Abstract
Background
Native Hawaiians have higher hypertension (HTN) and cardiovascular disease (CVD) rates than non-Hispanic whites, calling for culturally responsive interventions to close this gap.
Purpose
We tested the effects of a 6-month behavioral intervention, a cultural dance program based on hula (the customary dance of Hawai'i), for improving blood pressure (BP) and CVD risk among Native Hawaiians with uncontrolled HTN.
Methods
In a randomized controlled trial, we tested the effects of the hula-based intervention among 263 Native Hawaiians with uncontrolled HTN (systolic ≥ 140 or ≥ 130 mmHg if diabetes) and no CVD at enrollment. All participants received a brief culturally tailored heart health education before random assignment to the hula-based intervention (n = 131) or the education-only waitlist control (n = 132). Intervention received hula lessons and group-based activities for 6 months. Control received only 1-week education through 6 months.
Results
Intervention yielded greater reductions in systolic (−15.3 mmHg) and diastolic (−6.4 mmHg) BP than control (−11.8 and −2.6 mmHg, respectively) from baseline to 6 months (p < .05). At 6 months, 43% of intervention participants compared to 21% of controls achieved a HTN stage <130/80 mmHg (p < .001). The 10-year CVD risk reduction was two times greater for the intervention group than the control group based on the Framingham Risk Score calculator. All improvements for intervention participants were maintained at 12 months.
Conclusions
This trial represents one of the few rigorously conducted examinations of an Indigenous practice leveraged for health promotion, with implications for other ethnic populations.
Keywords: Hypertension, Cardiovascular disease, Native Hawaiian, Intervention, Dance, Exercise
Native Hawaiians with hypertension who participated in a cultural dance program show clinically meaningful improvements in blood pressure and cardiovascular disease risk than those who did not participate.
Introduction
Hypertension (HTN) affects 45% of the adult population in the USA [1]. It is a major risk factor for coronary heart disease and stroke [2], yet only half of the treated patients have their HTN under control [1]. Compared to non-Hispanic whites, Native Hawaiians, an indigenous US population, are 70% more likely to have HTN and 3–4 times more likely to have coronary heart disease and stroke, respectively [3, 4], and experience stroke an average of 10 years younger due to inadequate HTN control [5–9]. Pharmacologic interventions alone may be suboptimal for HTN control in Native Hawaiians [5, 6] who desire culturally responsive nonpharmacologic interventions [10–12]. The American Heart Association recommends culturally tailored interventions to improve cardiovascular disease (CVD) outcomes [13].
Nonpharmacologic HTN interventions have been focused on diet and/or physical activity [14, 15]. Improving physical activity reduces systolic blood pressure (BP) by 5–10 mmHg and diastolic BP by 1–6 mmHg in persons with HTN [16], comparable to dietary sodium reduction and weight loss [17–19]. Aerobic dance reduces systolic BP by 9–16 mmHg and diastolic BP by 3–5 mmHg [6, 20–23]. A pooled-analysis of 48,390 adults found moderate-intensity dancing associated with a 50% reduced risk in CVD mortality, possibly the result of periods of high-intensity exercise and social connectedness [24]. However, these studies were largely conducted with majority populations and based on Western dance forms [13, 25].
Hula is the traditional cultural dance of Hawai'i and, despite its misperception as entertainment for tourists, it is the premier cultural practice of Native Hawaiians, connecting them to their native language, history, and natural environment [26, 27]. Kumu hula, or hula masters, are teachers of this tradition. Hula training involves learning rhythmic and synchronized body movements that illustrate the poetry of accompanying songs or chants. There are 203 formal hula schools in Hawai'i, 619 throughout the continental US, and 455 internationally [28]. Aside from physical activity, the study of hula fosters knowledge of Hawaiian culture and strong social connections with other participants [12, 29]. A metabolic equivalents study showed hula achieved a metabolic equivalents of 5.7 for moderate-intensity and 7.6 for vigorous physical activity [30], consistent with national guidelines [31].
A prior 3-month hula-based HTN intervention called Ola Hou i ka Hula (translated as “restoring health through hula”) was tested in a small-scale randomized trial with 55 Native Hawaiians and Chuukese (Pacific Islanders from Micronesia) with previously uncontrolled HTN (systolic BP ≥ 140 or ≥ 130 mmHg for those with diabetes) [32]. The hula-based intervention included a culturally-tailored heart health education along with weekly hula classes. The intervention group showed a significant improvement in mean systolic BP (−18.3 mmHg) compared to a control group (7.6 mmHg) at 3 months, with 72% of the intervention participants achieving ≥ 10 mmHg reduction in systolic BP compared to 39% of controls. However, these improvements attenuated within 3 months postintervention [33].
In the present research, we conducted a fully powered, randomized controlled trial (RCT) to test the effects of a 6-month version of the Ola Hou i ka Hula program compared to an education-only waitlist control group in improving systolic BP control and 10-year CVD risk in adult Native Hawaiians with previously uncontrolled HTN (i.e., systolic BP ≥ 140 mm Hg). The original 3-month cultural program was expanded to 6 months to improve the long-term maintenance of any positive BP outcomes. We hypothesized the cultural dance program would lead to greater improvements in systolic BP (the primary outcome), diastolic BP, overall HTN control (e.g., achieving a systolic BP< 140 mm Hg), and estimated 10-year CVD risk from baseline to 6-month follow-up compared to an education-only waitlist control group. We further hypothesized the intervention group would maintain all improvements at a 12-month follow-up (i.e., 12 months from baseline, but 6 months after intervention cessation).
Methods
Study Design and Oversight
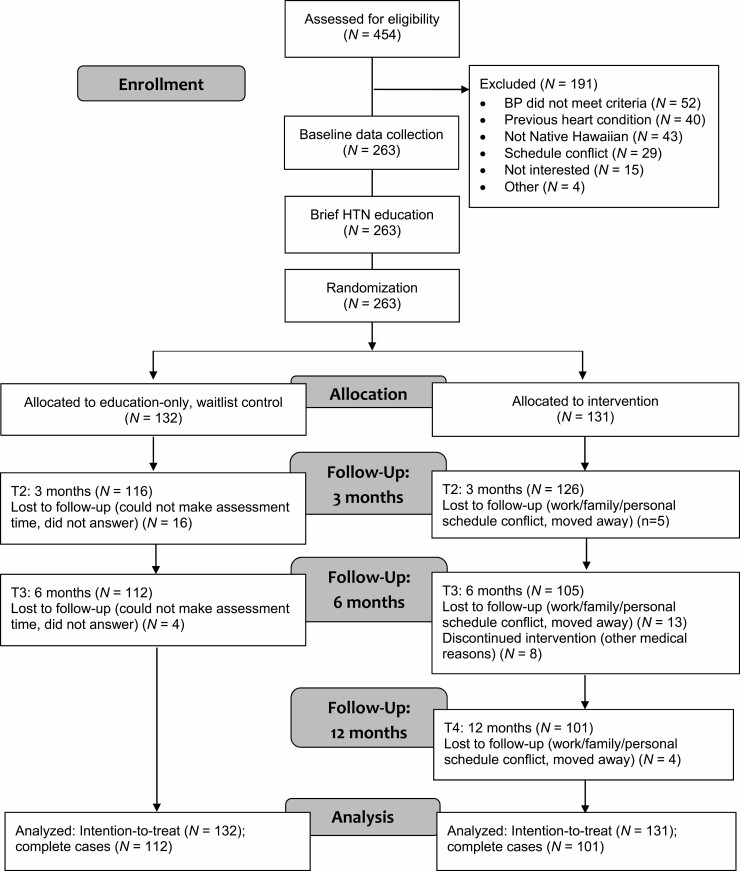
Figure 1 shows the CONSORT diagram of our 2-arm RCT with intervention and an education-only waitlist control group. A waitlist control rather than a concurrent control group was a mandate of our community partners and stakeholders to ensure all participants were eventually offered the hula-based intervention. All participants received the same brief heart health education curriculum before entering their randomly assigned group. We chose this approach to test the unique contribution of the hula intervention on BP above and beyond the effects of heart health education. Delivery of the HTN education to the waitlist control participants represents a highly conservative approach because it tests the effects of the hula-based intervention above and beyond the effect of HTN education.
