Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that severely impairs the quality of life of patients suffering of the disease. The disease is characterized by the appearance of inflammatory, deep and painful lesions in the skin areas containing apocrine sweat glands: armpits, groin and genitals. The HS clinical course is characterized by a recurrence of abscesses, sinuses that secrete bad-smelling mug contents and the appearance of skin scars. Many sufferers are diagnosed after a long period since the onset of wounds and treated with large amounts of systemic and topical antibiotics. Some are treated with retinoids, biological drugs and surgical operations. Patients undergo drainage and even surgical resection of the affected areas. Many patients use cannabis, and especially CBD oil as topical treatment and sub lingual. Using cannabis reduces inflammation, the number of lesions and shortens the duration of recovery. Many HS sufferers have other illnesses such as Crohn's, colitis and depression. The severity of depression is correlated with the severity of the disease, the location of the disease and the age of the outbreak. The burden of HS disease is extremely high, especially for patients with a severe disease. HS patients suffer from severe pain, bad-smelling pus discharge in the affected areas, social stigma, relationship difficulties, depression, and impaired work output. These factors, combined with the lack of control of the disease, often cause social isolation. In the lecture I will present data of 74 patients treated with medical cannabis I have been following in the past years.
Epidermal keratinocytes (KCs) are important modulators of skin inflammation through the secretion of cytokines, chemokines, arachidonic acid metabolites, and antimicrobial peptide. The endocannabinoid system has been shown to modulate inflammatory responses in various tissues including brain, gut, and skin. It consists of cannabinoid (CB) receptors, their lipid ligands (endocannabinoids), and the enzyme system that generates these ligands. CBD has been shown to reduce proliferation of sebocytes. It was also revealed that mainly CBD affects the multiple members of the transient receptor potential (TRP) ion channel family. many of these same TRP channels are intimately involved in cutaneous processes that include the initiation of pain, temperature, and itch perception, the maintenance of epidermal homeostasis.
The objective of the study was to evaluate the anti-proliferative and anti-inflammatory properties of the cannabinoids CBD, THC, caryophyllene and their combination integrated in a topical formulation preparation for the treatment of inflammatory skin diseases, mainly psoriasis.
Results
Anti-proliferation
The topical formulation showed significant reduction in the hyperproliferation (measured by BrdU method) in a comparable manner to the steroid control (dexamethasone).
MTT results showed no inhibition of cell viability, therefore the inhibition of proliferation does not result from cell toxicity.
Anti-inflammatory
OWC Topical formulation inhibited IL-8, a major inflammatory mediator that serves as proinflammatory mediator in psoriasis.
OWC Topical formulation inhibited IL-33. IL-33 induced by IL-17, a specific cytokine related to psoriasis.
Background
Severe Behavioral Problems (SBP) are a common contributor to morbidity and reduced quality of life in children with Intellectual Disability (ID). Medications commonly used for the treatment of SBP in ID are associated with a high risk of side effects. Innovative and safe interventions are urgently needed. Cannabidiol has some biological plausibility and may be an alternative to current medications for this patient group.
Observational studies suggest that cannabidiol may be safe and effective in managing SBP in children with ID, however well-designed studies are lacking to support its use. This pilot study aimed to investigate the feasibility of conducting a randomized placebo-controlled trial (RCT) of cannabidiol to reduce SBP in children with ID.
Methods
Double-blind, placebo-controlled, two-armed, parallel-design, randomized controlled trial of cannabidiol in children aged 8 − 16 years with ID and SBP. Participants were randomized 1:1 to receive either 98% cannabidiol in oil (Tilray Canada) or placebo orally for 8 weeks. The dose was up-titrated over 9 days to 20mg/kg/day in two divided doses with a maximum dose of 500mg twice/day. The feasibility and acceptability of all study components were assessed.
Results
Eight children were randomized, and all completed the full study protocol. There were no Serious Adverse Events or drop-outs. Protocol adherence for key study components was excellent: study visits 100% medication adherence 100%, blood tests 92%, and questionnaire completion 88%. Parents reported a high degree of acceptability with the study design. All parents reported they would recommend the study to other families with children with similar problems. There was an efficacy signal in favour of active drug.
