Abstract
Background
Exercise is safe and provides considerable benefits for patients with heart failure (HF) including improved function, quality of life, and symptoms. However, patients with HF have difficulty initiating and adhering to an exercise regimen. To improve adherence, our team developed Heart Failure Exercise and Resistance Training (HEART) Camp, a multicomponent, theory-driven intervention that was efficacious in a randomized controlled trial of long-term adherence to exercise in patients with HF. Identifying active components of efficacious interventions is a priority.
Purpose
The purpose of this study is to use mediation analysis to determine which interventional components accounted for long-term adherence to exercise in patients with HF.
Methods
This study included 204 patients with HF enrolled in a randomized controlled trial. Instruments measuring interventional components were completed at baseline, 6, 12, and 18 months. Hierarchical linear models generated slope estimates to be used as predictors in logistic regression models. Significant variables were tested for indirect effects using path analyses with 1,000 bootstrapped estimates.
Results
Significant mediation effects were observed for the interventional components of negative attitudes (β NA = 0.368, s.e. = 0.062, p < .001), self-efficacy (β SE = 0.190, s.e. = 0.047, p < .001), and relapse management (β RM = 0.243, s.e. = 0.076, p = .001).
Conclusions
These findings highlight improving attitudes, self-efficacy, and managing relapse as key interventional components to improve long-term adherence to exercise in patients with HF. Future interventions targeting adherence to exercise in patients with HF and other chronic illnesses should consider the incorporation of these active components.
Keywords: Heart failure, Exercise, adherence, Mediation analysis, Training
Improving negative attitudes, increasing self-efficacy, and resuming exercise after health setbacks are important to help individuals with heart failure adhere to exercise long-term
Introduction
The U.S. Department of Health and Human Service’s Physical Activity Guidelines for Americans recommends the incorporation of regular exercise into the lives of all adults, including those with existing chronic conditions [1]. The 2013 American College of Cardiology Foundation/American Heart Association Guideline for the Management of Heart Failure specifically recommends exercise training or regular physical activity as safe and effective for patients with heart failure (HF) [2]. Established health benefits of exercise in patients with HF include improvements in functional capacity, health-related quality of life, hospitalizations, and mortality [3–6].
Despite these known benefits, patients with HF have low rates of exercise initiation and difficulty with long-term adherence to exercise programs. Patients with HF experience troublesome symptoms including shortness of breath and fatigue that may be exacerbated by exercise. Exercise intolerance is common and patients with HF describe fear of exercise, lack of knowledge, and lack of self-efficacy in choosing an exercise program appropriate to their health status as barriers to adhering to an exercise program [7–9]. Interventions to promote adherence to regular exercise in patients with HF are limited [10]. Long-term adherence has been a challenge in the HF population in part because the course of the disease includes periods of exacerbation where exercise may not be tolerated followed by stabilization. To address these barriers, our team developed a multicomponent exercise intervention (Heart Failure Exercise and Resistance Training [HEART] Camp) based on social-cognitive and behavioral strategies that are supported in the literature.
Effective strategies that have been used to change exercise behavior in the general population include goal setting [11, 12], self-monitoring [13–16], frequent and prolonged contact [17–19], feedback and reinforcement [11, 12, 18, 20], self-efficacy enhancement [11, 15, 20], modeling [21], and problem solving and relapse prevention [12, 13, 22, 23]. This literature emphasizes that effective strategies for exercise behavior include both social-cognitive aspects (e.g., self-efficacy enhancement, modeling, and frequent and prolonged contact) as well as behavioral aspects (e.g., goal setting and feedback, self-monitoring, problem solving, and relapse prevention). The components of the HEART Camp intervention are targeted to improve knowledge, attitudes, self-efficacy, self-regulation, and social support for achieving long-term adherence to the recommended exercise guidelines for community-dwelling patients with stable HF. These components were selected with influence from Bandura’s social cognitive theory, the model of future-oriented motivation and self-regulation proposed by Miller and Brinkman [24], existing behavior intervention literature, and our prior experience with this intervention in the HF population. Miller and Brinkman expand aspects of social cognitive theory to the context of future goals and long-term behavior change [24].
Knowledge has been shown to drive adherence to other behavioral strategies in HF including medication adherence and self-management. According to Bandura [25], knowledge is a necessary component for problem solving and building self-efficacy. Arming patients with knowledge of the nuances of exercising with HF is key to preventing relapse after an acute HF event.
Attitudes toward exercise are important to initiating and sustaining an exercise program over time. Previous studies have shown that cardiac patients with a past history of exercise behaviors and positive attitudes toward exercise were more likely than those with a negative exercise history to adhere to exercise [26]. Furthermore, a negative emotional response to exercise was found in a qualitative study to adversely influence physical activity behaviors [27] in patients with HF.
Self-regulation, including self-monitoring, of health behaviors has been shown to be effective at increasing physical activity in patients with cardiovascular disease [28]. Goal-setting, individualized plans, and relapse management are key areas of self-regulation to be addressed in exercise interventions [29] and were each incorporated into the HEART Camp intervention [29].
Self-efficacy, or the confidence to complete a task or behavior, is widely incorporated into behavioral interventions as a foundational tenet for behavior change [25]. Self-efficacy is recognized as an important predictor of adherence to exercise and physical activity in healthy and chronically ill adults [30–33]. Interventions to improve adherence to self-care have shown self-efficacy to significantly improve behavioral outcomes in patients with cardiovascular disease [34].