Fig. 1.
CONSORT diagram of the randomized clinical trial.
Our study design and intervention implementation protocol were informed by a community-based participatory research (CBPR) approach described in detail elsewhere [33]. Briefly, CBPR is a necessary approach to conduct intervention research with Indigenous communities, which enhances community ownership, participation, and sustainability [34, 35]. It is also necessary for an intervention that is based on an Indigenous practice that requires cultural knowledge and endorsement [36, 37].
The University of Hawai'i at Mānoa and the Wai'anae Coast Comprehensive Health Center Institutional Review Boards approved our study protocol and materials and registered on ClinicalTrials.gov (NCT02620709) on November 23, 2015. An independent data safety monitoring board of five members, among them a physician with clinical trial experience, a cardiologist, and a biostatistician, conducted an ongoing review of safety and effectiveness data. Informed consent was obtained from all participants upon enrollment.
Participants and Sample Size Calculations
We recruited Native Hawaiians who met the following criteria: (1) physician-diagnosed HTN, (2) systolic BP ≥ 140 mmHg (or ≥ 130 mmHg for those with diabetes), (3) physician approval to participate in moderate physical activity, (4) no prior history of CVD, and (5) age 20 years or older. We excluded females who were pregnant and persons with a physical or mental health condition that would prevent full participation. No prior hula experience was required. The sample size calculation for this trial was based on a systolic BP mean difference of 7.5 mmHg (SD = 16.5) observed in the pilot trial between intervention and waitlist control, resulting in a target of 250 participants (125 per arm) to achieve 90% power for detecting this effect size, accounting for 20% attrition [33].
Enrollment and Implementation Sites
Between November 2015 and April 2019, enrollment and implementation occurred at the sites of six community-based organizations across three Hawai'i islands (O'ahu, Maui, and Hawai'i Island). Existing client or patient registries were used to identify prospective participants who were either mailed a flyer describing the study and/or phoned to inform them about the study. Recruitment also occurred via informational booths and health screenings at community events and cultural gatherings. The participating community-based organizations included a Hawaiian Homestead community center (Kula no na Po'e Hawai'i), two federally authorized Native Hawaiian Health Care Systems (Hui no ke Ola Pono and Ke Ola Mamo), a behavioral health service and training program (I Ola Lāhui), a federally qualified health center (Wai'anae Coast Comprehensive Health Center), and an independent health promotion organization (Mauli Ola).
All interested participants were screened for eligibility by a trained community researcher who also obtained informed consent from those eligible and willing to participate. Participants needed to obtain a written clearance from their physicians to participate and to confirm HTN and diabetes diagnosis and the absence of CVD (i.e., coronary artery disease, peripheral vascular disease, myocardial infarction, and ischemic stroke). The physician-clearance form also asked about any prescribed antihypertensive medication (i.e., class and dosage).
Randomization
Randomization was done using an online random assignment calculator by a project coordinator not involved in data collection or delivery of the intervention. Block randomization was done based on cohorts of 16–20 participants to ensure an equal or near-equal (for odd number cohorts) number assigned to the intervention group and control group within each community site over the accrual period, resulting in 14 cohorts in total. Thus, the number of participants randomly assigned to the hula-based intervention consisted of 8–10 participants per cohort with an equal or near-equal number randomly assigned to control per cohort. Participants and research staff involved in baseline data collection were initially blinded to the randomization assignments and unblinded upon completion of the pre-assignment heart health education.
Pre-Assignment Heart Health Education
Following the baseline assessments for each cohort, all participants received three 1-hr lessons of a culturally tailored heart health education curriculum adapted from national cardiology guidelines and modules [32, 38, 39]. The lessons were delivered in-person over 1 week in group settings by a trained community peer educator. The curriculum focused on: (1) signs, symptoms, complications of uncontrolled HTN, and benefits of physical activity, (2) understanding medications, and (3) heart-healthy eating including dietary sodium reduction. Upon completion, participants received their randomization assignments.
Experimental Intervention Group
Participants randomized to the intervention group received the 6-month Ola Hou i ka Hula program in two phases (Supplementary Material 1 summarizes these phases and activities). The first 3 months of the intervention involved 1-hr dance classes taught by the trained hula instructor, two times weekly (a total of 24 classes). All hula instructors completed a 6-hr dance protocol training led by a hula expert and research staff. The design of the first 3-month phase was informed by social cognitive theory to leverage the natural social support offered by the hula classes and to improve self-efficacy for behavior change [40]. Specifically, the hula lessons delivered in groups offered the opportunity for participants to engage in observational learning and achieve self-efficacy to make and sustain positive behavior change. More details can be found in Kaholokula et al. [32, 33].
Briefly, the hula classes were delivered in a group setting with hula lessons that emphasized Native Hawaiian values of mālama 'āina (land stewardship), lōkahi (living harmoniously with others and surroundings), hana pono (socially appropriate behaviors), and aloha (compassion toward others) to promote a positive group environment and social interactions. Also based on traditional hula training, the hula classes involved instructions in dance footwork, upper body movements, and learning poetry, stories, history, Hawaiian language, and meaning of the accompanying songs and chants. These foot and hand movements taught, as well as the tempo of the accompanying chant or music, was selected to accommodate persons of different adult age groups and levels of physical capabilities. Participants were encouraged to memorize the songs/chants and practice independently, or in self-arranged groups, utilizing their notes and those provided during the classes. Each class involved a 5−15-min warm-up period of walking and stretching, followed by dance and cultural instructions, with continuous dancing ranging between 10 and 40 min, and a 3−10-min cool-down period. Over these first 3 months, the classes progressively increased in intensity and duration in continuous dance from 10 min in Week 1 to 40 min by Week 12.
The second 3-month phase of the intervention, called Ma ka hana ka 'ike (“by doing one learns”), was designed for long-term maintenance of behavior change. This phase was informed by self-regulation theory [41], an extension of social cognitive theory, to emphasize a goal adoption strategy for sustained behavior change specific to HTN control [42, 43]. Specifically, self-regulation theory emphasizes behavioral goal adoption for self-directed change, implementation of productive actions to achieve goals, and maintenance strategies to promote sustainable change by developing behavioral capability, self-control, and self-efficacy. A total of 12 dance lessons taught by the hula instructor were offered for one 1-hr session each month and participants were encouraged to continue their dance practice at home and elsewhere (e.g., family and cultural gatherings). Consistent with the self-regulation theory, the other three 1-hr per week sessions (for a total of four sessions per month) were facilitated by a trained community peer educator, which included lessons on setting individual goals (i.e., specific, measurable, achievable, relevant, and time-based goals), value identification (e.g., aligning healthy goals to intrinsic values), and time management; brief review of the heart health educational materials with specific goal setting and self-monitoring and problem-solving activities (e.g., dietary sodium reduction); and peer-discussion groups to share experiences, personal strategies, and challenges to BP control. An additional educational lesson on managing negative emotions (e.g., identifying signs of depression, anxiety, and stress; management strategies, such as deep-breathing, seeking social support, and exercise) for emotional impulse control was provided to further develop emotional self-regulation skills.
