Abstract
Cannabis sativa L. (marijuana or hemp) is recognized worldwide for its psychoactive properties as well as for fiber production. This study focused on the evaluation of 3 droplet vitrification protocols for long-term conservation of shoot tips in liquid nitrogen (LN). Shoot tips (∼0.5 mm) were excised from 3- to 4-week-old in vitro-grown shoots of 3 cultivars (MX, VI-20, and B-5: high tetrahydrocannabinol [THC], high cannabidiol [CBD], and intermediate THC∼CBD, respectively) and pretreated on 5% dimethyl sulfoxide agar plates for 48 h. The shoot tips were then vitrified in LN using 3 separate cryoprotectant (plant vitrification solutions [PVS] #2, #3, and #4) droplets on an aluminum cryoplate. There was no significant difference between the regrowth of cryopreserved shoot tips exposed to PVS2 for 15 and 20 min, but regrowth of all 3 cultivars significantly declined after 20 min of exposure. Exposure duration of 15 min was adapted for subsequent experiments. Regrowth of cryopreserved MX was significantly higher with PVS2 (63%) than with PVS3 and PVS4 (≤5%). Regrowth of cryopreserved VI-20 was highest with PVS2 (57%) and significantly higher than with PVS3 and PVS4 (≤25%). The regrowth of cryopreserved shoot tips of B-5 was significantly different between all 3 protocols with PVS2 > PVS4 > PVS3. Both PVS2 and PVS4 produced regrowth above 55%, while regrowth with PVS3 was significantly lower (31%). These results indicate that 15–20 min of exposure to PVS2 are most suitable for cryopreservation of these varieties. This is the first report on protocol development for the cryopreservation of organized tissues of C. sativa L. for germplasm conservation.
Keywords: Conservation, Cultivars, Germplasm, Liquid nitrogen, Marijuana, Natural products
Introduction
Cannabis sativa is grown all over the world [1]. It has a long history of pharmacologic and therapeutic benefits due to the presence of the active cannabinoids, tetrahydrocannabinol (THC) and cannabidiol (CBD) [2, 3], and other constituents. Varieties with a high THC content are commonly referred to as marijuana, while those with a low THC content are known as hemp and are used for fiber [4]. In vitro methods for micropropagation and conservation have been reported [5, 6, 7]. The in vitro germplasm repository or genebank at the University of Mississippi, USA, was established in 2009 and has several collections of important varieties of C. sativa with different biochemical profiles [6, 7]. The University of Mississippi is currently the only federally approved institution to produce bulk C. sativa for research. A cryopreservation program (storage at ultra-low temperature, usually that of liquid nitrogen [LN] at −196°C) has recently been established to backup or secure medicinally important varieties against loss and to ensure the future availability of desired biochemically active compounds.
Cryopreservation of plant parts began in the late 1950s and early 1960s [8], and through many decades of research, it is now possible to achieve an ice-free intracellular environment necessary to maintain plants in stable metabolic condition during long-term storage in LN. Vitrification during cryopreservation involves the phase transition of water into an amorphous, metastable glass without crystal formation. Several vitrification mixtures have been used as cryoprotectants to increase the recovery of plants after cryopreservation [9, 10, 11, 12]. Vitrification has been applied to several types of medicinal plants with moderate to high recovery rates [13, 14]. The droplet vitrification technique was derived from the plant vitrification solution (PVS)-based vitrification technique [9] and the droplet freezing technique [15, 16]. These techniques involve the use of a sterile aluminum cryoplate [17], an important conductor of heat or thermal energy during immersion in LN [18, 19].
A cryopreservation technique was reported for C. sativa cell suspension cultures nearly 3 decades ago. Jekkel et al. [20] (1989) obtained 58% survival after cryopreservation of the suspension cultures using a controlled cooling rate of 2°C/min, transfer temperature of −10°C and 10% dimethyl sulfoxide (DMSO) as the cryoprotectant. There is no cryopreservation history for shoot tips of C. sativa, which are the preferable explants for clonal micropropagation of plants. The development of a simple and reliable protocol(s) for shoot tips of C. sativa would allow widespread use of cryopreservation for banking medicinally important cultivars with unique attributes. We evaluated the effects of 3 cryoprotectants for use with the droplet vitrification protocol for effective in vitro storage of Cannabis germplasm.
