Abstract
Objective
This research examined whether a cannabidiol (CBD)-opioid pharmacotherapy could attenuate cisplatin-induced tactile allodynia.
Methods
Mice (C57BL/6) were given 6 doses of 2.3 mg/kg cisplatin intraperitoneally (IP) on alternating days to induce tactile allodynia as quantified using an electric von Frey (eVF). Test groups in Experiment 1 received either vehicle, 0.1 or 2.5 mg/kg morphine, 1.0 or 2.0 CBD, or the 2 drugs in combination. Test groups in Experiment 2 received either vehicle, 0.1 or 2.5 mg/kg morphine, 1.0, 2.0, 3.0, or 4.0 mg/kg NB2111 (a long-acting CBD analogue), or the 2 drugs in combination. Drugs were administered IP 45 min before eVF assessment.
Results
Cisplatin produced tactile allodynia that was attenuated by 2.5 mg/kg morphine. Both CBD and NB2111 produced dose-dependent attenuation of tactile allodynia. CBD and NB2111, given in combination with sub-analgesic doses of morphine, produced attenuation of tactile allodynia equivalent to 2.5 mg/kg morphine.
Conclusions
While both CBD and NB2111, either alone or in combination with sub-analgesic doses of opioids, exhibited analgesic effects, NB2111 could be capable of superior analgesia over time by virtue of enhanced pharmacokinetics.
Keywords: Cisplatin, Cannabidiol, Cisplatin, Neuropathy, Analgesia, Allodynia
Introduction
Cisplatin is a common agent in chemotherapy used to treat a variety of cancers. Unfortunately, cisplatin has a dose-limiting effect, wherein 50–85% of all patients develop peripheral neuropathy 3–6 months into treatment. Cisplatin-induced neuropathy (CIN) presents in a “stocking and glove” distribution causing tingling paresthesia, numbness, and allodynia [1, 2]. Pain management for CIN includes anticonvulsant, antidepressant, and non-steroidal anti-inflammatory drugs. These drugs prove to be well tolerated in patients but show little efficacy in treating CIN [2, 3, 4]. While opioids can provide effective CIN pain relief, 76–96% of all patients report aversive side effects that include sedation, nausea, and fatigue which limit usefulness and diminish patients' quality of life [5, 6]. Added concerns of opioid therapy include tolerance, dose escalation, and dependence that can lead to withdrawal symptoms upon CIN resolution [7]. Collectively, these observations suggest a need to develop novel pharmacotherapies for CIN.
Cannabinoids (CB) are used in oncology settings to control nausea, weight loss, lack of appetite, and chemotherapy-related pain [8]. CB analgesia in both chronic and acute pain models is mediated through CB1 and CB2 receptors that are differentially expressed in the central and peripheral nervous systems [9, 10]. An emerging body of literature supports the notion that endocannabinoid systems may also modulate CIN. For example, CB1 and CB2 direct and indirect agonists attenuate tactile allodynia in rodent models of CIN [5, 11, 12]. However, like non-opioid therapies, CB compounds have modest efficacy and are of limited usefulness.
CB1 and opioid receptors are co-localized in pain pathways. Evidence suggests that a dual pharmacotherapy at these targets may increase CB-mediated analgesic effects [13, 14, 15, 16]. For example, the CB1 agonist THC shows synergistic effects with sub-analgesic doses of the μ-opi oid agonist morphine in a rat arthritic pain model [17]. However, the use of any CB1 agonist in oncology settings is unlikely due to these compounds increasing the proliferation and growth of some tumor cells [15]. Interestingly, other CB that show low affinity to CB receptors also show synergistic effects with low dose opioids. For example, cannabidiol (CBD) shows synergistic effects with sub-analgesic doses of morphine in an acute pain model (i.e., acetic acid writhing) but not against thermal pain [18]. Whether a combined CBD-opioid or a bio-engineered synthetic analogue of CBD-opioid pharmacotherapy could provide highly efficacious pain relief against CIN is unknown. In Experiment 1, we hypothesize a CBD-opioid interaction may enhance the analgesic effects against CIN allodynia. Further, in Experiment 2, we hypothesize a bio-engineered CBD-opioid pharmacotherapy could also produce robust analgesic effects compared to CBD alone and in combination with an opioid.