Intermittent fidelity checks of the intervention classes were conducted to ensure adherence to protocols and the quality of interaction between the hula instructor (or community peer educator) and the participants [44]. All fidelity checks of the hula lessons were done by the study’s hula expert using a standardized checklist and behavioral observation form. Fidelity checks of the other intervention components were performed by a trained research assistant using a standardized form. Immediate feedback was given to the hula instructor or community peer educator by the observer. Refresher training was also offered twice annually to review protocols.
Education-Only Waitlist Control Group
After the pre-assignment education sessions, no contact was made with participants randomly assigned to the education-only waitlist control group (referred to as “control group” hereafter) except to schedule the 3- and 6-month follow-up assessments. They were neither restricted nor encouraged to seek out other interventions, education, or help on their own. As with the intervention group, they were instructed to continue with their usual medical care. Participants in the control group were offered the hula-based program following their 6-month follow-up.
Outcome Measures
BP (systolic BP and diastolic BP in mmHg) was measured using a digital BP monitor (Omron© HEM-907-XL, Omron Healthcare) [45–47]. Standardized protocols were used, which involved a trained community researcher taking three BP measurements of each participant using appropriate cuff sizes [33, 38, 48]. The participant sat quietly for 5 min before the first BP measurement with feet flat on the floor and the arm in the BP cuff resting on a table at heart level. The average of the last two BP measurements was used for data analyses. Systolic BP was the primary outcome and diastolic BP a secondary outcome. Another secondary BP outcome was overall HTN control based both on systolic and diastolic BP and categorized into the following three HTN stages: <140/90, <135/85, and <130/80 mmHg.
The Framingham Risk Score-CVD (FRS-CVD) calculator was used to estimate the 10-year CVD risk based on guidelines from the American College of Cardiology and the American Heart Association [49]. It estimates the percent risk of a CVD event within the next 10 years and considers age, sex, systolic BP, high-density lipid-protein (HDL), total cholesterol, smoking and diabetes status, and pharmacologic treatment for HTN (yes vs. no). The “White” (vs. “Black”) race option (no other race option available) was used to estimate the percent risk. The FRS-CVD calculator age range is from 30 to 74; participants under 30 were assumed to be 30 and those over 74 were assumed to be 74 for calculations. Age, gender, and smoking status (i.e., never, former, and current smoker) for the FRS-CVD calculator was determined based on responses to a self-report questionnaire. Diabetes status was confirmed by the participants’ healthcare provider at enrollment. To assess total cholesterol and HDL, the Alere Cholestech LDX portable analyzer [50], which requires a finger stick drop of blood, was used [51, 52].
Self-report sociodemographic information, collected only at baseline, were (1) date of birth, (2) gender, (3) marital status, (4) highest educational attainment, (5) Native Hawaiian, and (6) other ethnic ancestries. Medical history and relevant health-related behaviors collected were (1) smoking history, (2) family history of CVD, (3) CVD risk factors (i.e., history of high cholesterol, HTN, and diabetes), and (4) prescribed HTN medications. Participants were asked to bring all of their prescribed medications to all scheduled assessments, but only the HTN medications were recorded, including class and dosage. To calculate body mass index (BMI), body weight in kg divided by height in meters squared and weight were measured in kg with an electronic scale (Tanita BWB800AS) and a stadiometer was used to measure the height (baseline only) in cm.
Data collection occurred at baseline, 3 months, and 6 months for all participants by a trained research assistant. Sociodemographic and behavioral measures were completed by the participant while clinical measures were taken of each participant by a research assistant. We included a 3-month follow-up assessment to examine the original 3-month component of the intervention and to determine the relative contribution of each intervention phase. A 12-month data collection (i.e., 12 months from baseline but 6 months after intervention cessation) visit was performed only for the intervention group to assess the maintenance of outcomes. Each participant received a $25 gift card upon completion of each assessment.
Data Analysis
We calculated means (M) and standard deviations (SD) for continuous variables and frequencies and percentages for categorical variables for descriptive data. Baseline differences between the study groups were done using a two-sample t-test or chi-squared or Fisher’s exact test. The primary outcome was systolic BP with diastolic BP, HTN stages (<140/90 mmHg, <135/85 mmHg, and <130/80 mmHg), and FRS-CVD risk score as secondary outcomes. For all outcomes analyses, we performed both intention-to-treat (ITT) and complete case (CCA) analyses based on multivariable linear modeling, comparing baseline to 3-month follow-up and 6-month follow-up data. For ITT, we used the last measured BP value or CVD risk score carried forward for missing data as recommended for clinical trials to provide an unbiased estimate of the efficacy of an intervention [53, 54]. We performed multivariable logistic regressions to calculate adjusted relative risk with 95% confidence intervals comparing three HTN stages (<140/90, <135/85, and <130/80 mmHg) between study groups. All outcome analyses (except that for the baseline to 12-month follow-up) adjusted for baseline value of the dependent variable, community site, and any of the measured variables not balanced at baseline. We adjusted for community site given possible variations in protocol adherence and differences in other characteristics unique to each site that could be confounders.
For the intervention group only, we performed the same set of analyses based on linear mixed-effects modeling (with time as a fixed effect) to examine change from 6 to 12 months to determine the maintenance of any BP improvements. We also calculated and compared retention between groups at 3 and 6 months and examined the correlation between the number of lessons attended for intervention participants and the degree of BP changes at 3, 6, and 12 months. The number of participants within each study group who were prescribed each class of HTN medication was also examined using the Cochran-Armitage Test for trend from baseline to 3 and 6 months. All analyses were performed using R version 3.6.0 (R Core Team, 2019) and SAS 9.4 using a probability value of <.05.
Results
Baseline Characteristics of Participants
We enrolled 263 participants (131 assigned to intervention and 132 to control). The balance was achieved in BP and other measured variables, except for weight (kg) for which the control was slightly heavier (Table 1). The average number of prescribed antihypertensive medication classes was balanced. A majority were female, but gender was evenly distributed.
Table 1.
