Abstract
Despite the burgeoning field of coronavirus disease-19 (COVID-19) research, the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralising antibodies remains unclear. This study validated two high-throughput immunological methods for use as surrogate live virus neutralisation assays and employed them to examine the half-life of SARS-CoV-2 neutralising antibodies in convalescent plasma donations made by 42 repeat donors between April and September 2020. SARS-CoV-2 neutralising antibody titres decreased over time but typically remained above the methods' diagnostic cut-offs. Using this longitudinal data, the average half-life of SARS-CoV-2 neutralising antibodies was determined to be 20.4 days. SARS-CoV-2 neutralising antibody titres appear to persist in the majority of donors for several months. Whether these titres confer protection against re-infection requires further study and is of particular relevance as COVID-19 vaccines become widely available.
Keywords: SARS-CoV-2, COVID-19, Neutralising antibodies, Half-life, Convalescent donor
Abbreviations: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; CPE, cytopathic effects; FcRn, neonatal Fc receptor; IgG, immunoglobulin G; MN, micro-neutralisation; nAb, neutralising antibody; NC, nucleocapsid; PPPT, post-positive PCR test; S1, spike 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. Background
As of mid-2021, the ongoing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in more than four million deaths worldwide [1] and continues to threaten the health and livelihood of millions. Scientific investigation continues apace to elucidate the mechanisms underlying the immune response in order to combat coronavirus disease-19 (COVID-19).
Insight into the persistence of SARS-CoV-2 neutralising antibodies (nAbs) in the plasma of convalescent individuals recovered from COVID-19 is critical for understanding population sero-prevalence and -protection, particularly as vaccines become increasingly available. While several studies have been undertaken to investigate this, an unclear picture remains as to whether antibody levels remain constant after infection [[2], [3], [4], [5], [6]] or decline substantially [[7], [8], [9], [10], [11], [12]]. This may in part be explained by the limited quantitative capacity of diagnostic assays developed rapidly over the past year [13], leading to challenges in the interpretation of results. Furthermore, sustained and regular follow-up testing of convalescent individuals over several months presents logistical obstacles that ultimately limit the granularity in which antibody persistence may be assessed.
In order to overcome these challenges and contribute to the understanding of SARS-CoV-2 nAb durability in plasma, this study aimed to validate two high-throughput immunological methods as surrogate assays for live virus neutralisation, and to subsequently use these methods to assess the half-life of SARS-CoV-2 nAbs in the plasma of individuals who have recovered from COVID-19.
2. Materials and methods
2.1. Human samples
Human plasma donations (N = 714) were made by 42 convalescent individuals with known high titres of SARS-CoV-2 antibodies who had volunteered to donate plasma for an investigational immune globulin which was in development as a potential treatment for COVID-19. Donors were initially identified upon presentation at donation centres across Germany and continental USA by providing an affirmative statement of suffering (but since recovering) from mild COVID-19 and confirming this via presentation of a medical certificate of positive viral PCR or antibody test. Donations were collected between April and September 2020 by the CSL Plasma donation centre network. All donors signed the CSL Behring general consent form for use of their plasma in research. All plasma donations were screened for nucleocapsid (NC) protein-binding IgG using the ARCH SARS-CoV-2 immunoglobulin G (IgG) diagnostic method (Cat. #06R8620, Abbott) run on an Abbott™ ARCHITECT™ Analyser, according to the manufacturer instructions.
2.2. Micro-neutralisation assay
Neutralising antibody titres were tested using a live virus assay as follows: plasma samples were prediluted 2.5-fold in DMEM (#D6546, Sigma), 2% foetal calf serum (#97068-088, VWR), 1% glutamine (#17-605E, Lonza) (inoculation medium), supplemented with citrate-dextrose (#C3821, Sigma) to a final concentration of 25%, followed by 10 serial two-fold dilutions in inoculation medium with 5% citrate-dextrose. Serial dilutions were mixed 1:1 with 1000 TCID50/mL SARS-CoV-2 virus (strain 2019-nCOV/Italy INM1 2nd P VERO E6 11.02.2020 [European Virus Archive GLOBAL (EVA-G)]) and incubated for one hour at 37 °C, 5% CO2. Each dilution was then applied in octuplicate to VERO E6 cells (Pasteur Institute, Molecular virology Lab) seeded at 105 cells/mL in a 96-well plate. Following incubation (1 h at 37 °C, 5% CO2), culture medium was added, and cells incubated for six days under the above conditions. Cells were then inspected for cytopathic effects (CPEs) and the number of plate wells exhibiting CPEs was recorded. The titre at which no CPE was observed in at least half of the octuplicates (50% micro-neutralisation titre, MN50) was then calculated according to the Spearman-Kärber method for each sample.
