Abstract
Peptides derived from proopiomelanocortin (POMC) are well-established neuropeptides and peptide hormones that perform multiple functions, including regulation of body weight. In humans and some animals, these peptides include α– and β–melanocyte-stimulating hormone (MSH). In certain rodent species, no β-MSH is produced from POMC because of a change in the cleavage site. Enzymes that convert POMC into MSH include prohormone convertases (PCs), carboxypeptidases (CPs), and peptidyl-α-amidating monooxygenase (PAM). Humans and mice with inactivating mutations in either PC1/3 or carboxypeptidase E (CPE) are obese, which was assumed to result from defective processing of POMC into MSH. However, recent studies have shown that selective loss of either PC1/3 or CPE in POMC-expressing cells does not cause obesity. These findings suggest that defects in POMC processing cannot alone account for the obesity observed in global PC1/3 or CPE mutants. We propose that obesity in animals lacking PC1/3 or CPE activity depends, at least in part, on deficient processing of peptides in non–POMC-expressing cells either in the brain and/or the periphery. Genetic background may also contribute to the manifestation of obesity.
Keywords: POMC, obesity, carboxypeptidase E, proprotein convertase, PC1/3, PC2
Proopiomelanocortin (POMC), first identified in 1977, functions in a variety of physiological processes ranging from analgesia to obesity (reviewed by Kumar et al) (1). POMC is highly expressed in the pituitary, where its products are released into the bloodstream, and in the hypothalamus, where POMC-derived peptides function as neuropeptides. Biologically active POMC products include endorphins such as β-endorphin, corticotropins such as adrenocorticotropin (ACTH), and melanotropins such as α–melanocyte-stimulating hormone (αMSH) (Fig. 1). Many of these peptides exist in multiple forms, such as the variably acetylated and truncated forms of β-endorphin. There are also tissue-specific differences in processing; for example, ACTH is the major form in the anterior pituitary, whereas the ACTH-derived peptides αMSH and corticotropin-like intermediate lobe peptide (CLIP) are the major forms in the hypothalamus and intermediate pituitary.
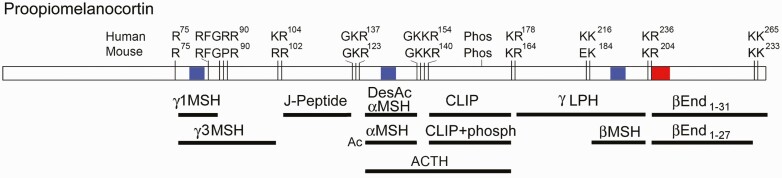
Figure 1.
Diagram of proopiomelanocortin (POMC) and major human POMC-derived peptides. Mice, rats, and some (but not all rodents) have differences in the consensus cleavages sites necessary to produce γ1MSH and βMSH, and as a result these 2 peptides are not detected in mouse hypothalamus or pituitary, although a shorter form of γ1MSH resulting from cleavage at an RxxR-F bond is detected in peptidomic studies of mouse brain and pituitary (2). A consensus site for Ser phosphorylation is present both in humans and mice (phosph), as well as N-glycosylation sites (not shown). The highly conserved N-terminal region has 2 intramolecular disulfide bonds (not shown). The numbers refer to the position within prePOMC (ie, the form containing the signal peptide). In addition to the indicated forms, other major forms include di-acetylated αMSH and acetylated β-endorphin. The opioid receptor-binding pentapeptide of β-endorphin (YGGFM) is highlighted in red, and the melanocortin receptor-binding tetrapeptide motif (HFRW) is highlighted in blue. αMSH, α–melanocyte-stimulating hormone; CLIP, corticotropin-like intermediate lobe peptide; End, endorphin; J-peptide, joining peptide; LPH, lipotropin.
Processing of POMC into mature peptides is accomplished by a series of enzymatic steps that occur in the endoplasmic reticulum (signal peptide cleavage, N-linked glycosylation, disulfide bond formation), Golgi (phosphorylation, modification of carbohydrate side chains), trans-Golgi network (peptidase activity), and secretory granules (peptidase activity, acetylation, amidation) (3). Two major endopeptidases are prohormone convertase 1 (PC1, also known as PC3, and commonly referred to as PC1/3) and PC2. These 2 enzymes are the only members of the 9-member proprotein convertase family that are primarily involved in the processing of prohormones and neuropeptide precursors, although other members (eg, furin) contribute to the processing of certain precursors (4). Except for β-endorphin 1-31, the products of PC-mediated cleavages contain C-terminal basic residues (eg, Lys, Arg) that are subsequently removed by a carboxypeptidase, primarily carboxypeptidase E (CPE). Certain peptides undergo further posttranslational modifications such as C-terminal amidation, which is catalyzed by peptidyl-α-amidating monooxygenase (PAM). This enzyme removes the carbon atoms from C-terminal Gly, leaving behind the amine moiety as the amide group (1). Whereas these enzymes are general enzymes that function in the production of many neuropeptides and peptide hormones, POMC-derived peptides are also modified by an as-yet unidentified acylating enzyme. The major acetylated peptides are αMSH and β-endorphin. Acetylation does not appear to affect the function of αMSH, but acetylation of β-endorphin eliminates the ability of this peptide to bind and activate opioid receptors (5, 6).
