Abstract
Background:
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are life-threatening spectra of mucocutaneous delayed hypersensitivity reactions. Prodromal viral-like symptoms are followed by a characteristic diffuse rash caused by keratinocyte apoptosis and epidermal detachment.
Cases:
Three adolescents were admitted with SJS/TEN and vulvovaginal involvement following initiation of Lamictal, Bactrim, and Phenobarbital. The patients received intravenous immunoglobulin and IV steroids. One patient received Etanercept. Topical emollients and strict perineal hygiene were initiated. No permanent sequelae were noted following vaginoscopy.
Summary and Conclusions:
Vulvovaginal involvement in SJS/TEN can occur and may result in permanent architectural changes. Early initiation of perineal hygiene, vaginal barrier creams, and menstrual suppression, if indicated, should be employed. Vaginoscopy may be used to document full recovery.
Background
Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are a rare but life-threatening spectra of mucocutaneous delayed hypersensitivity reactions. They most commonly occur secondary to medication exposures; most frequently anticonvulsants, sulfa containing antibiotics, allopurinol, and nonsteroidal anti-inflammatory drugs (NSAIDs) [1, 2, 3]. Sequelae of vulvovaginal involvement in SJS/TEN may result in severe, permanent architectural changes to the vagina, including total obliteration [4, 5, 6, 7, 8]. No standardized treatment exists but mainstays include withdrawal of the causative medication, supportive therapy, and immune mediating therapies including intravenous immunoglobulin (IVIG), steroids, and plasmapheresis.
We describe three adolescents who presented to our academic pediatric tertiary care center between June and September 2018 with diffuse maculopapular rash and bullae consistent with SJS/TEN after exposure to anticonvulsants (Lamictal, Phenobarbital) and Bactrim. Signed parental and patient consent for publication was obtained for each case.
Case 1
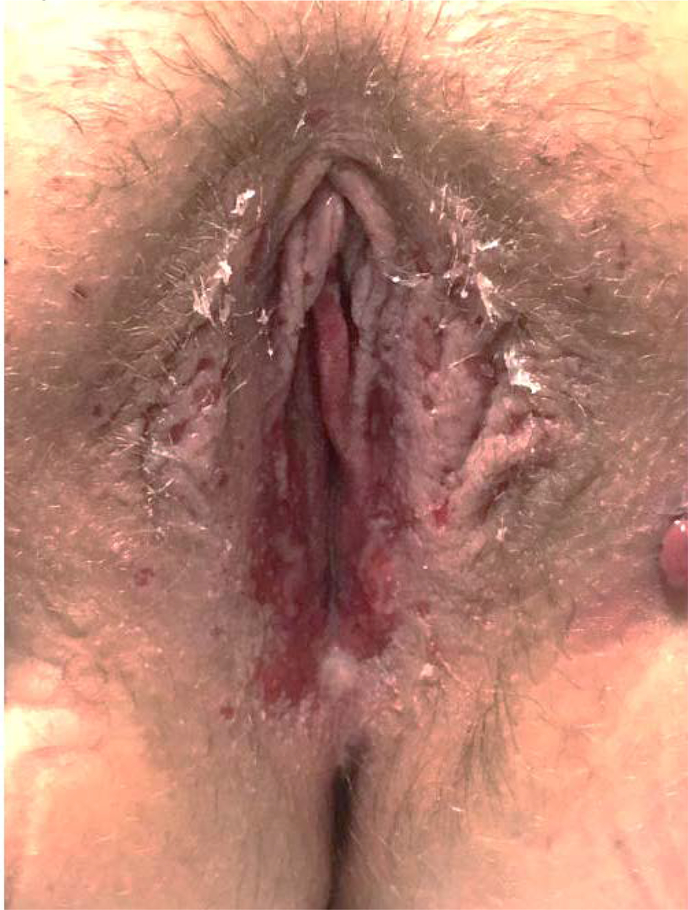
AA is a 15 year old with a history of juvenile myoclonic epilepsy who presented for a maculopapular rash while being transitioned from Levetiracetam to Lamictal. The rash developed on her chest, and shoulders. Lamictal was discontinued by her neurologist. A week later the rash spread to her face and genitals. She was started on prednisone for outpatient treatment but subsequently presented to the emergency department for evaluation of her worsening rash shortly thereafter. Dermatology consultation was obtained and the patient’s clinical findings were felt to be consistent with SJS/TEN. She received treatment with intravenous steroids and a three day course of IVIG. Gynecology was consulted because of the perineal distribution of the rash and patient discomfort. Gynecological exam was notable for significant bilateral blistering along the labia majora as well as in the vestibule between six and seven o’clock (Image 1).Recommendation was made for around the clock topical emollient and for perineal care after voids (cleansing the perineum with sterile water applied by a squirt bottle) to prevent agglutination. She was started on Norethindrone for menstrual suppression. Nine days after presentation her vulvovaginal findings were found to be resolved. While the patient did not follow up with Gynecology for a genitourinary examination, documentation in the dermatology clinic noted full resolution of her signs and symptoms.
