Abstract
V(D)J recombination is directed by recombination signal sequences. However, the flanking coding end sequence can markedly affect the frequency of the initiation of V(D)J recombination in vivo. Here we demonstrate that the coding end sequence effect can be qualitatively and quantitatively recapitulated in vitro with purified RAG proteins. We find that coding end sequence specifically affects the nicking step, which is the first biochemical step in RAG-mediated cleavage. The subsequent hairpin formation step is not affected by the coding end sequence. Furthermore, the coding end sequence effect can be ablated by prenicking the substrate, indicating that the coding end effect is specific to the nicking step. In reactions in which both 12- and 23-substrates are present, a suboptimal coding end sequence on one signal can slow down hairpin formation at the partner signal, a result consistent with models in which coordination between the signals occurs at the hairpin formation step. The coding end sequence effect on nicking and the coupling of the 12- and 23-substrates explains how hairpin formation can be rate limiting for some 12/23 pairs, whereas nicking can be rate limiting when low-efficiency coding end sequences are involved.
The exon that encodes the antigen-binding domain of the T-cell receptor or the immunoglobulin gene is assembled from germ line subexon elements V (variable), D (diversity), and J (joining) through a DNA rearrangement called V(D)J recombination. V(D)J recombination is directed by a recombination signal sequence (RSS) adjacent to each coding element. Each RSS contains a conserved palindromic heptamer that is immediately adjacent to the coding end sequence and an AT-rich nonamer separated from the heptamer by a nonconserved spacer of either 12 or 23 bp (12- or 23RSS). Recombination in vivo is coupled, in that it occurs strictly between a subexon element that has a 12RSS and one that has a 23RSS, a feature known as the “12/23 rule” (25). It has been shown that the consensus heptamer (5′-CACAGTG-3′) and nonamer (5′-ACAAAAACC-3′) are the optimal signal sequences for recombination. Mutations in heptamer or nonamer sequences or alteration of spacer length can markedly reduce recombination efficiency (10).
Initiation of V(D)J recombination requires the recombination activation genes, RAG1 and RAG2 (16, 22). RAG1 and RAG2 are the only lymphoid-specific factors required for V(D)J recombination because introduction of RAG protein expression vectors into nonlymphoid cells confers recombination activity to these cells (16, 21). RAG1 and RAG2 act together as the recombinase complex that recognizes the RSS and generates DNA double-strand breaks at the RSS-coding sequence junction. One recombination event results in four DNA ends, two signal ends, and two coding ends. The two coding ends are joined to form a coding joint, and the two signal ends are joined to form a signal joint. The broken DNA ends are joined through a pathway called nonhomologous DNA end joining, which is the major pathway to repair DNA double-strand breaks in mammalian cells (reviewed in reference 14).
Cell-free V(D)J recombination was achieved when purified recombinant RAG proteins became available, leading to a major step forward in the mechanistic understanding of the biochemistry of RAG-mediated cleavage (initiation) during V(D)J recombination. RAG-mediated cleavage occurs in two steps after RAG binding to the RSS (15). First, a nick is introduced at the 5′ end the heptamer adjacent to the coding sequence, leaving a 3′-hydroxyl group at the coding end and a 5′-phosphate group at the signal end. In the second step, the 3′-hydroxyl group at the coding end attacks the antiparallel strand in a direct transesterification reaction to create a covalently sealed hairpin structure at the coding end, leaving a 5′-phosphorylated blunt signal end. In vitro cleavage with purified recombinant RAG proteins is markedly influenced by the divalent cation present in the reaction (13, 18, 27). For an isolated signal substrate, Mg2+ only supports nicking, while Mn2+ supports both nicking and hairpin formation. Efficient hairpin formation can be seen with Mg2+ as the divalent cation only when both 12- and 23-signals are present in the reaction, and therefore cleavage with Mg2+ as the divalent cation mimics the in vivo situation in that cleavage is coupled in a 12/23 pair. RAG proteins plus DNA-bending proteins, such as HMG1, are sufficient to establish the 12/23 rule in vitro (13, 29). Ca2+ does not support either nicking or hairpin formation, but it does allow complex formation between the RAG complex and the DNA substrate containing the RSS (11). Therefore, Ca2+ is often used in electrophoretic mobility shift assays (EMSAs) (11, 12, 23, 24).
It was initially thought that the coding end sequence was neutral in V(D)J recombination because RSSs are necessary and sufficient to direct V(D)J recombination. However, direct testing showed that coding end sequence can affect the recombination frequency by up to 2 orders of magnitude (2, 3, 6, 7, 9). The coding end sequence effect in V(D)J recombination is at the cleavage stage, rather than at the rejoining of the broken DNA ends, because both coding joint and signal joint formation are similarly affected (9).
In this study, we determine the biochemical basis for this coding end sequence effect by using an in vitro cleavage assay. We find that the overall cleavage by RAGs can be affected by the coding end sequence in a manner that is qualitatively and quantitatively very similar to what has been demonstrated in vivo. Prenicking can fully eliminate this coding end sequence effect, confirming that the coding end sequence is influencing the nicking step only, without any impact on the subsequent hairpin formation step. Identification of the step at which the coding end sequence affects the RAG cleavage process permitted us to use a low-efficiency coding end on a 12-substrate to slow the coupled cleavage with a 23-substrate in a 12/23 system. Slowed nicking of the low-efficiency 12-substrate slows hairpin formation, but not nicking, of the partner 23-substrate, a finding consistent with the models in which hairpin formation is coupled (8, 29). This has implications for limitations on the overall rate of the RAG-mediated steps in vitro and in vivo and for the immune repertoire.
MATERIALS AND METHODS
DNA substrates.
