Abstract
ATP6AP2 expression is increased in the nephron during high-fat diet (HFD) and its knockout (ATP6AP2 KO) reduces body weight (WT) in mice. We evaluated the contribution of ATP6AP2 to urinary glucose (UG) and albumin (Ualb) handling during HFD. We hypothesized that nephron ATP6AP2 KO increases UG and Ualb and minimizes HFD-induced obesity. Eight-week-old male C57BL/6J mice with inducible nephron-specific ATP6AP2 KO and noninduced controls were fed either normal diet (ND, 12% kcal fat) or HFD (45% kcal fat) for 6 months. ATP6AP2 KO mice on ND had 20% (P < 0.01) lower WT compared with controls. HFD-fed mice had 41% (P < 0.05) greater WT than ND-fed control mice. In contrast, ATP6AP2 KO abrogated the increase in WT induced by HFD by 40% (P < 0.05). Mice on HFD had less caloric intake compared with ND controls (P < 0.01). There were no significant differences in metabolic rate between all groups. UG and Ualb was significantly increased in ATP6AP2 KO mice on both ND and HFD. ATP6AP2 KO showed greater levels of proximal tubule apoptosis and histologic evidence of proximal tubule injury. In conclusion, our results demonstrate that nephron-specific ATP6AP2 KO is associated with glucosuria and albuminuria, most likely secondary to renal proximal tubule injury and/or dysfunction. Urinary loss of nutrients may have contributed to the reduced WT of knockout mice on ND and lack of WT gain in response to HFD. Future investigation should elucidate the mechanisms by which loss of renal ATP6AP2 causes proximal tubule injury and dysfunction.
Keywords: obesity, kidney, ATP6AP2, (pro)renin receptor, proximal tubule
The prevalence of obesity continues to increase, with close to 40% of the United States population currently identified as obese (1). Renal disease is a common complication of obesity and a lack of therapeutic strategies to manage obesity-related kidney disease highlights the need to better understand the changes that occur in the kidney during obesity (2). These changes, which include hyperfiltration of albumin and glucose, coincide with alterations in proximal tubule function (3, 4). However, the mechanisms underlying these changes are largely unknown.
ATP6AP2, also known as the (pro)renin receptor (PRR), is expressed throughout the kidney, including the glomeruli, proximal and distal tubules, collecting ducts, and vasculature (5-8). It is expressed as a 39-kDa full-length transmembrane protein that can be cleaved to produce a 28 kDa circulating soluble form and an 8.9-kDa accessory subunit of the vacuolar H+-ATPase (9). ATP6AP2 plays an important physiologic role in the kidney through the reabsorption of sodium and water as well as acid secretion in the distal nephron (10-15). At the same time, renal ATP6AP2 has pathophysiologic functions that include promoting inflammation, fibrosis, and mitochondrial dysfunction in conditions such as diabetes and renal ischemia (16-18). Our laboratory recently demonstrated increased renal medullary ATP6AP2 expression in the setting of obesity and its contribution to renal sodium retention via upregulation of the sodium channel, αENaC, in the collecting duct (19). However, the function of renal proximal tubule ATP6AP2 and its influence on glucose and albumin homeostasis remains unknown. In this study, we demonstrate that nephron-specific ATP6AP2 knockout in mice causes proximal tubule injury with glucosuria and albuminuria that may explain the abrogation of HFD-induced obesity in this model.
Materials and Methods
Animal Care and Use
All study protocols were approved by the University of Virginia Animal Care and Use Committee and were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and guidelines as described by the NIH Guide for the Care and Use of Laboratory Animals. All studies utilized male inducible nephron-specific ATP6AP2 KO mice and noninduced littermate controls on a C57BL/6J background.
Genotyping
Inducible ATP6AP2 KO mice were generously provided by Drs. Donald Kohan and Nirupama Ramkumar at the University of Utah Health Sciences Center in Salt Lake City, UT. The development and verification of the nephron-specific ATP6AP2 KO was described previously (11). These mice are homozygous for Pax8-rTA and LC1 Cre and homozygous for floxed ATP6AP2. Genotyping was performed at 3 weeks of age using genomic DNA extracted from tail snips. Samples underwent PCR using the following primers as previously described (11):
PRR (600-bp flox, 400-bp wild-type products) forward: 5′- GGGGGGTAAATTGTTGATGAGTCTTGGAGCATAGC-3′; reverse 5′-GAAGCCCATGGACAGTGCAGCTACGTCTGGGATTCGA-3′. PAX-8-rtTA (600-bp product) forward: 5′-CCATGTCTAGACTGGACA AGA-3′; reverse: 5′-CTCCAGGCC ACATATGAT TAG-3′ LC-1 (480-bp product) forward: 5′- TCGCTGCATTACCGGTCGATGC-3′; reverse 5′-CCATGAGTGAACGAACCTGGTCG-3′.
PCR products were separated by electrophoresis on a 1% agarose gel.
ATP6AP2 Knockout Induction
ATP6AP2 KO was induced at 8 weeks of age by oral administration of 2 mg/mL doxycycline (Sigma, St. Louis, MO) in 2% sucrose water over 12 days. Littermate control mice were given 2% sucrose water over the same time period.