Image 1:
Case 1 at time of first vulvovaginal exam
Case 2
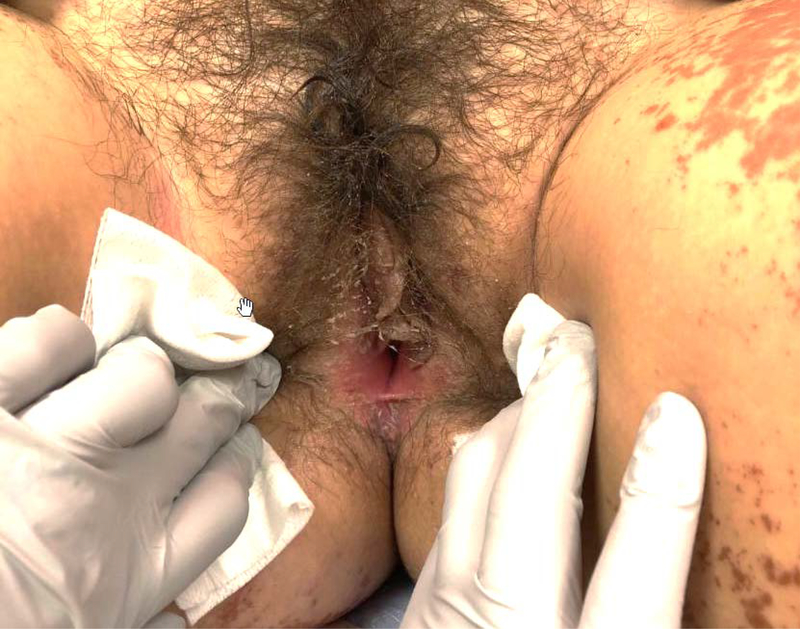
K.C. is a 13 year old with no prior significant past medical history who presented to our hospital with fever and subsequent development of a maculopapular and vesicular rash sparing her hands and feet following a course of Bactrim for suspected Methicillin Resistant Staphylococcus Aureus infection secondary to a spider bite. Dermatology was consulted and clinical findings were consistent with SJS/TEN. She received IVIG for two days. She was also noted to have elevated transaminases and IV steroids were subsequently started. Gynecology was consulted due to vulvar complaints and gynecological exam showed involvement of the mons, labia minora, and erythema of the vestibule with denuding of the epithelium of the inferior labia minora (Image 2). Recommendation was made for topical emollient and perineal care after voids to prevent agglutination. Significant improvement of her vulvovaginal findings was noted 10 days following presentation and she was discharged. Genitourinary examination and vaginoscopy performed approximately five weeks later showed no agglutination of the vulva or vagina.
Image 2:
Case 2 at time of first vulvovaginal exam
Case 3
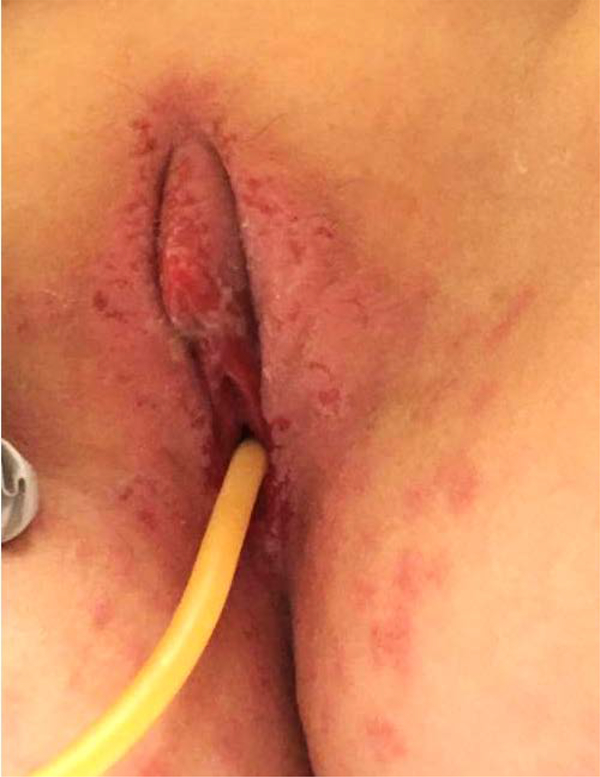
DL is a nine year old with a history of seizure disorders and developmental delay who was admitted to an outside hospital for status epilepticus and started on Phenobarbital. While hospitalized she developed a rash on her perineum and buttocks that was thought to be a candidal infection. She subsequently developed a diffuse mucocutaneous rash for which she was treated with Ceftriaxone, Doxycycline, and Penicillin due to initial concern for bacterial infection. The patient was subsequently transferred to our institution. Dermatology was consulted and clinical findings were consistent with SJS/TEN. The patient was started on Etanercept but developed transaminitis and transitioned to a three day course of IVIG and IV steroids. Gynecology was consulted due to the patient’s vulvovaginal complaints and exam revealed a desquamating rash of the mons, erythematous perineum and labia majora, as well as ulcerated lesions involving the labia minora (Image 3). Foley catheter was placed due to vulvar pain with urination. Recommendation was made for topical emollient and perineal care after voids to prevent agglutination. Significant improvement of her perineum and labia was noted two weeks after admission. Outpatient vaginoscopy showed no labial or vaginal agglutination.