All oligonucleotides were synthesized by Operon Technologies, Inc. (Alameda, Calif.), and purified by polyacrylamide gel electrophoresis under denaturing conditions. Each double-stranded DNA (dsDNA) substrate containing a single RSS (12 or 23) was constructed by annealing two complementary oligonucleotides. 12RSS substrates varying in coding end sequence were made with the following oligonucleotides (listed in pairs): (i) KY28, 5′-GAT CAG CTG ATA GCT ACC ACA GTG CTA CAG ACT GGA ACA AAA ACC CTG CT-3′, and KY29, 5′-TAG CAG GGT TTT TGT TCC AGT CTG TAG CAC TGT GGT AGC TAT CAG CTG AT-3′; (ii) KY30, 5′-GAT CAG CTG ATT TAA TTT CAC AGT GCT ACA GAC TGG AAC AAA AAC CCT GCT-3′, and KY31, 5′-TAG CAG GGT TTT TGT TCC AGT CTG TAG CAC TGT GAA ATT AAA TCA GCT GAT-3′; (iii) KY43, 5′-GAT CAG CTG AAA ATT AAA CAC AGT GCT ACA GAC TGG AAC AAA AAC CCT GCT-3′, and KY44, 5′-TAG CAG GGT TTT TGT TCC AGT CTG TAG CAC TGT GTT TAA TTT TCA GCT GAT-3′; and (iv) KY45, 5′-GAT CAG CTG ACG TAA TAA CAC AGT GCT ACA GAC TGG AAC AAA AAC CCT GCT-3′, and KY46, 5′-TAG CAG GGT TTT TGT TCC AGT CTG TAG CAC TGT GTT ATT ACG TCA GCT GAT-3′. 23RSS substrates were made by using the following oligonucleotides: (i) KY26, 5′-GAT CAG CTG AGG CCG GGC ACA GTG GTA GTA CTC CAC TCT CTG GCT GTA CAA AAA CCC TGC T-3′, and KY27, 5′-TAG CAG GGT TTT TGT ACA GCC AGA GAG TGG AGT ACT ACC ACT GTG CCC GGC CTC AGC TGA T-3′, and (ii) KY38, 5′-GAT CAG CTG ATT AAT TTC ACA GTG GTA GTA CTC CAC TCT CTG GCT GTA CAA AAA CCC TGC T-3′, and KY39, 5′-TAG CAG GGT TTT TGT ACA GCC AGA GAG TGG AGT ACT ACC ACT GTG AAA TTA ATC AGC TGA T-3′. Oligonucleotides used to construct the prenicked 12RSS were as follows: KY33, 5′-GAT CAG CTG ATA GCT AC-3′; KY34, 5′P-CAC AGT GCT ACA GAC TGG AAC AAA AAC CCT GCT-3′; and KY35, 5′-GAT CAG CTG ATT TAA TTT-3′ (P indicates 5′ phosphorylation).
Oligonucleotides were labeled with [γ-32P]ATP (3,000 Ci/mmol) (New England Nuclear Research Products, Boston, Mass.) by T4 polynucleotide kinase according to the manufacturer’s instructions (New England Biolabs, Beverly, Mass.). Unincorporated radioisotopes were removed by G-25 Sepharose (Amersham/Pharmacia, Piscataway, N.J.) spin-column chromatography. To anneal double-stranded substrate, the labeled oligonucleotide was heated at 95°C for 5 min with an equal molar amount of the complementary oligonucleotide and allowed to cool down slowly to room temperature.
Protein expression and purification.
Truncated mouse RAG proteins glutathione S-transferase (GST)-RAG1 (amino acids 330 to 1040) plus GST-RAG2 (amino acids 1 to 383) were coexpressed as GST fusion proteins in 293T cells and purified as previously described (1, 19, 28). C-terminal truncated mouse HMG1 was expressed in bacteria as a His6-tagged protein and purified over a Ni-nitriloacetic acid column (29).
In vitro cleavage assay.
A 10-μl reaction mixture containing 0.2 pmol of 32P-labeled substrate and equal amounts of unlabeled partner substrates in cleavage buffer (25 mM MOPS [morpholinepropanesulfonic acid], pH 7.0; 5 mM MgCl2; 30 mM KCl; 30 mM potassium glutamate; 1 pmol of HMG1). Cleavage was initiated by the addition of 200 ng of RAG proteins and incubated at 37°C. The reaction was stopped by the addition of 0.1% sodium dodecyl sulfate, 20 mM EDTA, and 10 μl of formamide. Samples were heated to 100°C for 3 min and put on ice immediately. Reaction products were separated on 15% denaturing polyacrylamide gels in 1× Tris-borate-EDTA (TBE) buffer. Gels were visualized by autoradiography by using a Molecular Dynamics PhosphorImager 445SI (Sunnyvale, Calif.) and quantified with ImageQuaNT software (version 4.1).
EMSA.
Exactly 0.2 pmol of 32P-labeled 12-substrate was mixed with 200 ng of RAG proteins in a 10-μl reaction mixture containing 25 mM MOPS (pH 7.0), 5 mM MgCl2, 30 mM KCl, 30 mM potassium glutamate, 1 pmol of HMG1, 0.1 mg of bovine serum albumin per ml, and 1 μM double-stranded nonspecific competitor DNA (35 bp). The reaction mixture was incubated for 10 min at 37°C. After the addition of 1 μl of 80% glycerol, 5 μl of the reaction mixture was loaded onto a 4% polyacrylamide gel, and electrophoresis (15 V/cm) was performed in the presence of 1× TBE at 4°C. Gels were visualized by autoradiography by using a Molecular Dynamics PhosphorImager 445SI and quantified with ImageQuaNT software.
RESULTS
Experimental strategy.
In order to examine the V(D)J recombination cleavage steps, we used oligonucleotide substrates that contain an RSS (12 or 23) and flanking sequences, as has been described previously (1, 12). The coding flank is 16 to 18 bp long, which is sufficient to support efficient cleavage (13). DNA substrates with the 12RSS are referred to as the 12-substrates, while DNA substrates with the 23RSS are referred to as the 23-substrates. The 12- and 23RSS used throughout this study are the consensus ones, which give optimal recombination efficiency (10) (Fig. 1A). The sequence that is directly adjacent to the heptamer is referred to as the coding end. Variations of the coding end sequence are designed according to our previous in vivo study (9). The coding end sequences of various 12- and 23-substrates used in this study are listed in Table 1.
FIG. 1.
Biochemical system to study RAG-mediated cleavage in V(D)J recombination. (A) Structure of a 12-substrate made by annealing two complementary oligonucleotides. The open triangle represents the consensus 12RSS. The shaded box represents the coding end sequence that is subject to variations. The name of each sequence motif is indicated above the DNA substrate. The 23-substrate differs from the 12-substrate only in spacer length (not shown). (B) 12/23-regulated in vitro cleavage occurs in two steps. First, substrates are nicked, and then hairpins form at the coding ends. Open and shaded triangles represent 12RSS and 23RSS, respectively.