Control of POMC processing to active peptide products is primarily mediated by the endopeptidases that are coexpressed in the various cell types that express POMC (4, 7, 8). Cell types that express only 1 of the 2 major PCs produce a different set of peptides than cells expressing both. For example, ACTH is a primary product in mouse anterior pituitary cells that express PC1/3 but not PC2, whereas intermediate pituitary cells that express both PC1/3 and PC2 further process ACTH into CLIP and des-acetyl-MSH-Gly-Lys-Lys-Arg, which is the precursor of αMSH (see Fig. 1). Neuronal POMC-expressing cells are not homogeneous but show considerable diversity (9). Single-cell RNA sequencing of neurons in mouse hypothalamus and hindbrain found that most POMC-positive cells express both PC1/3 and PC2, although a substantial number express only PC2, a smaller number express only PC1/3, and some do not express either PC (10, 11). Studies testing POMC with large amounts of PCs have demonstrated that PC2 is capable of cleaving all the sites within POMC, whereas PC1/3 has a more limited substrate preference and primarily cleaves mouse POMC on either side of ACTH. PC1/3 is able to also generate β-endorphin 1-31 and perform several other cleavages, albeit at a slower rate than PC2 (12-14). Thus, cleavage site preference is not all or none, but a function of the expression levels of each PC, and this is important to consider when interpreting the results of PC knockout mice described below.
While many of the POMC-processing events are highly conserved in mammals, certain processing differences occur between various species. For example, βMSH is a major product in humans (15) and is predicted to be produced in many other mammals and some rodents (eg, guinea pigs, rabbits, chinchilla) because of the presence of a Lys-Lys PC2 consensus cleavage site in γLPH (see Fig. 1). However, βMSH is not produced in all rodents because the Lys-Lys cleavage site is not present in some species (eg, mice, rats, hamsters) (see Fig. 1).
Peptide products derived from POMC exert their actions primarily though the actions of 2 different classes of receptors, melanocortin receptors (MCRs) and endorphin/opioid receptors (6). There are 5 melanocortin receptors, each with distinct functions and tissue distributions (16-19). Except for MC2R, which is expressed mainly in the adrenal medulla and binds selectively to ACTH, all the other MCRs bind all MSH peptides (α, β, and γ) as well as ACTH, albeit with slight differences in affinity (6, 16). Of the MCRs, only MC3R and MC4R are highly expressed in the brain, and MC4R is especially abundant in hypothalamic areas associated with feeding/body weight regulation. In addition to binding ACTH and αMSH, the form of MSH lacking the acetyl group (des-acetyl MSH) also binds to MC4R with high affinity. In contrast, only unacetylated forms of β-endorphin bind to the various opioid receptors, of which there are 3: μ, δ, and κ (20). Although β-endorphin is often considered to bind selectively to μ opioid receptors, it also binds to δ opioid receptors with comparable affinity, and to κ opioid receptors with approximately 10-fold lower affinity (5, 20). Both the 1-31 and the 1-27 forms of β-endorphin bind with comparable affinity and are agonists (5), despite older claims that the 1-27 form is an antagonist (21). All 3 types of opioid receptors are abundantly expressed in the brain as well as in the periphery.
The Role of Proopiomelanocortin-derived Peptides in Obesity
Multiple lines of evidence from animal studies and human genetic analyses have implicated POMC-derived peptides in obesity (6, 17). POMC knockout mice exhibit adult-onset obesity, resulting from a combination of elevated feeding and changes to metabolism that reduce energy expenditure (22). Humans bearing mutations that eliminate the expression of most of the POMC molecule are also obese (23). Human POMC mutations that affect the formation of βMSH result in obesity, whereas mutations that affect the formation of αMSH are not generally associated with obesity, but have been reported (24-26). Certain human mutations in POMC fail to produce βMSH because of alterations of the cleavage site between βMSH and β-endorphin. While β-endorphin may contribute to the feeding/body weight effects of POMC (27), animal studies with β-endorphin show conflicting results (6). Similar to work in humans, studies in dogs found that βMSH plays an important role in the regulation of body weight. Certain breeds (eg, Labrador retrievers) have a deletion in POMC that removes the βMSH sequence, and the resulting animals are prone to obesity (28). This deletion affects only βMSH production, and the level of αMSH in Labrador retrievers is normal (28). Thus, while αMSH is a major contributor to body weight regulation in mice and rats, βMSH plays a more important role in humans and other mammals.