Conclusions
This is the first study to investigate cannabidiol for SBP in children with ID. The findings suggest that the study protocol is feasible and acceptable to patients with ID and SBP and their families. This pilot study informed the design for a planned full-scale RCT of cannabidiol in children with ID and SBP which has received competitive funding from the Australian Government's Medical Research Future Fund.
Introduction
Cannabis Sativa products are known to be therapeutically effective since ancient times L. despite its pharmacological typification was made in the mid-20th century. Its use was recently regulated in Uruguay.
Objectives
to analyse a preliminary clinical-therapeutic experience with MC with high content of cannabidiol (CBD).
Methods
epidemiological, observational and retrospective study of a 355-patient cohort who spontaneously consulted to learn about CM at a private clinic, between August 2016 and December 2017.
Demographic data, medical records, expectation and previous experience with cannabis were collected in the first intervie In most cases w. cannabis with high content of CBD was prescribed (5,25% CBD and 0,2% THC).
In subsequent consultations, access to access to MC was investigated and both response to it and adverse effects were studied by means of analogue scales. The study used descriptive statistics.
Results
in the cohort studied, women with an average age of 67 years old and university studies prevaile The following conditions motivated consultations: neurological (38%) d. rheumatic or bone degenerative diseases (37%), neoplasms (13%), psychiatric diseases (4%) and miscelánea (8%). In most cases (60,6%) patients stated symptoms improved and only 16,3% of the populaiton studied presented mild adverse effects. High costs and difficulties in accessing MC were the reasons for not starting or abandoning treatment.
Conclusions
our preliminary study reflects the positive therapeutic response and non significant adverse effects to MC with high content of CB 60,6% of patients treated referred improvement in their symptoms. Decisive factors for a successful treatment point at the need to make access to MC easier by improving management to obtain it and reducing costs for a greater accessibility.
The opioid crisis has directed a quest for safer alternatives in pain managemen In the past decade nearly forty countries have legalised the medical use of cannabis and/or certain derivatives (MC). Recognising the potential in MC in the draft guideline of the National Institute for Clinical Excellence further research with cannabinoids in general and in cannabidiol in particular was encouraged.
Acute low back pain (ALBP) consumes significant resources in Emergency/Urgent Care settings therefore, a safer alternative to opiates would be the best interest of both patients and healthcare provider At present high quality evidence proving/disproving the use of MC in ALBP is sparse due to heterogeneity in dosing, constitution, indication and method of administration.
We designed a multicentre, prospective study series including observational and double blind randomised, non-inferiority interventional trials to assess whether oral administration of CBD is at least as effective than regular care with NSAIDs in treating lower back pai The main outcome measures in this study are Patient Reported Outcomes (PROs) like Numeric Rating Scale Pain Catastrophising Scale and Pain Disability Assessment Scal Robust statistical analysis will be implemented to avoid chance findings (Table 1. for PICO in/exclusion, statistical analysis of the study)
Table 1.
Summary of the epidemiological articles (for Abstract no ID 36).