TABLE 1.
DNA substrate coding end sequences and efficiencya
| DNA substrate | Coding end sequence | Efficiency |
|---|---|---|
| KY28/29 | 5′-TAGCTAC-12RSS-3′ | High |
| KY30/31 | 5′-TTTAATTT-12RSS-3′ | Low |
| KY43/44 | 5′-AAATTAAA-12RSS-3′ | Intermediate |
| KY45/46 | 5′-CGTAATAA-12RSS-3′ | Intermediate |
| KY26/27 | 5′-GGCCGGG-23RSS-3′ | High |
| KY38/39 | 5′-TTAATTT-23RSS-3′ | Low |
12/23 regulated cleavage (Fig. 1B) is carried out as described previously (1, 12). Each reaction contains equal molar amounts of a 12-substrate and a 23-substrate. Only one of them is 5′ labeled at the coding flank. Therefore, only the nicked and hairpin products of the labeled substrate are detectable on a denaturing polyacrylamide gel. Also, only one of the two substrates (12 or 23, but not both) undergoes coding end sequence variation in an individual experiment.
Coding end sequence affects the overall RAG-mediated cleavage in vitro.
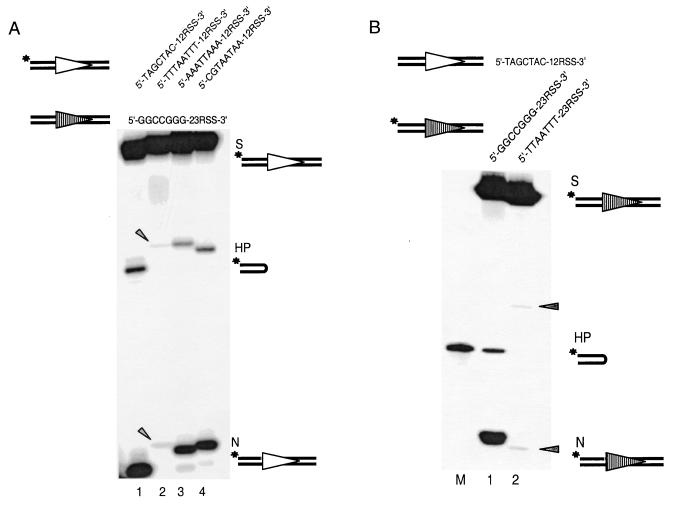
Coding end sequence can affect the initiation frequency of V(D)J recombination of extrachromosomal substrates inside the cell (9). We first tested whether the coding end sequence effect can be reproduced in vitro by using a well-defined RAG cleavage assay (1, 12). Four 12-substrates with different coding end sequences were selected based on our previous cellular studies (9), including one high-efficiency (5′-TAGCTAC-12RSS-3′), one low-efficiency (5′-TTTAATTT-12RSS-3′), and two intermediate-efficiency (5′-AAATTAAA-12RSS-3′ and 5′-CGTAATAA-12RSS-3′) coding end sequences. Each of these different 12-substrates was paired with the same 23-substrate. The coding end sequence for the 23-substrate is 5′-GGCCGGG-23RSS-3′, which gives optimal recombination frequency inside the cell (9). The organization of coding end sequences on our oligonucleotide substrates directly corresponds to those for the extrachromosomal substrates. We found that the optimal coding end sequence found in vivo also has the most efficient cleavage in our biochemical cleavage assay in vitro (Fig. 2A, lane 1). The suboptimal coding end substrate yields only minimal amounts of nicking and hairpin products (Fig. 2A, lane 2). The two intermediate coding end sequences show intermediate cleavage efficiency (Fig. 2A, lanes 3 and 4). Hereafter, we refer to the 12-substrate with an optimal coding end sequence as the “12-high” substrate, while the substrate with a coding end sequence with the lowest recombination efficiency is the “12-low” substrate. The difference in hairpin formation between the 12-high substrate and the 12-low substrate is 20-fold after 20 min. This difference is very similar to the 34-fold difference observed in vivo for signal joint formation with corresponding RSS and coding end sequences (9). Therefore, the coding end sequence effect observed for wild-type RAG proteins in cells can be faithfully reproduced in a fully defined biochemical system.
FIG. 2.
Coding end sequence effect seen in vivo can be reproduced in vitro. (A) Coding end sequence effects on the processing of 12-substrates. The 12-substrate is 5′ labeled for each individual reaction. As a result, only the nicked and hairpin products of the 12-substrate are detectable on the gel. The coding end sequence is varied here only on the 12-substrate, including high-efficiency (lane 1), low-efficiency (lane 2), and intermediate-efficiency (lanes 3 and 4) 12-substrates. The top strand sequences of the 12- and 23-substrates are indicated above the gel. Open and shaded triangles represent 12RSS and 23RSS, respectively. Asterisks indicate the radioactively labeled positions. The 12-high (lane 1) and 12-low (lane 2) have a 1-bp difference in length due to the different lengths of the coding end sequence. As a result, the nicking and hairpin products of the 12-high are one and two nucleotides shorter, respectively, than those of the 12-low. Oligonucleotide markers corresponding to each N and HP species are not shown but were run on each gel. S, substrate; HP, hairpin; N, nicking. The arrowheads indicate the positions of faintly visible nicked and hairpinned products for the 12-low substrate. (B) Coding end sequence variation on 23-substrates. M is a size marker corresponding to the hairpin product of 23-high (lane 1) because of its aberrant mobility on the denaturing gel. The mobility of the hairpin product of the 23-low (lane 2) is normal; therefore, no marker is shown for this hairpin product. All other symbols are as in Fig. 2A. The arrowheads indicate the positions of faintly visible nicked and hairpinned products for the 23-low substrate.
Next, we asked whether this coding end effect also applies to the 23-substrate. Similarly, we compared the cleavage of two 23-substrates that differ only in coding end sequence, 5′-GGCCGGG-23RSS-3′ versus 5′-TTAATTT-23RSS-3′. Each was paired with the 12-high substrate (5′-TAGCTAC-12RSS-3′). Again, nicking and hairpin formation of the former is much more efficient than that of the latter (Fig. 2B, compare lanes 2 and 3). The magnitude of the effect is comparable to that observed for the 12-substrates. It is useful to note that 5′-GGCCGGG-RSS-3′ and 5′-TAGCTAC-RSS-3′ showed equally high efficiency in the cellular assay, as well as in the in vitro cleavage assay (data not shown). We therefore conclude that coding end sequence can markedly affect RAG-mediated cleavage and that this effect applies to both 12- and 23-substrates.