Methods
Animals
Male C57BL/6 mice (25–30 g; Envigo, Indianoplis, IN, USA) were housed 5 per polycarbonate tub with soft bedding in a temperature- and humidity-controlled vivarium. The mice were maintained under a 12-h light/dark cycle with lights on at 06: 00. Food and water were available ad libitum. Animals acclimated to the vivarium 1 week prior to experimental manipulations. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Mississippi and collected at the University (protocols 13-017 and 15-022).
Behavioral Measures
An electronic von Frey (eVF; Topcat Metrology Ltd., Little Downham, UK) quantified the development of tactile allodynia during CIN induction and served as the endpoint in analgesic screening. The animals were placed into an elevated clear Plexiglas enclosure (3.81 × 11.43 × 11.43 cm) with a metal rod floor. After an acclimation period of 15 min, a von Frey filament was applied to the mid-plantar region of the hind paw and withdrawal thresholds were recorded. The filaments were alternatingly applied to the left and right hind paws at 3-min intervals for a total of 4 measurements per paw. The average score of these 8 tests served as the dependent measure.
Test Compounds
Experiment 1
Cisplatin (Tocris, Ellisville, MO, USA) was dissolved in 0.9% saline to yield dosages of 2.3 mg/kg/mL. Lactated Ringer's solution (0.25 mL; Abbott laboratories, Chicago, IL, USA) was used to hydrate the mice to prevent kidney and liver damage associated with repeated cisplatin administration. Due to the efficacy of alleviating allodynia, morphine served as the gold standard analgesic. Morphine sulfate (Research Biochemicals International, Natick, MA, USA) was dissolved in 0.9% saline to yield dosages of 0.1 and 2.5 mg/kg/mL. CBD 1.0 and 2.0 mg/kg/mL was chosen for this experiment as previous studies in our lab have shown it to provide analgesic properties. CBD 1.0 and 2.0 mg/kg/mL (NIDA Drug Supply Program) solutions were prepared by dissolving CBD in 5% ethanol, 5% cremophor, and injectable water. All test compounds were administered intraperitoneally (IP). Sample sizes ranged from 5 for the drug group of 2.5 mg/kg morphine and 2.0 mg/kg CBD to 9–14 animals for all other drug groups.
Experiment 2
Morphine sulfate was dissolved in 0.9% saline to yield dosages of 0.1 and 2.5 mg/kg/mL. NB2111 (Nemus Bioscience Inc., Costa Mesa, CA, USA) 1.0–4.0 mg/kg/mL was dissolved in 5% ethanol, 5% cremophor, and injectable water. Doses of NB2111 were chosen based on the doses used in Experiment 1. All test compounds were administered IP. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Mississippi (protocol 15-022). Sample sizes ranged from 9 to 11 animals per drug group.
Synthesis of NB2111 (CBD-Mono-Val-Mono-Hemisuccinate)
NB2111 was synthesized by Elsohly Laboratories, Inc., Oxford, MS, USA. CBD was dissolved in dichloromethane (DCM) and a catalytic amount of 4-dimethylaminopryridine (DMAP). Boc-valine (1.1 eq.) was dissolved in DCM and 1.1 eq. of dicyclohexylcarbodiimide (DCC). The CBD/DMAP solution was then added to Boc-valine/DCC solution. Thin layer chromatography (10% ethyl acetate (EtOAc)/90% hexane) indicated the completion of reaction. The product was then purified using silica gel and gradient-elution (beginning at 0% EtOAc/100% hexane and increasing to 3% EtOAc/97% hexane) to yield pure CBD-mono-val-boc. CBD-mono-val-boc was dissolved in tetrahydrofuran with excess HCl(g) removed with N2(g) to form CBD-mono-val. CBD-mono-val was confirmed by mass spectrometry. CBD-mono-val was dissolved in DCM and a catalytic amount of DMAP. Succinic anhydride (1.1 eq.) and triethylamine was added to the CBD-mono-val/DMAP solution. CBD-mono-val-mono-hemisuccinate (NB2111) was purified using silica gel and gradient-elution (beginning at 0% EtOAc/100% hexane and increasing to 30% EtOAc/70% hexane) as pure compound. NB2111 was confirmed by mass spectrometry.