The influence of socialization on behavior is uniquely emphasized by social cognitive theory. Social support has been shown to increase the efficacy of exercise interventions across chronic illness populations [29]. In a systematic review of qualitative literature, encouragement and support from friends and family was positively associated with activity levels [27]. All of these components were incorporated into the HEART Camp intervention to help patients with HF adhere to exercise long-term.
The HEART Camp intervention was tested for efficacy in a 2-group, 18 month randomized controlled trial (RCT) of patients with HF [35]. This trial was efficacious with more subjects in HEART Camp intervention group adhering to exercise at 18 months compared to enhanced usual care. Detailed descriptions of the trial protocol [36] and primary and secondary study outcomes [35, 37] have been published previously. The trial, registered at clinicaltrials.gov (NCT01658670), was designed with quantitative measures of each interventional component and a specific aim to test mediation effects and determine active components of the HEART Camp intervention. The primary outcome of the HEART Camp study was long-term adherence to exercise. Adherence was defined as participation in 80% of the goal of 150 min of moderate intensity exercise per week. At 18 months, 35.2% of the intervention group was adherent compared to 19.3% of the control group. Cohen’s h (difference in arcsine transformations) was 0.36 indicating a moderate effect size for the primary outcome of adherence.
To date, there is no known published research focused on identifying interventional components that contribute to long-term adherence to exercise in patients with HF. Therefore, the purpose of this study was to examine the influence of interventional components of HEART Camp on long-term adherence to exercise in patients with HF enrolled in a RCT using mediation analysis. We hypothesized that knowledge, attitudes, self-efficacy, self-regulation, and social support would significantly mediate the effect of HEART Camp on long-term adherence to exercise in patients with HF.
Methods
This study tested the mediation effects of interventional components on long-term adherence to exercise in participants enrolled in a 2-group, 18-month RCT.
Design
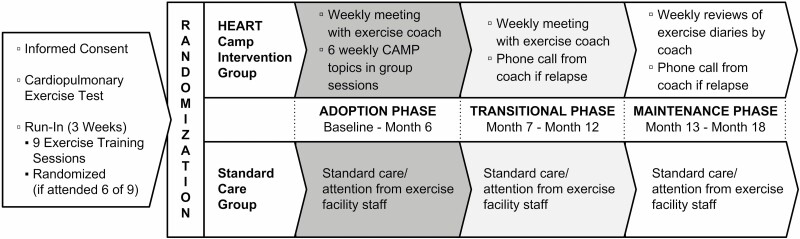
In brief, this multi-site RCT utilized a prospective, two-group, repeated measures design. All participants were required to undergo cardiopulmonary stress testing prior to exercise and a minimum of six supervised training sessions in a cardiac rehabilitation setting. Participants were then randomized to the intervention group or an enhanced usual care group after assuring safety to exercise and an exercise orientation in cardiac rehabilitation. Both groups received paid access to an exercise facility for the entire 18 months of the study. The intervention group also received support from an exercise coach that was tapered over the 18 months. Data were collected at baseline, 6, 12, and 18 months. The study model as originally published will be shown in Fig. 1 with permission from the publisher.
Fig. 1.
Study model. Study model was previously published by BMC in Pozehl BJ, Duncan K, Hertzog M, et al. Study of adherence to exercise in heart failure: the HEART camp trial protocol. BMC Cardiovasc Disord. 2014;14:172. Published 2014 Nov 29. doi:10.1186/1471-2261-14-172.
Sample
Participants (N = 204) were enrolled by trained research assistants from two urban medical centers in two Midwestern states after approval from institutional review boards for all sites. Table 1 shows inclusion/exclusion criteria. Participants that reported current or past exercise in the prior 6 months of 3 or more times per week were excluded. All participants documented written informed consent. Screening and randomization details have been published previously [36].
Table 1.
Study Inclusion/Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| HF with preserved or reduced ejection fraction confirmed with echocardiography and clinical evaluation | Evidence of decompensated HF |
| Adults, 19 years of age or oldera | Unstable angina |
| Able to speak and read English | MI, CABG, or biventricular pacemaker implantation in previous 6 weeks |
| Telephone access | Any orthopedic or neuromuscular disorder that would preclude participation |
| Stable, guideline-directed pharmacologic therapy for ≥30 subsequent days | Current participation in an exercise program 3 or more times per week in the previous 8 weeks |
| CPX results that precluded safe exercise | |
| Plans to move >50 miles from the exercise site in the subsequent year | |
| VO2peak in women >21 ml/kg-1/min−1 or in men >24 ml/kg−1/min−1 | |
| Current or planned pregnancy |
CABG coronary artery bypass graft; CPX cardiopulmonary stress test; MI myocardial infarction; VO2peak peak oxygen uptake.
a Nineteen years of age is the age of majority in one of the states of data collection.
HEART Camp Intervention
The intervention incorporated five theoretically driven components: knowledge, attitudes, self-efficacy, self-regulation, and social support, based on Social Cognitive Theory [25] and Miller and Brickman’s model of Future-Oriented Motivation and Self-Regulation [24]. These components are described in more detail below and were incorporated throughout three interventional phases that were selected based on our prior work [35, 38–40] including adoption (0–6 months), transition (7–12 months), and maintenance (13–18 months). During the adoption phase, participants received educational training that focused on HF pathophysiology, exercise and HF, nutrition, medications and exercise, and attitudes about exercise. Participants also met individually with an exercise coach weekly by phone during the adoption and transition phases to review participants’ exercise diaries, and further reinforce strategies for self-regulation of exercise, relapse prevention, and exercise goal setting. In the maintenance phase, participants continued to submit diaries to exercise coaches on a weekly basis. Exercise coaches contacted any participants who did not submit an exercise diary for the week.