Diet Treatment and Organ Collection
Mice at 10 weeks of age from knockout and control groups were randomly assigned to either standard chow diet (ND) (12% kcal fat) (Teklad, Indianapolis, IN) or high-fat diet (HFD) (45% kcal fat from lard) (Research Diets, D12451, New Brunswick, NJ) for a period of 6 months, creating 4 treatment groups (n = 5-7): normal diet control (ND), normal diet ATP6AP2 knockout (NDKO), high-fat diet control (HFD), and high-fat diet ATP6AP2 knockout (HFDKO). All mice had ad libitum access to tap water throughout diet treatment. At the end of the 6-month period mice were euthanized under anesthesia with ketamine and xylazine. Kidneys were decapsulated, weighed, and sectioned. Samples for protein analysis were separated into cortical and medullary components and were snap frozen at −80 °C for later analysis while samples for histology and immunofluorescence were processed as described below.
Histology, Immunohistochemistry, and Immunofluorescence
Kidney samples for histology and immunohistochemistry were fixed for 24 hours in 10% buffered formalin (Thermo Scientific, Waltham, MA) prior to transfer to 70% ethanol. Samples then underwent paraffin embedding and sectioning followed by automated hematoxylin and eosin (H&E) or periodic acid Schiff (PAS) staining in the UVA Research Histology Core. For immunohistochemistry, staining was performed on a robotic platform (Ventana discover Ultra Staining Module, Ventana Co., Tucson, AZ, USA). Tissue sections (4 µm) were deparaffinized using EZ Prep solution (Ventana). A heat-induced antigen retrieval protocol set for 64 minutes was carried out using Cell Conditioner 1 (Ventana). Endogenous peroxidases were blocked with peroxidase inhibitor (CM1) for 8 minutes before incubating the section with cleaved PARP 1 antibody (1:200, ab32064, RRID:AB_777102; http://antibodyregistry.org/AB_777102, Abcam) for 60 minutes at room temperature. Antigen-antibody complex was then detected using a DISCOVERY OmniMap anti-rabbit multimer RUO detection system and DISCOVERY ChromoMap DAB Kit (Ventana Co.). All the slides were counterstained with hematoxylin subsequently; they were dehydrated, cleared, and mounted for the assessment. Positive staining was then quantified as the average number of positive nuclei per high-powered field in 5 separate high-powered fields per mouse.
For immunofluorescence, kidney samples were fixed for 2 hours in 4% paraformaldehyde (Alfa Aesar, Haverhill, MA). Tissue was then washed in phosphate-buffered saline, treated with 100 mM tris-HCl (pH 7.4) for 1 hour, then treated with 30% sucrose overnight prior to freezing in OCT. Eight-micron frozen sections were made, permeabilized with 0.3% TritonX in TBS, blocked with 1% milk, and probed with rabbit anti-ATP6AP2 antibody (1:100, HPA003156, RRID:AB_1078245; http://antibodyregistry.org/AB_1078245, Sigma) and goat anti-villin antibody (1:100, SC7672, RRID:AB_2215973; http://antibodyregistry.org/AB_2215973, Santa Cruz Biotechnology, Dallas, TX) overnight at 4 °C. Sections were then exposed to Alexa-647 donkey anti-rabbit antibody (1:500, A31573, RRID:AB_2536183; http://antibodyregistry.org/AB_2536183, Invitrogen, Carlsbad, CA) and Alexa-594 donkey anti-goat antibody (1:500, A11055, RRID:AB_2534102; http://antibodyregistry.org/AB_2534102, Invitrogen) for 1 hour at room temperature before washing and mounting in Fluoromount (Electron Microscopy Sciences, Harfield, PA). Images were taken using an Olympus IX81 spinning disk confocal microscope using a 60× plan apochromat water immersion objective with a numeric aperture of 1.2. Background staining was gated using sections exposed to secondary antibody only and images were quantified with the FIJI version of Image J. Proximal tubule–specific expression of ATP6AP2 was quantified by using the Sync Windows function and picking proximal tubules that expressed villin in the brush border in 1 channel and measuring the Integrated Intensity of the ATP6AP2 in the entire single proximal tubule. Ten different tubules were quantified from 5 different regions of the renal cortex.