Coding end sequence affects nicking.
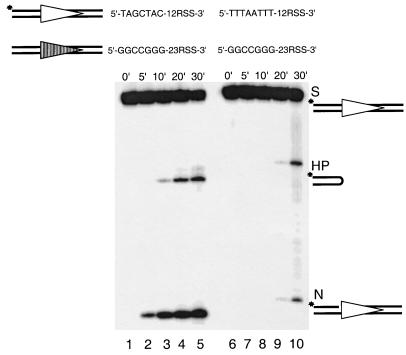
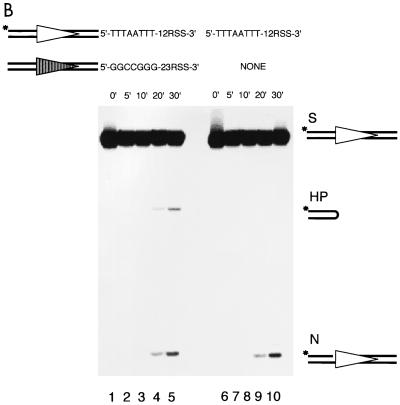
Biochemical cleavage occurs in two steps: nicking and hairpin formation. Each of these steps could be affected by the coding end sequence. We first examined coding end sequence effects on nicking as a function of time. The 12-high substrate and the 12-low substrate were 5′ labeled at the coding flank. Each of these was paired with the same 23-high substrate. The nicking and hairpin products detected on the gel result from the processing of the 12-substrate. The nicking product of the 12-high substrate could be readily detected by as early as 5 min (Fig. 3A, lane 2). In contrast, nicking product of the 12-low substrate is barely detectable until 20 min (Fig. 3A, lane 9). This clearly indicates that nicking is greatly affected by coding end sequence variations.
FIG. 3.
Coding end sequence effect on nicking as a function of time. The left panel shows the cleavage of the 12-high (labeled) paired with the 23-high over a period of 30 min (lanes 1 through 5). The right panel (lanes 6 through 10) shows the cleavage of the 12-low (labeled) paired with the same 23-high as in the left panel. The time points are shown above the gel. All other symbols are as in previous figures.
Interestingly, when the 12-high substrate is paired with the 23-high substrate, the hairpin product of the 12-high substrate is a small fraction (20% at 20 min) of the nicking product for each time point (Fig. 3A, lanes 2 to 5). This suggests that hairpin formation can be a slow step relative to nicking. However, when the 12-low substrate is paired with the 23-high substrate, the hairpin product of the 12-low substrate is almost equal to the corresponding nicked product (Fig. 3A, compare the nicked versus the hairpinned product in lanes 9 and 10). This indicates that hairpin formation on the 12-low substrate is no slower than the nicking step, which suggests that nicking is the rate-limiting step here. Also, we consistently see a marked increase in the hairpin formation for the 12-low substrate from 20 to 30 min. One possibility to explain this would be that the cleavage reaction is coordinated at the hairpin formation step (29). Therefore, the accumulation of a vast excess of nicked 23-substrates, though undetectable on the gel because it is unlabeled, would increase, by mass action, the hairpin formation of the paired 12-low substrate. To further explore this, we studied the timing of coupling between the two paired substrates (see below).
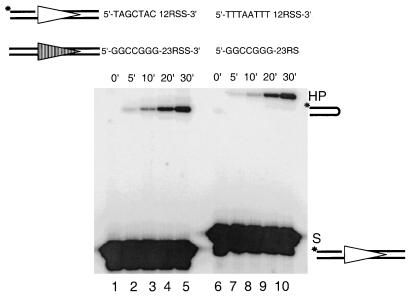
Coding end sequence does not affect hairpin formation of prenicked substrates.
To study specifically the coding end sequence effect on hairpin formation, we bypassed the nicking step by prenicking the 12-substrate. This was performed by annealing three oligonucleotides to form a dsDNA with a nick on the top strand separating the coding end from the heptamer of the RSS (Fig. 4, compare the 12-substrate versus the 23-substrate). The coding flanks of the 12-substrates were 5′ labeled. When each of the prenicked 12-substrates was paired with the 23-high substrate, we found that the conversion to hairpin product occurs at the same rate for prenicked 12-high substrate and prenicked 12-low substrate, regardless of the difference in their coding end sequences (Fig. 4, compare lanes 1 through 5, and lanes 6 through 10). Therefore, the coding end sequence does not affect the conversion of the nicked product to hairpin product. This indicates the coding end sequence exerts its effect at the nicking step and that this effect is fully eliminated by artificially prenicking the target.
FIG. 4.
Elimination of coding end sequence effect by prenicking the 12-substrates. The absence of a dash between the coding end and the 12RSS (top row) indicates the position of a nick introduced by annealing three oligonucleotides. The nicked products become the substrates in this experiment as indicated by “S.” The left panel shows the hairpin formation of a prenicked 12-high paired with an intact 23-high over a period of 30 min. The right panel shows the cleavage of a prenicked 12-low paired with the same intact 23-high over the same period of time. All other symbols are as in previous figures.
Coding end sequence does not affect the binding of RAG proteins to the RSS.
The elimination of the coding end sequence effect by prenicking indicates that coding end sequence only affects the nicking step. A logical prediction would be that the prior step, namely, binding of the RAG proteins to the 12- and 23-substrates, is entirely unaffected. To confirm this, we tested RAG-DNA interaction by an EMSA. The EMSA was performed by using precisely the same conditions as the cleavage assay except for the addition of a 50-fold molar excess of nonspecific dsDNA to prevent nonspecific RAG-DNA interactions. A band with retarded mobility corresponding to the RAG-DNA complex can be clearly detected (Fig. 5). This complex is resistant to nonspecific dsDNA competitors but sensitive to specific competition with cold probe (data not shown), indicating a specific protein-DNA interaction. Addition of an equal amount of cold 23-substrate resulted in a reduction in the signal intensity rather than formation of a synaptic complex migrating at a different position (12). The intensity of the RAG-DNA complex formed by RAG proteins with the 12-high substrate is comparable to that with the 12-low substrate (Fig. 5, compare lanes 3, 4, and 5 with lanes 8, 9, and 10, respectively). No difference was found when we compared RAG-DNA binding by EMSA with other substrates that varied in coding end sequence (data not shown). Furthermore, no difference was observed for the binding of RAGs to the prenicked 12-high substrate compared to the intact 12-high substrate (data not shown). These findings indicate that the coding end sequence does not affect the binding of the RAG proteins to the DNA substrate.