Materials and Methods
Plant Material and Growth Conditions
The plant materials (shoots) used for this study were collected from the indoor-grown collections of plants produced at the National Center for Natural Products Research (NCNPR), Coy Waller Laboratory Complex, University of Mississippi, USA. The shoots were taken from plants of elite cultivars of C. sativa L.; MX, characterized with a high THC content, VI-20, with high CBD content; and B-5, with intermediate levels of THC and CBD content. These indoor-grown plants were raised under climatic control conditions (temperature: 75 ± 3°F, and relative humidity: ∼60%).
Sterilization
The shoots were pre-rinsed for 2 min in running tap water, followed by surface disinfection in 15% commercial bleach (Clorox, regular bleach [5.25% v/v]) with 0.1% Tween 20 for 18 min and then rinsed thoroughly with sterile distilled water (500 mL) for 3 min. The shoots were further treated with 0.2% mercuric chloride for 3 min. Shoots were rinsed 3 times with sterile distilled water before planting in growth medium.
Growth Medium Composition
After the surface sterilization of shoots, they were transferred to growth medium containing Murashige and Skoog (MS) mineral salts [21] formulated as a commercial powder (Caisson Laboratories Inc.) and MS vitamins mixture, with 0.12 mgL−1 meta-topolin (Caisson Laboratories Inc.), 8 gL−1 agar powder (Caisson Laboratories Inc.), 30 gL−1 anhydrous sucrose (Caisson Laboratories Inc.), and 1 gL−1 activated charcoal. The pH of the medium was adjusted to 5.7 before the addition of the agar and activated charcoal. The medium was sterilized by autoclaving at 121°C, for 15 min, and dispensed after autoclaving into sterile Magenta GA7 boxes.
Culture Conditions
All shoot cultures were transferred to a culture room with controlled environmental conditions of 25 ± 2°C at a 16-h photoperiod. The shoots were grown under photon flux of 52 μmol/m2/s (LI-250A, LI-COR® Biosciences, USA) provided by cool white fluorescent bulbs. The shoots were subcultured on fresh growth medium every 3–4 weeks.
Cryopreservation Procedure
Pretreatment Procedure
Shoot tips (∼0.5 mm) consisting of a meristematic dome with 1 or 2 attached leaf primordia were excised from 3-week-old shoot cultures and held for 48 h on a pretreatment medium consisting of MS basal medium with 0.3 M sucrose, 8 gL−1 agar, and 5% DMSO (Sigma-Aldrich Co.) at pH 5.7 prior to all experimentation. The DMSO was filter sterilized using membrane filters (0.45 μm, 150-mL analytical filter unit) and added to a cooled pretreatment medium after autoclaving. About 25 mL medium was dispensed into each Petri dish. The shoot tip cultures were placed in the grow room under the controlled conditions described above. After the pretreatment, shoot tips of each variety were cryopreserved, with shoot tips treated but not exposed to LN used as the controls.
Composition of Cryoprotectants
The cryoprotectants (vitrification solutions) consisted of PVS2 (30% glycerol, 15% ethylene glycol, 15% DMSO in liquid MS medium with 0.4 M sucrose [w/v], pH 5.8) [9], PVS3 (40% sucrose, 40% glycerol in liquid MS medium [w/v], pH 5.8) [11], and PVS4 (0.6 M sucrose, 3.8 M glycerol, and 20% ethylene glycol in liquid MS [w/v], pH 5.8) [22].
Effect of Cryoprotectants
The optimal exposure durations for PVS2, PVS3, and PVS4 were determined. Shoot tips of each variety (12 per treatment) were pretreated as described above and transferred to 1 mL loading solution (LS) (2 M glycerol in 0.4 M sucrose MS medium [v/v], pH 5.8) [22] in 1.2 mL cryovials for 20 min at 25 ± 1°C. The LS was removed and the shoot tips were individually transferred to wells on a sterile aluminum cryoplate (7 × 37 × 0.5 mm, depth of well is 0.75 mm) with 3- to 5-µL droplets of cryoprotectant solutions per well. Each aluminum cryoplate had 12 wells with 1 shoot tip per well. Cryoprotectants were applied at 25 ± 1°C for 10, 15, 20, 25, and 30 min. Following each of these exposure durations, 12 shoot tips per treatment per replicate were rinsed in liquid MS medium containing 1.2 M sucrose as described below and then cultured in the growth medium (control). Also, 12 shoot tips per treatment per replicate were plunged rapidly in LN for 10–15 min, followed by rapid rewarming in a 45°C water bath for 1 min before the samples were rinsed in a solution of liquid MS with 1.2 M sucrose and then planted on above growth medium in 24-cell culture plates. This experiment was done with 3 replicates for each variety.