Electron Microscopy
Uninephrectomized mice underwent cannulation of the cardiac left ventricular cavity followed by perfusion with fixative containing 4% paraformaldehyde and 0.5% glutaraldehyde. The kidney was then extracted and placed in the same fixative solution before transfer to the Advanced Microscopy Facility for further processing. Samples were washed in distilled water (4 × 5 minutes), postfixed for 1 hour in 1% osmium-tetroxide, dehydrated through a graded ethanol series followed by transition into 100% acetone, and infiltrated with epoxy resin polymerized for 48 hours at 60 °C Ultrathin sections (70-80 nm) were prepared with a Diatome diamond knife on a Leica Ultracut UCT ultramicrotome, collected on 200 mesh copper grids (EMS), and contrast stained using a double-lead procedure (Daddow) as follows: 5 minutes in lead citrate; 15 minutes in uranyl acetate (3.0% in 50.0% acetone); and a final 5 minutes in lead citrate. Thin sections were examined in a JEOL 1230 transmission electron microscope (Japan Electron Optics Limited, Tokyo, Japan),
Measurement of Body Weight, Body Composition, 24-Hour Food Consumption, Urine Composition, and Energy Expenditure
Standard 24-hour metabolic cage studies were conducted during the sixth month of diet. Mice were weighed and then placed in individual metabolic cages. Food in each cage was weighed at the beginning and end of the 24-hour period to determine calorie consumption normalized to mouse body weight. Urine was collected during the 24-hour period to determine urine volume. Urine aliquots were stored at −80 °C for later analysis. To determine energy expenditure, mice were placed in Oxymax metabolic chambers (Columbus Instruments) for 72 hours. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured every 15 minutes. The mice were allowed to adjust to cages for the first 12 hours of each session and measurements from hours 12 to 60 of the session were utilized for data analysis. VO2 measurements were normalized to %lean body mass. Body composition was measured using an EchoMRI-100H body composition analyzer (EchoMRI, Houston, Tx).
Western Blot
Renal cortical tissue was homogenized in T-PER Tissue Protein Extraction Reagent (Thermofisher) with Halt Protease Inhibitor Cocktail and EDTA (Thermofisher). Homogenate was then centrifuged at 12 000g for 10 minutes at 4 oC and supernatant used for all assays. BCA assay was used to determine protein concentration and 25 ug of protein for each sample loaded on gels. Samples were mixed with Laemmli buffer and separated by electrophoresis on a Criterion TGX Stain-Free gel (Bio-rad). Protein was transferred to a polyvinyldene difluoride membrane (Millipore) using a Trans-blot Turbo Transfer System (Bio-rad) and total protein was imaged on a Chemidoc MP system (Bio-rad). Membranes were then blocked with 2% bovine serum (BSA)/2% goat serum in 1× TBST with 0.1% Tween for 1 hour at room temperature. Membranes were incubated with primary antibody overnight at 4 °C in the same BSA/serum solution before 1-hour room temperature incubation with secondary antibody in BSA/serum/TBST. Immunoreactive bands were detected by chemiluminescence using a Chemidoc MP imager. Densitometric analysis of all images was performed using Image Lab Software (Bio-rad). The following antibodies were used for Western blotting: anti-ATP6AP2 (1:3000, HPA003156, RRID:AB_1078245, Sigma), anti-podocin (1:1000, P0372, RRID:AB_261982; http://antibodyregistry.org/AB_261982, Sigma), anti-rabbit-HRP (1:3000, ab97051, RRID:AB_10679369; http://antibodyregistry.org/AB_10679369, Abcam, Cambridge, UK).
Urine Glucose and Urine Albumin to Creatinine Ratio
Urine glucose concentrations were measured by colorimetric assay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. Urine glucose excretion was calculated by multiplying the urine glucose concentration for each sample by 24-hour urine volume. Urine albumin to creatinine ratio was determined by dividing the urine albumin concentration obtained with the Albuwell M Mouse Albumin Elisa (Exocell, Newton Square, PA) by the urine creatinine concentration measured by colorimetric assay (Cayman).
Statistical Analysis
Comparison between treatment groups were carried out using Student’s 2-tailed t test for comparisons between 2 treatment groups and 2-way ANOVA with Tukey test for comparisons between multiple groups. Data are expressed throughout as mean ± standard error of the mean and P values < 0.05 were considered statistically significant.
Results
Renal Cortical Expression of ATP6AP2
Prior studies by our group and others demonstrated that the same Pax8-rtTA/LC-1 system was effective in reducing ATP6AP2 expression in the renal medulla but expression in the proximal tubule had not been explored (11, 19). We therefore performed immunofluorescence staining for ATP6AP2 in frozen kidney sections with villin used as a proximal tubule marker. As demonstrated in Fig. 1, ATP6AP2 staining colocalized with villin in control ND mice and was reduced by 95.3% in knockout mice. Renal cortical expression of ATP6AP2 was also evaluated by Western blot (Fig. 2). Compared with ND, renal cortical protein expression of ATP6AP2 did not change with HFD in control mice. However, renal cortical ATP6AP2 expression was decreased by 62% and 66% (P < 0.05) in NDKO and HFDKO mice respectively, when compared with ND and HFD control mice. Thus, proximal tubule ATP6AP2 expression was significantly reduced in ND and HFD ATP6AP2 KO mice.
Figure 1.
Renal proximal tubule ATP6AP2 expression. (A) Immunofluorescence is shown for ATP6AP2 in green and proximal tubule marker villin in red. DNA is stained with Hoechst dye shown in blue. White scale bar represents 10 µm. (B) Quantification of proximal tubule ATP6AP2 expression. Abbreviations: ND, normal diet control; NDKO, ATP6AP2 knockout. *P < 0.05 compared with control.
Figure 2.