Intervention Components and Measurement Tools
Demographics and clinical characteristics
Self-reported demographic information was collected at baseline from each subject including age, race, sex, and marital status. Baseline clinical variables extracted from the medical record included left ventricular ejection fraction (LVEF), type of HF (preserved vs. reduced LVEF), New York Heart Association (NYHA) functional class and medications. NYHA functional classes were developed in the 1960s by the Criteria Committee of the New York Heart Association as a tool for categorizing functional limitations in patients with HF. NYHA Class I is assigned to those without functional limitations, NYHA Class II includes those with slight physical function limitation, but some shortness of breath is noted with exercise. NYHA Class III patients experience significant limitations in function and experience exercise intolerance with activities. Lastly, NYHA Class IV corresponds to patients that experience symptoms at rest [41].
Adherence
Meeting 80% of a recommended behavior is commonly used to define adherence in exercise trials [3]. For this study, participants were considered adherent to exercise recommendations if they completed 80% of the 150-min guideline (i.e., 120 min/week) recommended by the Heart Failure Society of America 2010 Comprehensive Heart Failure Practice Guideline [42]. Minutes of moderate intensity exercise, averaged across the 4 weeks prior to each measurement point, were obtained from exercise diaries completed by participants daily and validated with data obtained from a heart rate monitor worn by participants during exercise.
Interventional components
The five interventional components: knowledge, attitudes, self-regulation, self-efficacy, and social support and the measures used to determine the mediation effects are described below. Each component was measured at baseline, and at 6, 12, and 18 months. Knowledge of exercise training and general HF-related knowledge was delivered and reinforced via supervised exercise training sessions, weekly coach meetings, and six-group education sessions. Knowledge was measured using the Physical Activity and Heart Disease I.Q. tool, a 12-item True/False tool developed by the National Heart, Lung, and Blood Institute of the National Institutes of Health to assess knowledge of exercise in cardiovascular conditions and how exercise affects the heart [43].
Attitudes including addressing participant safety and perceived benefits and barriers. We incorporated baseline safety measures including cardiopulmonary stress tests to prescribe an individualized exercise prescription at baseline, used heart rate monitors during exercise and monitored rating of perceived exertion, and held weekly phone discussions with the exercise coach and participant to identify strategies to overcome perceived barriers and reinforce benefits to exercise. Attitudes were measured using the Attitudes Toward Physical Activity/Exercise tool. This tool includes 14 items that assess positive (6 items) and negative attitudes (8 items) toward exercise. Cronbach’s alpha has been previously reported at 0.74 for the positive attitude subscale and 0.82 for the negative attitude subscale [44]. For this study, we found α = 0.85 (negative attitudes) and α = 0.82 (positive attitudes).
Interventional components to improve self-efficacy included: (a) Enactive mastery experiences, whereby participants demonstrated mastery of all study equipment, (b) Vicarious experiences by interacting with peers during group educational sessions, (c) Verbal persuasion including educational materials presented in paper form, and (d) Physiological and affective states whereby participants learned to assess and recognize their HF symptoms in relation to exercise and strategies to manage symptoms during exercise. Self-efficacy was measured with the Barriers Self-Efficacy Scale that examines confidence with exercise behavior. Internal consistency for the BARSE was previously reported as excellent (α = 0.92–0.94) [45]. In this sample we recorded α =0.93.
Self-regulation skills were taught during weekly exercise coaching. Participants were taught to self-monitor during exercise using their heart rate monitor, rating of perceived exertion, and symptoms. Exercise coaches addressed these self-regulation skills: goal setting, self-monitoring, problem solving, and barriers management. Self-regulation was measured using the Physical Activity Self-Regulation Scale (PASR) which has been previously validated to measure specific skills necessary for self-regulation [46]. The PASR includes subscales that measure self-monitoring, goal setting, eliciting support, reinforcement, time management, and relapse prevention. Internal consistency in our sample was α = 0.92 (overall scale) and α = 0.73–0.88 (subscales).
Lastly, participants were part of six-group sessions where they had the opportunity to meet peers with similar HF issues. We measured social support using the Revenson Support Scale [47]. This scale measures positive support and problematic support using 20 items. Internal consistency has been reported to range from 0.64 to 0.93 [47]. For the problematic subscale α = 0.70 and the positive subscale α = 0.94 for our sample.
Statistical Analysis
Descriptive statistics were calculated on all study variables. The direct effect of the intervention on adherence was reported in a previous paper [35], with chi-square analyses indicating significant differences in adherence at 12 and 18 months (see Fig. 2 path c). As outlined by Baron and Kenny [48], for a variable to be a mediator the intervention must have a significant effect on that variable (path a) and the variable must then have a significant effect on the outcome (path b). The effect of the intervention on potential mediators was first determined using longitudinal multilevel models (MLM), often called hierarchical linear models or mixed models for change. As opposed to listwise deletion, MLM uses maximum likelihood estimation, which utilizes all available data. Variables found to be non-normally distributed were transformed prior to analysis. The MIXED procedure in SAS version 9.4 was used for this analysis. A separate model was performed for each outcome variable. The models included the intercept, group effect, time, and the time by group interaction, which indicates if the groups significantly differ in change. The variables that were found to be significantly or marginally (p < .10) affected by the intervention were retained for the next step in the analysis.