Testing of Vitrification Procedures on C. sativa
The droplet vitrification procedure of Panis et al. [23] was followed. The successive steps for the cryopreservation of C. sativa shoot tips of each variety were as follows: (1) pretreatment of shoot tips in 5% DMSO plate for 48 h. (2) LS was added to shoot tips as described above for 20 min. (3) The shoot tips (1 per well) were transferred into sterile aluminum cryoplates with droplets of each cryoprotectant (#2, #3, and #4). Each treatment had 20 shoot tips per treatment per replicate and was held in the cryoprotectant for 15 min. (4) Aluminum cryoplates with shoot tips were transferred to a cryovial, closed, and rapidly plunged in LN for 10–15 min. (5) Cryopreserved shoot tips were rapidly rewarmed in 45°C water for 1 min. (6) Shoot tips were rinsed by serial dilution in liquid MS medium containing 1.2 M sucrose for 3–5 min. (7) Cryopreserved shoot tips were planted in the standard growth medium described above. The controls (12 shoot tips) had the same treatments but were not plunged into LN. Each cultivar and treatment was tested separately.
Data Collection
Data on the regrowth of shoot tips were recorded 8 weeks after rewarming. Regrowth was recorded for each originally cryopreserved shoot tip that remained green, enlarged, and resumed growth with production of leaves and shoots. All data are presented as percentage of total number of original shoot tips that recovered from cryopreservation ± SE of means. Data were analyzed using ANOVA (SAS version 9.2 for Windows; SAS Institute Inc., Cary, NC, USA), followed by means separation using Duncan's multiple range test. Differences in means were considered significant at p ≤ 0.05.
Results
Effect of Cryoprotectants Duration on the Regrowth of Cryopreserved Shoot Tips
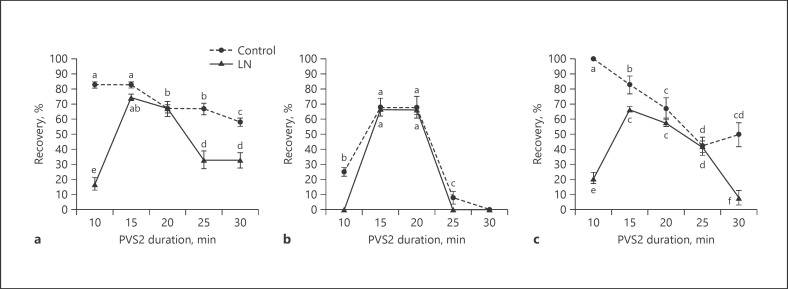
There was no significant difference between the regrowth of cryopreserved shoot tips exposed to PVS2 for 15 and 20 min (Fig. 1). However, there was generally a significant decline in regrowth beyond 20 min in PVS2. Variety MX (Fig. 1a) had more regrowth at extreme exposure (30 min) than other cultivars. Exposure durations of 10, 25, and 30 min resulted in 0% regrowth following cryopreservation of variety VI-20 (Fig. 1b). Variety B-5 (Fig. 1c) appeared more tolerant to PVS2 with better regrowth rates after cryopreservation than variety VI-20. The cultivars had comparatively poor regrowth with PVS3 and PVS4 (data not shown).
Fig. 1.