2.3. Phadia™ EliA™ SARS-CoV-2-Sp1 IgG (EliA S1-IgG) assay
Plasma samples were tested on the Phadia System (ThermoFisher Scientific, Uppsala, Sweden) at 37 °C. SARS-CoV-2 spike 1 (S1) antigen (AA14-681, expressed in mammalian cells) was adsorbed onto irradiated polystyrene EliA wells. Antibodies bound to the S1 antigen were detected fluorometrically with an anti-human IgG-β-galactosidase conjugate and EliA Development Solution (10-9441-01, Phadia). Six different concentrations of purified human IgG, calibrated against World Health Organization (WHO)-IRP67/86, were used to quantify the IgG antibody concentration. Quantification of the novel first WHO international standard anti-SARS-CoV-2 immunoglobulin preparation 20/136 revealed that 1 EliA unit (U)/mL equals 4 International Units per mL (IU/mL). The method is CE approved and received FDA EUA approval in January 2021. For diagnostic purposes and to focus on high specificity, the cut-off of the EliA S1-IgG assay was set at 10 EliA U/mL (lower limit of equivocal zone set to 7 EliA U/mL). Sensitivity was observed at 97.6% (80/82) (95% CI: 91.5–99.3%) >15 days post-symptom onset. Specificity was determined with a set of 340 serum samples collected before December 2019 from healthy blood donors and was observed at 99.4% (338/340) (95% CI: 97.9–99.8%). To address questions depending on high sensitivity rather than on high specificity, including longitudinal studies of patients with a known infection history, the cut-off can be set to 97.9% specificity at 0.7 EliA U/mL, the detection limit of the test. This modification resulted in a sensitivity of >99% in an internal study with 694 longitudinal samples 2–27 weeks post-symptom onset (data not shown).
2.4. Angiotensin-converting enzyme 2 (ACE2) receptor binding inhibition assay
Plasma samples were tested on the Phadia System at 37 °C. Biotinylated S1 antigen was coated on streptavidin-immobilised EliA wells. Antibodies bound to the biotinylated-S1 antigen were detected fluorometrically with a human ACE2 receptor-β-galactosidase conjugate (Phadia) and EliA Development Solution (10-9441-01, Phadia). The ACE2 receptor binding inhibition method follows the principle of a competitive inhibition assay, measuring the inhibition of SARS-CoV-2-ACE2 receptor binding by antibodies present in diluted plasma samples. Six different concentrations of a recombinant neutralising anti-SARS-CoV-2 antibody were used to quantify the antibody concentration. According to the manufacturer's recommendations, antibody values greater than 0.3 μg/mL were considered positive.
2.5. Statistical analysis
GraphPad Prism (v.8.1.1) was used for calculation of the Pearson r correlation co-efficient between both EliA S1-IgG and ACE2 receptor binding inhibition assay methods, and the cell-based neutralisation assay. The software was also used to calculate half-life values for each donor for both EliA S1-IgG and ACE2 receptor binding inhibition assay methods using an unconstrained one-phase exponential decay model. All performance calculations of EliA S1-IgG and ACE2 receptor binding inhibition assay methods were performed using Analyse-it for Microsoft Excel (v.4.60).
3. Results
3.1. Baseline donor demographics
In total, 42 donors who had provided plasma donations 4–35 times from 32 to 168 days post-positive PCR test (PPPT) were selected, with a total of 714 donations analysed (Supplementary Table 1). The median age of donors was 47 years old (range 22–62 years), 61.9% were male and 64.2% of donors made their donations in the United States (Supplementary Table 1). While all selected donors returned a positive screening value using the Abbott ARCHITECT SARS-CoV-2 IgG method at initial donation, by the time of their last donation, approximately 26% of donors tested negative with this assay (Supplementary Fig. 1).