The primary effects of POMC-derived peptides on feeding/body weight are thought to be mediated by MSH peptides acting on the melanocortin 4 receptor (MC4R) (18, 29, 30). Mouse studies show that intracerebroventricular (i.c.v.) administration of MC4R agonists blocks feeding (eg, an anorexic effect). These agonists include αMSH, the major MSH peptide in the mouse brain, as well as other agonists such as des-acetyl αMSH (6). Although des-acetyl αMSH was less effective in vivo, this presumably reflects its lower stability in the brain due to the presence of aminopeptidases (6). ACTH-1-24 injected i.c.v. also reduced feeding behavior in rats (31). Whereas MC4R agonists block feeding, antagonists of MC4R stimulate feeding (6). These antagonists include the naturally occurring agouti-related peptide, which has been reported to be a competitive antagonist, an inverse agonist, and an allosteric modulator (32, 33). Historically, the agouti obesity syndrome was described more than 50 years ago (34) and is thought to be due to effects on melanocortin receptors (35). Genetic studies have implicated MC4R as a major contributor to obesity, with 2% to 5% of morbidly obese humans found to have loss-of-function mutations in this receptor (36). Recently, an αMSH agonist (setmelanotide) was approved by the Food and Drug Administration for treatment of obesity resulting from rare mutations in POMC, MC4R, the leptin receptor, or PC1/3 (16, 37).
Taken together, it is clear that POMC peptides, and especially the MSH peptides, play a critically important role in obesity. Whereas in humans and dogs the principal melanocortin is βMSH, in mice and several other rodents αMSH plays a primary role because βMSH is not produced.
Mutations in POMC-processing Enzymes Are Associated With Obesity in Mice
The various mouse mutations in POMC-processing enzymes are summarized in Table 1, which shows that only select mouse models of processing enzyme insufficiency result in obesity (eg, global Cpe; Pcsk1 N222D). The contribution of hypothalamic αMSH to obesity in these various mouse models is unclear, with some models reporting reductions and others no change. It is important to note that upregulation of Pomc gene and protein expression has been reported in many of these mouse models; this may result in physiological compensation that increases levels of bioactive melanocortins and/or extended precursors. It should also be mentioned that some studies focus on pituitary αMSH, whereas others measure hypothalamic peptide. A third complexity is the fact that commercial radioimmunoassays may not be able to distinguish between extended forms of αMSH and αMSH itself, while peptidomics measure authentic αMSH as well as other forms (eg, des-acetyl-MSH, C–terminally-extended MSH) (40, 46). Nonetheless, the general lack of correlation between αMSH levels and obesity is perplexing and does not support the hypothesis that defective POMC processing to αMSH is the major causal mechanism underlying obesity. Potential explanations to account for this discrepancy are described below, along with discussion of specific mutations.
Table 1.
Mouse models of processing enzyme insufficiency
| Enzyme | Mouse modela | Description | Obesityb | Relative αMSH levels | Reference |
|---|---|---|---|---|---|
| CPE | |||||
| Cpe fat (S244P) | Global point mutant | Yes | Reduced > 10-fold (hypothalamus and pituitary) | (38) | |
| Cpe knockout | Global | Yes | Reduced > 10-fold (hypothalamus and pituitary) | (39) | |
| CpeFlox::PomcCre knockout | Floxed; conditional | No | Reduced > 10-fold (hypothalamus and pituitary) | (40) | |
| PC1/3 | |||||
| Pcsk1 knockout | Global | Noc | Normal (pituitary) | (41) | |
| Pcsk1Flox::PomcCre knockout | Floxed; conditional | No | Normal (hypothalamus) | (42) | |
| Pcsk1-N222D | Point mutant | Yes | Reduced by half (hypothalamus) | (43) | |
| Pcsk1-N221D | Point mutant | No | Normal (hypothalamus) | (44) | |
| Pcsk1-V96L;exon3 del | Exon3 splicing mutant and point mutant | Yes | Not measured | (45) | |
| PC2 | Pcsk2 knockout | Global | No | Reduced > 10-fold (below detection limit in pituitary and hypothalamus) | (46-49) |
| PAM | Pam knockout, heterozygous | Global | No | Normal (hypothalamus) | (50) |
Abbreviations: αMSH, α-melanocyte-stimulating hormone; CPE, carboxypeptidase E; PAM, peptidyl-α-amidating monooxygenase; PC1/3, prohormone convertase 1.
a Homozygous unless indicated.
b Obesity on normal chow diet.
c Depends on mouse strain.