Renal cortical ATP6AP2 expression. Representative Western blot for renal cortical ATP6AP2 expression after 6 months on ND vs HFD and with and without ATP6AP2 knockout (upper panel) and quantification of signals on Western blots (lower panel). Data presented as mean ± SEM and normalized to ND. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 5), NDKO (N = 7), HFD (N = 6), and HFDKO (N = 6).
Body Weight and Body Composition
As shown in Fig. 3A, control mice fed HFD for 6 months had a 41% (P < 0.01) greater body weight compared with control ND-fed mice. NDKO mice demonstrated a 20% (P < 0.05) lower body weight compared with ND control and ATP6AP2 KO completely abolished the weight gain observed in the HFD control group, resulting in a 40% (P < 0.01) lower body weight in HFDKO compared with control mice on HFD. To investigate these findings further, we measured adiposity as a percentage of body weight in the different groups using echo MRI (Fig. 3B). Control mice on HFD showed a greater body fat percentage of 15.6% (P < 0.01) compared with control mice on ND, reflecting increased adiposity on HFD. ATP6AP2 KO mice had reduced adiposity in HFD-fed mice compared with their HFD-fed controls, resulting in a 15.9% lower body fat content (P < 0.01) in HFDKO vs HFD. Nephron-specific ATP6AP2 KO thus prevented diet-induced increases in body weight and adiposity.
Figure 3.
Mouse body weight and body composition. (A) Body weights of mice after 6 months on diet, (B) Percent body fat mass of mice as measured by echo MRI at 6 months on diet. Data presented as mean ± SEM. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 5), NDKO (N = 7), HFD (N = 6) and HFDKO (N = 6).
Energy Consumption and Expenditure
Given the observed lower body weight in ATP6AP2 KO, we measured 24-hour caloric intake and normalized it to body weight (Fig. 4A). After 6 months on HFD diet, control mice demonstrated 50.5% less caloric consumption (P < 0.01) while ATP6AP2 KO mice had greater caloric consumption by 114% compared with HFD control (P < 0.01). To further investigate the observed differences in mouse body weight and adiposity, we utilized metabolic cages to assess metabolic rates (Fig. 4B). Neither nocturnal nor diurnal oxygen consumption (VO2) was significantly affected by HFD and there was no significant difference in VO2 between knockout mice on either diet vs their respective controls. Similarly, there was no difference in carbon dioxide production (VCO2) between treatment groups. However, the respiratory exchange ratio (RER = VCO2/VO2) was lower in HFD-fed controls compared with ND controls, consistent with higher fatty acid oxidation in HFD-fed mice. ATP6AP2 KO partially prevented the decrease in RER. Taken together, these results demonstrate that the lower body weight in ATP6AP2 KO mice was not explained by lower caloric intake or greater energy expenditure.
Figure 4.
Energy consumption and energy expenditure. (A) 24-hour caloric consumption normalized to mouse body weight (bw), (B) Metabolic cage data including VO2, VCO2, and RER divided into diurnal (top) and nocturnal (bottom) periods and normalized to mouse lean body mass. Data presented as mean ± SEM. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 5), NDKO (N = 7), HFD (N = 6) and HFDKO (N = 6).
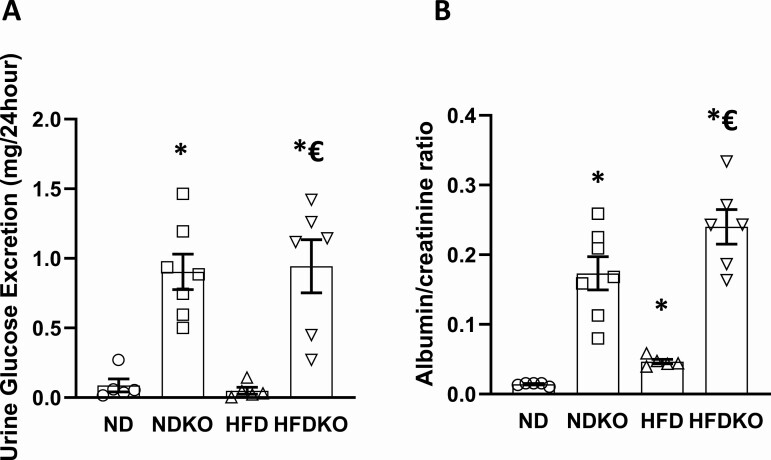
Urine Glucose and Albumin Excretion
As previously reported and attributed to downregulation of aquaporin-2 (11, 19), nephron-specific ATP6AP2 KO mice had greater urine output on both ND and HFD that was compensated for by increased water intake (Supplemental Figure 1A (20)). When measuring 24-hour glucose excretion, we found no significant difference between control mice on ND and those on HFD, while ATP6AP2 KO resulted in dramatically greater glucose excretion (P < 0.01) in ND and HFD mice (P < 0.01) (Fig. 5A). The higher glucose excretion was not due solely to polyuria as urinary glucose concentrations were also greater in knockout mice on HFD compared with HFD controls (Supplemental Figure 1B (20)). The urinary glucose loss did not affect blood glucose levels, suggesting a possible compensatory mechanism such as increase in liver gluconeogenesis. Albumin/creatinine ratios were significantly greater in control mice on HFD compared with ND control mice (P < 0.01) (Fig. 5B). Interestingly, ATP6AP2 KO resulted in higher albumin/creatinine ratios in NDKO mice compared with ND controls (P < 0.01) and in HFDKO mice compared with HFD controls (P < 0.01). The dramatically greater glucosuria and albuminuria with nephron-specific ATP6AP2 KO may thus contribute to the drastically lower weight in this animal model.