| Title, Year, Author | Primary and secondary outcomes | Conclusion of the investigators |
|---|---|---|
| Marijuana Use Patterns Among Patients with Inflammatory Bowel Disease 2013 Allegretti |
Prevalence Perception and clinical characteristics of medicinal use. |
Data suggest that at least one-third of patients with IBD would consider participating in a trial investigating the role of cannabis in IBD, again emphasizing the growing interest in using marijuana in this disease. |
| Patterns of cannabis use in patients with Inflammatory Bowel Disease: A population based analysis 2015, Weiss and Freidenberg |
Prevalence Depression Index, Maladaptive coping (smoking, alcohol, marijuana), weight perception, dietary habits |
Patients with IBD experience symptom control and pain relief with marijuana use. However, the motivations amongst this cohort of patients in unknonwn, these results are important when considering alternative therapies for patients with severe IBD. |
| Cannabis Use Provides Symptom Relief in Patients with Inflammatory Bowel Disease but Is Associated with Worse Disease Prognosis in Patients with Crohn's Disease. Storr 2014, Canada |
Abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%), and diarrhoea (28.6%), although side effects were frequent. The use of Cannabis for more than 6 months at any time for IBD symptoms was a strong predictor of requiring surgery and for | Cannabis use is common in patients with IBD and subjectively improved pain and diarrheal symptoms. However, Cannabis use was associated with higher risk of surgery in patients with Crohn's disease. Patients using Cannabis should be cautioned about potential harm, until clinical trials evaluate efficacy and safety. |
| Prevalence and Patterns of Marijuana Use in Young Adults With Inflammatory Bowel Disease 2017 Phatak et al. |
Prevalence Prevalence of medicinal use, view on legalisation |
High rate of marijuana use was seen in the cohort of young adults with IBD. Majority of patients report symptom improvement with marijuana use, but do not inform their physicians. physician's awareness of potential marijuana use could prevent a false impression regarding the efficacy of prescribed therapy (like pain relief, nausea and poor appetite). Lack of awareness of substance use leads to poor counsel them about possible adverse effects. |
| Profiles of Patients Who Use Marijuana for Inflammatory Bowel Disease 2017 Kerling et al. |
Prevalence Perceived benefit of medical marijuana |
Marijuana may be filling an unmet medical need for some patients. |
| Cannabis Use in Persons With Inflammatory Bowel Disease and Vulnerability to Substance Misuse 2019 Hansen et al. |
Prevalence Substance misuse risk factor |
Individuals using cannabis for IBD symptoms show increased vulnerability to substance misuse. Before prescribing and endorsing cannabis as a therapeutic option for IBD, gastroenterologists should find mechanisms for identifying at-risk individuals and establishing pathways for substance use disorder and/or comorbid mental health treatment. |
| Medicinal Cannabis for Inflammatory Bowel Disease: A Survey of Perspectives, Experiences, and Current Use in Australian Patients Benson et al. 2020 |
Prevalence Perceived benefit of medical marijuana |
Most users (92.7%) endorsed cannabis as effective in symptom management. Cannabis-using ulcerative colitis patients reported better quality of life than nonusers on some measures. |
Multidisciplinary approach will be needed to explore all clinical aspects of MC use in ALBP to avoid the serious consequences (like ones of heavy-handed opioid use) using important patient centred outcomes like iatrogenic harm, crowding length of stay, applicability of validated scoring systems and patient satisfaction.
Proposal for Talk on Cannabinoids and Beauty and Skin Disorders for 2020
I will first give a brief overview of what the endocannabinoid system is, and how it is involved in the skin. Next I will discuss the very recent scientific studies behind CBD and beauty claims and cannabinoids and anti-aging, acne, itch, eczema, and psoriasis. I will include a summary of what products are already on the market and where that market is projected to extend to.
Parallel is a leading, global company that is pioneering human well-being through the benefits of cannabinoids, engaging in the discovery, advancement, and commercialization of proprietary cannabis-based therapies. We are currently developing multiple best-in-class differentiated cannabis-based formulations and delivery platforms, which will be the focus of this tal These includes. a pulmonary delivery platform based on a novel fine-mist inhaler (FMI), providing precise, discrete and predictable dosing, maximizing cannabinoid delivery into the lungs. When compared to vapes and commercially available cannabis pressurized inhalers in laboratory settings Parallel's FMI displayed reduced throat deposition (<20%), with a substantial portion of the emitted cannabinoids ending up in the lungs (77%), and specifically in the alveoli, which is critical for achieving efficient pulmonary delivery and improved efficacy. We have also developed novel oral delivery platforms “DROPS”, which can be used as beverage additives or fully integrated into ultra-rapid onset, ready-to-drink beverages. In contrary to “DROPS” vast majority of commercially available cannabinoid beverages contained substantially lower cannabinoid levels than that declared on the label. “DROPS”-infused beverages also display stable cannabinoid levels over time in extreme temperatures with a loss of <5% potency over time, whereas all other tested beverages showed a loss of >20%. Finally, the particle size measured with “DROPS”-infused beverages remained stable over time and was significantly smaller (<100 nm) compared to those of other tested products, a key attribute towards achieving rapid onset and superior bioavailability. Those delivery platforms will provide patients with safe predictable, and efficacious cannabis-based therapies to address specific diseases and symptomatic needs and improve their quality of life.