Cisplatin Induction and Drug Efficacy Screening Procedure
The cisplatin induction protocol is summarized in Table 1. Mice received 6 IP injections of cisplatin (2.3 mg/kg/mL) on alternating days with lactated Ringer's solution on intervening days over a 12-day period. Baseline eVF measurements were taken prior to enrollment in the study to ensure balanced group assignments. To monitor the progression of tactile allodynia, additional eVF measurements were taken on Ringer's solution Days 3 and 6 prior to daily injections. On Ringer's solution Day 6, eVF measurements revealed significantly lower paw withdrawal thresholds indicative of neuropathy. Drug efficacy screening was conducted 2 days later to minimize the potential effect that repeated eVF testing may have on our CIN endpoint. The mice were counterbalanced and assigned to drug groups. All test compounds were delivered IP 45 min prior to eVF testing.
Table 1.
Timeline of the cisplatin induction protocol and behavioral testing
| Day 1 | von Frey baseline |
| Cisplatin Day 1 | |
| Day 2 | Ringer's solution Day 1 |
| Day 3 | Cisplatin Day 2 |
| Day 4 | Ringer's solution Day 2 |
| Day 5 | Cisplatin Day 3 |
| Day 6 | Ringer's solution Day 3 |
| Day 7 | Cisplatin Day 4 |
| Day 8 | Ringer's solution Day 4 |
| Day 9 | Cisplatin Day 5 |
| Day 10 | Ringer's solution Day 5 |
| Day 11 | Cisplatin Day 6 |
| Day 12 | von Frey final |
| Ringer's solution 6 | |
| Day 13 | Drug- and test-free holiday |
| Day 14 | Drug probe test |
| von Frey |
Statistical Analyses
Data were analyzed with SPSS software using one-way ANOVAs for both between-group (e.g., drug treatment conditions) and within-group (i.e., pre- versus post-cisplatin administration) comparisons. Post-hoc comparisons for group differences used Fisher's least significant difference (LSD). Significance testing was set at p < 0.05.
Results
Experiment 1
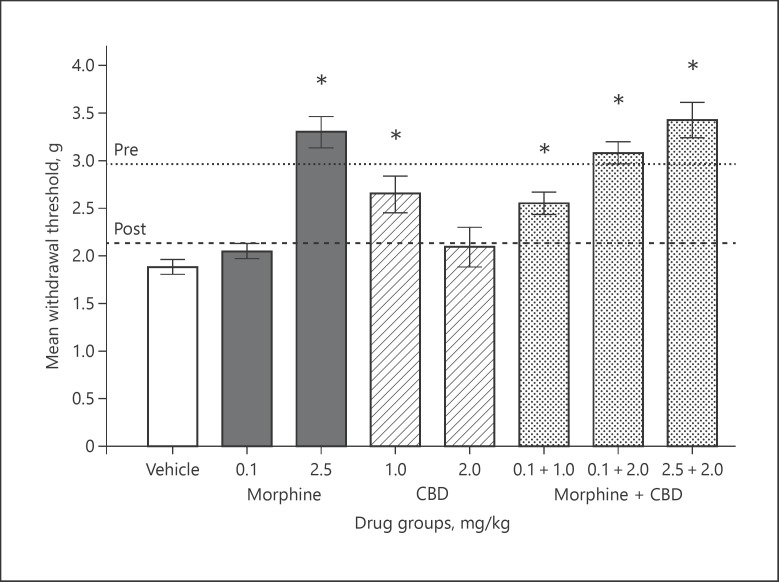
The effects of the various test compounds on cisplatin-induced tactile allodynia are summarized in Figure 1. Baseline eVF responses prior to and after cisplatin administration are shown as dotted and dashed lines, respectively. Following the cisplatin induction protocol, all mice showed lower response thresholds indicative of tactile allodynia. A one-way ANOVA of these data revealed a significant decrease in paw withdrawal following the cisplatin protocol: F(1,71) = 136.03, p < 0.0001.
Fig. 1.