Regrowth rates of shoot tips of Cannabis sativa cultivars MX (a), VI-20 (b), and B-5 (c) exposed to plant vitrification solution #2 (PVS2) for 10–30 min at 25 ± 2°C prior to cryopreservation in liquid nitrogen (LN). The controls had all treatments except LN. Bars with the same letter are nonsignificant at p ≤ 0.05
Effect of Cryoprotectants on the Droplet Vitrification of C. sativa Cultivars
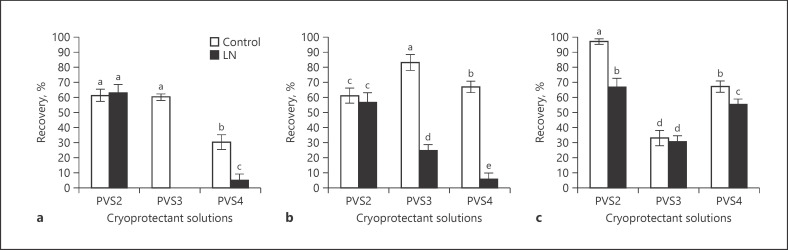
Regrowth percentage of MX shoot tips following LN was 63% with PVS2 treatment (Fig. 2a), while it was less than 10% with PVS4, and there was no regrowth from PVS3, although the control was high. Regrowth of control shoot tips without LN was significantly better with PVS2 (61%) and PVS3 than with PVS4 (30%); however, there was no significant difference between PVS2 and PVS3 (Fig. 2a).
Fig. 2.
Regrowth of shoot tips of Cannabis sativa cultivars MX (a), VI-20 (b), and B-5 (c) exposed to plant vitrification solutions (PVS) #2, #3, and #4 for 15 min at 25 ± 1°C prior to droplet vitrification cryopreservation in liquid nitrogen (LN). The controls had all treatments except exposure to LN. Bars with the same letter are nonsignificant at p ≤ 0.05.
Regrowth of VI-20 shoot tips following cryopreservation was highest with PVS2 (57%). This was significantly higher than with PVS3 (25%) and PVS4 (6%). The nonfrozen shoot tips had regrowth of 61–80% for all 3 cultivars (Fig. 2b).
The regrowth of cryopreserved B-5 shoot tips was significantly different with PVS2 > PVS4 > PVS3. Both PVS2 and PVS4 had regrowth above 50%, but with PVS3 regrowth was low (31%) (Fig. 2c). The regrowth of noncryopreserved B-5 shoot tips treated with PVS2 was significantly better than with other treatments (Fig. 2c).
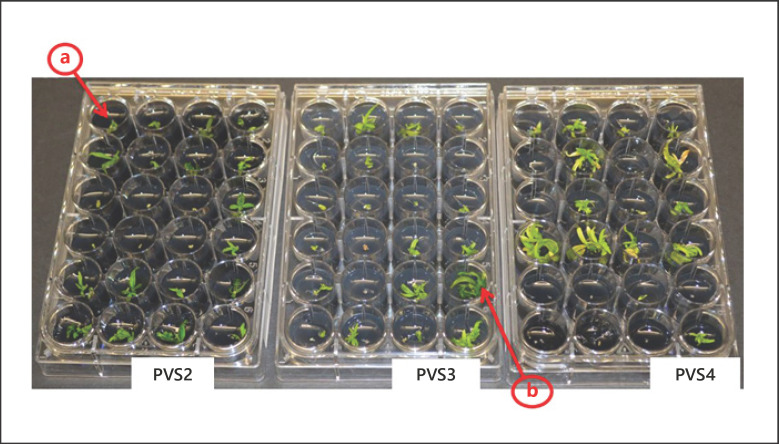
There was no intermediary callus formation during recovery from any of the cultivars, but a few low-quality shoot tips were observed that remained green and/or enlarged (≥3 mm in length) but did not undergo differentiation (Fig. 3a).
Fig. 3.
Regrowth after 6 weeks of rewarming of cryopreserved shoot tips (∼0.5 mm) of Cannabis sativa cultivar B-5. Shoot tips were cryopreserved by droplet vitrification using plant vitrification solutions (PVS) #2, #3, and #4. a Survival indicated cryopreserved shoot tips that remained green and/or enlarged but did not develop. b Regrowth of cryopreserved shoot tips with differentiated leaves and shoots.