Baseline characteristics of participants by study group
| Characteristic* | Intervention (N=131) | Waitlist Control (N=132) | p value† |
|---|---|---|---|
| Age (years) | 58.1 ± 13.7 | 57.9 ± 12.6 | 0.88 |
| Female | 111 (84.7) | 109 (82.6) | 0.64 |
| Community site | 0.56 | ||
| Site 1 | 7 (5.3) | 10 (7.6) | |
| Site 2 | 44 (33.6) | 44 (33.3) | |
| Site 3 | 18 (13.7) | 16 (12.1) | |
| Site 4 | 12 (9.2) | 8 (6.1) | |
| Site 5 | 20 (15.3) | 14 (10.6) | |
| Site 6 | 30 (22.9) | 40 (30.3) | |
| Education level | 0.97 | ||
| Less than high school | 1 (0.8) | 1 (0.8) | |
| High School | 50 (38.5) | 48 (36.9) | |
| Some college | 51 (39.2) | 50 (38.5) | |
| College | 28 (21.5) | 31 (23.8) | |
| Marital status | 0.13 | ||
| Never married | 29 (22.1) | 27 (20.5) | |
| Currently married | 50 (38.2) | 66 (50.0) | |
| Disrupted marital status | 52 (39.7) | 39 (29.5) | |
| Other medical conditions | |||
| High cholesterol | 64 (48.9) | 69 (52.3) | 0.58 |
| Diabetes | 52 (39.7) | 66 (50.0) | 0.09 |
| Heart condition | 3 (2.3) | 6 (4.5) | 0.50 |
| Other medical conditions | 18 (13.7) | 25 (18.9) | 0.25 |
| No other medical condition | 36 (27.5) | 28 (21.2) | 0.24 |
| Prescribed HTN medication | 114 (87.0) | 113 (85.6) | 0.74 |
| No. of HTN medications | 1.31 ± 0.88 | 1.40 ± 0.87 | 0.37 |
| Smoking history | 0.10 | ||
| Never | 69 (52.7) | 79 (59.8) | |
| Former | 44 (33.6) | 45 (34.1) | |
| Current | 18 (13.7) | 8 (6.1) | |
| Weight, kg | 93.5 ± 19.0 | 99.7 ± 28.1 | 0.04 |
| Height, cm | 163.6 ± 7.8 | 164.4 ± 9.3 | 0.48 |
| Body-mass-index, kg/m2 | 34.9 ± 6.4 | 36.7 ± 9.3 | 0.07 |
| Systolic blood pressure, mmHg | 144.1 ± 16.9 | 145.1 ± 13.9 | 0.60 |
| Diastolic blood pressure, mmHg | 85.2 ± 12.6 | 84.5 ± 10.4 | 0.64 |
| High-density lipoproteins, mg/dL‡ | 48.8 ± 14.6 | 46.5 ± 15.1 | 0.24 |
| Total cholesterol, mg/dL‡ | 174.0 ± 31.4 | 177.3 ± 42.3 | 0.48 |
* Data are shown as mean ± standard deviation or number (percentages); numbers for some variables may not equate to total sample size because of missing data.
† Group difference p values based on two-sample independent t-tests and Chi-squared or Fisher’s exact tests, as appropriate.
‡ Sample size reduced to 247 for high-density lipoproteins (123 intervention; 124 control) and 250 for total cholesterol (126 intervention; 124 control) due to unreliable data.
Retention and Adverse Events
Retention at the 3 months was better for intervention at 96% compared to control at 88% (p = .02). At 6 months, it was 80% and 85% (p = .40), respectively, and 77% at 12 months for intervention. There were no adverse events reported.
Comparison of Primary and Secondary BP Outcome
Based on ITT, the intervention had a significantly greater reduction in systolic BP at 3 and 6 months than control (Table 2). Systolic BP reduced an average of −14.7 and −15.3 mmHg for intervention versus −11.5 and −11.8 mmHg for control at 3 and 6 months, respectively. The mean group-difference in systolic BP was 3.6 mmHg (95% CI: −6.0, −1.2) at 3 months and 3.9 mmHg (95% CI: −6.4, −1.4) at 6 months. CCA yielded similar systolic BP outcome at 6 months (Table 2), with a mean group-difference of 5.7 mmHg (95% CI: −7.1, −2.0).
Table 2.
Comparison of blood pressure (BP) outcomes from baseline to 3- and 6-month follow-up between intervention and control group
| Outcome | Baseline to 3 months | Baseline to 6 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline M ± SD | 3-month M ± SD | Within-group difference M ± SD | Between-group difference (95% CI) | p* | N | Baseline M ± SD | 6-month M ± SD | Within-group difference M ± SD | Between-group difference (95% CI) | p* | |
| Systolic BP | ||||||||||||
| Intention-to-treat | −3.6 (−6.0, −1.2) | 0.04 | −3.9 (−6.4, −1.4) | 0.04 | ||||||||
| Intervention | 131 | 144.1 ±16.9 | 129.4 ± 16.4 | −14.7 ± 19.0 | 131 | 144.1 ± 16.9 | 128.9 ± 16.3 | −15.3 ± 18.8 | ||||
| Control | 132 | 145.1 ± 13.9 | 133.6 ± 19.1 | −11.5 ± 19.1 | 132 | 145.1 ± 13.9 | 133.3 ± 18.7 | −11.8 ± 19.5 | ||||
| Complete cases | −2.6 (−5.4, 0.1) | 0.17 | −5.7 (−8.5, −2.8) | 0.01 | ||||||||
| Intervention | 126 | 144.3 ± 17.1 | 129.0 ± 16.5 | −15.3 ± 19.1 | 105 | 145.2 ± 17.9 | 128.2 ± 15.6 | −16.9 ± 18.5 | ||||
| Control | 116 | 144.2 ± 13.3 | 131.2 ± 18.1 | −13.1 ± 19.8 | 112 | 144.2 ± 12.8 | 132.6 ± 17.0 | −11.5 ± 18.1 | ||||
| Diastolic BP | ||||||||||||
| Intention-to-treat | −3.5 (−4.9, −2.2) | 0.04 | −4.0 (−5.7, −2.2) | 0.02 | ||||||||
| Intervention | 131 | 85.2 ± 12.6 | 78.5 ±13.0 | −6.7 ± 12.6 | 131 | 85.2 ± 12.6 | 78.8 ± 10.9 | −6.4 ±11.8 | ||||
| Control | 132 | 84.6 ± 10.4 | 81.2 ± 12.6 | −3.3 ± 10.9 | 132 | 84.6 ± 10.4 | 81.9 ± 11.3 | −2.6 ± 11.3 | ||||
| Complete cases | −3.3 (−4.8, −1.8) | 0.08 | −4.6 (−6.6, −2.5) | 0.01 | ||||||||
| Intervention | 126 | 85.1 ± 12.7 | 78.2 ± 13.1 | −7.0. ± 12.8 | 105 | 85.5 ± 12.5 | 78.1 ± 10.1 | −7.4 ± 11.7 | ||||
| Control | 116 | 84.4 ± 10.7 | 80.6 ± 13.0 | −3.8 ± 11.6 | 112 | 84.8 ± 10.6 | 81.8 ± 10.8 | −2.9 ± 11.9 |
*p values are based on between-group comparisons using a multivariable linear regression model with the BP change (follow-up value minus baseline value) as the dependent variable, adjusting for the baseline BP value, weight (kg), and community site. Intervention effect estimates are the difference in means and 95% confidence intervals (CI), net same adjustments.
Based on ITT, the intervention also had a significantly greater reduction in diastolic BP at 3 and 6 months compared to control (Table 2). Diastolic BP decreased an average of −6.7 and −6.4 mmHg for intervention versus −3.3 and −2.6 mmHg for control at 3 and 6 months, respectively. The mean group difference in diastolic BP was 3.5 (95% CI: −4.9, −2.2) at 3 months and 4.0 (95% CI: −5.7, −2.2) at 6 months. CCA yielded the same positive diastolic BP outcome at 6 months (Table 2), with a mean group-difference of 4.6 mmHg (95% CI: −7.0, −2.3).