Carboxypeptidase E
One of the earliest single-gene mutations found to cause obesity in mice was named fat. The fat mutation spontaneously arose in the early 1970s (51). In 1995 the mutation was mapped to the Cpe gene and found to result from an inactivating point mutation in CPE (38). Disruption of the Cpe gene by removal of critical exons (39) or by insertion of a neo cassette that eliminates production of CPE yielded a similar phenotype as the fat mouse (39, 40). In addition to adult-onset obesity, mice lacking CPE activity show many other phenotypes, including anxiety- and depressive-like behaviors, altered thermoregulation, severely reduced fertility, and hyperglycemia (38, 39, 52-54). These additional phenotypes reflect the broad role of CPE in processing many peptide precursors in addition to POMC (2). Some of the phenotypes such as hyperglycemia are highly dependent on background strain and sex, mainly developing in male mice on the BKS background (54), while other phenotypes such as impaired fertility and obesity were noted in all mouse strains examined (38, 39, 52-54). However, while obesity was found in global Cpe null mice regardless of background strain, the age of onset and severity of the obesity may be modified by genetic background, with modifier genes reported on mouse chromosomes 11 and 14 (54). Two case reports of human CPE mutations that eliminate enzyme activity show similar phenotypes as the mice, including morbid obesity (55, 56). The obesity phenotype in mice lacking CPE activity was originally attributed to defective processing of αMSH, because levels of the mature form of αMSH are reduced approximately 95% in mice lacking CPE (57). Similarly, in humans the defect in CPE was thought to result from decreased αMSH and/or βMSH in the brain (55, 56). However, mice with a disruption of Cpe in Pomc-expressing cells are not obese, causing a reevaluation of these assumptions (40).
The reduction of αMSH levels in the hypothalami of CpeFlox::PomcCre knockout mice is comparable to the reduction of this peptide seen in global Cpe null mice (40). Thus, if the greater than 90% reduction in αMSH levels is not sufficient to cause obesity in Pomc conditional mice, then it is highly unlikely that the decreased level of this peptide in global Cpe null mice is responsible for their obesity. While this seems to contradict the numerous studies that have established an important role for αMSH in body weight regulation in mice, and the related βMSH peptides in humans and other mammals, there is a simple explanation: in mice lacking CPE, the large reduction in the level of the mature form of αMSH is accompanied by a dramatic increase in the level of C-terminally extended forms (eg, αMSH with GKKR on the C-terminus) (40). If these extended forms are biologically active, then functional MC4R agonists are present in CpeFlox::PomcCre null mice. In this scenario, αMSH does not require C-terminal trimming of the basic residues and C-terminal amidation for bioactivity. Although this has not been directly tested, it is clear that the amidated C-terminus of αMSH is not required for receptor activation based on the similar affinities of αMSH and ACTH for MC4R (6), and the effectiveness of i.c.v. ACTH-1-24 in blocking feeding (31). Furthermore, structural analysis of MC4R in complex with peptide ligands shows a strong interaction between the internal receptor-binding tetrapeptide motif of the peptide and MC4R, while the N- and C-termini of the peptide do not contribute to binding (29). Thus, the lack of obesity in POMC cell-specific CPE knockout mice likely reflects the bioactivity of C-terminally–extended forms of MSH peptides. If this supposition is correct, then obesity in mice with a global loss of CPE activity does not result from defective production of αMSH, but presumably from the absence of another peptide or peptides that are also CPE products (40).
Prohormone Convertase 1
PC1/3, encoded by the PCSK1 gene, is widely distributed within neuronal and endocrine cells throughout the body, where it contributes to the processing of a large number of physiologically important peptide precursors, including POMC, most hypothalamic neuropeptide precursors, and prohormones such as proinsulin (reviewed in Shakya and Lindberg) (58). Common PCSK1 polymorphisms are associated with obesity in a variety of different human populations (59). Severe inactivating mutations of PCSK1 are rare but are associated with 2-fold higher odds of obesity in heterozygous carriers (60). In the homozygous state, these rare inactivating mutations represent a serious medical condition in neonates due to an as-yet unexplained persistent diarrhea that results in failure to thrive and requires parenteral nutrition (61). This intestinal absorption defect also lessens the obesity phenotype, though these patients often also exhibit obesity. While for obvious reasons brain αMSH has never been measured in patients with inactivating PCSK1 mutations, levels of a variety of different peptide precursors are elevated in patient blood (61-63). New cases of severe pediatric PCSK1 insufficiency are reported each year (reviewed in (58, 59); see also (64, 65)).