3.2. Validation of immunological assays for use as surrogate live virus neutralisation assays
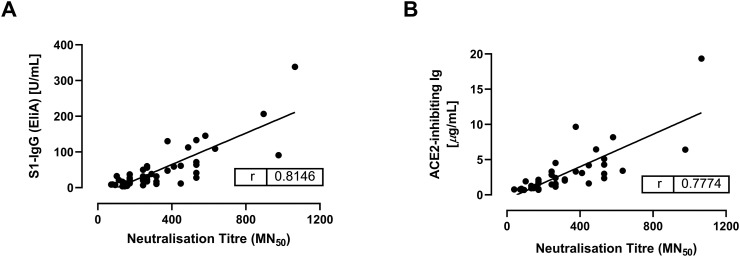
For a robust longitudinal analysis of SARS-CoV-2 antibody titres, we chose two methods designed for use on Phadia fully automated, high-throughput random access serological analysers, namely the EliA S1-IgG and the ACE2 receptor binding inhibition assays. In order to validate these assays as surrogates for live virus neutralisation, we first analysed a subset of 47 samples (from 12 donors with an average of four donations each) using both methods and then correlated the results against those generated with a live virus neutralisation assay for the same samples. We observed a strong positive correlation for both methods with Pearson r values of 0.81 and 0.78 for the EliA S1-IgG and ACE2 receptor binding inhibition assays, respectively (Fig. 1 ).
Fig. 1.
Correlation of the concentration of surrogate nAb titre (assessed by ACE2 receptor binding inhibition assay and EliA S1-IgG methods) against neutralisation titre (assessed by cell-based, live virus neutralisation assay). Each datapoint represents one donation. A linear regression was fitted to each graph.
ACE2, angiotensin-converting enzyme 2; Ig, immunoglobulin; IgG, immunoglobulin G; MN, micro-neutralisation; nAb, neutralising antibody; r, Pearson's r correlation coefficient; S1, spike 1.
3.3. Assessing the longevity of SARS-CoV-2 nAbs
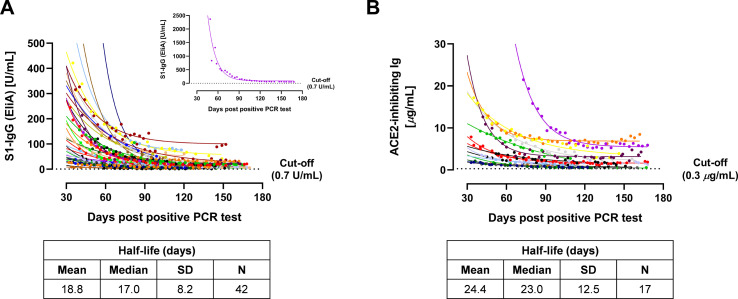
The EliA S1-IgG and ACE2 receptor binding inhibition assay methods were then applied to the entire sample set. The total number of donations tested was 568 and 357 for the EliA S1-IgG and ACE2 receptor binding inhibition assays, respectively. For both assays, we observed a sharp decline in the titre of surrogate nAbs over the course of the study, particularly within the first 60 days PPPT. However, titres tended to level out at around 90 days PPPT and did not decrease below the diagnostic cut-off values by the time of last donation, indicating that most infected individuals retained sero-positive antibodies for a significant period following viral infection (Fig. 2 [complete donor set], Supplementary Fig. 2 [individual donors]).
Fig. 2.
Convalescent plasma donation testing for SARS-CoV-2 nAbs using EliA S1-IgG (A) and ACE2 receptor binding inhibition assay (B) methods, plotted against the number of days post-positive PCR test. Each donor is shown in a different colour, and colours are preserved between graphs. Datapoints for each donor (that is, donations) were fitted using a one-phase decay exponential regression, which allowed for the derivation of the half-life of SARS-CoV-2-related antibodies to be calculated in each case. Exceptionally high EliA S1-IgG results were observed for initial donations from donor #24 (pink), and are therefore called out as an inset to A. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
ACE2, angiotensin-converting enzyme 2; Ig, immunoglobulin; IgG, immunoglobulin G; nAb, neutralising antibody; S1, spike 1; SD, standard deviation.