Association between Weight Change and Systolic BP Outcome
We examined the association between weight and systolic BP changes at 6 months. Pearson correlation coefficients between body weight change (kg) and systolic BP change was r = .05 (p = .58) for intervention and r = .12 (p = .22) for control. CCA yielded no significant difference in weight loss between intervention (M = −0.15 kg; SD = 3.22) and control (M = −0.48 kg; SD = 5.50) at 6 months [t(179) = .54, p = .59]. ITT yielded similar nonsignificant results.
Comparison of Hypertension Stages
A greater number of intervention versus control participants improved their BP stages at 6 months (Table 3). Most notable is that, based on ITT, 42.7% of intervention participants achieved <130/80 mmHg compared with 21.2% of control participants (RR = 1.8, 95% CI: 1.4, 2.2), which was similar for CCA (see Table 3).
Table 3.
Comparison of three hypertension stages at 6-month follow-up between intervention and control group
| Hypertension stage | Intervention | Control | Relative risk (95% CI) | p* |
|---|---|---|---|---|
| Intention-to-treat | N = 131 | N = 132 | ||
| <140/90 mm Hg | 94 (71.8%) | 81 (61.4%) | 1.1 (0.9, 1.3) | 0.13 |
| <135/85 mm Hg | 74 (56.5%) | 55 (41.5%) | 1.3 (1.1, 1.5) | 0.02 |
| <130/80 mm Hg | 56 (42.7%) | 28 (21.2%) | 1.8 (1.4, 2.2) | <0.001 |
| Complete cases | N = 105 | N = 112 | ||
| <140/90 mm Hg | 78 (74.3%) | 70 (62.5%) | 1.2 (1.0, 1.3) | 0.04 |
| <135/85 mm Hg | 61 (58.1%) | 47 (42.0%) | 1.4 (1.1, 1.6) | <0.001 |
| <130/80 mm Hg | 47 (44.8%) | 23 (20.5%) | 2.0 (1.5, 2.5) | <0.0001 |
Data are shown as number (%) within-group and relative risk (95% confidence intervals) between-group.
* p values are based on between-group comparisons using a multivariable logistic regression model, adjusting for baseline systolic BP, weight (kg), and community site. Intervention effect estimates are relative risk and 95% confidence intervals (CI), net same adjustments.
Comparison of Estimated 10-Year CVD Risk
Cholesterol data from 21 participants (11 intervention and 10 control) were excluded from analyses due to unreliable readings at baseline. Table 1 shows the HDL and total cholesterol baseline data and Supplementary Material 2 summarizes the data between baseline to 6 months. ITT indicated that intervention achieved a significantly greater reduction in estimated 10-year CVD percent risk at 6 months compared to control (Table 4). The FRS-CVD yielded an average of −4.9% for intervention versus −2.7% for control, with a mean group-difference of 2.8% (95% CI: −4.1, −1.4). CCA yielded similar results, with an average of −6.4% for intervention and −2.8% for control, with a mean difference of 4.3% (95% CI: −6.0, −2.5).
Table 4.
Comparison of 10-year cardiovascular disease risk based on the Framingham Risk Score—Cardiovascular Disease (FRS-CVD) calculator from baseline to 6-month follow-up between intervention and control group.
| FRS-CVD | N † | Baseline M ± SD | 6-month M ± SD | Within-group change M ± SD | Intervention effect (95% CI) | p* |
|---|---|---|---|---|---|---|
| Intention-to-treat | −2.8 (−4.1, −1.4) | 0.02 | ||||
| Intervention | 120 | 19.6% ± 15.8 | 14.5% ± 10.8 | −5.2% ± 10.9 | ||
| Control | 122 | 19.9% ± 14.4 | 17.4% ± 15.3 | −2.5% ± 9.5 | ||
| Complete cases | −4.3 (−6.0, −2.5) | <0.01 | ||||
| Intervention | 87 | 21.0% ± 16.3 | 14.3% ± 9.6 | −6.7% ± 10.8 | ||
| Control | 97 | 19.2% ± 13.7 | 16.6% ± 14.6 | −2.6% ± 9.5 |
*p values are based on between-group comparisons using a multivariable linear model with the prevent risk change (follow-up value minus baseline value) as the dependent variable, adjusting for baseline weight (kg), and community site. Intervention effect estimates are the difference in means and 95% confidence intervals (CI), net same adjustments.
†Sample size reduced due to missing cholesterol data at baseline.
Postintervention Maintenance of BP and CVD Risk Outcomes
For the intervention participants, there was a small, nonsignificant mean increase from 6 to 12 months in systolic BP of 1.5 mmHg (SD = 14.5) and diastolic BP of 0.8 mmHg (SD = 9.2) based on ITT (N = 101). A majority of the intervention participants remained below 140/90 mmHg (69.5%) and 135/85 mmHg (55%), with 38.2% below 130/80 mmHg. CCA yielded similar BP results at 12 months. Based on ITT (N = 120) and CCA (N = 74), intervention showed a small, nonsignificant FRS-CVD mean risk change of 0.74% (SD = 5.8; p = .34) and 0.30% (SD = 6.3; p = .51) at 12 months, respectively.
Intervention Exposure-Response Association
The average number of lessons attended by intervention participants was 14.2 (SD = 7.0; maximum lessons = 24) for the first 3-month phase, 6.6 (SD = 4.0; maximum lessons = 12) for the second 3-month phase, and 21.8 (SD = 9.5; maximum = 36) over the 6-month intervention period. A majority of participants attended at least two-thirds of the sessions. We found no significant association between the number of lessons attended and changes in systolic BP within any of the two intervention phases (r = −.08, p < .37; r = .05, p < .61) or in combination (r = −.14, p < .17).
Antihypertensive Medication
To account for any medication changes during the study, we compared antihypertensive medications between and within groups. Supplementary Material 3 shows the class and distribution of antihypertensive medications across groups at baseline and each follow-up assessment. We found no significant changes in the distribution of the participants’ antihypertensive medication classes from baseline to 6 months based on the Cochran-Armitage test for trend analysis of CCAs and no significant changes at 12 months for intervention participants. We also found no significant change from baseline in the average number of antihypertensive medications between intervention and control at 3 months (M = −.04, SD = .64 and M = −.11, SD = .72, respectively, p = .38) and at 6 months (M = −.15, SD = .73 and M = −.11, SD = .73, respectively, p = .66).
Discussion
Our study is one of the first RCT of a cultural practice leveraged to address CVD disparities in an Indigenous population. Supporting our primary hypothesis, the 6-month cultural dance program based on hula was superior to control in reducing systolic BP among Native Hawaiians with previously uncontrolled HTN. Supporting our secondary hypotheses, the intervention was also superior to control in improving diastolic BP, HTN stage, and 10-year risk for CVD. We also found that the intervention participants maintained these improvements at 12 months, as hypothesized. These BP improvements are not likely due to changes in weight or antihypertensive medication. Also worth noting is the high retention of participants in both the intervention and control group at 6 months, 80% and 85%, respectively, and 77% at 12 months for intervention, which exceeds national recommendations (≥70%) for attrition in RCTs as a criterion in judging the effectiveness of an intervention [55].