FIG. 5.
RAG-RSS interaction is not affected by coding end sequence variation. RAG-RSS interaction is examined by EMSA. The RAG-DNA complex is indicated. The 12-high (5′-TAGCTAC-12RSS-3′) substrate interacts with the RAG complex (lane 3–5) comparably as does the 12-low (5′-TTTAATTT-12RSS) (lanes 8 to 10). Quantitation of the shifted bands (RAG-DNA complexes) shows a shift of 22% of the total radioactivity for the 12-high substrate (lane 4) and of 24% for the 12-low substrate (lane 9).
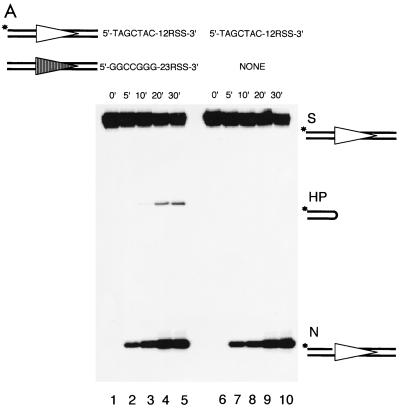
Presence of the 23-substrate does not affect the nicking efficiency of the paired 12-substrate.
It has not been clear when the 12/23 RSS synapsis occurs relative to the nicking step in V(D)J recombination (5, 8, 14, 20). Recently, synapsis of a 12- and a 23RSS was demonstrated with Ca2+ by EMSA (12). If this synapsis prior to nicking is the physiological point of 12/23 synapsis in RAG protein-mediated cleavage, it might have an impact on the nicking step. To test the possibility that the observed coding end sequence effect on nicking might actually be exerted on a synapsis step prior to nicking, we compared the nicking of a 12-high or a 12-low substrate in the presence or absence of a 23-substrate (23-high). As expected, efficient hairpin formation of the 12-substrate occurs only in the presence of a 23-substrate (Fig. 6A, lanes 1 through 5 and Fig. 6B, lanes 1 through 5). Although limited hairpin formation of the 12-high can be detected in the absence of a 23-substrate upon prolonged exposure (data not shown), a result consistent with a previous study (18), efficient hairpin formation absolutely requires the presence of both signals, indicating that our cleavage system is 12/23 regulated. When we compared the nicking efficiency in these cases, we found that the nicked product of the 12-substrate accumulates in the absence of any 23-substrate (Fig. 6, lanes 6 through 10) at the same rate as in the presence of a 23-substrate (Fig. 6, lanes 1 through 5). This clearly shows that the presence of the 23-substrate only promotes hairpin formation; it has no effect on the nicking of the 12-substrate, regardless of whether it is a 12-high or a 12-low. This suggests that 12/23 synapsis, even if it occurs prior to nicking, does not influence the nicking of either individual substrate. We do not know whether coding end sequence affects the formation of a 12/23 synaptic complex without further evidence. However, given that the nicking occurs equally well whether the 12- and 23-substrates are both present or just present individually, the coding end sequence effect on nicking cannot be influenced by any effect on the 12/23 synapsis. Therefore, the coding end sequence effect on nicking is specific to the nicking step.
FIG. 6.
Presence of the 23-substrate has no impact on nicking of the 12-substrate. (A) The left panel shows the cleavage of the 12-high in the presence of the 23-high over a period of 30 min (lanes 1 through 5). The right panel shows the cleavage of the same 12-high in the absence of any 23-substrates over the same period of time (lanes 6 through 10). (B) The left panel shows the cleavage of the 12-low in the presence of the 23-high over a period of 30 min (lanes 1 through 5). The right panel shows the cleavage of the same 12-low in the absence of any 23-substrates over the same period of time (lanes 6 through 10). Note the accumulation of the nicked products at the bottom of the gel. All other symbols are as in previous figures.
Coding end sequence variation on one substrate affects the hairpin formation but not the nicking of the partner substrate.
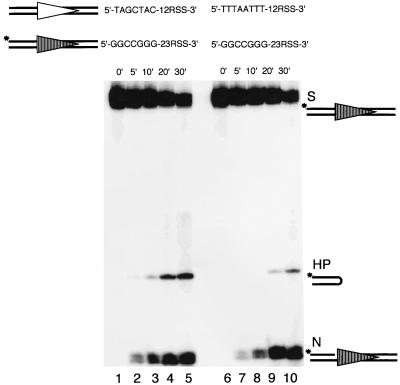
Synchronization between 12- and 23-signals at the hairpin formation step has been inferred recently by using an oligonucleotide substrate that contains both a 12- and a 23-signal on the same molecule (13, 29). This inference is based on the fact that single hairpin formation is much less frequent than double-hairpin formation (13, 29) and that mutations on one RSS block the hairpin formation on both signals (13). However, it is uncertain whether the apparent synchronization in hairpin formation is just a simple temporal association or a mechanistic coordination between the 12- and 23RSSs. Mutation of one RSS would not necessarily reveal this coordination either. Our finding of the coding end sequence effect on nicking provides us with a unique opportunity to study these possibilities. We can intentionally slow down the nicking of one substrate by using a suboptimal coding end sequence (leaving both the 12- and 23RSS unmutated) and then examine the nicking and hairpin formation of the partner substrate.