Discussion
One of the important keys to a successful cryopreservation by vitrification is to optimize the dehydration process in cryoprotectant solution in order to prevent injury by chemical toxicity or osmotic shock resulting in increased regrowth. We examined the regrowth responses of cryopreserved shoot tips of all 3 Cannabis varieties over a range of exposure durations to PVS2, a cryoprotectant generally known to produce time-dependent regrowth following cryopreservation [19, 24], and found that the cryopreserved shoot tips of these varieties produced significantly higher regrowth at 15 and 20 min compared to other durations (Fig. 1). Many cryopreserved medicinal plants have optimal regrowth when exposed for 20 min to PVS2 [14, 25]. However, 10–25 min exposure duration to PVS2 at 25°C was shown to be optimal for several herbaceous plant species [24, 26]. It is clear from these studies that the acquisition of tolerance to PVS2 is necessary for survival after cryopreservation and also that the exposure duration may be genotype dependent. Our data detail the specific regrowth responses of both cryopreserved and noncryopreserved shoot tips of C. sativa exposed to PVS2 and the role of cryoprotection in Cannabis recovery from LN. Exposure of shoot tips of these cultivars for a longer time (> 30 min) to PVS3 and PVS4 may be necessary to achieve sufficient cryoprotection and improved regrowth. Kim et al. [27] (2009) evaluated 90–150 min exposure of garlic explants to PVS3 and found that 150 min was optimal for cryopreservation of garlic by droplet vitrification.
The MX is a unique cultivar with high THC content [2]. This implies that the cultivar is of great importance for drug production. The ability to successfully cryopreserve this cultivar with over 60% regrowth, using the PVS2 protocol (Fig. 2a), provides long-term security for this and other important varieties processed for in vitro conservation. Overall, the PVS2 droplet vitrification customized for Cannabis spp. produced a moderate regrowth of 57–67% of cryopreserved shoot tips. This result indicates that this technique could be applied on all 3 varieties targeted for long-term conservation. This project is part of an ongoing effort to cryopreserve all the varieties of C. sativa held at the NCNPR, University of Mississippi. The droplet vitrification technique using PVS2 has been applied to many medicinal plant types, including Byrsonima intermedia [28] and Atractylodes macrocephala [29]. The significance of each critical step of this vitrification procedure has been reviewed [19, 30].
An important observation was made during the recovery of cryopreserved shoot tips. The majority of the cryopreserved shoot tips of these varieties had as much as 83% survival (data not shown), but in many cases this did not translate or equate to regrowth (Fig. 3b). Similar observations were made during the cryopreservation of Lilium lancifolium by droplet vitrification [31]. The authors recorded 84% survival, while the regrowth was 68%. Kim et al. [12] (2006) reported 60.7% survival and a regrowth of 50.2% for garlic shoot tips and also 69.1% survival and a regrowth of 30.8% for Chrysanthemum shoot tips following cryopreservation by the PVS2 droplet technique. Leunufna and Keller [32] (2005) obtained higher survival (67–70%) compared to regrowth (30–50%) of cryopreserved Dioscorea spp. following a modified PVS2 droplet vitrification technique. A low regrowth compared to survival rate is not peculiar to droplet vitrification alone. Preetha [25] (2013) reported 50–60% survival and 30–40% regrowth after cryopreservation of the medicinal plant Kaempferia galanga L. by the regular PVS2 vitrification technique. The causes of this phenomenon (low rates of differentiation of cells and tissues) during vitrification or droplet vitrification methods are unclear, however; the major factors affecting the growth and development of cells or tissues after cryopreservation include the physiological status of the donor plant [33] and the explant to be cryopreserved [34], size and position of shoot tips [35], and origin of material [36]. Due to the regulatory nature of C. sativa, these factors and mechanisms have not been fully explored. Research on different aspects of cryopreservation is ongoing in our laboratory and will be communicated in the future. This study was initiated in the hope to achieve protocol development for cryopreservation of Cannabis tissues. The results are encouraging.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have declared no conflict of interest.
Acknowledgements
This project was supported in part by the National Institute of Drug Abuse (NIDA). Contract # N01DA-15-7793.
