To the editor:
Patients with end-stage renal disease (ESRD) develop inefficient immune responses upon vaccination and have a high risk of developing severe coronavirus disease 2019 (COVID-19). The globally expanding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant of concern (VOC), B.1.6.17.2/Delta, evades immune responses and might constitute a particular threat to these patients.1, 2, 3
Herein, we evaluated the efficacy of a third dose (second boost) by BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) mRNA vaccines (Supplementary Table S1 and Supplementary Figure S1) in ESRD patients with no response/low response (NR/LR) after prime-boost BNT162b2 vaccination and compared with ESRD with high response (HR) following the regular prime-boost vaccination. Enzyme-linked immunosorbent assay, pseudovirus neutralization assay, and flow cytometry were applied to assess humoral and cellular immunity against the spike (S) protein of SARS-CoV-2 wild type (WT-S) and the Delta-VOC (Delta-VOC-S) before and 3 to 5 weeks following the last booster vaccination.
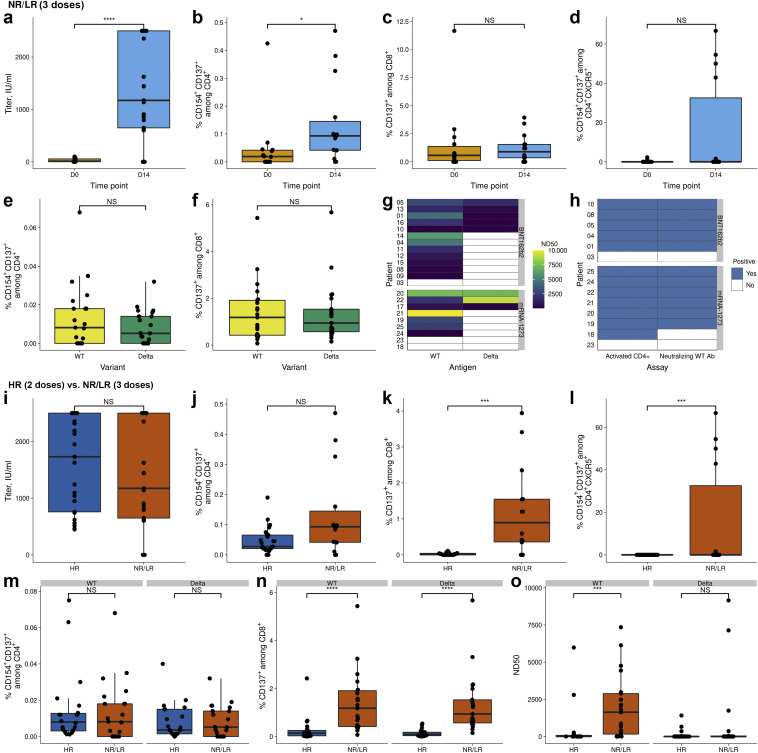
In NR/LR, 20 of 23 patients developed high-binding WT-S antibody titers (Figure 1 a and Supplementary Figure S2A), with neutralizing capacity in 19 of 22 patients. The third vaccination led to an increase in WT-S protein-reactive CD4+ T cells (Figure 1b) without differences between the applied vaccines (Supplementary Figure S2B and C). The higher frequency of S-reactive T follicular helper (Tfh) cells was the only difference observed in mRNA-1273–boosted patients (Supplementary Figure S2D).
Figure 1.
The effect of a third severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination boost. Patients with no response/low response (NR/LR) after a regular prime-boost SARS-CoV-2 mRNA vaccination (BNT162b2; Pfizer–BioNTech) scheme were given a second boost (3 doses). Patients with antibody titres >250 IU/ml after the first boost served as control group (high response [HR], 2 doses). (a–h) Comparison within NR/LR. (a) Antibody titers before and 3 to 5 weeks after the second SARS-CoV-2 mRNA vaccine boost. (b–d) Analysis of vaccine-reactive T-cell immunity following stimulation with SARS-CoV-2 Spike (S)-protein overlapping peptide pools. (b) The frequency of antigen-specific CD4+ T cells. (c) The frequency of antigen-specific CD8+ T cells. (d) The frequency of activated T follicular helper (Tfh) cells, as defined by CXC chemokine receptor 5 (CXCR5) expression. (e,f) Analysis of T-cell immunity following stimulation with (Delta-variant of concern [VOC]–S) peptides (Delta) and corresponding peptides from wild type (WT-S; Wuhan-1 isolate). (e) The frequency of antigen-specific CD4+ T cells. (f) The frequency of antigen-specific CD8+ T cells. (g) A comparison of neutralizing antibodies against pseudoviruses bearing WT-S or Delta-VOC-S. (h) The correlation between the activation of CD4+ T cells and neutralization. White indicates no detection of humoral (antibody) or cellular (T-cell) immunity. (i–o) A comparison between HR (2 doses) and NR/LR (3 doses). (i) Antibody titers 3 to 5 weeks after the second SARS-CoV-2 mRNA vaccine boost. (j,k) Analysis of vaccine-reactive T-cell immunity following stimulation with SARS-CoV-2–S-protein overlapping peptide pools. (j) The frequency of antigen-specific CD4+ T cells. (k) The frequency of antigen-specific CD8+ T cells. (l) The frequency of activated Tfh cells, as defined by CXCR5 expression. (m–o) The analysis of T-cell immunity following stimulation with Delta-VOC-S peptides (Delta) and the corresponding peptides from WT-S (Wuhan-1 isolate). (m) The frequency of antigen-specific CD4+ T cells. (n) The frequency of antigen-specific CD8+ T cells. (o) Neutralizing antibodies against pseudoviruses bearing WT-S or Delta-VOC-S. The box plots indicate the 75th, 50th, and 25th quantiles, and the whiskers indicate 1.5× the interquartile range. ∗P ≤ 0.05, ∗∗∗P ≤ 0.001, ∗∗∗∗P ≤ 0.0001. D0, day 0; D14, day 14; ND50, 50% neutralization dose; NS, not significant.