Mean paw withdrawal in grams of force (±SEM). Dotted and dashed lines depict baseline responses pre- and post-cisplatin administration protocol prior to drug efficacy screening. Vertical bars represent mean responses on the drug efficacy screening day for drug treatment conditions. Doses are in mg/kg and delivered IP 45 min prior to testing. * Denotes significant attenuation of tactile allodynia compared to the vehicle group (p < 0.05). Sample sizes were n = 5 for the drug group of 2.5 mg/kg morphine and 2.0 mg/kg CBD, and n = 9–14 animals for all other drug groups.
On the drug efficacy screening day, vehicle-treated mice continued to show tactile allodynia. A sub-analgesic dose of morphine (0.1 mg/kg) did not affect eVF responses, whereas 2.5 mg/kg morphine fully attenuated tactile allodynia. CBD produced a modest attenuation of CIN at the 1.0 but not at the 2.0 mg/kg dose. The sub-analgesic dose of morphine given in combination with 2.0 mg/kg CBD attenuated tactile allodynia comparable to 2.5 mg/kg morphine. This enhanced CBD-opioid effect was modestly enhanced with 2.5 mg/kg morphine.
A one-way ANOVA of these data revealed a significant main effect for the drug: F(7,71) = 15.72, p < 0.0001. Fisher's LSD demonstrated that the mean withdrawal thresholds were significantly higher in the vehicle than in the 2.5 mg/kg morphine, the 1.0 mg/kg CBD, the 0.1 mg/kg morphine in combination with 1.0 and 2.0 mg/kg CBD, and the 2.5 mg/kg morphine in combination with 2.0 mg/kg CBD groups (p values ≤ 0.0001).
Experiment 2
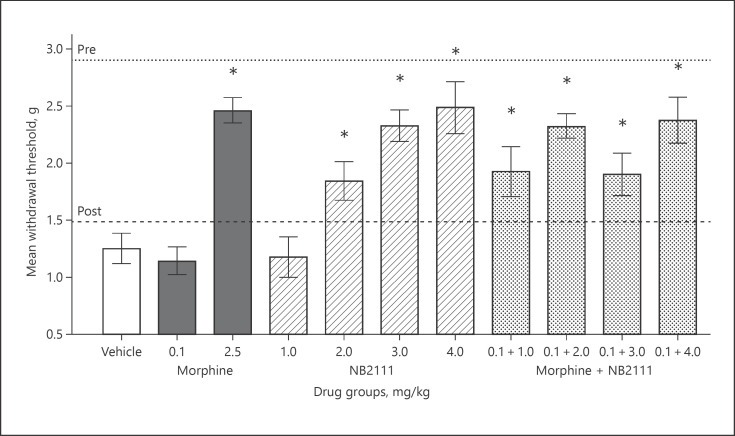
Efficacy screening of these test compounds on cisplatin-induced tactile allodynia are summarized in Figure 2. Baseline eVF responses prior to and after cisplatin administration are shown as dotted and dashed lines, respectively. Following the cisplatin induction protocol, all mice showed lower response thresholds indicative of tactile allodynia. A one-way ANOVA of these data revealed a significant decrease in paw withdrawal following the cisplatin protocol: F(1,99) = 601.36, p < 0.0001.
Fig. 2.
Mean paw withdrawal in grams of force (±SEM). Dotted and dashed lines depict baseline responses pre- and post-cisplatin administration protocol prior to drug efficacy screening. Vertical bars represent mean responses on the drug efficacy screening day for drug treatment conditions. Doses are in mg/kg and delivered IP 45 min prior to testing. * Denotes significant attenuation of tactile allodynia compared to the vehicle group (p < 0.05). Sample sizes were n = 9–11.
On the drug efficacy screening day, vehicle-treated mice continued to show tactile allodynia. A sub-analgesic dose of morphine (0.1 mg/kg) did not affect eVF responses, whereas 2.5 mg/kg morphine fully attenuated tactile allodynia. NB2111 given alone at 3.0 and 4.0 mg/kg produced a dose-dependent attenuation of tactile allodynia equal to 2.5 mg/kg morphine. The combination of a sub-analgesic dose of morphine and NB2111 shifted this dose response curve to the left, where 2.0 mg/kg NB2111 attenuated tactile allodynia equal to that of 2.5 mg/kg morphine.