References
- 1.Small E, Marcus D. Hemp: A new crop with new uses for North America. In: Janick J, Whipkey A, editors. Trends in new crops and new uses ASHS Press. Alexandria (VA): Alexandria (VA); pp. pp. 284–326. [Google Scholar]
- 2.Chandra S, Lata H, Mehmedic Z, Khan IA, ElSohly MA. Assessment of cannabinoids content in micropropagated plants of Cannabis sativa and their comparison with conventionally propagated plants and mother plant during developmental stages of growth. Planta Med. 2010 May;76((7)):743–50. doi: 10.1055/s-0029-1240628. [DOI] [PubMed] [Google Scholar]
- 3.Zuardi AW. History of cannabis as a medicine: a review. Br J Psychiatry. 2006 Jun;28((2)):153–7. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 4.Slade D, Mehmedic Z, Chandra S, ElSohly M, Is cannabis becoming more potent? In: Marijuana and Madness. 2nd ed. Castle D, Murray RM, D'Souza DC, editors. Cambridge University Press; 2012. pp. pp. 35–54. [Google Scholar]
- 5.Mandolino G, Ranalli P. Advances in biotechnological approaches for hemp breeding and industry. In: Ranalli P, editor. Advances in hemp research. New York: Haworth Press; 1999. pp. pp. 185–208. [Google Scholar]
- 6.Lata H, Chandra S, Mehmedic Z, Khan I, ElSohly MA. Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L. In Vitro Cell Dev Biol Plant. 2009;45((1)):12–9. [Google Scholar]
- 7.Lata H, Chandra S, Mehmedic Z, Khan IA, ElSohly MA. In vitro germplasm conservation of high Δ9-tetrahydrocannabinol yielding elite clones of Cannabis sativa L.under slow growth conditions. Acta Physiol Plant. 2012;34((2)):743–50. [Google Scholar]
- 8.Sakai A. Survival of the twig of woody plants at -196 o C. Nature. 1960;185((4710)):392–4. [Google Scholar]
- 9.Sakai A, Kobayashi S, Oiyama I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990 Jun;9((1)):30–3. doi: 10.1007/BF00232130. [DOI] [PubMed] [Google Scholar]
- 10.Sakai A, Kobayashi S, Oiyama I. Survival by vitrification of nucellar cells of navel orange (Citrus sinensis var. brasiliensis Tanaka) cooled to −196 oC. J Plant Physiol. 1991;137((4)):465–70. doi: 10.1007/BF00232130. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa S, Sakai A, Amano Y, Matuzawa T. Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 1993;91((1)):67–73. [Google Scholar]
- 12.Kim HH, Lee JK, Yoon JW, Ji JJ, Nam SS, Hwang HS, et al. Cryopreservation of garlic bulbil primordia by the droplet-vitrification procedure. Cryo Letters. 2006 May-Jun;27((3)):143–53. [PubMed] [Google Scholar]
- 13.Uchendu EE, Brown DW, Saxena PK. Cryopreservation of shoot tips and cotyledons of the north american ginseng (Panax quinquefolius l.) Cryo Letters. 2011 Nov-Dec;32((6)):463–72. [PubMed] [Google Scholar]
- 14.Uchendu EE, Shukla MR, Reed BM, Saxena PK. An efficient method for cryopreservation of St John's wort and tobacco: role of melatonin. Acta Hortic. 2014;((1039)):233–41. [Google Scholar]
- 15.Kartha KK, Leung NL, Mroginski LA. In vitro growth responses and plant regeneration from cryopreserved meristems of cassava (Manihot esculenta Crantz) Z Pflanzenphysiol. 1982;107((2)):133–40. [Google Scholar]
- 16.Schafer-Menuhr A, Schumacher HM, Mix-Wagner G. Cryopreservation of potato cultivars: design of a method for routine application in genebanks. Acta Hortic. 1997;((447)):477–82. [Google Scholar]
- 17.Matsumoto T. Cryopreservation of plant genetic resources: conventional and new methods. Rev Agric Sci. 2017;5((0)):13–20. [Google Scholar]
- 18.Panis B, Piette B, Swennen R. Swennen: Droplet-vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci. 2015;168((1)):45–55. [Google Scholar]
- 19.Sakai A, Engelmann F. Vitrification, encapsulation-vitrification and droplet-vitrification: a review. Cryo Letters. 2007 May-Jun;28((3)):151–72. [PubMed] [Google Scholar]