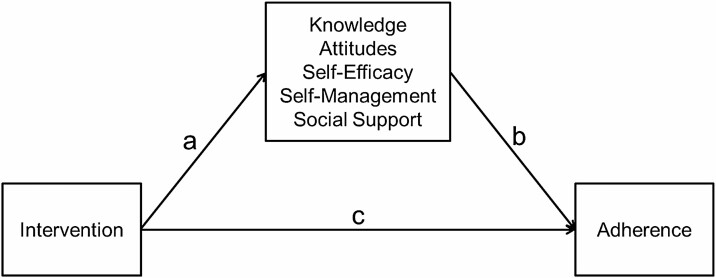
Fig. 2.
Mediation model testing components accounting for change as a result of the intervention.
A second requirement for a variable to be a mediator is that the mediator variable has a significant effect on the outcome of adherence (path b). If the intervention is affecting change, then changes on these variables should predict adherence. During the previous multilevel models, individual slope estimates on the outcomes, using all available data, were saved using empirical Bayes’ slope estimates (model-predicted estimates of change). These values were entered as predictors in logistic regression models predicting adherence to exercise at 18 months.
The variables that significantly predicted adherence (both a and b paths were significant) were then tested for significant indirect effects in Mplus following the bootstrapping procedure (with 1,000 bootstrapped estimates) recommended by Preacher and Hayes [49].
Results
The sample consisted of 204 participants with an average age of 60.4 (±11.5 years). Sample demographics and clinical characteristics are shown in Table 2. Additional details of the demographic characteristics of the sample were previously reported [35]. No significant differences were observed on any baseline demographic or clinical variables across groups. As reported, the HEART Camp group had significantly greater adherence at 12 and 18 months [35]. Of the 204 participants, 188 completed adherence data at 6 months (92% retention), 178 at 12 months (87%), and 159 at 18 months (78%).
Table 2.
Sample Characteristics: Demographic and Clinical Variables
| Characteristic | Intervention | Enhanced Usual Care | P |
|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | ||
| N | 102 | 102 | |
| Lincoln, NE (51, 50%) | Lincoln, NE (51, 50%) | ||
| Detroit, MI (51, 50%) | Detroit, MI (51, 50%) | ||
| Demographic variables | |||
| Age | 59.8 ± 12.6 | 60.9 ± 10.3 | .482 |
| Female | 45 (44.1%) | 46 (45.1%) | .888 |
| Married | 52 (51.0%) | 61 (59.8%) | .205 |
| Nonwhite | 51 (50.0%) | 44 (43.1%) | .326 |
| Clinical variables | |||
| HFrEF | 85 (83.3%) | 80 (78.4%) | .373 |
| HFpEF | 17 (16.7%) | 22 (21.6%) | |
| NYHA functional class | |||
| I | 5 (4.9%) | 11 (10.8%) | .248 |
| II | 54 (52.9%) | 59 (57.8%) | |
| III | 42 (41.2%) | 31 (30.4%) | |
| IV | 1 (1.0%) | 1 (1.0%) | |
| Beta-blocker medication | 99 (97.1) | 100 (98.0) | .651 |
| ACEI/ARB medication | 92 (90.2%) | 83 (81.4%) | .071 |
| LVEF, % | 39.3 ± 12.1 | 40.5 ± 14.0 | .504 |
| BMI, kg/m2 | 35.0 ± 8.6 | 34.7 ± 7.8 | .828 |
ACEI angiotensin-converting enzyme inhibitor; ARB Angiotensin receptor blocker; BMI body mass index; HFpEF heart failure with preserved ejection fraction; HFrEF heart failure with reduced ejection fraction; LVEF left ventricular ejection fraction; NYHA New York Heart Association.
Main Effects on Potential Mediators (Path a)
Intervention effects were tested on the potential mediators using longitudinal MLM. Several variables were transformed (log or power transform) to normalize the outcomes. No significant time by group interactions were found for the Physical Activity and Heart Disease IQ knowledge measure or either subscale of the social support measure (Revenson Support Scale). Table 3 presents parameter estimates for the effect of the intervention over time for each potential moderator variable. A significant effect was observed for the Negative Attitudes subscale on the Attitudes Toward Physical Activity/Exercise scale (p = .016). There were significant effects observed on overall scores on the Physical Activity Self-Regulation Scale (p = .033), as well as three of the subscales: Eliciting Support (p = .007), Relapse Management (p = .025), and Time Management (p = .049). Non-significant effects were observed for PASR Reinforcements (p = .098) and the Barriers Self-Efficacy Scale (p = .051). All effects observed were in the hypothesized direction, so these seven variables were retained for further analyses.
Table 3.
Main Effects on Potential Mediators (Path a)
| Variable | β | S.E. | P |
|---|---|---|---|
| Negative attitudes | −0.054 | 0.022 | .016 |
| Positive attitudes | 1.170 | 1.762 | .507 |
| Barriers Self-Efficacy Scale (BARSE) | 2.209 | 1.124 | .051 |
| Physical Activity and Heart Disease (NHLBI) | −8.369 | 19.780 | .673 |
| PASR self-monitoring | 0.070 | 0.135 | .606 |
| PASR goal setting | 0.159 | 0.129 | .221 |
| PASR eliciting social support | 0.368 | 0.135 | .007 |
| PASR reinforcements | 0.193 | 0.116 | .098 |
| PASR time management | 0.272 | 0.138 | .049 |
| PASR relapse prevention | 0.275 | 0.122 | .025 |
| Physical Activity Self-Regulation Scale (PASR12) | 1.283 | 0.595 | .033 |
| RSS–Problematic Support | −0.025 | 0.025 | .314 |
| RSS–Positive Support | 0.370 | 0.637 | .563 |
Effects of Change in Mediators on Adherence
Individual slope estimates were calculated for the mediators that were found to be affected by the intervention. Logistic regressions were performed with slope estimates predicting adherence to exercise at 18 months (see Table 4). No effects were observed for the PASR subscales of Eliciting Reinforcements or Time Management. The remaining variables significantly predicted adherence, including Negative Attitudes Toward Physical Activity (p < .001), Barriers Self-Efficacy Scale (p < .001), Physical Activity Self-Regulation (PASR) Eliciting Support (p = .004), PASR Relapse Management (p < .001), and overall PASR (p = .013). In each case, participants who improved on the mediators during the study were more likely to be adherent to exercise recommendations at 18 months.