Medical Cannabis (MC) is proving to be a clinically effective treatment in the management of veterans with complex physical and mental health issues. However the standardization of dosing regimens remains controversial. Whereas lower dosages and single strains MC are often effective in civilian populations, this is not the case in the veteran population. Veterans have complex medical issues which require MC regimens composed of multiple strains and multiple formulations administered at key clinical times throughout the day. Data regarding these highly effective MC regimens in the Canadian veteran population will be reviewed as well as their potential for the reduction of the adverse effects of polypharmacy and opioid sparing potential.
Background
Medicinal cannabis (MC) has received increased research attention over recent decades and had been used successfully in chronic pain, muscle spasticity, chemotherapy-induced nausea and vomiting, and intractable childhood epilepsy and PTSD. Yet its potential applications in the field of neuropsychiatry are lesser known.
Methods
As a part of consulting work, we assessed condition of 478 adults with various neuropsychiatric illnesses, such as Autistic Spectrum/PD Disorder, Chronic Headache multiple sclerosis, adulthood epilepsy, Head/Brain Traumas including hypoxic/ischemic injury, Gilles de la Tourette syndrome, Parkinson's and Alzheimer diseases, dementias etc., who applied to the Ministry of Health in order to obtain a license for the MC. Unfortunately only about 75% were succeeded in this so they consisted the study group. Quality of Life Scale & Clinical Global Impression-Improvement Scale (CGI-I) were used. We followed-up them (in terms of periodical evaluation) for a period of about five years.
Results
At baseline almost all patients used also conventional medications (such as antidepressants, anticonvulsants etc.). Majority of them were cannabis-naïve. MC of different properties and potency was provided by several companies. The ways of delivery: spray oil, inhalation. The MC daily dosage was flexible in dose 1.5–2.5 gr/day. In most cases a significant improvement in Quality of Life (up to 50%) and in CGI-I (up to 60%) scores was observed. Under this combine treatment the patients reported a discontinuation or lowering the dosage of conventional medications. Improved patients belonged to group with multiple comorbidities. No exacerbations or serious adverse events were reported.
Discussion & Conclusions
This naturalistic observational study represents a first attempt to assess and to monitor the effectiveness and safety of the MC in various neuropsychiatric illnesses, focusing on the quality of life. The results show good tolerability and other benefits of such flexible combine approach especially in patients with multiple clinical comorbidities.
The leak of ability the patient to have a sustained therapeutic regiment with maintained cultivar or other MC products (due to MoH failed reform), presented a serious restrain. Further investigations are needed to substantiate our observations and to elaborate the most effective and safe therapeutic approaches to this difficult-to-treat group.
We are conducting a novel, online research study to examine the impact of human genetic variation on the medical risks and benefits of cannabis. Understanding biological factors that contribute to cannabis use and its medically relevant effects is important to guide effective and safe decisions about use. Cannabis is the most commonly used illicit drug in multiple countries and is increasingly used both recreationally and for its expected medical benefits. In the United States for example acceptance of cannabis use has rapidly evolved, and while it remains illegal at the federal level, a majority of U.S. states have now voted to legalize cannabis use in at least some contexts and forms. Our study conducts recruitment and assessments entirely online which is efficient and cost-effective. Moreover our novel approach meets social distancing and safety needs during the current COVID-19 pandemic by enabling human participant research without in-person interactions. Our study design leverages online tools and the growing popularity of the consumer genomics industry. This project immediately addresses a high-priority substance cannabis, and establishes a platform for studying emerging research needs including, potentially, a range of therapeutic effects of the plant. We expect to begin recruiting participants this fall.