Although the mean systolic BP differences of 3.9 mmHg (ITT) and 5.7 mmHg (CCA) between intervention and control participants could be termed modest effect sizes, they represent the effects of the hula program over and above the culturally tailored heart health education provided upfront to all. This assumption is supported by the fact that the mean difference in systolic BP in our pilot trial of the original 3-month version of our hula-based program was greater than what we found in this larger trial at 3 months. This may be because the waitlist control group in the pilot trial did not receive any culturally tailored heart health education or any other attention. Thus, the coupling of the hula-based program with the culturally tailored heart health education, as originally designed, yields significantly greater benefits than heart health education alone, and arguably above and beyond usual care as well, given that all participants needed to be under physician care to qualify for participation.
The overall systolic BP improvements of −15.3 mmHg (ITT) and −16.9 mmHg (CCA) yielded by the 6-month hula-based program, which includes the heart health education, is sizable compared to other nonpharmacologic interventions [56]. Other aerobic dance interventions show an average systolic BP improvement of −12 mmHg [20]. Traditional physical activity-based interventions improve systolic BP by −5 and −10 mmHg [16, 57]. Dietary interventions improve systolic BP by −6 and −11 mmHg [58, 59]. Weight loss interventions produce systolic BP improvements of −3 to −8 mmHg [60]. Prior research shows a 5 mm Hg reduction in systolic BP can lower a person’s risk for ischemic heart disease by 21%, stroke by 34%, and all-cause mortality by 7% [61, 62].
To examine the clinical significance of the BP improvements, we investigated three HTN stages based on clinical guidelines in place at the start of our study in 2014 [63] and the new guidelines in 2017 [64]. A BP of less than 140/90 mmHg is the recommended target for individuals with HTN but without diabetes and CVD, and less than 130/80 mmHg for those with diabetes [64]. We found that participants of our hula-based cultural dance program were 80% more likely to achieve a BP stage below 130/80 mmHg than control participants, 43% versus 21%, respectively. At 12 months, the intervention group maintained these improvements with a 70% remaining under 140/90 mmHg.
Using the FRS-CVD to estimate the 10-year CVD risk, a validated and popular CVD risk calculator [49], we found nearly a two times greater decrease in 10-year CVD risk for the intervention participants than those in the control condition, but we interpret these results with caution. The FRS-CVD was validated primarily with non-Hispanic whites and blacks, and no validation studies included Native Hawaiians or other Pacific Islanders. The FRS-CVD may underestimate the 10-year CVD risk among Indigenous populations [65]. Although, compared to other CVD risk calculators, the FRS-CVD better predicts CVD risk in other Indigenous populations [66]. Still, caution should be taken in interpreting our findings on CVD risk until further data are available regarding the clinical utility of the FRS-CVD calculator, or any other CVD risk calculator for that matter, with Native Hawaiian and other Pacific Islander populations.
The BP improvements in our study do not appear to be due to weight loss or changes in antihypertensive medication. The high BMI among our study participants is consistent with the prevalence of obesity among Native Hawaiians in general (43%) [67]. It is well-established that exercise interventions for HTN management can produce significant reductions in BP independent from changes in body weight [68, 69]. The possible mechanism for which exercise, independent of weight loss, improves BP includes a reduction in vascular resistance, arterial resistance, and sympathetic activity and improvement in endothelial function, baroreflex sensitivity, and sodium handling. [68–70]. It is also unlikely that the BP improvements in our study were due to any changes in antihypertensive medication from baseline to 6-month follow-up. About 86% of our participants in both the intervention and control were on antihypertensive medication at baseline, and there were no notable changes in these medications prescribed during the 6-month intervention period within or across study groups.
Another possible explanation for the BP improvements we found, aside from the benefits of exercise, might be due to the socio-cultural aspects of hula, especially in light of our finding that the number of hula lessons received was not associated with BP outcomes. Although not found here, a dose-effect has been found in other trials of lifestyle intervention with Native Hawaiians (46, 48). Thus, the benefits of our cultural dance program may be due in part to socio-cultural aspects, such as activating Native Hawaiian collective values and forming social relationships with other group members, which are not dose-dependent. The hula lessons were group-based and emphasized positive interactions with the other group members. For example, our preliminary examination of the 3-month version of the hula-based program found a significant inverse association between systolic BP and social functioning [32] and a decrease in perceptions of racism among the intervention participants [33]. Other studies have found an association between higher social support and lower BP [71, 72] and better HTN control [73, 74] in other populations. A combination of increased physical activity, positive social interactions, and factors related to promoting a positive cultural identity possibly contributed to BP improvements found among the intervention participants of this study. Further studies are needed to elucidate the mediators of the intervention effects on BP to better understand the relative influence of physical activity and socio-cultural factors.
Our application of self-regulation theory [41] to improve long-term maintenance of HTN-related behavior changes appears successful and consistent with previous HTN studies [42, 43, 75]. The long-term maintenance of health-promoting behaviors, such as regular physical activity, associated with positive BP outcomes is often challenging for many people who return to baseline BP levels within 6 months postintervention [76]. Our 12-month results suggest otherwise for our cultural dance program, and that activities based on self-regulation theory can help with long-term positive behavior change. Again, further studies of our hula-based intervention are necessary to tease out the effects of factors related to self-regulation, socio-cultural factors, and physical activity on BP.
One limitation worth noting about our trial is the relatively low number of male participants who represented only 16% of our entire sample. We made a special effort to recruit men including having a male hula instructor and having Native Hawaiian male community leaders of culturally based programs promote and refer potentially eligible men to our study. Although this strategy contributed to the number of men enrolled in our study, we did not achieve a gender-balance. Our challenge in recruiting Native Hawaiian men was not due to eligibility issues, but most likely due to a lack of interest or willingness to participate in an intervention and/or the lack of male community researchers and educators on the project. A majority of community health workers and advocates are female in the Native Hawaiian community. It may also be that the practice of hula is more attractive to Native Hawaiian females than their male counterparts, even though hula, traditionally, was a ubiquitous practice among men [27]. There are also many all-male hula schools in Hawai'i. However, the overrepresentation of Native Hawaiian females in a clinical trial is not unusual. Our prior RCTs of other lifestyle interventions with Native Hawaiians also resulted in a greater than 80% female participation [45, 46, 77]. An investigation into the reasons why Native Hawaiian men are less likely to participate in RCTs is necessary to improve their recruitment into future trials of lifestyle interventions.
In conclusion, our findings have strong implications for other high-risk but understudied ethnic populations, especially other Indigenous groups (e.g., American Indians and Alaska Natives), who also bear a disproportionate CVD risk compared to non-Hispanic whites. Although hula as a cultural practice originated with Native Hawaiians, our study has shown that Indigenous cultural practices can be successfully leveraged for health promotion. The use of culturally responsive approaches for high-risk racial and ethnic populations is strongly recommended to “close the gap” in health disparities [13, 25, 36, 37]. We suspect that the benefits of a culturally-based intervention extend beyond physical activity and to the benefits offered by the social and cultural engagement aspects of such an intervention [11, 12], a concept that is supported by other studies seeking to decrease CVD risk [78–81]. As suggested by our high-retention, culturally based interventions, versus traditional medical or behavioral interventions, are likely to resonate more strongly with certain ethnic groups because of alignment with their cultural values and preferences, thus making them more relevant, accessible, and sustainable [37, 82].