Figure 5.
Urine glucose and albumin excretion. (A) 24-hour urine glucose excretion, (B) Urine albumin to creatinine ratio. Data presented as mean ± SEM. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 5), NDKO (N = 7), HFD (N = 5) and HFDKO (N = 5).
To confirm that the differences in urine glucose and albumin excretion were due to the ATP6AP2 KO and not to exposure to doxycycline, we treated a small additional cohort of mice without the Pax8-rtTA cre with the same doxycycline or sucrose water control regimens as the experimental cohort (Supplemental Figure 3 (20)). Unlike the knockout mice, doxycycline treated wild-type mice did not demonstrate polyuria, natriuresis, glucosuria, or albuminuria observed in the ATP6AP2 KO mice when compared with controls.
Kidney Histology
Since the renal proximal tubule is the major site for glucose and albumin reabsorption, we evaluated the health status of this nephron segment by examining renal tissue sections for histologic differences between wild-type and ATP6AP2 KO mice. Representative light microscopy images from control and ATP6AP2 KO mice on ND and HFD are shown in Fig. 6A-6F. ATP6AP2 KO mice showed a mild to moderate degree of proximal tubular epithelial apoptosis, tubular atrophy, and tubular damage while no such changes were seen in control mice on either diet. Knockout mice also had visible tubular dilatation consistent with observed polyuria. To further characterize these changes, we performed Masson trichrome staining to visualize fibrosis (Fig. 7A-7D) and quantitative immunohistochemistry for the apoptosis marker PARP1 (Fig. 7E-7I). Control mice on either diet had no significant interstitial fibrosis while ATP6AP2 KO mice demonstrated mild to moderate interstitial fibrosis. Renal cortical PARP1 staining was significantly greater in ATP6AP2 knockout mice on both ND and HFD compared with their respective controls with most of the staining present in the proximal tubules. Notably, no significant glomerular damage was detectable by PAS staining in ATP6AP2 KO mice (Supplemental Figure 4 (20)). To further rule out podocyte injury as a cause for the observed albuminuria, we evaluated renal cortical podocin expression (Fig. 8A) and performed electron microscopy of podocytes (Fig. 8B-8E). No significant differences were seen in podocin levels between any of the treatment groups suggesting that tubular ATP6AP2 KO did not cause podocyte injury. Electron microscopy similarly did not show evidence of podocyte effacement in any of the treatment groups. Instead, these results suggest that the observed glucosuria and albuminuria were related to proximal tubule injury and possibly tubular dysfunction.
Figure 6.
Renal Histology. (A-B) Representative light microscopy (H&E) images of kidneys from control mice on ND and HFD respectively (400× magnification) (C-D) H&E images of kidneys from ATP6AP2 knockout mice on ND and HFD respectively showing apoptotic cells with nuclear debris (arrows) (400× magnification), (E) PAS image of kidney from ATP6AP2 knockout mouse on ND showing tubular atrophy with flattened tubular epithelium and shrunken tubules (400× magnification) (PAS), (F) PAS image of kidney from ATP6AP2 knockout mouse on ND showing loss of tubular nuclei and dissolution of cytoplasm (arrows) (PAS) (400× magnification).
Figure 7.
Renal interstitial fibrosis and apoptosis. (A-D) Masson trichrome staining of kidney sections from (A) ND, (B) NDKO, (C) HFD, and (D) HFDKO mice respectively (200× magnification). (E-H) Immunohistochemistry for PARP1 in (E) ND, (F) NDKO, (G) HFD, (H) HFDKO respectively (400× magnification). (I) Quantitation of PARP1 immunostaining expressed as positive nuclei per 400× high-powered field. Data expressed as mean ± SEM. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 3), NDKO (N = 4), HFD (N = 3), HFDKO (N = 3).
Figure 8.
Podocyte health. (A) Representative Western blot for renal cortical podocin expression. Data presented as mean ± SEM and normalized to ND. *P < 0.05 compared with ND, €P < 0.05 compared with HFD. ND (N = 5), NDKO (N = 7), HFD (N = 6) and HFDKO (N = 6). (B-E) Electron microscopy images of podocytes for (B) ND, (C) NDKO, (D) HFD, (E) HFDKO mice respectively. Scale bars represent 2 micrometers. Podocyte foot processes are indicated by arrows.