Consistent with these findings, a one-way ANOVA of these data revealed a significant effect for the drug: F(10,99) = 9.76, p < 0.0001. Fisher's LSD demonstrated that mean withdrawal thresholds were significantly higher compared to vehicle in the 2.0–4.0 mg/kg NB2111 groups and the drug combination of 0.1 mg/kg morphine and 1.0–4.0 mg/kg NB2111 groups (p values ≤ 0.0001).
Discussion
Experiment 1
The results from Experiment 1 demonstrate that a 6-dosing protocol of 2.3 mg/kg cisplatin over a 12-day period leads to robust tactile allodynia in mice, a hallmark sign of CIN. Further, tactile allodynia persisted for several days after the last cisplatin administration as evidenced by continued reduced paw withdrawal thresholds in vehicle-treated mice on the drug efficacy screening day. These findings are consistent with the literature that this and other cisplatin administration protocols produce CIN in rodents [5, 19].
Mice receiving 2.5 mg/kg morphine displayed a robust attenuation of tactile allodynia. This finding is consistent with the literature that opioid agonists produce analgesia in a wide variety of pain models including CIN [6]. Mice receiving 1.0 mg/kg CBD displayed a modest but significant attenuation of tactile allodynia relative to placebo but only 60% of the response seen with 2.5 mg/kg morphine. Doubling the dose of CBD (2.0 mg/kg) provided no significant enhancement of analgesia relative to placebo or morphine.
A sub-analgesic dose of morphine (0.1 mg/kg) did not further alter the anti-allodynic properties of 1.0 mg/kg CBD given alone. However, this sub-analgesic dose of morphine greatly enhanced the efficacy of 2.0 mg/kg CBD producing an effect equivalent to that of 2.5 mg/kg morphine alone. Finally, the effective morphine dose of 2.5 mg/kg did not further potentiate the 2.0 mg/kg CBD. Collectively, these findings demonstrate that the modest efficacy of CBD can be greatly enhanced with sub-analgesic doses of an opioid agonist. While literature exists that demonstrates an analgesic synergy between CBD and morphine in models of inflammatory pain [18], this is the first report in the literature to describe such findings generalizable to a murine model of CIN.
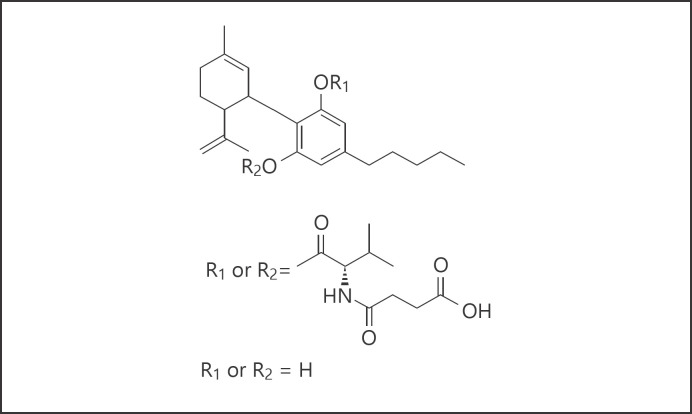
The potential of combining sub-analgesic doses of opioids with CBD to treat CIN in oncology settings is not without several translational challenges. Foremost among these may be the poor absorption of CBD when given by enteral administration related to the hydrophobic nature of native CB molecules that can hinder transmembranous transport. Through chemical modification, our research has led to the development of NB2111, a synthetic, bio-engineered CBD analog (see Fig. 3) that possess several characteristics to suggest this compound may provide longer lasting effects in clinical populations due to enhanced bioavailability. Pilot data has shown this compound is readily absorbed within 30 min of administration compared to CBD and that stable and biologically relevant blood levels persist beyond 8–12 h after administration. However, whether NB2111 shows biological activity like CBD in the CIN model is unknown. The purpose of Experiment 2 is to study the efficacy of NB2111 to attenuate tactile allodynia in a murine CIN model both in combination of sub-analgesic doses of morphine and without.
Fig. 3.
Chemical structure of NB2111, cannabidiol-mono-valinate-mono-hemisuccinate; molecular formula C30H43N6; molecular weight 513.68.