Supplementary Material
Acknowledgments
In addition to the authors, the following were members of the KāHOLO (Hula Optimizing Lifestyle Options) Project: Adrienne Dillard and Cappy Solatorio (Kula no nā Po'e Hawai'i), Donna Palakiko and Corin Kim (Ke Ola Mamo), Aukahi Austin Seabury and Geri Kaleponi (I Ola Lāhui), Monica Esquivel (Wai'anae Coast Comprehensive Health Center), Joey Gonsalves, Suzette Kaho'ohanohano, and Marriza Warren (Hui No Ka Ola Pono), Kahau Vegas (Ke Ola Mamo and Wai'anae Coast Comprehensive Health Center) and Napua Casson, Stacy Haumea, Desmon Haumea, and Wes Sumida (UH-JABSOM). We would like to recognize and thank our External Scientific Advisory Committee members—Cy Bridges (hula master); Karina Walters (University of Washington); Barbara Howard (MedStar Health Research Institute); and Dedra Buchwald (Washington State University)—for their guidance on this study. The KāHOLO Project was funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL126577) and the National Institute on Minority Health and Health Disparities of the National Institutes of Health (U54MD007584). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute and the National Institute of Minority Health and Health Disparities of the National Institutes of Health.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Joseph Keawe'aimoku Kaholokula, Mele Look, Tricia Mabellos, Hyeong Jun Ahn, So Yung Choi, Ka'imi A. Sinclair, Thomas A. Wills, Todd B. Seto, Māpuana de Silva on behalf of the Kā-HOLO Project declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7:e008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. [DOI] [PubMed] [Google Scholar]
- 3.Office of Minority Health. Heart Disease and Native Hawaiians/Pacific Islanders. Available at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=79. Accessibility verified August 8, 2020.
- 4.Schiller J, Lucas J, Ward B, Peregoy J. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2012;10:1–207. [PubMed] [Google Scholar]
- 5.Nakagawa K, Koenig MA, Asai SM, Chang CW, Seto TB. Disparities among Asians and native Hawaiians and Pacific Islanders with ischemic stroke. Neurology. 2013;80:839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa K, Koenig MA, Seto TB, Asai SM, Chang CW. Racial disparities among Native Hawaiians and Pacific Islanders with intracerebral hemorrhage. Neurology. 2012;79:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aluli NE, Jones KL, Reyes PW, Brady SK, Tsark JU, Howard BV. Diabetes and cardiovascular risk factors in Native Hawaiians. Hawaii Med J. 2009;68:152–157. [PMC free article] [PubMed] [Google Scholar]
- 8.Aluli NE, Reyes PW, Brady SK, et al. All-cause and CVD mortality in Native Hawaiians. Diabetes Res Clin Pract. 2010;89:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mau MK, Sinclair K, Saito EP, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in native Hawaiians and other Pacific Islanders. Epidemiol Rev. 2009;31:113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaholokula JK, Saito E, Mau MK, Latimer R, Seto TB. Pacific Islanders’ perspectives on heart failure management. Patient Educ Couns. 2008;70:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Look MA, Kaholokula JK, Carvhalo A, Seto T, de Silva M. Developing a culturally based cardiac rehabilitation program: the HELA study. Prog Community Health Partnersh. 2012;6:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maskarinec GG, Look M, Tolentino K, et al. Patient perspectives on the Hula Empowering Lifestyle Adaptation Study: benefits of dancing hula for cardiac rehabilitation. Health Promot Pract. 2015;16:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havranek EP, Mujahid MS, Barr DA, et al. ; American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council . Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 14.Wexler R, Aukerman G. Nonpharmacologic strategies for managing hypertension. Am Fam Physician. 2006;73:1953–1956. [PubMed] [Google Scholar]
- 15.Vooradi S, Mateti U. A systemic review on lifestyle interventions to reduce blood pressure. J Health Res Rev. 2016;3:1–5. [Google Scholar]
- 16.Semlitsch T, Jeitler K, Hemkens LG, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. 2013;43:1009–1023. [DOI] [PubMed] [Google Scholar]
- 17.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Arch Intern Med. 2008;168:571–580. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 19.Matyas E, Jeitler K, Horvath K, et al. Benefit assessment of salt reduction in patients with hypertension: systematic overview. J Hypertens. 2011;29:821–828. [DOI] [PubMed] [Google Scholar]
- 20.Conceição LS, Neto MG, do Amaral MA, Martins-Filho PR, Oliveira Carvalho V. Effect of dance therapy on blood pressure and exercise capacity of individuals with hypertension: a systematic review and meta-analysis. Int J Cardiol. 2016;220:553–557. [DOI] [PubMed] [Google Scholar]
- 21.Maruf FA, Akinpelu AO, Salako BL. Effects of aerobic exercise and drug therapy on blood pressure and antihypertensive drugs: a randomized controlled trial. Afr Health Sci. 2013;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruf FA, Akinpelu AO, Salako BL. A randomized controlled trial of the effects of aerobic dance training on blood lipids among individuals with hypertension on a thiazide. High Blood Press Cardiovasc Prev. 2014;21:275–283. [DOI] [PubMed] [Google Scholar]
- 23.Aweto HA, Owoeye OB, Akinbo SR, Onabajo AA. Effects of dance movement therapy on selected cardiovascular parameters and estimated maximum oxygen consumption in hypertensive patients. Nig Q J Hosp Med. 2012;22:125–129. [PubMed] [Google Scholar]
- 24.Merom D, Ding D, Stamatakis E. Dancing participation and cardiovascular disease mortality: a pooled analysis of 11 population-based British Cohorts. Am J Prev Med. 2016;50:756–760. [DOI] [PubMed] [Google Scholar]
- 25.Walters KL, Johnson-Jennings M, Stroud S, et al. Growing from our roots: Strategies for developing culturally grounded health promotion interventions in American Indian, Alaska Native, and Native Hawaiian communities. Prev Sci. 2018;21(Suppl 1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaeppler AL.Hula Pahu. Honolulu, HI: Bishop Museum Press; 1993. [Google Scholar]
- 27.Stillman AK.Sacred Hula: The Historical Hula ‘Āla‘apapa. Honolulu HI: Bishop Museum Press; 1998. [Google Scholar]
- 28.Halau Hula (Hula Schools). Available at http://www.mele.com/resources/hula.html. Accessibility verified April 9, 2020.
- 29.Look MA, Maskarinec GG, de Silva M, Seto T, Mau ML, Kaholokula JK. Kumu hula perspectives on health. Hawaii J Med Public Health. 2014;73:21–25. [PMC free article] [PubMed] [Google Scholar]
- 30.Usagawa T, Look M, de Silva M, et al. Metabolic equivalent determination in the cultural dance of hula. Int J Sports Med. 2014;35:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haskell WL, Lee IM, Pate RR, et al. ; American College of Sports Medicine; American Heart Association . Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. [DOI] [PubMed] [Google Scholar]
- 32.Kaholokula JK, Look M, Mabellos T, et al. Cultural dance program improves hypertension management for native hawaiians and pacific islanders: a pilot randomized trial. J Racial Ethn Health Disparities. 2017;4:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaholokula JK, Look MA, Wills TA, et al. ; Kā-HOLO Project . Kā-HOLO Project: a protocol for a randomized controlled trial of a native cultural dance program for cardiovascular disease prevention in Native Hawaiians. BMC Public Health. 2017;17:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchwald D, Mendoza-Jenkins V, Croy C, McGough H, Bezdek M, Spicer P. Attitudes of urban American Indians and Alaska Natives regarding participation in research. J Gen Intern Med. 2006;21:648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. Am J Public Health. 2010;100(Suppl 1):S40–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamoto SK, Kulis S, Marsiglia FF, Steiker LK, Dustman P. A continuum of approaches toward developing culturally focused prevention interventions: from adaptation to grounding. J Prim Prev. 2014;35:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaholokula JK, Ing CT, Look MA, Delafield R, Sinclair K. Culturally responsive approaches to health promotion for Native Hawaiians and Pacific Islanders. Ann Hum Biol. 2018;45:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mau MKL, Lim E, Kaholokula JK, et al. A randomized controlled trial to improve heart failure disparities: the Mālama Pu'uwai (caring for heart) Study. Open Access J Clin Trials. 2017;9:65–74. [Google Scholar]
- 39.Heart Failure Society of America: Heart Failure Education Modules. Available at http://www.hfsa.org/heart_failure_education_modules.asp. Accessibility verified March 28, 2014.