In mice, some but not all Pcsk1 mutations are associated with obesity. The Pcsk1 N222D point mutation, which was produced by chemical mutagenesis, causes males to be approximately 32% heavier, and females to be approximately 68% heavier than wild-type littermates at 26 weeks (43). Owing to its position next to an important calcium binding site, N222D is critical for maximal enzyme activity, and N222D PC1/3 mutant mice exhibit approximately 50% reduced hypothalamic αMSH (43). Mutation of the nearby catalytically more benign site N221D does not result in obesity, nor does the N221D mutant mouse exhibit any reduction in hypothalamic αMSH (44). Chemical mutagenesis resulting in another inactivating mutation (V96L, which affects exon3 splicing) also resulted in obese mice (45). In contrast, the global Pcsk1 knockout mouse is not obese in the C57BL/6j mouse strain (66). These mice have normal levels of αMSH (41). However, when these same knockout mice were back-crossed for more than 20 generations into the CD1 strain, female PC1/3 null mice averaged 48 g at 16 weeks, while wild-type controls averaged 36 g at this same age, and male mice showed no difference in body weight (R. Day, personal communication July 2, 2021). Recently, conditional Pcsk1Flox::PomcCre mice that lack PC1/3 specifically in POMC-expressing cells were generated; these mice are neither obese nor do they exhibit alterations in hypothalamic αMSH (42). While a compensatory increase in PC2 may be responsible for restoring hypothalamic αMSH levels (42), it is unclear why PC2 apparently does not substitute for PC1/3 in other Pcsk1-insufficient mouse models (eg, the N222D mouse). It is possible that differential proteotoxicity caused by misfolding contributes to the magnitude of biological effects since the PC1/3 N222D and the V96L mutations are far more proteotoxic than the N221D mutation or the PC1/3 knockout (45, 67, 68).
Prohormone Convertase 2
PC2, encoded by Pcsk2, was knocked out in 1997 (69). In a study that used radioimmunoassays to detect peptides, these global PC2 null mice were found to lack detectable levels of αMSH in the hypothalamus and pituitary (46). This was confirmed using peptidomics analysis, with levels of αMSH and des-acetyl-αMSH reduced below the detection limit (eg, > 95% reduction) in Pcsk2 knockout mice (48, 49). Despite this radical reduction in αMSH, PC2 knockout mice are not obese, perhaps in part because extended forms of αMSH such as ACTH are increased in these mice (46, 47). The major phenotype of the Pcsk2 null, aberrant glucose handling, appears to result from a total lack of processing of proglucagon to mature glucagon, a peptide hormone required for release of tissue sugar stores (69). Circulating levels of ACTH are also extremely high in the PC2 null, resulting in mildly increased corticosterone levels (70). Interestingly, this same phenotype is also visible in the knockout of the obligate PC2 binding partner 7B2, but only when both null mouse models are obtained in the same substrain background (70). Double knockouts of both PC2 and 7B2, which represent a mixed 129Sv:C57Bl/6j background, were extremely obese (4). These data support the idea that background strain differences play a huge role in phenotype determination in these 2 nulls, and likely also affect differential results obtained in other obesity-related mouse models. In humans, polymorphisms in PCSK2 are associated with alterations in glucose handling (reviewed in Shakya and Lindberg) (58); however, inactivating mutations in PCSK2 have not been reported.
Peptidyl-α-Amidating Monooxygenase
The amidating enzyme PAM is required to generate the mature form of αMSH; in the absence of PAM, the Gly-extended precursor form of amidated peptides accumulates. Homozygous Pam knockout mice die in utero between embryonic age days 14.5 and 15.5 with severe edema and cardiovascular deficits, presumably due to the absence of amidated peptides derived from proadrenomedullin (50). Heterozygous Pam knockout mice are viable, and have normal levels of αMSH and other amidated peptides (50). Aberrations in glucose handling and percentage of body fat were detected in 10-month-old heterozygous PAM knockout mice, but total body weight was comparable to wild-type controls (50).
Summary
It has been firmly established that POMC-derived MSH peptides that stimulate melanocortin receptors, especially MC4R, play a critical role in obesity. It has also been established that the POMC-processing enzymes PC1/3, PC2, and CPE are involved in the production of αMSH. Since certain mouse lines with mutations in POMC-processing enzymes are obese, it was assumed that obesity resulted from the greatly reduced levels of αMSH. However, this assumption now appears to be incorrect for several reasons. First, global PC2 knockout mice are not obese, despite their extremely low levels of αMSH. Second, recent studies using PomcCre mice deficient in CPE indicate that hypothalamic αMSH deficiency cannot explain the occurrence of obesity in mice with global processing enzyme loss. Third, the finding of increased body weight in PC1/3 nulls on certain background strains, but not others, clearly implicates background factors in the manifestation of obesity. Taken together, these data strongly suggest that even within the context of hypothalamic POMC, placing the sole focus on αMSH as the underlying mediator of processing enzyme-related obesity is incorrect. Indeed, the fact that loss of either PC1/3 or CPE in POMC-expressing cells does not result in obesity strongly implicates other cell types in the obesity phenotype.
