappel J.A., Wetter D.A. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207–215. doi: 10.1016/j.mayocp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Ortega-Quijano D., Fernandez-Nieto D., Jimenez-Cauhe J., Cortes-Cuevas J.L., Marcos-Mencia D., Rodriguez-Dominguez M., et al. Association between COVID-19 and chilblains: a case-control study. J Eur Acad Dermatol Venereol. 2021;35:e359–e361. doi: 10.1111/jdv.17195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salje H., Tran Kiem C., Lefrancq N., Courtejoie N., Bosetti P., Paireau J., et al. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson C.Y., Flanders W.D., Strickland M.J., Honein M.A., Howards P.P. Potential sensitivity of bias analysis results to incorrect assumptions of nondifferential or differential binary exposure misclassification. Epidemiology. 2014;25:902–909. doi: 10.1097/EDE.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumley S.F., Wei J., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73:e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcomer S.R., Kulldorff M., Xu S., Daley M.F., Fireman B., Lewis E., et al. Bias from outcome misclassification in immunization schedule safety research. Pharmacoepidemiol Drug Saf. 2018;27:221–228. doi: 10.1002/pds.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberg C.R., Umbach D.M., Greenland S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am J Epidemiol. 1994;140:565–571. doi: 10.1093/oxfordjournals.aje.a117283. [DOI] [PubMed] [Google Scholar]
- 26.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon D.E., Gallman A.E., Hruza G.J., Rosenbach M., Lipoff J.B., Desai S.R., et al. Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis. 2021;21:313–314. doi: 10.1016/S1473-3099(20)30986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., á Steig B., Gaini S., et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1792. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salmon-Ceron D., Slama D., De Broucker T., Karmochkine M., Pavie J., Sorbets E., et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect. 2021;82:e1–e4. doi: 10.1016/j.jinf.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghadam P., Frumholtz L., Jaume L., De Masson A., Jachiet M., Begon E., et al. Frequency of relapse and persistent cutaneous symptoms after a first episode of chilblain-like lesion during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2021;35:e566–e568. doi: 10.1111/jdv.17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poizeau F., Barbarot S., Le Corre Y., Brenaut E., Samimi M., Aubert H., et al. The long-term outcome of patients with chilblains associated with SARS-CoV-2. Acta Derm Venereol. 2021 doi: 10.2340/00015555-3930. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.