For this experiment, the 23-high substrate is labeled and paired with either the 12-high substrate or the 12-low substrate. Therefore, only the nicking and hairpin products of the 23-substrate are detectable. We found that the nicking of the 23-high substrate progresses at the same rate, regardless of whether it is paired with the 12-high substrate or 12-low substrate (Fig. 7, compare lanes 1 through 5 with lanes 6 through 10). Therefore, the coding end sequence does not affect the nicking of the partner substrate. This is in agreement with our previous finding that nicking is independent of the 12/23 synapsis (Fig. 6). However, the hairpin formation of the 23-high substrate is markedly delayed when paired with the 12-low substrate (Fig. 7, compare lanes 1 through 5 with lanes 6 through 10). This result illustrates two important points. First, the delay of hairpin formation on the 23-substrate by a sequence change on the 12-substrate indicates that hairpin formation is the coupled step during RAG-mediated cleavage. Second, in a 12/23-regulated cleavage reaction, hairpin formation of the 23-substrate requires not only its own nicking but also the nicking of the 12-substrate. This indicates a coordination of the events between the two signals.
FIG. 7.
Coding end sequence variation at the 12-substrate affects the processing of the partner 23-substrate in trans. The coding end sequence is varied on the 12-substrate, but the 23-substrate is radiolabeled. The left panel shows the cleavage of the 23-high paired with the 12-high over a period of 30 min (lanes 1 through 5). The right panel shows the same 23-high paired with the 12-low over the same period of time (lanes 6 through 10). The hairpin product of the 23-high runs abnormally fast; however, no marker is shown in parallel because it has been shown before in Fig. 2B. All other symbols are as in previous figures.
Similarly, when a 12-high was paired with a 23-low the hairpin formation of the 12-high, but not the nicking of the 12-high, was markedly delayed relative to the nicking and hairpin formation of the same 12-high paired with a 23-high (data not shown).
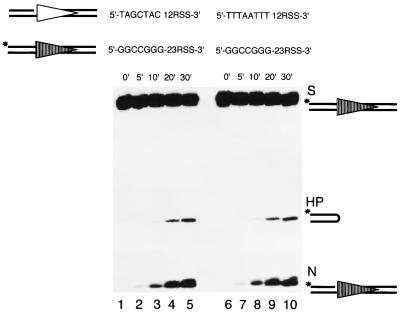
Prenicking relieves the trans effect on hairpin formation by coding end sequence.
To further test our hypothesis that nicking on both signal substrates is required for coupled hairpin formation, we repeated the previous experiment with prenicked 12-substrates. The labeled 23-high substrate was paired with either a prenicked 12-high substrate or a prenicked 12-low substrate. We found that hairpin formation of the 23-high substrate accumulates at the same rate regardless of whether it is paired with a prenicked 12-high substrate or a prenicked 12-low substrate (Fig. 8, compare lanes 1 through 5 to lanes 6 through 10). Similarly, the hairpin formation of a 12-high occurred with the same efficiency when paired with a prenicked 23-high or a prenicked 23-low (data not shown). This finding confirms our hypothesis that pairing of one nicked 12-substrate and one nicked 23-substrate is the prerequisite for coupled hairpin formation. In this regard, the functional synapsis of the 12/23 RSS is not two intact substrates but rather two nicked RSS substrates.
FIG. 8.
Prenicking of the 12-substrate eliminates its trans effect on the hairpin formation of the partner 23-substrate in trans. The left panel shows the cleavage of the 23-high paired with a prenicked 12-high over a period of 30 min (lanes 1 through 5). The right panel shows the cleavage of the same 23-high paired with a prenicked 12-low over the same period of time (lanes 6 through 10). All other symbols are as in previous figures.
DISCUSSION
Our study demonstrates that the coding end sequence plays an important role in determining the efficiency of RAG-mediated cleavage, and this permits insight into the coordination between the 12- and 23-substrates at the hairpin formation step. Nicking, the first biochemical step, is affected by coding end sequence variation (Fig. 3). This effect is specific to nicking because prenicking relieves the effect (Fig. 4). This also indicates that hairpin formation is not affected. Coding end sequence does not affect the binding of RAG proteins to the RSS (Fig. 5). The presence of both 12- and 23-substrates to permit synapsis has no effect on nicking (Fig. 6). Slowed nicking of one substrate affects the hairpin formation but not the nicking of the partner substrate (Fig. 7). Prenicking of the 12-substrate eliminates its trans effect on the hairpin formation of the paired 23-substrate (Fig. 8).
Functional definition of coding end sequences that affect V(D)J recombination.
The first in vivo findings that coding end sequence could quantitatively affect the efficiency of V(D)J recombination were performed with extrachromosomal substrates in pre-B cells from wild-type mice (9). It was found that the effect on signal joints was as much as 34-fold. The least-efficient coding ends were those ending with 5′-TTT-RSS-3′. Subsequent studies confirmed this observation (2, 3, 6, 7), and one indicated that 5′-TT-RSS-3′ was sufficient for the inhibition (7).
Mutant forms of RAG proteins also showed sensitivity to coding end sequence in the cellular assay (17), but the “good” and “bad” coding end sequences defined by using the mutant RAG proteins were quite different from those found with wild-type RAG proteins. The wild-type RAG proteins showed little sensitivity to the good and bad coding end sequence defined by the mutant RAG1 (13, 17). Unfortunately, the good and bad coding end sequence definition based on the mutant RAG1 has been used in many subsequent studies, even though wild-type RAG proteins do not show sensitivity to them. Not surprisingly, biochemical studies based on the definition with mutant RAG1 often generate conflicting results. For example, cleavage differences were seen under nonphysiological conditions when Mn2+ was the divalent cation (4, 13), while no difference could be observed for coupled cleavage with Mg2+. Even with Mn2+, this coding end sequence effect only applies to the 12RSS and not to the 23RSS (13).
The results described in the current study unify the in vivo extrachromosomal studies (performed with wild-type RAG proteins) with the purified biochemical system by using paired 12/23 substrates (also performed here with wild-type core RAG proteins). Both the qualitative and quantitative correlations between the cellular and the biochemical studies are very strong. The previous cellular studies indicated that the coding end sequence affected the efficiency of both signal joint and coding joint formation (9). Based on this, we inferred that the cleavage phase of V(D)J recombination (rather than rejoining) was the most likely stage at which coding end sequence effects had their impact. The biochemical studies reported here confirm that the primary coding end sequence effects occur in the cleavage phase of V(D)J recombination. Furthermore, the data here show that it is the nicking step at which this effect is seen, and the magnitude of this effect corresponds to the magnitude seen in vivo for signal joint formation.
Changes in the coding end sequence of a 12-substrate can affect the processing of a partner 23-substrate, indicating coupling in trans between the substrates.