Table 4.
Effects of Change in Mediators on Adherence (Path b)
| Variable | Β | S.E. | P |
|---|---|---|---|
| Negative attitudes | −26.275 | 5.611 | <.001 |
| Barriers Self-Efficacy Scale (BARSE) | 0.380 | 0.081 | <.001 |
| PASR eliciting social support | 2.618 | 0.920 | .004 |
| PASR reinforcements | 1.167 | 1.239 | .346 |
| PASR time management | 0.100 | 0.067 | .135 |
| PASR relapse prevention | 3.302 | 0.926 | <.001 |
| Physical Activity Self-Regulation Scale (PASR12) | 0.736 | 0.298 | .013 |
Indirect Effects of the Intervention
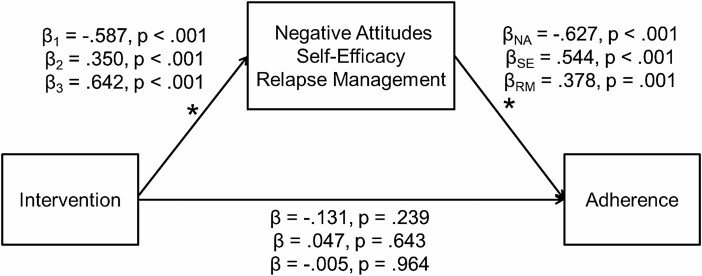
Path analyses testing the indirect effects of the five variables retained indicated that three variables demonstrated significant indirect effects. Significant effects were observed for Negative Attitudes Toward Physical Activity (β = 0.368, s.e. = 0.062, p < .001), the Barriers Self-Efficacy Scale (β = 0.190, s.e. = 0.04, p < .0017), and PASR Relapse Management (β = 0.243, s.e. = 0.076, p = .001). Standardized model estimates for each of the three models, respectively, are presented in Fig. 3.
Fig. 3.
Path models of indirect effects. Note: Beta values are standardized regression coefficients.
The intervention significantly decreased negative attitudes, increased self-efficacy, and increased relapse management, which in turn improved adherence to exercise. In each of the models, the direct effect of the intervention is no longer significant, thus providing evidence of mediation. These effects were further verified by logistic regressions accounting for the intervention and each mediator in a separate model. In each case, the intervention is no longer a significant predictor of adherence. Therefore, negative attitudes, self-efficacy, and relapse management significantly mediated the effect of the intervention on adherence to exercise [49].
Discussion
Long-term adherence to exercise is a challenge for patients with HF. Few studies have tested an intervention to improve long-term adherence to exercise in HF. To the best of our knowledge, no studies have examined interventional components to identify active components within the intervention in this population. Findings from this study demonstrate that the HEART Camp intervention positively impacted long-term adherence to exercise through the theoretically based interventional components. Self-efficacy, negative attitudes, and relapse management were significant mediators of the intervention on long-term adherence.
Consistent with previous research, self-efficacy mediated the treatment effects on adherence to exercise in this sample of patients with HF. Previous studies have demonstrated self-efficacy as a significant mediator in exercise/physical activity interventions targeting individuals who are adolescents [50], healthy adults [51, 52], elderly [45], those with breast cancer [46] or diabetes [53], and those who are overweight [54] or obese [44]. Furthermore, the length of the intervention (short-6 months or less vs. long-12 months or greater) appears to affect whether self-efficacy mediates the intervention effects on exercise/physical activity behavior. Self-efficacy is a significant mediator in long-term exercise/physical activity interventions, but not in the short-term interventions. These findings indicate that individuals’ confidence in their ability to exercise builds over time.
For patients with HF, developing self-efficacy and confidence with exercise training is particularly challenging. When any individual, who has not been exercising, begins a moderate intensity exercise-training program the “normal” physiologic response (signs and symptoms) is a sense of shortness of breath and fatigue. This normal physiologic response to exercise overlaps with the fatigue and shortness of breath that are hallmark signs and symptoms of worsening HF. Therefore, for patients with HF, we believe it takes longer (12 months or longer) to develop the appropriate level of self-efficacy and be confident in their ability to distinguish a “normal” exercise response from “abnormal” HF response (signs and symptoms). This differentiation is particularly important as patients with HF experience the “ups and downs” of their chronic condition while establishing an exercise program. This extended experiential period of time, with HEART Camp coach support, may be necessary for patients with HF to develop adequate self-efficacy to sustain adherence long-term. As mentioned, self-efficacy may require 12 months or longer to build and ultimately impact adherence, even in healthy adults [38, 39]. HF patients experience symptoms of dyspnea and fatigue that are coupled with exercise-related fear, lack of knowledge, and low skill level [7–9]. These issues compromise and lengthen the process of achieving self-efficacy for exercise in patients with HF. To overcome these challenges, exercise should be recommended to patients early in their HF trajectory and support for exercise adherence is needed over a longer period.