Because PC1/3 and CPE process many neuropeptide and endocrine precursors into mature forms, we propose that the obese phenotype in all CPE null and some PC1/3 mutant mice is due to deficient precursor processing of one or more non-POMC peptides in the central nervous system and/or endocrine system. In this scenario, processing enzymes in non–POMC-expressing cell types must be inactivated to produce an obese phenotype. The situation may be yet more complex; these other pathways may potentially feed back to affect POMC-related hypothalamic pathways, creating a complex interplay of peripheral and central hormonal signals ultimately resulting in the hyperphagia and metabolic changes that fuel obesity. This latter possibility is supported by the recent Food and Drug Administration approval of the MC4R agonist setmelanotide for treatment of humans with mutations in the PCSK1 gene; this implies that the obese phenotype of humans with impaired PC1/3 activity can be rescued, as least in part, by stimulation of MC4R. However, the degree of weight reduction is not publicly known, because data from clinical trials involving PCSK1 patients have not yet been published. It is also not known if the obesity seen in humans or mice with inactivating mutations in CPE can be partially rescued with setmelanotide; setmelanotide efficacy in CPE-deficient mouse models would indicate if melanotropin deficiency exists despite the presence of the extended bioactive MCR agonists.
In conclusion, while POMC-derived peptides such as αMSH have taken center stage as the major anorexigenic peptides in mice, the development of obesity in mice with mutations in POMC-processing enzymes is most likely due to altered production of other peptides and/or to background factors. In this regard, it is interesting to note that one group has found that pancreatic β-cell–specific elimination of either CPE or PC1/3 using an Ins1Cre driver does not produce obesity (Y. C. Chen, A. J. Taylor, and C. B. Verchere, personal communication July 6, 2021), while another group has observed profound obesity in Pcsk1-floxed mice generated with the PdxCre driver, where Pdx represents a transcription factor required for pancreatic development (D. T. Meier and M. Y. Donath, personal communication May 31, 2021). A more complete understanding of the peptidergic differences between these 2 different pancreatic Pcsk1 floxed mouse models, as well as studies using additional Cre drivers and background strains to generate conditional knockout mouse models, will shed light on the specific roles of peptides other than αMSH in mediating processing enzyme-related obesity.
Acknowledgments
We thank M. J. Martin, U. Hochgeschwender, R. Day, A.J. Taylor, C.B. Verchere, J. W. M. Creemers, and D.T. Meier for constructive comments.
Financial Support: This work was supported by the National Institutes of Health (NIH grant No. DA042351 to I.L.).
Glossary
Abbreviations
- αMSH
α–melanocyte-stimulating hormone
- ACTH
adrenocorticotropin
- CLIP
corticotropin-like intermediate lobe peptide
- CPE
carboxypeptidase E
- i.c.v.
intracerebroventricular
- MCR
melanocortin receptor
- PAM
peptidyl-α-amidating monooxygenase
- PC
prohormone convertase
- PC1/3
prohormone convertase 1
- POMC
proopiomelanocortin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Kumar D, Mains RE, Eipper BA. 60 Years of POMC: from POMC and α-MSH to PAM, molecular oxygen, copper, and vitamin C. J Mol Endocrinol. 2016;56(4):T63-T76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fricker LD. Analysis of mouse brain peptides using mass spectrometry-based peptidomics: implications for novel functions ranging from non-classical neuropeptides to microproteins. Mol Biosyst. 2010;6(8):1355-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fricker LD. Neuropeptides and Other Bioactive Peptides: From Discovery to Function. Morgan & Claypool Life Sciences; 2012. [Google Scholar]
- 4. Hoshino A, Lindberg I.. Peptide Biosynthesis: Prohormone Convertases 1/3 and 2. Morgan & Claypool Life Sciences; 2012. [Google Scholar]
- 5. Gomes I, Sierra S, Lueptow L, et al. Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci U S A. 2020;117(21):11820-11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harno E, Gali Ramamoorthy T, Coll AP, White A. POMC: the physiological power of hormone processing. Physiol Rev. 2018;98(4):2381-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274(30):20745-20748. [DOI] [PubMed] [Google Scholar]
- 8. Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011;768:23-57. [DOI] [PubMed] [Google Scholar]
- 9. Quarta C, Claret M, Zeltser LM, et al. POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat Metab. 2021;3(3):299-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowsett GKC, Lam BYH, Tadross JA, et al. A survey of the mouse hindbrain in the fed and fasted states using single-nucleus RNA sequencing. Mol Metab. 2021;53:101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam BYH, Cimino I, Polex-Wolf J, et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol Metab. 2017;6(5):383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjannet S, Rondeau N, Day R, Chrétien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991;88(9):3564-3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem. 1993;268(3):1763-1769. [PubMed] [Google Scholar]