Models in which nicking precedes 12/23 synapsis have been proposed earlier by others (8, 12, 27), based on the fact that Mg2+ allows nicking but not hairpin formation on an isolated RSS substrate. However, experimental data concerning whether 12/23 synapsis stimulates nicking has been limited. Data from Eastman and Schatz (5) with a body-labeled PCR fragment containing two signals (12 × 12, 12 × 23, or 23 × 23) showed a consistent twofold-higher level in the total nicking for the 12 × 23 substrate relative to alternative substrates of equivalent length. Our data demonstrates that 12/23 synapsis is unnecessary for nicking on individual substrates and that the nicking efficiency is not affected by the 12/23 synapsis (Fig. 6). Based on these data, we conclude that 12/23 synapsis does not have a significant impact on the nicking step.
Consistently, we demonstrated that hairpin formation, but not nicking, of the 23-substrate can be affected by a change of the coding end sequence on the 12-substrate in a 12/23-regulated reaction (Fig. 7). The temporal synchronization in hairpin formation during RAG-mediated steps was shown previously, based on the fact that the majority of the hairpin product of a recombination substrate containing two signals (12 and 23) is double hairpinned rather than single hairpinned (13, 29). In these studies, however, the efficiency of the coding end sequence at the 12- or 23-RSS was not considered, and therefore the apparent synchronization of the hairpin formation could have been a fortuitous result of their both having moderate to high efficiencies for nicking and hairpinning.
Our current study provides an improved approach to examining the actual coordination between signals at the hairpin formation step. Slowed nicking of a 12-substrate also slows down the hairpin formation but not the nicking of the paired 23-substrate. This clearly demonstrates a high degree of coordination between the two signals at the hairpin formation step. This also indicates that the nicking of a 12-substrate is a prerequisite for the hairpin formation of the paired 23-substrate. It is, therefore, conceivable that the physiological point at which two signals communicate with each other is at the stage when both signals have been nicked.
Recently, it was shown that blockage of hairpin formation at one RSS failed to block the hairpin formation at the other RSS (13). This was done by the introduction of a phosphorothioate linkage on the bottom strand (Fig. 1A) at a position where hairpin formation normally occurs, which specifically blocks hairpin formation at that position. This indicates that the completion of hairpin formation is not communicated between the two RSSs, even though it leaves open the question of whether the initiation of hairpin formation is coordinated. For example, would blockage of the nicking at one RSS affect the hairpin formation at the other RSS? Blocking hairpin formation by a phosphorothioate linkage is still compatible with nicking on the top strand. Phosphorothioate linkages could be considered for blockage of nicking (e.g., if it is substituted on the top strand), as was done for hairpinning (13, 26). However, we find that, unlike blocking of hairpinning, it is not possible to block nicking by using phosphorothioate linkages because this results in nicking at alternative positions instead (30).
Either nicking or hairpin formation can limit the rate of cleavage in V(D)J recombination in vivo and in vitro because of the communication between sites.
Using substrates that have moderate- to high-efficiency coding ends, generally the hairpin formation step appears to be slower than the nicking step. Large amounts of nicked product are generated before smaller amounts are converted to the hairpin product. However, with low-efficiency coding ends, the nicking step becomes very slow. In a biochemical system with both 12- and 23-substrates, the very slow nicking of the 12-low substrate, for example, makes the nicking of the 12-substrate appear to be the rate-limiting step. The 23-high substrate is nicked quite rapidly, as usual. However, without a nicked 12-low substrate to pair with, the coordinated hairpin formation is slowed. The small amount of nicked 12-low substrate that is generated, pairs with a marked excess of nicked 23-high substrate, and the pair is rapidly converted to the two corresponding hairpin products. Effectively then, the slowly nicked 12-low substrate is determining the overall rate. These observations are relevant to the corresponding events in vivo because the in vitro coding end effects match those observed for the same coding and RSS sequences tested in cells with wild-type RAG proteins.
Relevance of coding end sequence effects to the antigen receptor repertoire.
The coding end sequence effect we observed is consistent with a biased antigen receptor repertoire. For example, the coding end sequence, 5′-TTTAATTT-RSS-3′, is the most unfavorable one in both our cellular and biochemical assays. In fact, 5′-T-heptamer-3′ is significantly underrepresented in our genomic antigen receptor loci. Of 96 coding ends associated with the 12RSS that have been examined, only 10 have T-heptamer configuration. Seven of these ten elements determine the first nucleotide of a codon, suggesting that they are preserved for other selective reasons (9).
We have not yet performed an extensive survey to fully determine the efficiency of all possible coding ends. For the last three nucleotides adjacent to the heptamer, there are 64 possible coding end sequence combinations. When taken as a 12/23 pair, the number is 4096. However, this biochemical assay system provides an effective and biologically relevant method to assess some of these combinations.
The recombination frequency of any given V, D, or J elements is affected by many factors, such as cellular selection, chromatin accessibility, and quality of the RSS. Our study shows that coding end sequence is another major component that should be taken into consideration. Also, when a cryptic recombination site that may contribute to chromosome translocations during lymphoid tumorigenesis is to be analyzed, not only the nucleotide sequences resembling the RSS but also the flanking sequences (coding ends) should be evaluated.
One could speculate that coding end sequence might also affect the efficiency of the hairpin opening or rejoining of the broken ends. Previous cellular studies by us and others (2, 3, 6, 7, 9) indicated that both signal joint and coding joint formation are affected by coding end sequence. This indicates that most of the quantitative impact of the coding end sequence occurs before or during cleavage. Nevertheless, smaller quantitative effects may exist at later steps, and it will be quite interesting to examine these possibilities.
ACKNOWLEDGMENTS
We thank Patricia Cortes for RAG expression constructs. We also thank Chih-Lin Hsieh and members of our laboratory, including Robert Tracy and Zarir Karanjawala, for reviewing the manuscript.
This research was supported by National Institutes of Health grants and a Leukemia Society of America Scholar Award to M.R.L. M.R.L. is the Rita and Edward Polusky Basic Cancer Research Professor.