Cellular immunity against WT-S and Delta-VOC-S was comparable irrespectively of helper or cytotoxic T cells or vaccine type (Figure 1e and f and Supplementary Figure S2E and F). In contrast, only 8 had neutralizing antibodies against Delta-VOC-S (Figure 1g). A clear association between cellular and humoral immunity was observed for each patient (Figure 1h). More important, when comparing the data obtained from NR/LR following the third dose with ESRD HR after the second dose, overall, superior results in cellular immunity and WT neutralizing capacity were observed. Although S-binding antibody titers and S-reactive CD4+ T cells were comparable between both cohorts (Figure 1i and j), WT neutralizing capacity and S-WT– and Delta-reactive CD8+ T cells and S-reactive Tfh cells were significantly higher in NR/LR after the second booster (third dose) compared with HR requiring only 1 booster (2 doses; Figure 1g, h, n, and o).
Our data demonstrate that patients with ESRD can benefit from a second vaccination boost by improving their cellular and humoral immunity not only to the vaccination-specific strain but also against the globally expanding Delta-VOC.
Footnotes
Supplementary Methods.
Figure S1. Comparison of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adaptive immunity to the wild-type (WT) variant or Delta variant of concern (VOC) in hemodialysis patients requiring a second vaccine booster. Patients with no/low titers after a regular prime-boost SARS-CoV-2 mRNA vaccination (BNT162b2; Pfizer–BioNTech) scheme were given a second boost (2 boosts). (A) Antibody titers before and 3 to 5 weeks after the final SARS-CoV-2 mRNA vaccine boost. (B–D) Analysis of vaccine-reactive T-cell immunity following stimulation with SARS-CoV-2 spike (S)-protein overlapping peptide pools. (B) Frequency of SARS-CoV-2 S-reactive CD4+ T cells. (C) Frequency of SARS-CoV-2 S-reactive CD8+ T cells. (D) Frequency of SARS-CoV-2 S-reactive Tfh cells as defined by CXC chemokine receptor 5 (CXCR5) expression. (E,F) Analysis of T-cell immunity following stimulation with Delta-VOC–S peptides (Delta) and corresponding peptides from WT-S (Wuhan-1 isolate). (E) The frequency of antigen (WT or Delta)–reactive CD4+ T cells. (F) Frequency of WT or Delta–reactive CD8+ T cells. The box plots indicate the 75th, 50th, and 25th quantiles, and the whiskers indicate 1.5× the interquartile range.
Figure S2. Gating strategy to identify spike (S)-protein reactive T cells. Peripheral blood mononuclear cells (PBMCs) were incubated for 16 hours with overlapping peptide pools (OPPs) of the complete severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wild-type (WT) S-protein. Brefeldin A was added after 2 hours. The reactivity to the Delta variant of concern (VOC) mutations was evaluated using OPP peptides covering the Delta VOC mutations and the corresponding WT peptides. Stimulation with peptide diluent and Staphylococcus aureus enterotoxin B (SEB) as polyclonal stimulus served as negative and positive controls, respectively. Cells were acquired using a Cytoflex flow cytometer.
Table S1. Patient characteristics. Patients with no/low titers after a regular prime-boost severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination (BNT162b2; Pfizer–BioNTech) scheme were given a second boost. Patients not requiring this additional boost (binding antibody titers > 250 IU/ml) serve as a control group (1 boost).
Supplementary References.
Supplementary Material
References
- 1.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thieme C, Blazquez-Navarro A, Safi L, et al. Impaired humoral but substantial cellular immune response to variants of concern B1.1.7 and B.1.351 in hemodialysis patients after vaccination with BNT162b2. J Am Soc Nephrol, in press. [DOI] [PMC free article] [PubMed]
- 3.Blazquez-Navarro A., Safi L., Meister T.L., et al. Superior cellular and humoral immunity toward SARS-CoV-2 reference and alpha and beta VOC strains in COVID-19 convalescent as compared to the prime boost BNT162b2-vaccinated dialysis patients. Kidney Int. 2021;100:698–700. doi: 10.1016/j.kint.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.