3.4. Calculation of SARS-CoV-2 nAb half-life
The quantitative nature of the methods allowed for the fitting of an exponential regression and calculation of the half-life for each convalescent donor. With the EliA S1-IgG method, the mean (standard deviation) half-life was 18.8 (8.2) days, while the ACE2 receptor binding inhibition assay gave a mean half-life of 24.4 (12.5) days. By combining results generated from both assays, the mean half-life across the donor set was 20.4 (9.8) days (Supplementary Table 2).
4. Discussion
In this study, SARS-CoV-2 nAb titres declined with time but rarely reached baseline, even at 168 days. Crucially, all donors remained sero-positive according to cut-off values in the two diagnostic assays applied in this study and returned a positive diagnostic result for their final donation. This builds upon earlier reports of SARS-CoV-2 antibody persistence in plasma. Similar to the rapid decrease in nAb titre remarked here, Seow et al. observed that the neutralisation titre of 65 convalescent subjects decreased markedly 94 days post-onset of symptoms [9]. This was supported by Long and co-workers [8], who showed a waning S1- and NC-binding IgG titre in more than 90% of convalescent study participants, with several participants being classified as seronegative (via neutralisation assay) eight weeks post discharge. Paralleling our findings, Lau et al. [7] observed a decrease over time that followed a bi-exponential trend with a rapid decline in nAb titre 30–40 days post-onset of symptoms, followed by a more gradual decrease over time. Several additional studies of SARS-CoV-2 nAbs support an initial decline in nAb titre but most indicate that lower titres and/or detectable neutralising activity are measurable up to 6–10 months post-onset, though there are discrepancies between these studies with respect to how disease severity impacts antibody persistence [[10], [11], [12],14]. Notably, Betton et al. highlighted that decreases in IgG titres in serological assays may not necessarily cause a loss of neutralising capacity [10], therefore careful interpretation of nAb titre data is required, especially given the temporal and methodological differences between studies.
Our data complements and builds upon previous studies by providing granularity and quantitative capability which allowed exponential curve fittings and half-life calculations. We found that the half-life of SARS-CoV-2 antibodies in COVID-19 convalescent plasma was approximately 20 days. Mean half-life values determined by each assay were somewhat different (18.8 versus 24.4 days for EliA S1-IgG and the ACE2 receptor binding inhibition assays, respectively); this may be a result of sample size, as fewer donor half-lives were calculated with the ACE2 receptor binding inhibition assay, though it is interesting to note that this assay typically delivered higher half-life values than its EliA S1-IgG counterpart. This could, in part, be a result of the difference in assay sensitivity and specificity. Given that both assays demonstrated good correlation with live virus neutralisation assays and considering inter-donor variability, we anticipate that the mean value of approximately 20 days for SARS-CoV-2 nAb half-life is a dependable estimation.
To our knowledge, this is the first study to calculate the half-life of SARS-CoV-2 nAbs from sequential plasma samples from the same donors. Other studies have estimated nAb half-life using modelling techniques, including Vanshylla et al. who used regression modelling to calculate the half-life of nAb activity from two donation timepoints from 342 individuals, reporting a half-life of 6.7 weeks for serum SARS-CoV-2 nAbs [15]. Their study also supports the longitudinal findings of our data, suggesting that, although nAb titres decline over time, neutralising function remains detectable many months after initial infection. Similarly, Yamayoshi et al. used a linear mixed model to estimate serum nAb half-life at approximately 5.7 weeks after peak titre and 10.3 weeks after 30 days post-onset, suggesting that the rate of antibody decay decreases over time [16]. Likewise, using a mixed effects modelling approach with 158 samples collected over three timepoints up to 149 days post-symptom onset, Wheatley et al. found that a two-phase decay model best represented the decline in nAb titres, suggesting that the half-life increased significantly over time [17].
The variability in reported half-lives of SARS-CoV-2 nAbs may be a result of the heterogeneity of samples, methods and modelling used to determine them. In our study, the half-life of SARS-CoV-2 nAbs was able to be determined through a combination of: (1) having collected sequential donations made over several months by COVID-19 convalescent donors together with; (2) quantitative, high-throughput binding assays which we demonstrated as having strong correlation to SARS-CoV-2 live virus neutralisation. The minimum of four donations per donor provides an extra element of granularity in determination of SARS-CoV-2 nAb half-life compared with other studies which have estimated half-life based on pooled samples from various donors or few timepoints from unique donors. Furthermore, the EliA S1-IgG assay utilised the novel SARS-CoV-2 IgG WHO standard and therefore conforms with global guidelines for COVID-19 diagnostics. Thus, the data reported here should provide a well-founded reflection of the persistence of SARS-CoV-2 nAbs. This may be further supported by the fact that our half-life calculation is within the range of the half-life of IgG in normal human plasma (23 days) [18] which indicates that such antibodies may be subject to FcRn-mediated IgG recycling [19].