REFERENCES
- 1.Besmer E, Mansilla-Soto J, Cassard S, Sawchuk D J, Brown G, Sadofsky M, Lewis S M, Nussenzweig M C, Cortes P. Hairpin coding end opening is mediated by the recombination activating genes RAG1 and RAG2. Mol Cell. 1998;2:817–828. doi: 10.1016/s1097-2765(00)80296-8. [DOI] [PubMed] [Google Scholar]
- 2.Boubnov N V, Wills Z P, Weaver D T. V(D)J recombination coding junction formation without DNA homology: processing of coding end termini. Mol Cell Biol. 1993;13:6957–6968. doi: 10.1128/mcb.13.11.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boubnov N V, Wills Z P, Weaver D T. Coding sequence composition flanking either signal element alters V(D)J recombination efficiency. Nucleic Acids Res. 1995;23:1060–1067. doi: 10.1093/nar/23.6.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuomo C A, Mundy C L, Oettinger M A. DNA sequence and structure requirements for cleavage of V(D)J recombination signal sequences. Mol Cell Biol. 1996;16:5683–5690. doi: 10.1128/mcb.16.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastman Q M, Schatz D G. Nicking is asynchronous and stimulated by synapsis in 12/23 rule-regulated V(D)J cleavage. Nucleic Acids Res. 1997;25:4370–4378. doi: 10.1093/nar/25.21.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezekiel U R, Engler P, Stern D, Storb U. Asymmetric processing of coding ends and the effect of coding end nucleotide composition on V(D)J recombination. Immunity. 1995;2:381–389. doi: 10.1016/1074-7613(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 7.Ezekiel U R, Sun T, Bozek G, Storb U. The composition of coding joints formed in V(D)J recombination is strongly affected by the nucleotide sequence of the coding ends and their relationship to the recombination signal sequences. Mol Cell Biol. 1997;17:4191–4197. doi: 10.1128/mcb.17.7.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellert M. Recent advances in understanding V(D)J recombination. Adv Immunol. 1997;64:39–64. doi: 10.1016/s0065-2776(08)60886-x. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein R M, Lieber M R. Coding end sequence can markedly affect the initiation of V(D)J recombination. Genes Dev. 1993;7:1459–1469. doi: 10.1101/gad.7.7b.1459. [DOI] [PubMed] [Google Scholar]
- 10.Hesse J E, Lieber M R, Mizuuchi K, Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989;3:1053–1067. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- 11.Hiom K, Gellert M. A stable RAG1-RAG2-DNA complex that is active in V(D)J cleavage. Cell. 1997;88:65–72. doi: 10.1016/s0092-8674(00)81859-0. [DOI] [PubMed] [Google Scholar]
- 12.Hiom K, Gellert M. Assembly of a 12/23 paired signal complex: a critical control point in V(D)J recombination. Mol Cell. 1998;1:1011–1019. doi: 10.1016/s1097-2765(00)80101-x. [DOI] [PubMed] [Google Scholar]
- 13.Kim D R, Oettinger M A. Functional analysis of coordinated cleavage in V(D)J recombination. Mol Cell Biol. 1998;18:4679–4688. doi: 10.1128/mcb.18.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieber M R. Pathologic and physiologic double-strand breaks: roles in cancer, aging, and the immune system. Am J Pathol. 1998;153:1323–1332. doi: 10.1016/s0002-9440(10)65716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBlane J F, van Gent D C, Ramsden D A, Romeo C, Cuomo C A, Gellert M, Oettinger M A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 16.Oettinger M A, Schatz D G, Gorka C, Baltimore D. Rag-1 and Rag-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 17.Sadofsky M J, Hesse J E, van Gent D C, Gellert M. RAG-1 mutations that affect the target specificity of V(D)J recombination: a possible direct role of RAG-1 in site recognition. Genes Dev. 1995;9:2193–2199. doi: 10.1101/gad.9.17.2193. [DOI] [PubMed] [Google Scholar]
- 18.Santagata S, Aidinis V, Spanopoulou E. The effect of Me2+ cofactors at the initial stages of V(D)J recombination. J Biol Chem. 1998;273:16325–16331. doi: 10.1074/jbc.273.26.16325. [DOI] [PubMed] [Google Scholar]
- 19.Sawchuk D, Weis-Garcia F, Malik S, Besmer E, Bustin M, Nussenzweig M, Cortes P. V(D)J recombination: modulation of RAG1 and RAG2 cleavage activity on 12/23 substrates by whole cell extract and DNA-bending proteins. J Exp Med. 1997;185:2025–2032. doi: 10.1084/jem.185.11.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schatz D G. V(D)J recombination moves in vitro. Semin Immunol. 1997;9:149–160. doi: 10.1006/smim.1997.0068. [DOI] [PubMed] [Google Scholar]
- 21.Schatz D G, Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988;53:107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- 22.Schatz D G, Oettinger M A, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- 23.Swanson P C, Desiderio S. V(D)J recombination signal recognition: distinct, overlapping DNA-protein contacts in complexes containing RAG1 with and without RAG2. Immunity. 1998;9:115–125. doi: 10.1016/s1074-7613(00)80593-2. [DOI] [PubMed] [Google Scholar]
- 24.Swanson P C, Desiderio S. RAG-2 promotes heptamer occupancy by RAG-1 in the assembly of a V(D)J initiation complex. Mol Cell Biol. 1999;19:3674–3683. doi: 10.1128/mcb.19.5.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 26.vanGent D C, Mizuuchi K, Gellert M. Similarities between initiation of V(D)J recombination and retroviral integration. Science. 1996;271:1592–1594. doi: 10.1126/science.271.5255.1592. [DOI] [PubMed] [Google Scholar]
- 27.vanGent D C, Ramsden D A, Gellert M. The RAG1 and RAG2 proteins establish the 12/23 rule in V(D)J recombination. Cell. 1996;85:107–113. doi: 10.1016/s0092-8674(00)81086-7. [DOI] [PubMed] [Google Scholar]
- 28.Weis-Garcia F, Besmer E, Sawchuk D J, Yu W, Hu Y, Cassard S, Nussenzweig M, Cortes P. V(D)J recombination: in vitro coding joint formation. Mol Cell Biol. 1997;17:6379–6385. doi: 10.1128/mcb.17.11.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West R B, Lieber M R. The RAG-HMG1 complex enforces the 12/23 rule of V(D)J recombination specifically at the double-hairpin formation step. Mol Cell Biol. 1998;18:6408–6415. doi: 10.1128/mcb.18.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu, K., M. R. Lieber. Unpublished data.