Self-reported attitudes toward exercise are noted to impact exercise adherence and have been found to impact whether individuals choose to engage in exercise or avoid it [47, 48]. While these prior studies were completed in healthy adolescents and adults, we hypothesized that attitudes would be particularly important in the HF population. Studies have indicated patients with HF fear exercise and lack knowledge which inherently leads to more negative attitudes and less positive attitudes toward exercise. Negative attitudes are predominant compared to positive attitudes prior to patients with HF initiating an exercise program as patients’ fear and concern of “exercise with HF” override positive attitudes. Furthermore, negative attitudes have been noted as a stronger predictor of physical activity compared to positive attitudes [49]. Patients in our intervention group also experienced a reduction in their depression and anxiety from baseline compared to enhanced usual care [37]. Depression and anxiety reduction may have supported the reduction of negative attitudes. Findings from our study support this previous research indicating the importance of attitudes and especially negative attitudes. Building self-efficacy and the capacity to persist with exercise may have also resulted in the reduction of negative attitudes [51]. They may be particularly important in chronically ill populations of older adults that make up a majority of the HF population. Many of these individuals have not engaged in routine exercise prior to developing HF and have little experience with exercise since having HF. The multicomponent intervention in the HEART Camp trial was successful in alleviating fear for exercise and subsequently played a role in improving exercise adherence.
Long-term adherence to exercise is difficult for healthy adults and is an even greater challenge for individuals with chronic illness. Over the trajectory of HF, patients will experience periods of worsening HF resulting in exercise relapse. Greater relapse has been reported in chronically ill diabetic patients resulting in feelings of perceived guilt [52]. While this has not been studied in patients with HF, the HEART Camp exercise coaches were instructed to proactively address relapse and assist participants throughout the 18-month intervention with return to exercise if relapse occurred. Worsening HF and the need to adjust/abstain from exercise during times of HF exacerbation were discussed in the pre-exercise educational sessions. Participants were told that relapse was anticipated. They were encouraged to return to exercise slowly, as they had when initially starting to exercise, if relapse occurred. Our findings emphasize the importance of relapse management as an interventional strategy in long-term adherence to exercise for patients with HF.
We did not record a mediation effect of the knowledge interventional component. Knowledge has been previously shown as necessary, but insufficient alone to instigate or sustain behavior change [55, 56]. However, we also felt these findings may be attributed to the measure used to quantify knowledge. The true and false items of the Physical Activity and Heart Disease IQ instrument tested knowledge of how exercise affects the heart. Our intervention focused on teaching the knowledge and skills required to exercise with HF. The six sessions of cardiac rehabilitation were meant to give the participant one-on-one guidance while learning and beginning to exercise. This included information about how to evaluate their response to exercise and adjust intensity accordingly. Finally, it is important to recognize that knowledge gained from education is a necessary but not sufficient prerequisite for HF patients to learn self-regulation skills [53].
Social support has been identified as a significant mediator in an intervention to increase physical activity in healthy adults [54]. Our findings did not show social support as a significant mediator. In retrospect, the intervention may have lacked adequate social support. We had six educational sessions that were meant to provide social support from peers, but group membership changed weekly resulting in a lack of consistent social support. Additionally, the social support occurred only in the first 6 weeks of the study. There may have been an inadequate dose of social support with only 6 weeks out of the 18-month study period. Alternatively, these findings may have been driven by our social support measure. The Revenson Support Scale is not specific to physical activity behavior [47]. Other studies reporting generic social support measures did not capture mediation effects for exercise interventions [54]. Future studies should consider the provision of social support for the entire period of the intervention.
Our study is among the first to examine the effect of active interventional components to promote exercise adherence. However, findings may not be generalizable beyond patients with HF in the Midwest. Future exercise interventions should test mechanistic components to validate these findings in future clinical trials. Based on our findings, interventional components that improve self-efficacy, attitudes, and relapse management are important to improve long-term adherence to exercise in patients with HF. Our analyses were limited by a lack of published findings identifying clinically meaningful differences. This will be a focus of our analyses moving forward and we hope to identify benchmarks in future studies.
In conclusion, this study identified self-efficacy, negative attitudes, and relapse management as mediating interventional components important to promoting long term exercise adherence in patients with HF. The burdensome symptoms of HF including shortness of breath and fatigue make it more difficult for patients with HF to adhere to recommended exercise. Identification of active components within an efficacious exercise intervention for patients with HF is important to improve long-term adherence to exercise and ultimately, patient outcomes. Future exercise intervention studies should carefully consider the active interventional components as imperative to moving science forward in this area.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL112979, Pozehl, PI).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflicts of interest.
Authors’ Contributions: The HEART Camp intervention was originally conceptualized by B.P., J.N., and the HEART Camp research team. P.D. assisted with data collection. W.W.A. and K.K. conducted the data analyses and drafted the initial manuscript. All authors contributed to the interpretation of results, the development of the manuscript and reviewed the manuscript in its final form.
Ethical Approval: This study was conducted in accordance with the Declaration of Helsinki or comparable ethical standards, and all procedures involving research study participants were approved by the University of Nebraska Medical Center's Institutional Review Board.
Informed Consent: Written informed consent was obtained from all individual participants included in the study.