Image 3:
Case 3 at time of first vulvovaginal exam
Discussion
SJS/TEN are a spectrum of syndromes that most commonly occur as adverse drug reactions. These syndromes are characterized by diffuse cutaneous and mucous membrane involvement due to keratinocyte apoptosis resulting in detachment of the dermis from the epidermis [3]. The characteristic rash is typically preceded by a prodromal period of fever and viral-like symptoms, such as sore throat, photophobia, myalgias, and arthritis. The skin lesions start as erythema and then progress to non-blanching erythematous-to-purpuric lesions that then progress to frank bullae and skin sloughing. The mucous membrane involvement mirrors the cutaneous findings and can affect the eyes, mouth, and up to 70% of cases may involve the vulvovaginal mucosa [4].
These syndromes are further defined by body surface area (BSA) affected, with SJS affecting up to 10% BSA and TEN more than 30% BSA [9]. The majority of cases can be directly attributed to medication with up to 50% associated with the use of anticonvulsants including Carbamazepine, Phenytoin, and Phenobarbital. Other medications that are commonly associated include sulfonamide antibacterials, allopurinol, and OXICAM containing NSAIDS [3]. The sequelae of this disease can be severe, with a reported 1-year mortality rate of approximately 24–49% based upon the amount of cutaneous involvement and in severe cases mortality rate of approximately 80% within 60 days [10,12].
Although SJS/TEN is a rare condition in both adult and pediatric populations, vulvovaginal involvement is common and long-lasting sequelae may include atrophy, strictures, synechiae, stenosis of the vaginal introitus, dyspareunia, and obliteration of the vagina [13, 14, 7]. The mainstay of vulvovaginal management is supportive. The simple interventions of aggressive perineal hygiene were enough to prevent the most common vulvovaginal sequelae in our series and should be initiated immediately and may be combined with the placement of an indwelling catheter depending on the extent and progression of the bullae [18]. The simplicity of this intervention is particularly useful in settings where pediatric gynecological services are unavailable or unable to see the patient in a timely fashion.
While no specific articles discuss menstruation in the setting of SJS/TEN, it is reasonable for older adolescents to discuss and initiate menstrual suppression to aid in comfort with menstrual hygiene and to prevent agglutination. Some evidence does suggest employing topical or intravaginal steroids, depending on the severity and progression of the disease, particularly in older patients who may also opt for the use of vaginal dilators. In younger girls or non-sexually active adolescents vaginoscopy is a simple tool to confirm clinical improvement or to diagnose any of the previously mentioned findings [7]. All three of the patients in our case series underwent genitourinary examination and/or vaginoscopy, confirming the absence of gynecological sequelae.
Management of a patient with SJS or TEN can be difficult, as there is no standardized treatment beyond basic management. For any patient with SJS or TEN, the pillars of care include withdrawal of the causative medication, supportive care with meticulous wound care, and evaluation by appropriate specialists. Given the widespread involvement and multiple organ systems that can be compromised, these conditions require multi-specialty coordination of care. Any patient suspected of having SJS/TEN should be seen by dermatology (to confirm the diagnosis), gynecology for patients with a vulva and vagina (for management and evaluation of vulvovaginal involvement), and ophthalmology (for management and evaluation of ocular involvement). Patients should be admitted to a burn center, if possible, as those patients cared for at these facilities have a much lower mortality rate [15]. Other therapies that have been tried include systemic steroids, IVIG, cyclosporine, TNF-a inhibitors, and plasmapheresis [9, 16, 17]. There is currently significant conflicting evidence surrounding the use of these agents and a complete discussion is beyond the scope of this report.