From a qualitative viewpoint, our results also agree with studies that demonstrate sustained SARS-CoV-2 antibody levels in convalescent individuals for a significant period of time [[2], [3], [4], [5], [6]], albeit with titres maintained following a sharp decline. Interestingly, a study in Wuhan supports the notion of long-term maintenance of nAbs. In this study, 532 of 9542 randomly selected individuals were found to be positive for SARS-CoV-2 pan-immunoglobulins. Using data from the 335 individuals who attended all three follow-up visits, the authors did not capture a dramatic initial decline in nAb titres as seen in our study, perhaps due to participants being randomly selected rather than being followed-up post-symptom onset, meaning they could be at later stages of infection upon testing. Importantly, however, in the 40% of individuals who had developed nAbs, neither nAb titres nor the proportion of those with nAbs significantly declined over three timepoints between April and December 2020 [6], suggesting that nAbs are long-lasting. The findings of our study can support in defining this timeframe more accurately. Our study supports the notion of initial high titres of nAb undergoing a rapid decline but residual titres remaining detectable for an extended period of time. This correlates with a typical immune response in which short-lived, antibody-producing cells initially generate high titres of nAb with a half-life comparable to that of typical human IgG. Following the decay of these antibodies after approximately 20 days, long-lived plasma cells may remain to produce the low levels of nAb which were detected up to 168 days here.
The protective titre of SARS-CoV-2 nAbs has recently been estimated to be 28.6% of the mean convalescent titre using the ‘protective neutralisation classification model’, which estimates protective titre levels independently of any assumption of the distribution of neutralisation titres [20]. Based on this model, we would estimate that approximately 64% of the donors in this study would retain a protective titre. Nevertheless, while antibody-mediated viral neutralisation is integral to protection, other immune responses such as T- and B-cell memory also play a role. As such, it is difficult at this point to say with certainty whether such levels are indeed protective and further data is required to prove this observation.
Interestingly, similar observations of declining yet retained nAb titre have previously been made for SARS-CoV, albeit over the course of several years [[21], [22], [23]]. Whether this also occurs for recovered COVID-19 patients remains to be determined. Of particular interest to future studies will be changes in absolute and relative levels of nAb titre upon re-infection as well as upon vaccination, together with analysis of clinical symptoms associated with such situations.
While this was a longitudinal analysis, the duration of study was limited due to the ongoing nature of the pandemic. We have not reported results beyond 168 days but continue to collect plasma from donors for future analysis in order to establish the long-term dynamics of SARS-CoV-2 nAbs. Moreover, donations were limited geographically and by disease severity, with only individuals who had experienced mild COVID-19 being selected. An extension of donor sampling to more diverse populations with individuals who have experienced different severities of COVID-19 would be beneficial to establish a global representation of SARS-CoV-2 nAb kinetics, particularly as there are conflicting reports on the importance of disease severity in the dynamics of the immune response. It is important to note that the findings from this study cannot be extrapolated to examine the persistence of anti-SARS-CoV-2 antibodies occurring following vaccination, as donor samples were collected prior to the rollout of vaccines. Indeed, variability in characteristics of antibody responses has already been observed between vaccine induced- and natural immunity [[24], [25], [26]]. Further studies are needed to determine the impact of vaccination on anti-SARS-CoV-2 immunoglobulin titre persistence.
5. Conclusion
In conclusion, using two high-throughput immunological methods with strong correlation to live virus neutralisation, this study demonstrates that although SARS-CoV-2 nAb titres decline post-infection, they remain above baseline and are maintained for at least five months with a half-life of approximately 20 days. This indicates that SARS-CoV-2 nAb titres may persist for a significant period post-infection which may impact re-infection and vaccination outcomes.