Issues of women's hormonal health and pain management are often overlooked and are poorly managed by standard medical treatments. In part because the issues are complex and require a multimodal treatment approach, which is not pharmacologically available through current drug-based medicine practices. Medical Cannabis is a very promising treatment, which allows for multifactorial treatment targets depending on the strain of medical cannabis and formulation used. More specifically strains can be chosen clinically to target inflammation, microcirculation, neuroprotection, neurodegeneration, and other basic mechanisms of homeostasis that enhance the management of women's health issues. Furthermore the varied formulations including, edibles, topicals, ingestibles, ans nasal sprays, as well as rectum and vaginal suppositories, provide an advantage over other standard medication formulations. Data from clinical cases of endometriosis fibromyalgia syndrome, post-partum depression, sleep deprivation, and enhancement of sexual pleasure will be reviewed.
In recent years the Cannabis sativa genome is at the centre of research efforts due to the species' large array of secondary metabolism products and their potential to interact with mammalian systems. Removal of legislative barriers has enabled the publication of multiple genome assemblies using various technologies for sequencing and assembly mostly using highly heterozygous plants. In this work we demonstrate a pan-genome comparison of multiple cannabis genomes of hemp and drug type cultivars. We have de-novo assembled two new heterozygous elite-line genomes with fully phased high accuracy assemblies and compared them to four public reference-level non-phased assemblies and several commercial clones at WGS-level assembly. This comparison was carried out in a pan-genome structure based on a common coordinate system of the CBDRx reference genome. We have aligned and ordered multiple genome assemblies with phased haplotypes and created uniform chromosome mapping. We have generated a non-redundant dataset of 43000 transcripts and mapped it to each haplotype. This enabled identification of allelic variation and novel homologues of important genes for cannabinoid biosynthesis as well as an accurate comparison of copy number, present-absent and structural variations, identification of highly conserved gene region duplications and identification of novel candidate genes. We have identified hyper-variable regions and massive genome rearrangements that may hold great significance to cannabis and hemp research and breeding.
Background
Pain perception in Inflammatory Bowel Disease (IBD) is a complex somatic and psychological phenomenon. Mapping of the latter might be helpful to define the appropriate treatment mix. Anxiety Mood Disorders and certain maladaptive coping strategies are well documented in IBD. Unlike smoking and alcohol consumption cannabis use is less thoroughly researched: only non-contemporaneous evidence exists.
Objectives
The authors of the present review aimed to assess if there was a statistically significant association between marijuana use and adult IBD (vs unselected controls) and if there was one to determine the most important patterns/characteristics. The search window was intentionally set to the past 7 years because since 2013 there have been significant changes both in legal and medical recognition of cannabis/cannabinoids.
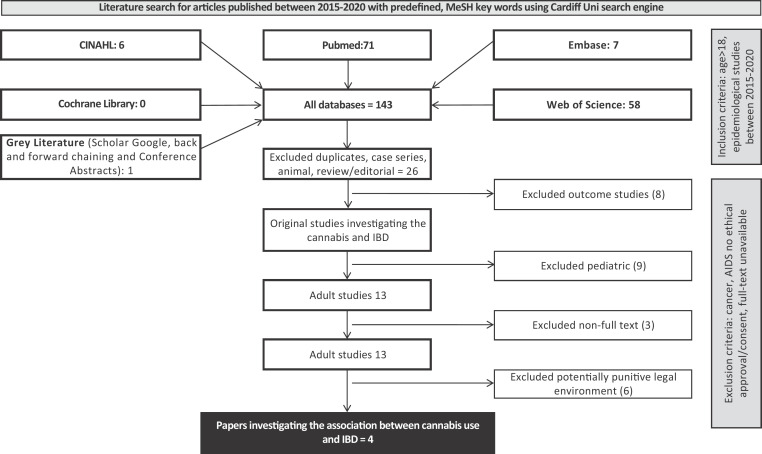
Search strategy
Embase, CINAHL, Web of Science, PubMed, and the Cochrane Library were researched using preselected keywords for full-text epidemiology focused studies published in English between 2013 and 2020.