References
- 1.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor CM, Whellan DJ, Lee KL, et al. ; HF-ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piña IL, Lin L, Weinfurt KP, et al. ; HF-ACTION Investigators . Hemoglobin, exercise training, and health status in patients with chronic heart failure (from the HF-ACTION randomized controlled trial). Am J Cardiol. 2013;112:971–976. [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Davos C, Francis DP, Coats AJ; ExTraMATCH Collaborative . Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin J, Williams WR, Hutchison S. Multidisciplinary management of elderly patients with chronic heart failure: Five year outcome measures in death and survivor groups. Eur J Cardiovasc Nurs. 2009;8:34–39. [DOI] [PubMed] [Google Scholar]
- 7.Jaarsma T, Abu-Saad HH, Dracup K, Halfens R. Self-care behaviour of patients with heart failure. Scand J Caring Sci. 2000;14:112–119. [PubMed] [Google Scholar]
- 8.Rodriguez KL, Appelt CJ, Switzer GE, Sonel AF, Arnold RM. “They diagnosed bad heart”: A qualitative exploration of patients’ knowledge about and experiences with heart failure. Heart Lung. 2008;37:257–265. [DOI] [PubMed] [Google Scholar]
- 9.Pihl E, Fridlund B, Mårtensson J. Patients’ experiences of physical limitations in daily life activities when suffering from chronic heart failure; A phenomenographic analysis. Scand J Caring Sci. 2011;25:3–11. [DOI] [PubMed] [Google Scholar]
- 10.Deka P, Pozehl B, Williams MA, Yates B. Adherence to recommended exercise guidelines in patients with heart failure. Heart Fail Rev. 2017;22:41–53. [DOI] [PubMed] [Google Scholar]
- 11.Albright CL, Pruitt L, Castro C, Gonzalez A, Woo S, King AC. Modifying physical activity in a multiethnic sample of low-income women: One-year results from the IMPACT (Increasing Motivation for Physical ACTivity) project. Ann Behav Med. 2005;30:191–200. [DOI] [PubMed] [Google Scholar]
- 12.Eakin EG, Bull SS, Riley KM, Reeves MM, McLaughlin P, Gutierrez S. Resources for health: A primary-care-based diet and physical activity intervention targeting urban Latinos with multiple chronic conditions. Health Psychol. 2007;26:392–400. [DOI] [PubMed] [Google Scholar]
- 13.Carels RA, Darby LA, Cacciapaglia HM, Douglass OM. Reducing cardiovascular risk factors in postmenopausal women through a lifestyle change intervention. J Womens Health. 2004;13:412–426. [DOI] [PubMed] [Google Scholar]
- 14.Kumanyika SK, Shults J, Fassbender J, et al. Outpatient weight management in African-Americans: The Healthy Eating and Lifestyle Program (HELP) study. Prev Med. 2005;41:488–502. [DOI] [PubMed] [Google Scholar]
- 15.Perry CK, Rosenfeld AG, Bennett JA, Potempa K. Heart-to-heart: Promoting walking in rural women through motivational interviewing and group support. J Cardiovasc Nurs. 2007;22:304–312. [DOI] [PubMed] [Google Scholar]
- 16.Yancey AK, McCarthy WJ, Harrison GG, Wong WK, Siegel JM, Leslie J. Challenges in improving fitness: Results of a community-based, randomized, controlled lifestyle change intervention. J Womens Health. 2006;15:412–429. [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Champagne CM, Harsha DW, et al. ; Writing Group of the PREMIER Collaborative Research Group . Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. [DOI] [PubMed] [Google Scholar]
- 18.Marcus BH, Napolitano MA, King AC, et al. Telephone versus print delivery of an individualized motivationally tailored physical activity intervention: Project STRIDE. Health Psychol. 2007;26:401–409. [DOI] [PubMed] [Google Scholar]
- 19.Toobert DJ, Strycker LA, Glasgow RE, Barrera M Jr, Angell K. Effects of the mediterranean lifestyle program on multiple risk behaviors and psychosocial outcomes among women at risk for heart disease. Ann Behav Med. 2005;29:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus BH, Bock BC, Pinto BM, Forsyth LH, Roberts MB, Traficante RM. Efficacy of an individualized, motivationally-tailored physical activity intervention. Ann Behav Med. 1998;20:174–180. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. J Consult Clin Psychol. 1998;66:777–783. [DOI] [PubMed] [Google Scholar]
- 22.Green BB, McAfee T, Hindmarsh M, Madsen L, Caplow M, Buist D. Effectiveness of telephone support in increasing physical activity levels in primary care patients. Am J Prev Med. 2002;22:177–183. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs AD, Ammerman AS, Ennett ST, et al. Effects of a tailored follow-up intervention on health behaviors, beliefs, and attitudes. J Womens Health. 2004;13:557–568. [DOI] [PubMed] [Google Scholar]
- 24.Miller RB, Brickman SJ. A Model of future-oriented motivation and self-regulation. Educ Psychol Rev. 2004;16(1): 9–33. [Google Scholar]
- 25.Bandura A.Self-Efficacy the Exercise of Control. New York, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- 26.Byun W, Ozemek C, Riggin K, Strath S, Kaminsky L. Correlates of objectively measured physical activity in cardiac patients. Cardiovasc Diagn Ther. 2014;4:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tierney S, Mamas M, Skelton D, et al. What can we learn from patients with heart failure about exercise adherence? A systematic review of qualitative papers. Health Psychol. 2011;30:401–410. [DOI] [PubMed] [Google Scholar]
- 28.Ferrier S, Blanchard CM, Vallis M, Giacomantonio N. Behavioural interventions to increase the physical activity of cardiac patients: A review. Eur J Cardiovasc Prev Rehabil. 2011;18:15–32. [DOI] [PubMed] [Google Scholar]