- 20.Jekkel Z, Heszky LE, Ali AH. Effect of different cryoprotectants and transfer temperatures on the survival rate of hemp (Cannabis sativa L.) cell suspension in deep freezing. Acta Biol Hung. 1989;40((1-2)):127–36. [PubMed] [Google Scholar]
- 21.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15((3)):473–97. [Google Scholar]
- 22.Sakai A. Development of cryopreservation techniques. In: Engelmann F, Takagi H, editors. Cryopreservation of Tropical Plant Germplasm- Current Research Progress and Application. Rome: JIRCAS, Tsukuba and IPGRI; 2000. pp. pp. 1–7. [Google Scholar]
- 23.Panis B, Piette B, Andre E, van den Houwe I, Swennen R. Droplet-vitrification: the first generic cryopreservation protocol for organized plant tissues? Acta Hortic. 2011;((908)):157–63. [Google Scholar]
- 24.Matsumoto T, Sakai A, Yamada K. Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep. 1994 May;13((8)):442–6. doi: 10.1007/BF00231963. [DOI] [PubMed] [Google Scholar]
- 25.Preetha TS, Hemantha Kumar AS, Krishnan PN. Shoot tip cryopreservation by vitrification in Kaempferia galanga L.An endangered, overexploited medicinal plant in Tropical Asia. IOSR J Pharm Biol Sci. 2013;8((3)):19–23. [Google Scholar]
- 26.Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A. Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L.) Schott) by vitrification. 1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep. 1997;16((9)):594–9. doi: 10.1007/BF01275498. [DOI] [PubMed] [Google Scholar]
- 27.Kim HH, Lee YG, Shin DJ, Ko HC, Gwag JG, Cho EG, et al. Development of alternative plant vitrification solutions in droplet-vitrification procedures. Cryo Letters. 2009 Sep-Oct;30((5)):320–34. [PubMed] [Google Scholar]
- 28.Silva LC, Paiva R, Swennen R, Andre E, Panis B. Shoot-tip cryopreservation by droplet vitrification of Byrsonima intermedia A. Juss.: a woody tropical and medicinal plant species from Brazilian cerrado. Cryo Letters. 2013 Jul-Aug;34((4)):338–48. [PubMed] [Google Scholar]
- 29.Zhang JM, Huang B, Lu XX, Volk GM, Xin X, Yin GK, et al. CRYOPRESERVATION OF IN VITRO-GROWN SHOOT TIPS OF CHINESE MEDICINAL PLANT Atractylodes macrocephala KOIDZ. USING A DROPLET-VITRIFICATION METHOD. Cryo Letters. 2015 May-Jun;36((3)):195–204. [PubMed] [Google Scholar]
- 30.Sakai A, Hirai D, Niino T. Development of PVS-based vitrification and encapsulation-vitrification protocols. In: Reed BM, editor. Plant Cryopreservation: A Practical Guide. NY: Springer; 2008. pp. pp. 33–58. [Google Scholar]
- 31.Chen XL, Li JH, Xin X, Zhang ZE, Xin PP, Lu XX. Cryopreservation of in vitro-grown apical meristems of Lilium by droplet-vitrification. S Afr J Bot. 2011;77((2)):397–403. [Google Scholar]
- 32.Leunufna S, Keller ER. Cryopreservation of yams using vitrification modified by including droplet method: effects of cold acclimation and sucrose. Cryo Letters. 2005 Mar-Apr;26((2)):93–102. [PubMed] [Google Scholar]
- 33.Reed BM. Plant Cryopreservation: A Practical Guide. New York: Springer Science Business Media LLC; 2008. [Google Scholar]
- 34.Dereuddre J, Fabre J, Bassaglia C. Resistance to freezing in liquid nitrogen of carnation (Dianthus caryophyllus L. var Eolo) apical and axillary shoot tips excised from different aged in vitro plantlets. Plant Cell Rep. 1988 May;7((3)):170–3. doi: 10.1007/BF00269315. [DOI] [PubMed] [Google Scholar]
- 35.Azimi M, O'Brien C, Ashmore S, Drew R. M, O'Brien C, Ashmore S, Drew R: Cryopreservation of Papaya Germplasm. Acta Hortic. 2005;((692)):43–50. [Google Scholar]
- 36.Baek HJ, Kim HH, Cho EG, Chae YA, Engelmann F. Importance of explant size and origin and of preconditioning treatments for cryopreservation of garlic shoot apices by vitrification. Cryo Letters. 2003 Nov-Dec;24((6)):381–8. [PubMed] [Google Scholar]