Experiment 2
The results from Experiment 2 parallel those seen with Experiment 1 and show that this cisplatin administration protocol produces robust tactile allodynia in mice, a hallmark sign of CIN.
As in Experiment 1, mice receiving 2.5 mg/kg morphine displayed a robust attenuation of tactile allodynia. NB2111 produced a robust dose-dependent attenuation of tactile allodynia using 1 mg/kg up to 4 mg/kg, with the response equivalent to 2.5 mg/kg morphine at the 3.0 and 4.0 mg/kg doses. Further, this NB2111 dose response function shifted to the left by the addition of a sub-analgesic dose of morphine. This drug combination achieved a maximum effect at the 2.0 mg/kg NB2111 dose that was as efficacious as 2.5 mg/kg morphine alone. Collectively, these findings demonstrate that (1) NB2111 alone produces robust analgesia equal to opioids against CIN and (2) these NB2111 effects can be achieved at lower doses when combined with sub-analgesic doses of an opioid agonist.
Conclusion
Challenges in pain management in oncology settings lead to unnecessary suffering, diminished quality of life, and, in some instances, decreased life expectancy due to patients' forgoing continued chemotherapy treatment related to intractable pain. Current therapies against CIN are either only modestly effective or are fully effective but poorly tolerated. NB2111 may be useful in pain management as NB2111 is a biologically active analog of CBD that exhibits comparable efficacy to opioids in this CIN murine model. Collectively, these findings strongly indicate continued study of NB2111 in the management of pain in oncologic settings and, perhaps, other etiologies associated with neuropathic pain.
Statement of Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Mississippi and collected at the University (protocols 13-017 and 15-022).
Disclosure Statement
Kenneth J. Sufka, Waseem Gul, and Hannah M. Harris declare no conflicts of interest. Mahmoud ElSohly serves as an unpaid consultant on the Advisory Board of Nemus Biosciences Inc., Costa Mesa, CA, USA. This study was sponsored by Nemus Bioscience, Inc., Costa Mesa, CA, USA.
Acknowledgments
The authors wish to acknowledge Jontae D. Warren for his assistance and expertise in electronic von Frey data collection.
References
- 1.Paice JA. Chronic treatment-related pain in cancer survivors. Pain. 2010;152:84–89. doi: 10.1016/j.pain.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf S, Barton D, Kottschade L, Grothey A., Lopriniz C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Miltenburg NC, Booger W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40:872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol Res. 2012;67:94–109. doi: 10.1016/j.phrs.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toth C, Au S. A prospective identification of neuropathic pain in specific chronic polyneuropathy syndromes and response to pharmacological therapy. Pain. 2008;138:657–666. doi: 10.1016/j.pain.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Dougherty PM, Abdi S. Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol Oncol. 2015;136:453–459. doi: 10.1016/j.ygyno.2015.01.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285:6–12. doi: 10.1016/j.canlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Chiou LC, Hu SS, Ho Y. Targeting the cannabinoid system for pain relief? Acta Anaesthesiol Taiwan. 2013;51:161–170. doi: 10.1016/j.aat.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Pisanti S, Picardi P, D'Alessandro A, Laezza C, Bifulco M. The endocannabinoid signaling system in cancer. Trends Pharmacol Sci. 2013;34:273–282. doi: 10.1016/j.tips.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Vera G, Cabezos PA, Martín MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol Biochem Behav. 2013;105:205–212. doi: 10.1016/j.pbb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA, Seybold VS. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci. 2012;32:7091–7101. doi: 10.1523/JNEUROSCI.0403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson-Poe AR, Pocius E, Herschbach M, Morgan MM. The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids. Pharmacol Biochem Behav. 2013;103:444–449. doi: 10.1016/j.pbb.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall W, Christie M, Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncol. 2005;6:35–42. doi: 10.1016/S1470-2045(04)01711-5. [DOI] [PubMed] [Google Scholar]
- 15.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 16.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 17.Cox ML, Haller VL, Welch SP. Synergy between Δ9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Neelakantan H, Tallarida RJ, Reichenbach ZW, Tuma RF, Ward SJ, Walker EA. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol. 2014;26:304–314. doi: 10.1097/FBP.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and the changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2012;116:224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]