Funding
Funding for this work was provided by CSL Behring AG and Thermo Fisher Scientific ImmunoDiagnostics Phadia GmbH.
Declaration of Competing Interest
TB, SM, TH, PS and NR are employees of CSL Behring, AG. RJ, MW and TS are employees of CSL Plasma. CK, AH, UK are employees of CSL Behring Innovation, GmbH. JSP, LS and DF are employees of Thermo Fisher Scientific ImmunoDiagnostics Phadia, GmbH.
Acknowledgements
The authors would like to acknowledge the following for logistical support, laboratory analysis, and statistical input: Sara Stinca, Margit El-Azhari, Wilfried Meyers, Clélia Dental, Carrie Chastain and Stéphanie Mateos. Editorial assistance was provided Meridian HealthComms Ltd., funded by CSL Behring.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108871.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Health Organization Coronavirus Disease (COVID-19) Pandemic 2021. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from:
- 2.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnleitner S.T., Prelog M., Jansen B., Rodgarkia-Dara C., Gietl S., Schonegger C.M., et al. Maintenance of neutralizing antibodies over ten months in convalescent SARS-CoV-2 afflicted patients. Transbound Emerg Dis. 2021 doi: 10.1111/tbed.14130. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andrell J., et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med (N Y). 2021;2(3):281–295. doi: 10.1016/j.medj.2021.02.001. (e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Z., Ren L., Yang J., Guo L., Feng L., Ma C., et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397(10279):1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau E.H.Y., Tsang O.T.Y., Hui D.S.C., Kwan M.Y.W., Chan W.H., Chiu S.S., et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021;12(1):63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 9.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betton M., Livrozet M., Planas D., Fayol A., Monel B., Vedie B., et al. Sera neutralizing activities against severe acute respiratory syndrome coronavirus 2 and multiple variants 6 months after hospitalization for coronavirus disease 2019. Clin. Infect. Dis. 2021;73(6) doi: 10.1093/cid/ciab308. (e1337-e44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen K.W., Linderman S.L., Moodie Z., Czartoski J., Lai L., Mantus G., et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Medicine. 2021;2(7) doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh J.Y., Kwak J.E., Yang J.S., Hwang S.Y., Yoon J.G., Seong H., et al. Longitudinal assessment of anti-SARS-CoV-2 immune responses for six months based on the clinical severity of COVID-19. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuce M., Filiztekin E., Ozkaya K.G. COVID-19 diagnosis -a review of current methods. Biosens. Bioelectron. 2021;172:112752. doi: 10.1016/j.bios.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dispinseri S., Secchi M., Pirillo M.F., Tolazzi M., Borghi M., Brigatti C., et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021;12(1):2670. doi: 10.1038/s41467-021-22958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanshylla K., Di Cristanziano V., Kleipass F., Dewald F., Schommers P., Gieselmann L., et al. Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe. 2021;29(6):917–929. doi: 10.1016/j.chom.2021.04.015. (e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamayoshi S., Yasuhara A., Ito M., Akasaka O., Nakamura M., Nakachi I., et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine. 2021;32:100734. doi: 10.1016/j.eclinm.2021.100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.X., et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021;12(1):1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo E.D., Hansen R.J., Balthasar J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004;93(11):2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 19.Kim J., Hayton W.L., Robinson J.M., Anderson C.L. Kinetics of FcRn-mediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin. Immunol. 2007;122(2):146–155. doi: 10.1016/j.clim.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 21.Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y., et al. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirology. 2006;11(1):49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L., Xie J., Sun J., Han Y., Zhang C., Fan H., et al. Longitudinal profiles of immunoglobulin G antibodies against severe acute respiratory syndrome coronavirus components and neutralizing activities in recovered patients. Scand. J. Infect. Dis. 2011;43(6–7):515–521. doi: 10.3109/00365548.2011.560184. [DOI] [PubMed] [Google Scholar]
- 23.Cao W.C., Liu W., Zhang P.H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 24.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaquero S.T., de Campos-Mata L., María Ramada J., Díaz P., Navarro-Barriuso J., Ribas-Llaurado C., et al. SARS-CoV-2 naïve and recovered individuals show qualitatively different antibody responses following mRNA vaccination. medRxiv. 2021 Preprint article on. [Google Scholar]
- 26.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;100208 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material