Discussion
This study demonstrates that nephron-specific deletion of ATP6AP2 prevents diet-induced weight gain while also producing significant glucosuria and albuminuria in the setting of mild to moderate proximal tubule damage. Metabolic evaluation indicates that the loss of urinary metabolites is a significant contributor to the weight differences between knockout and control mice. ATP6AP2 KO mice did not have reduced caloric intake, ruling out caloric deficit as the cause of impaired weight gain. Similarly, the lack of difference in VO2 across treatment groups indicates that differences in metabolic rate do not explain why ATP6AP2 knockout mice on both diets gained less weight than their control counterparts. Although the knockout mice in this study had greater urine output, they compensated with increased water intake (Supplemental Figure 1A (20)), suggesting that fluid loss was also not a cause of lower weight. Rather with NDKO and HFDKO mice excreting 10 and 14 times as many calories in urine, respectively, as controls on the same diets, it is likely that the observed glucosuria and albuminuria played a significant role in preventing weight gain in the ATP6AP2 knockout mice.
At the same time, it is not clear whether the degree of glucosuria and albuminuria reported in this study can fully explain the weight differences between control and knockout mice. Our study does not exclude all other possible sources of caloric loss in the knockout. We did not measure fecal caloric content, but we did not observe any gastrointestinal abnormalities such as diarrhea in the knockout mice. Also note that Pax8, the gene that drives cre expression in our knockout mice, is expressed in other metabolically active tissues including liver, thyroid, and pancreas. However, prior recombination studies with this model found only limited recombination in the liver (11), and reporter mouse studies of the Pax8-rtTA cre mice noted only limited periportal cre expression and detected no thyroid cre expression (21). Furthermore, the aforementioned lack of difference in metabolic rates in our knockout mice do not support thyroid dysfunction as a cause of lower weight in the knockout mice. Additionally, the loss of other urine metabolites could have contributed to impaired weight gain. For example, increased fatty acid bound to albumin in urine on HFD (2) which could represent an additional caloric loss. Future studies will investigate other potential contributors to impaired weight gain with nephron-specific ATP6AP2 KO.
Greater glucosuria and albuminuria indicate that ATP6AP2 KO caused significant proximal tubule dysfunction. Reabsorption of filtered glucose occurs almost entirely in the proximal tubule (22). It is less likely that increased glomerular glucose filtration contributed to the glucosuria since we did not observe significant glomerular damage or differences in blood glucose levels between controls and knockout. (Supplemental Figure 2 (20)). It possible that increased liver gluconeogenesis could be contributing to maintain normal blood glucose in the knockout. Our recent studies have shown reduced renal gluconeogenesis in this knockout model compared with controls (23). Therefore, reduced glucose reabsorption by the proximal tubule best explains the observed glucosuria. Similarly, the majority of filtered albumin is likewise reabsorbed by the proximal tubule and defects in proximal tubule albumin absorption cause albuminuria (24, 25). Although glomerular injury and increased albumin filtration could also cause albuminuria, this is unlikely in this case for several reasons. First, while we observed structural proximal tubule abnormalities, we did not find histologic changes in the glomerulus. Second, as noted by Ramkumar et al, the Pax8-rtTA cre utilized in our model is not expressed in the glomerulus suggesting no glomerular ATP6AP2 KO in this model (11, 21). Third, podocin, a marker of podocyte health (17), was not reduced in the ATP6AP2 KO mice, and electron microscopy did not show podocyte effacement, demonstrating that podocyte integrity was not compromised. Thus, decreased resorptive function of the proximal tubule with ATP6AP2 KO is the most likely cause for urinary losses of glucose and albumin.
It remains unclear, however, whether the reported impairment in proximal tubule function is due to the observed structural proximal tubule injury or to a direct effect of ATP6AP2 KO on glucose and albumin transport. The carboxy terminal fragment of ATP6AP2 is a necessary component of the vacuolar H+-ATPase and loss of ATP6AP2 results in impaired acidification of intracellular vesicles, decreased autophagy, and even cell death in multiple cell types (15, 26-28). This alone could explain the histologically visible tubular damage in the knockout mice. At the same time, receptor-mediated endocytosis of albumin requires endosomal acidification by the H+-ATPase acting in concert with chloride channel 5 (CLC5) (29). Pharmacologic inhibition of the H+-ATPase in the proximal tubule decreases receptor-mediated protein endocytosis in the proximal tubule, resulting in albuminuria (30). Recent in vitro studies of megalin, a primary component of the receptor complex that reabsorbs albumin, also found that ATP6AP2 deletion impaired megalin-mediated endocytosis in immortalized human proximal tubule cells (31). Similarly, our laboratory has previously found that ATP6AP2 knockdown reduces mTOR activation in the kidney and loss of mTOR activity in the proximal tubule produces a “Fanconi-like” syndrome with albuminuria and glucosuria due to reduced activity and membrane insertion of SGLT-2 (32). Future investigations will elucidate how ATP6AP2 regulates receptor-mediated endocytosis of albumin as well as the expression and activity of glucose transporters in the proximal tubule.
The results of this study are novel in that they indicate an essential role for ATP6AP2 in maintaining the normal structure and function of the renal proximal tubule. Interestingly, prior studies using the same nephron-specific ATP6AP2 KO model had reported no morphologic abnormalities in the renal cortex or medulla (11, 12). However, it is not clear whether these studies assessed morphologic changes over as long a duration as the current study, and the authors did not report on apoptosis. In contrast, Trepiccione et al reported that an inducible Pax8-cre ATP6AP2 KO model, similar to the Pax8/LC-1 model in this study, had increased interstitial fibrosis on trichrome staining but noted no morphologic changes by light microscopy (15). Our study clearly shows that nephron-specific ATP6AP2 KO results in structural damage to the proximal tubule.