Results
Out of 143 records seven articles qualified. Cannabis use prevalence in IBD patients who “ever used” varied between 54-70% (vs 46-60% in unselected population) and “current use” varied between 6.8-25% (vs 8.6-14%). The predictive value of certain psychological factors was equivocal.
Discussion
Our review determined increased prevalence however, the wide variability/range suggests the need for more focused/stratified research and analysis. The controversial role of psychologic factors implied that the somatic anti-inflammatory effect of cannabinoids might be amplified or on the contrary, attenuated by those covariates.
Conclusion
Dynamic, repeated psychometric analysis alongside medical assessment might help to better understand cannabis use in this complex syndrome and inform the multidimensional management of adult IBD.
Fig. 1.
(For Abstract no ID 36)
Glioma is the most common primary malignant brain tumor with poor survival and limited therapeutic options. The non-psychoactive phytocannabinoid cannabidiol (CBD) has been shown to be effective against glioma; however the molecular target and mechanism of action of CBD in glioma are poorly understood. Here we investigated the molecular mechanisms underlying the antitumor effect of CBD in preclinical models of human glioma. Our results showed that CBD induced autophagic rather than apoptotic cell death in glioma cells. We also showed that CBD induced mitochondrial dysfunction and lethal mitophagy arrest leading to autophagic cell death. Mechanistically calcium flux induced by CBD through TRPV4 activation played a key role in mitophagy initiation. We further confirmed TRPV4 levels correlated with both tumor grade and poor survival in glioma patients. Tran-scriptome analysis and other results demonstrated that ER stress and the ATF4-DDIT3-TRIB3-AKT-mTOR axis downstream of TRPV4 were involved in CBD-induced mitophagy in glioma cells. Lastly CBD and temozolomide combination therapy in patient-derived neurosphere cultures and mouse orthotopic models showed significant synergistic effect in both controlling tumor size and improving survival. Altogether these findings showed for the first time that the antitumor effect of CBD in glioma is caused by lethal mitophagy and identified TRPV4 as a molecular target and potential biomarker of CBD in glioma. Given the low toxicity and high tolerability of CBD we therefore propose CBD should be tested clinically for glioma, both alone and in combination with temozolomide.
Delivery of cannabis to the lungs is typically performed by smoking or vaping. Since the dose depends on the inhalation pattern accurate dosing is limited. In addition the heating vaping or burning of the cannabis changes its active ingredients further complicating the ability to control the actual cannabinoids and terpenes inhaled by the patient. We developed a unique hand held, patented, gas nebulizer that can convert viscous fluids (up to 80cP) into aerosol with micron size droplets (fig 1). We adapted a capsule with a proprietary oil free cannabis formulation that contains up to 1.1 ml, which is more than needed for a single day use. Dosing will be dynamic and personalized using a smartphone app. This technology has the capability to administer accurate and reproducible dosage of cannabinoids to the plasma while ensuring accurate composition of cannabinoids and terpenes since no heating is involved in the creation of aerosol.
Fig. 1.

(For Abstract no ID 32)
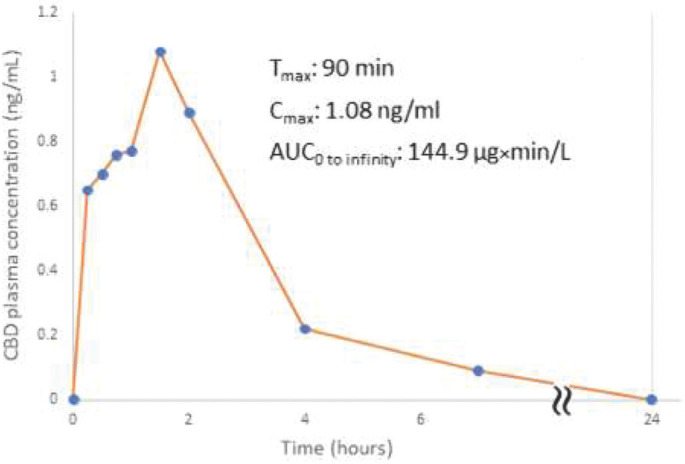
The CBD concentration in our oil-free CBD formulation was 10 mg/ml (synthetic CBD, Cerilliant C-0145). Formulation stability was verified to be at least 1 month. Each puff was produced from 50 µl of the formulation. Droplet size distribution was measured using laser diffraction (Spraytec) and the median mass aerodynamic diameter (MMAD) was less then 5 µm (fig 2) as required by FDA for inhalation. Next Generation Impactor (Copley) which simulates droplet deposition in the respiratory system indicated CBD deposition of 83%. Initial pre-clinical studies in anesthetized pigs indicated delivery of CBD into the plasma (fig 3)
Fig. 2.