In all three of the above cases high suspicion and early intervention successfully prevented any serious complications of SJS/TEN. Ultimately, the initiation of IVIG, steroids, and strict perineal hygiene resulted in rapid improvement in gynecological symptoms. The gynecologic interventions described above are not only simple but they are also well tolerated by patients and may be continued outpatient. It is important to note that pediatric gynecologic care may not be readily available in many medical centers and our interventions can be easily initiated in the absence of a pediatric gynecologist.
References:
- 1.Emans SJH, & Laufer MR (Eds.). (2011). Emans, Laufer, Goldstein’s pediatric & adolescent gynecology (6th ed). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins Health. [Google Scholar]
- 2.Pliskow S (2013). Severe gynecologic sequelae of Stevens-Johnson syndrome and toxic epidermal necrolysis caused by ibuprofen: a case report. The Journal of Reproductive Medicine, 58(7–8), 354–356. [PubMed] [Google Scholar]
- 3.Mockenhaupt M (2011). The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Review of Clinical Immunology, 7(6), 803–813; quiz 814–815. 10.1586/eci.11.66 [DOI] [PubMed] [Google Scholar]
- 4.Meneux E, Wolkenstein P, Haddad B, Roujeau JC, Revuz J, & Paniel BJ (1998). Vulvovaginal involvement in toxic epidermal necrolysis: a retrospective study of 40 cases. Obstetrics and Gynecology, 91(2), 283–287. [DOI] [PubMed] [Google Scholar]
- 5.Meneux E, Paniel BJ, Pouget F, Revuz J, Roujeau JC, & Wolkenstein P (1997). Vulvovaginal sequelae in toxic epidermal necrolysis. The Journal of Reproductive Medicine, 42(3), 153–156. [PubMed] [Google Scholar]
- 6.Hart R, Minto C, & Creighton S (2002). Vaginal adhesions caused by Stevens-Johnson syndrome. Journal of Pediatric and Adolescent Gynecology, 15(3), 151–152. [DOI] [PubMed] [Google Scholar]
- 7.de Jesus LE, Dekermacher S, Manhães CR, Faria LM, & Barros ML (2012). Acquired labial sinechiae and hydrocolpos secondary to Stevens-Johnson syndrome. Urology, 80(4), 919–921. 10.1016/j.urology.2012.06.051 [DOI] [PubMed] [Google Scholar]
- 8.Boyraz G, Basaran D, Salman MC, Ozgul N, & Yuce K (2017). Vaginal Reconstruction for Vaginal Obliteration Secondary to Stevens Johnson Syndrome: A Case Report and Review of Literature. Oman Medical Journal, 32(5), 436–439. 10.5001/omj.2017.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, & Harr T (2018). Current Perspectives on Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Clinical Reviews in Allergy & Immunology, 54(1), 147–176. 10.1007/s12016-017-8654-z [DOI] [PubMed] [Google Scholar]
- 10.Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197–1204. doi: 10.1038/jid.2012.510) [DOI] [PubMed] [Google Scholar]
- 11.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115(2):149–153. doi: 10.1046/j.1523-1747.2000.00061.x [DOI] [PubMed] [Google Scholar]
- 12.Guégan S, Bastuji-Garin S, Poszepczynska-Guigné E, Roujeau JC, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126(2):272–276. doi: 10.1038/sj.jid.5700068 [DOI] [PubMed] [Google Scholar]
- 13.Lee HY, Walsh SA, & Creamer D (2017). Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. British Journal of Dermatology, 177(4), 924–935. 10.1111/bjd.15360 [DOI] [PubMed] [Google Scholar]
- 14.Wilson EE, & Malinak LR (1988). Vulvovaginal sequelae of Stevens-Johnson syndrome and their management. Obstetrics and Gynecology, 71(3 Pt 2), 478–480. [PubMed] [Google Scholar]
- 15.Palmieri TL, Greenhalgh DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehabil. 2002;23(2):87–96. doi: 10.1097/00004630-200203000-00004 [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Tang S, Li S, Pan Y, & Ding Y (2019). Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a systemic review. The Journal of Dermatological Treatment, 1–8. 10.1080/09546634.2019.1577548 [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, & Mockenhaupt M (2017). Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-analysis. JAMA Dermatology, 153(6), 514–522. 10.1001/jamadermatol.2016.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhuri TK, & Kremer C (2010). Early gynaecological assessment following Stevens-Johnson syndrome/toxic epidermal necrolysis. Journal of Obstetrics and Gynaecology: The Journal of the Institute of Obstetrics and Gynaecology, 30(8), 871–872. 10.3109/01443615.2010.501415 [DOI] [PubMed] [Google Scholar]