Earlier reports on the physiologic role of renal epithelial ATP6AP2 focused primarily on its role in renal ion handling, largely in the distal nephron. Ramkumar et al noted a urinary concentration defect with nephron-wide ATP6AP2 KO due to downregulation of aquaporin 2 (11), and mice with a collecting duct specific knockout had similar abnormalities (14, 33). Our laboratory and others also reported that ATP6AP2 regulates urinary sodium excretion by modifying collecting duct expression of ENaC (10, 19, 34, 35) while Trepiccione et al demonstrated impaired urinary acidification by intercalated cells in the absence of ATP6AP2 (15). In the proximal tubule, Ramkumar et al meanwhile showed no significant effect of ATP6AP2 deletion on expression of the sodium transporter NHE3 (12). To our knowledge, ours is therefore the first in vivo study to implicate ATP6AP2 in proximal tubule absorption of larger molecules such as glucose and albumin.
The current study has some notable limitations. First, the use of a Pax8-rTA Cre, which affects the entire renal tubule, makes it difficult to definitively determine tubule-specific effects as there can be crosstalk between different segments of the nephron (36). Future development of proximal tubule–specific ATP6AP2 KO mice would help to confirm our results. Our findings also differ to some degree from previous reports of inducible nephron-specific ATP6AP2 KO mice. Ramkumar et al reported no difference in weight between wild-type and ATP6AP2 KO mice on normal diet, and our own laboratory did not find a significant difference in weight between wild-type and knockout mice following 10 weeks of high-fat diet (11, 19). However, closer examination of the data in these studies shows a trend toward lower weight in the knockout groups. As the current study followed mice for a considerably longer time period, differences may have become more apparent. For currently unclear reasons, Trepiccione et al also reported no glucosuria in their nephron-wide inducible ATP6AP2 KO mouse (15).
While our study indicates that nephron-wide ATP6AP2 KO has detrimental effects on the proximal tubule, reducing reabsorption in the proximal tubule could also be beneficial in the setting of obesity. Obesity and hyperlipidemia induce proximal tubule lipotoxicity and cellular dysfunction through increased uptake of fatty acids bound to albumin (37). This has led to speculation that reducing proximal tubular cell endocytosis might in fact be protective when albumin filtration is increased (38). Indeed, renal knockout of megalin in the setting of obesity reduces proximal tubule endocytosis of albumin bound fatty acids and prevents autolysosome dysfunction, hypertrophy, and tubulointerstitial fibrosis (39). However, ATP6AP2 increases renal inflammation and fibrosis in diabetes and ischemia (16, 18) and it was recently reported that the soluble form of ATP6AP2 promotes a fibrotic response in proximal tubule cells in vitro under TGF-β stimulation (40). The balance between beneficial and detrimental roles of renal ATP6AP2 in obesity therefore requires further clarification.
In summary, nephron-specific deletion of ATP6AP2 prevents diet-induced weight gain concomitant with significant glucosuria and albuminuria in the presence of structural damage to the renal proximal tubule.
Acknowledgments
The authors would like to thank Brandon Kemp and the laboratory of Dr. Robert Carey for their assistance in performing the dual immunofluorescence studies in this project. We also express our thanks to Dr. Bruce Gaylinn for performing the Oxymax metabolic cage studies.
Financial Support: This study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016 as well as National Institutes of Health grants DK078757; DK114875; HL091535 to Helmy Siragy.
Glossary
Abbreviations
- ATP6AP2 KO
ATP6AP2 ([pro]renin receptor) knockout
- BSA
bovine serum albumin
- ENaC
epithelial sodium channel
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- HFDKO
high-fat diet ATP6AP2 knockout
- KO
knockout
- ND
normal diet (standard chow)
- NDKO
normal diet ATP6AP2 knockout
- PAS
periodic acid Schiff
- PRR
(pro)renin receptor
- RER
respiratory exchange ratio
- WT
body weight
- Ualb
urinary albumin
- UG
urinary glucose
- VCO2
carbon dioxide production
- VO2
oxygen consumption
Additional Information
Disclosures : Authors declare no conflict of interest.