(For Abstract no ID 32)
Fig. 3.
(For Abstract no ID 32)
Our data indicate the feasibility of accurate and reproducible systemic administration of medical cannabis using a hand held nebulizer. Further testing is needed before first-in-human trials can be performed.
Investors and operators alike have been misinformed about the opportunities for Medical Cannabis in Europe and have paid dearly. Our e-presentation summarizes a recent published KOMAND research report that describes the most important considerations for the European medical cannabis market and introduces a blueprint for identifying the best- and worst-positioned strategies. The European medical cannabis market is steadily growing while heavily regulated under existing pharmaceutical legislation. Having sound business facts and scientific data adhering to proven business principles and adopting smart strategies are the key to success.
Medical indications that are potential targets for cannabis treatment are expanding steadily but scientific evidence from clinical trials, the gold standard for medical adoption, takes time to accumulate, disseminate and shape into official clinical guidelines. Strategies exist for using indirect evidence of efficacy and safety for cannabis medicines to accelerate this medical adoption process. Evidence from official pilot studies discretionary prescribing, open-label trials, and even surveys of gray-market cannabis use (sometimes collectively called Real World Evidence) are adding to the body of scientific knowledge about medical cannabis. To date European market forecasts have been overstated and are unusable for serious business decision making. KOMAND has developed and implemented a 2-level forecasting approach which uses leading countries as benchmarks for market projections and then more sophisticated forecasts for a deeper analysis of country-level fundamentals and qualitative factors. Such forecasts are important for developing realistic go-to-market strategies in a nascent medical market.
Finally, before committing capital to a company, it is wise to take an investment in strategy approach. In this new industry choosing a viable strategic position is imperative for survival and then eventual success. In this report KOMAND introduces its 6-ArchTM model for identifying the best strategies for a European context. European outcomes will not mirror the successes and failures seen in North America.
Fig. 1.
(For Abstract no ID 51)
Numbers refer to abstract numbers
Avisar, I. ID 35
Baruch, Y. ID 24
Bierut, L. ID 50
Cranswick, N. ID 22
Culverhouse, R. ID 50
Di-Cori, T. ID 32
Earleywine, M. ID 50
Efron, D. ID 22
Freeman, J. ID 22
Galzerano, J. ID 23
Greenspan, S. ID 27
Guo, Y. ID 50
Gyarmathy, V. ID 36, ID 114
Holmes, J. ID 33
Huang, T. ID 34
Jacknin, J. ID 37
Kanizsai, P. ID 36
Lee, K. ID 22
Levy, O. ID 33
Lin, S. ID 50
Love, R. ID 26
Makriyiannis, N. ID 51
Moscovitch, A. ID 27
Mulraney, M. ID 22
Payne, J. ID 22
Prakash, C. ID 22
Reznik, I. ID 111
Rivas, R. ID 27
Ruark, M. ID 33
Saccone, N. ID 50
Taylor, K. ID 22
Temtsin Krayz, G. ID 32
Thirlwell, C. ID 26, ID 27
Toledo, E. ID 32
Unger, T. ID 51
Valdes, S. ID 33
Velazquez, M. ID 23
Weisshaus, O. ID 28
Williams, K. ID 22
Xantus, G. ID 36, ID 114
Xu, T. ID 34
Yan, C. ID 34
Yucel, T. ID 33
Yuhan, W. ID 33