- 14. Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994;269(26):17440-17447. [PubMed] [Google Scholar]
- 15. Kirwan P, Kay RG, Brouwers B, et al. Quantitative mass spectrometry for human melanocortin peptides in vitro and in vivo suggests prominent roles for β-MSH and desacetyl α-MSH in energy homeostasis. Mol Metab. 2018;17:82-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeo GSH, Chao DHM, Siegert AM, et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol Metab. 2021;48:101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baldini G, Phelan KD. The melanocortin pathway and control of appetite-progress and therapeutic implications. J Endocrinol. 2019;241(1):R1-R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kühnen P, Krude H, Biebermann H. Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol Med. 2019;25(2):136-148. [DOI] [PubMed] [Google Scholar]
- 19. Dores RM, Liang L, Davis P, Thomas AL, Petko B. 60 Years of POMC: melanocortin receptors: evolution of ligand selectivity for melanocortin peptides. J Mol Endocrinol. 2016;56(4): T119-T133. [DOI] [PubMed] [Google Scholar]
- 20. Fricker LD, Margolis EB, Gomes I, Devi LA. Five decades of research on opioid peptides: current knowledge and unanswered questions. Mol Pharmacol. 2020;98(2):96-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicolas P, Li CH. Beta-endorphin-(1-27) is a naturally occurring antagonist to etorphine-induced analgesia. Proc Natl Acad Sci U S A. 1985;82(10):3178-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5(9):1066-1070. [DOI] [PubMed] [Google Scholar]
- 23. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155-157. [DOI] [PubMed] [Google Scholar]
- 24. Challis BG, Pritchard LE, Creemers JWM, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11(17):1997-2004. [DOI] [PubMed] [Google Scholar]
- 25. Lee YS, Challis BG, Thompson DA, et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3(2):135-140. [DOI] [PubMed] [Google Scholar]
- 26. Dubern B, Lubrano-Berthelier C, Mencarelli M, et al. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatr Res. 2008;63(2):211-216. [DOI] [PubMed] [Google Scholar]
- 27. Appleyard SM, Hayward M, Young JI, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144(5):1753-1760. [DOI] [PubMed] [Google Scholar]
- 28. Raffan E, Dennis RJ, O’Donovan CJ, et al. A deletion in the canine POMC gene is associated with weight and appetite in obesity-prone Labrador retriever dogs. Cell Metab. 2016;23(5):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Israeli H, Degtjarik O, Fierro F, et al. Structure reveals the activation mechanism of the MC4 receptor to initiate satiation signaling. Science. 2021;372(6544):808-814. [DOI] [PubMed] [Google Scholar]
- 30. Brouwers B, de Oliveira EM, Marti-Solano M, et al. Human MC4R variants affect endocytosis, trafficking and dimerization revealing multiple cellular mechanisms involved in weight regulation. Cell Rep. 2021;34(12):108862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vergoni AV, Poggioli R, Marrama D, Bertolini A. Inhibition of feeding by ACTH-(1-24): behavioral and pharmacological aspects. Eur J Pharmacol. 1990;179(3):347-355. [DOI] [PubMed] [Google Scholar]
- 32. Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799-802. [DOI] [PubMed] [Google Scholar]
- 33. Pritchard LE, Armstrong D, Davies N, et al. Agouti-related protein (83-132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J Endocrinol. 2004;180(1):183-191. [DOI] [PubMed] [Google Scholar]
- 34. Wolff GL, Pitot HC. Influence of background genome on enzymatic characteristics of yellow (A v –, A vy –) mice. Genetics. 1973;73(1):109-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385(6612):165-168. [DOI] [PubMed] [Google Scholar]
- 36. Anderson EJ, Çakir I, Carrington SJ, et al. 60 Years of POMC: regulation of feeding and energy homeostasis by α-MSH. J Mol Endocrinol. 2016;56(4):T157-T174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Markham A. Setmelanotide: first approval. Drugs. 2021;81(3):397-403. [DOI] [PubMed] [Google Scholar]
- 38. Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10(2):135-142. [DOI] [PubMed] [Google Scholar]
- 39. Cawley NX, Zhou J, Hill JM, et al. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145(12):5807-5819. [DOI] [PubMed] [Google Scholar]
- 40. Fricker LD, Tashima AK, Fakira AK, Hochgeschwender U, Wetsel WC, Devi LA. Neuropeptidomic analysis of a genetically defined cell type in mouse brain and pituitary. Cell Chem Biol. 2021;28(1):105-112.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan H, Nanno D, Che FY, et al. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry. 2005;44(12):4939-4948. [DOI] [PubMed] [Google Scholar]