- 40.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. [DOI] [PubMed] [Google Scholar]
- 41.Bandura A. The primacy of self-regulation in health promotion. Appl Psychol-Int Rev. 2005;54:245–254. [Google Scholar]
- 42.Baghianimoghadam M, Aivazi S, Mzloomy SS, Baghianimoghadam B. Factors in relation with self-regulation of hypertension, based on the model of goal directed behavior in Yazd city. J Med Life. 2011;4:30–35. [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor SD, Bagozzi RP, Gaither CA. Decision making and effort in the self-regulation of hypertension: testing two competing theories. Br J Health Psychol. 2005;10:505–530. [DOI] [PubMed] [Google Scholar]
- 44.Bellg AJ, Borrelli B, Resnick B, et al. ; Treatment Fidelity Workgroup of the NIH Behavior Change Consortium . Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. [DOI] [PubMed] [Google Scholar]
- 45.Kaholokula JK, Mau MK, Efird JT, et al. A family and community focused lifestyle program prevents weight regain in Pacific Islanders: a pilot randomized controlled trial. Health Educ Behav. 2012;39:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend CK, Miyamoto RE, Antonio M, et al. The PILI@Work Program: a translation of the diabetes prevention program to Native Hawaiian-serving worksites in Hawai'i. Transl Behav Med. 2016;6:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mau MK, Kaholokula JK, West MR, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Pilot project. Prog Community Health Partnersh. 2010;4:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaholokula JK, Wilson RE, Townsend CK, et al. Translating the diabetes prevention program in native Hawaiian and pacific islander communities: the PILI ‘Ohana Project. Transl Behav Med. 2014;4:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 50.Donato LJ, Deobald GR, Wockenfus AM, Hornseth JM, Saenger AK, Karon BS. Comparison of two point of care devices for capillary lipid screening in fasting and postprandial adults. Clin Biochem. 2015;48:174–176. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair KA, Makahi EK, Shea-Solatorio C, Yoshimura SR, Townsend CK, Kaholokula JK. Outcomes from a diabetes self-management intervention for Native Hawaiians and Pacific People: Partners in Care. Ann Behav Med. 2013;45:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ing CT, Zhang G, Dillard A, et al. Social support groups in the maintenance of glycemic control after community-based intervention. J Diabetes Res. 2016;2016:7913258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCoy CE. Understanding the Intention-to-treat Principle in Randomized Controlled Trials. West J Emerg Med. 2017;18:1075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amico KR. Percent total attrition: a poor metric for study rigor in hosted intervention designs. Am J Public Health. 2009;99:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahim S, Smith GD. Lowering blood pressure: a systematic review of sustained effects of non-pharmacological interventions. J Public Health Med. 1998;20:441–448. [DOI] [PubMed] [Google Scholar]
- 57.Carpio-Rivera E, Moncada-Jiménez J, Salazar-Rojas W, Solera-Herrera A. Acute effects of exercise on blood pressure: a meta-analytic investigation. Arq Bras Cardiol. 2016;106:422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacks FM, Svetkey LP, Vollmer WM, et al. ; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 59.Juraschek SP, Miller ER 3rd, Weaver CM, Appel LJ. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J Am Coll Cardiol. 2017;70:2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harsha DW, Bray GA. Weight loss and blood pressure control (Pro). Hypertension. 2008;51:1420–5; discussion 1425. [DOI] [PubMed] [Google Scholar]
- 61.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 62.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. [DOI] [PubMed] [Google Scholar]
- 63.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 64.Whelton PK, Carey RM, Aronow WS, et al. 2017ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 65.Kumar A, Shariff M. Atherosclerostic cardiovascular disease risk score: Are Indians underestimating the risk of cardiovascular disease? Indian Heart J. 2019;71:364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. [DOI] [PubMed] [Google Scholar]
- 67.Galinsky AM, Zelaya CE, Simile C, Barnes PM.. Health conditions and behaviors of native Hawaiian and pacific islander persons in the United States, 2014. Vital Health Stat. 2017;3:1–99. [PubMed] [Google Scholar]
- 68.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sports Med. 2004;34:307–316. [DOI] [PubMed] [Google Scholar]
- 69.Swift DL, Lavie CJ, Johannsen NM, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15:659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowen KS, Uchino BN, Birmingham W, Carlisle M, Smith TW, Light KC. The stress-buffering effects of functional social support on ambulatory blood pressure. Health Psychol. 2014;33:1440–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schulz U, Pischke CR, Weidner G, et al. Social support group attendance is related to blood pressure, health behaviours, and quality of life in the Multicenter Lifestyle Demonstration Project. Psychol Health Med. 2008;13:423–437. [DOI] [PubMed] [Google Scholar]
- 73.Lu X, Juon HS, He X, Dallal CM, Wang MQ, Lee S. The association between perceived stress and hypertension among Asian Americans: does social support and social network make a difference? J Community Health. 2019;44:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merz EL, Roesch SC, Malcarne VL, et al. Social support, simpatía, and hypertension prevalence in hispanics/latinos: findings from the HCHS/SOL sociocultural ancillary study. J Lat Psychol. 2016;4:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor SD, Bagozzi RP, Gaither CA, Jamerson KA. The bases of goal setting in the self-regulation of hypertension. J Health Psychol. 2006;11:141–162. [DOI] [PubMed] [Google Scholar]
- 76.Hinderliter AL, Sherwood A, Craighead LW, et al. The long-term effects of lifestyle change on blood pressure: One-year follow-up of the ENCORE study. Am J Hypertens. 2014;27:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ing CT, Miyamoto RES, Fang R, et al. Comparing weight loss-maintenance outcomes of a worksite-based lifestyle program delivered via DVD and face-to-face: a randomized trial. Health Educ Behav. 2018;45:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell CN, Thorpe RJ Jr, Laveist TA. Race/Ethnicity and hypertension: the role of social support. Am J Hypertens. 2010;23:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cornwell EY, Waite LJ. Social network resources and management of hypertension. J Health Soc Behav. 2012;53:215–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med. 2003;25:1–7. [DOI] [PubMed] [Google Scholar]
- 81.Yang YC, Boen C, Mullan Harris K. Social relationships and hypertension in late life: evidence from a nationally representative longitudinal study of older adults. J Aging Health. 2015;27:403–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanley LR, Swaim RC, Kaholokula JK, et al. The imperative for research to promote health equity in indigenous communities. Prevention Science. 2017;21(Suppl 1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.