- 29.Greaves CJ, Sheppard KE, Abraham C, et al. ; IMAGE Study Group . Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CS, Sloane R, Morey MC. Developing predictors of long-term adherence to exercise among older veterans and spouses. J Appl Gerontol. 2020;39:1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caetano LCG, Pacheco BD, Samora GAR, Teixeira-Salmela LF, Scianni AA. Self-efficacy to engage in physical exercise and walking ability best predicted exercise adherence after stroke. Stroke Res Treat. 2020;2020:2957623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang KM, Dindoff K, Arnold JM, Lane J, Swartzman LC. What matters to patients with heart failure? The influence of non-health-related goals on patient adherence to self-care management. Patient Educ Couns. 2015;98:927–934. [DOI] [PubMed] [Google Scholar]
- 33.Martin KA, Sinden AR. Who will stay and who will go? A review of older adults’ adherence to randomized controlled trials of exercise. J Aging Phys Act. 2001;9(2):91–114. [Google Scholar]
- 34.Kashani M, Eliasson AH, Walizer EM, et al. Early empowerment strategies boost self-efficacy to improve cardiovascular health behaviors. Glob J Health Sci. 2016;8:55119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozehl BJ, McGuire R, Duncan K, et al. Effects of the HEART camp trial on adherence to exercise in patients with heart failure. J Card Fail. 2018;24:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pozehl BJ, Duncan K, Hertzog M, et al. Study of adherence to exercise in heart failure: The HEART camp trial protocol. BMC Cardiovasc Disord. 2014;14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman JF, Kupzyk KA, Artinian NT, et al. The influence of the HEART Camp intervention on physical function, health-related quality of life, depression, anxiety and fatigue in patients with heart failure. Eur J Cardiovasc Nurs. 2020;19:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pozehl B, Duncan K, Hertzog M. The effects of exercise training on fatigue and dyspnea in heart failure. Eur J Cardiovasc Nurs. 2008;7:127–132. [DOI] [PubMed] [Google Scholar]
- 39.Pozehl B, Duncan K, Hertzog M, Norman JF. Heart Failure Exercise and Training Camp: Effects of a multicomponent exercise training intervention in patients with heart failure. Heart Lung. 2010;39:S1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pozehl B, Duncan K, Krueger S, VerMaas P. Adjunctive effects of exercise training in heart failure patients receiving maximum pharmacologic therapy. Prog Cardiovasc Nurs. 2003;18:177–183. [DOI] [PubMed] [Google Scholar]
- 41.Criteria Committee NYHA, Inc. Diseases of the heart and blood vessels. Nomenclature and criteria for diagnosis. 6th ed. Boston, MA: Little, Brown and Co; 1964. [Google Scholar]
- 42.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1–194. [DOI] [PubMed] [Google Scholar]
- 43.National Heart, Lung, and Blood Institute. Physical activity & heart disease I.Q. [NIH Publication No. 96–3795]. Available at http://www.uky.edu/~hadleyr/downloads/phy_act.htm. Accessibility verified December 28, 2020.
- 44.Nelson TD, Benson ER, Jensen CD. Negative attitudes toward physical activity: Measurement and role in predicting physical activity levels among preadolescents. J Pediatr Psychol. 2010;35:89–98. [DOI] [PubMed] [Google Scholar]
- 45.McAuley E, Mailey EL, Mullen SP, et al. Growth trajectories of exercise self-efficacy in older adults: Influence of measures and initial status. Health Psychol. 2011;30:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Umstattd MR, Motl R, Wilcox S, Saunders R, Watford M. Measuring physical activity self-regulation strategies in older adults. J Phys Act Health. 2009;6(suppl 1):S105–S112. [DOI] [PubMed] [Google Scholar]
- 47.Revenson TA, Schiaffino KM, Majerovitz SD, Gibofsky A. Social support as a double-edged sword: The relation of positive and problematic support to depression among rheumatoid arthritis patients. Soc Sci Med. 1991;33:807–813. [DOI] [PubMed] [Google Scholar]
- 48.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 49.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 50.Taymoori P, Lubans D. Mediators of behavior change in two tailored physical activity intervenitons for adolescent girls. Psychol Sport Exerc. 2008;9:605–619. [Google Scholar]
- 51.Anderson ES, Winett RA, Wojcik JR, Williams DM. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: Social support, self-efficacy, outcome expectations and self-regulation in the guide-to-health trial. J Health Psychol. 2010;15:21–32. [DOI] [PubMed] [Google Scholar]
- 52.Anderson-Bill ES, Winett RA, Wojcik JR, Williams DM. Aging and the social cognitive determinants of physical activity behavior and behavior change: Evidence from the guide to health trial. J Aging Res. 2011;2011:505928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dyck D, De Greef K, Deforche B, et al. Mediators of physical activity change in a behavioral modification program for type 2 diabetes patients. Int J Behav Nutr Phys Act. 2011;8:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roesch SC, Norman GJ, Villodas F, Sallis JF, Patrick K. Intervention-mediated effects for adult physical activity: A latent growth curve analysis. Soc Sci Med. 2010;71:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arlinghaus KR, Johnston CA. Advocating for behavior change with education. Am J Lifestyle Med. 2018;12:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corace K, Garber G. When knowledge is not enough: Changing behavior to change vaccination results. Hum Vaccin Immunother. 2014;10:2623–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]