- 42. Shakya M, Gahlot S, White A, Verchere CB, Low MJ, Lindberg I. Mice lacking PC1/3 expression in POMC-expressing cells do not develop obesity. Endocrinology. 2021;162(6):bqab055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet. 2006;15(11):1884-1893. [DOI] [PubMed] [Google Scholar]
- 44. Jarvela TS, Shakya M, Bachor T, White A, Low MJ, Lindberg I. Reduced stability and pH-dependent activity of a common obesity-linked PCSK1 polymorphism, N221D. Endocrinology. 2019;160(11):2630-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muhsin NIA, Bentley L, Bai Y, Goldsworthy M, Cox RD. A novel mutation in the mouse Pcsk1 gene showing obesity and diabetes. Mamm Genome. 2020;31(1-2):17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J Neurochem. 2003;86(3):556-563. [DOI] [PubMed] [Google Scholar]
- 47. Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology. 2004;145(2):519-528. [DOI] [PubMed] [Google Scholar]
- 48. Pan H, Che FY, Peng B, Steiner DF, Pintar JE, Fricker LD. The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. J Neurochem. 2006;98(6):1763-1777. [DOI] [PubMed] [Google Scholar]
- 49. Zhang X, Pan H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J Neurochem. 2010;112(5):1168-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Czyzyk TA, Ning Y, Hsu MS, et al. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol. 2005;287(2):301-313. [DOI] [PubMed] [Google Scholar]
- 51. Coleman DL, Eicher EM. Fat (fat) and tubby (tub), two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered. 1990;81(6):424-427. [DOI] [PubMed] [Google Scholar]
- 52. Rodriguiz RM, Wilkins JJ, Creson TK, et al. Emergence of anxiety-like behaviours in depressive-like Cpe(fat/fat) mice. Int J Neuropsychopharmacol. 2013;16(7):1623-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC. Deficiencies in pro-thyrotropin-releasing hormone processing and abnormalities in thermoregulation in Cpefat/fat mice. J Biol Chem. 2002;277(50):48587-48595. [DOI] [PubMed] [Google Scholar]
- 54. Collin GB, Maddatu TP, Sen S, Naggert JK. Genetic modifiers interact with Cpe(fat) to affect body weight, adiposity, and hyperglycemia. Physiol Genomics. 2005;22(2):182-190. [DOI] [PubMed] [Google Scholar]
- 55. Alsters SI, Goldstone AP, Buxton JL, et al. Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS One. 2015;10(6):e0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Durmaz A, Aykut A, Atik T, et al. A New cause of obesity syndrome associated with a mutation in the carboxypeptidase gene detected in three siblings with obesity, intellectual disability and hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol. 2021;13(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab. 2010;299(2):E189-E197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shakya M, Lindberg I. Mouse models of human proprotein convertase insufficiency. Endocr Rev. 2021;42(3):259-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stijnen P, Tuand K, Varga TV, Franks PW, Aertgeerts B, Creemers JW. The association of common variants in PCSK1 with obesity: a HuGE review and meta-analysis. Am J Epidemiol. 2014;180(11):1051-1065. [DOI] [PubMed] [Google Scholar]
- 60. Akbari P, Gilani A, Sosina O, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021;373(6550):eabf8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martín MG, Lindberg I, Solorzano-Vargas RS, et al. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology. 2013;145(1):138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303-306. [DOI] [PubMed] [Google Scholar]
- 63. Jackson RS, Creemers JW, Farooqi IS, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112(10):1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aerts L, Terry NA, Sainath NN, et al. Novel homozygous inactivating mutation in the PCSK1 gene in an infant with congenital malabsorptive diarrhea. Genes (Basel). 2021;12(5):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qian Y, Wu B, Liu R, et al. Case report: complete maternal uniparental isodisomy of chromosome 5 (iUPD(5)mat) with PCSK1 nonsense variant in an infant with recurrent diarrhea. Front Genet. 2021;12:668326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhu X, Zhou A, Dey A, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. 2002;99(16):10293-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prabhu Y, Blanco EH, Liu M, et al. Defective transport of the obesity mutant PC1/3 N222D contributes to loss of function. Endocrinology. 2014;155(7):2391-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stijnen P, Brouwers B, Dirkx E, et al. Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity. Int J Obes (Lond). 2016;40(6):973-981. [DOI] [PubMed] [Google Scholar]
- 69. Furuta M, Yano H, Zhou A, et al. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A. 1997;94(13):6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peinado JR, Laurent V, Lee SN, et al. Strain-dependent influences on the hypothalamo-pituitary-adrenal axis profoundly affect the 7B2 and PC2 null phenotypes. Endocrinology. 2005;146(8):3438-3444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.