Data Availability
Some datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
- 1. Palmer MK, Toth PP. Trends in lipids, obesity, metabolic syndrome, and diabetes mellitus in the United States: an NHANES analysis (2003-2004 to 2013-2014). Obesity (Silver Spring). 2019;27(2):309-314. [DOI] [PubMed] [Google Scholar]
- 2. de Vries AP, Ruggenenti P, Ruan XZ, et al. ; ERA-EDTA Working Group Diabesity . Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417-426. [DOI] [PubMed] [Google Scholar]
- 3. Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92(2):313-323. [DOI] [PubMed] [Google Scholar]
- 4. Nizar JM, Shepard BD, Vo VT, Bhalla V. Renal tubule insulin receptor modestly promotes elevated blood pressure and markedly stimulates glucose reabsorption. JCI Insight. 2018;3(16). doi: 10.1172/jci.insight.95107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C, Siragy HM. Autophagy upregulates (pro)renin receptor expression via reduction of P62/SQSTM1 and activation of ERK1/2 signaling pathway in podocytes. Am J Physiol Regul Integr Comp Physiol. 2017;313(1):R58-R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akhtar S, Siragy HM. Pro-renin receptor suppresses mitochondrial biogenesis and function via AMPK/SIRT-1/ PGC-1α pathway in diabetic kidney. PLoS One. 2019; 14(12):e0225728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang J, Siragy HM. Sodium depletion enhances renal expression of (pro)renin receptor via cyclic GMP-protein kinase G signaling pathway. Hypertension. 2012;59(2):317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109(11):1417-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ichihara A. (Pro)renin receptor and vacuolar H(+)-ATPase. Keio J Med. 2012;61(3):73-78. [DOI] [PubMed] [Google Scholar]
- 10. Quadri S, Siragy HM. (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J Hypertens. 2016;34(3):486-494; discussion 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramkumar N, Stuart D, Calquin M, et al. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol. 2015;309(1):F48-F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramkumar N, Stuart D, Mironova E, et al. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol. 2016;311(1): F186-F194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peng K, Lu X, Wang F, et al. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2017;312(2):F245-F253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prieto MC, Reverte V, Mamenko M, et al. Collecting duct prorenin receptor knockout reduces renal function, increases Na+ excretion and mitigates renal responses in ANGII induced hypertensive mice. Am J Physiol Renal Physiol. 2017;313(6): F1243-F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trepiccione F, Gerber SD, Grahammer F, et al. Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol. 2016;27(11):3320-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quadri SS, Culver S, Siragy HM. Prorenin receptor mediates inflammation in renal ischemia. Clin Exp Pharmacol Physiol. 2018;45(2):133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Siragy HM. High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-β-catenin-snail signaling pathway. Plos One. 2014;9(2):e89233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li C, Matavelli LC, Akhtar S, Siragy HM. (Pro)renin receptor contributes to renal mitochondria dysfunction, apoptosis and fibrosis in diabetic mice. Sci Rep. 2019;9(1):11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quadri SS, Culver S, Ramkumar N, Kohan DE, Siragy HM. (Pro)Renin receptor mediates obesity-induced antinatriuresis and elevated blood pressure via upregulation of the renal epithelial sodium channel. Plos One. 2018;13(8):e0202419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Culver S, Akhtar S, Rountree-Jablin C, Keller S, Cathro H, Siragy H. Data from renal ATP6AP2 in obesity. Center for Open Science Digital Repository. Deposited August 20, 2021. doi: 10.17605/OSF.IO/WQY3J [DOI] [PMC free article] [PubMed]
- 21. Traykova-Brauch M, Schönig K, Greiner O, et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med. 2008;14(9):979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;79(Suppl 120):S1-S6. [DOI] [PubMed] [Google Scholar]
- 23. Akhtar S, Culver SA, Siragy HM. Novel regulation of renal gluconeogenesis by Atp6ap2 in response to high fat diet via PGC1-α/AKT-1 pathway. Sci Rep. 2021;11(1):11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006;69(3):440-449. [DOI] [PubMed] [Google Scholar]
- 25. Christensen EI, Kristoffersen IB, Grann B, Thomsen JS, Andreasen A, Nielsen R. A well-developed endolysosomal system reflects protein reabsorption in segment 1 and 2 of rat proximal tubules. Kidney Int. 2021;99(4):841-853. [DOI] [PubMed] [Google Scholar]
- 26. Kinouchi K, Ichihara A, Sano M, et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res. 2010;107(1):30-34. [DOI] [PubMed] [Google Scholar]
- 27. Oshima Y, Kinouchi K, Ichihara A, et al. Prorenin receptor is essential for normal podocyte structure and function. J Am Soc Nephrol. 2011;22(12):2203-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riediger F, Quack I, Qadri F, et al. Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol. 2011;22(12):2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marshansky V, Ausiello DA, Brown D. Physiological importance of endosomal acidification: potential role in proximal tubulopathies. Curr Opin Nephrol Hypertens. 2002;11(5):527-537. [DOI] [PubMed] [Google Scholar]
- 30. Takano M, Nakanishi N, Kitahara Y, Sasaki Y, Murakami T, Nagai J. Cisplatin-induced inhibition of receptor-mediated endocytosis of protein in the kidney. Kidney Int. 2002;62(5):1707-1717. [DOI] [PubMed] [Google Scholar]
- 31. Sun Y, Goes Martini A, Janssen MJ, et al. Megalin: a novel endocytic receptor for prorenin and renin. Hypertension. 2020;75(5):1242-1250. [DOI] [PubMed] [Google Scholar]
- 32. Grahammer F, Ramakrishnan SK, Rinschen MM, et al. mTOR Regulates Endocytosis and Nutrient Transport in Proximal Tubular Cells. J Am Soc Nephrol. 2017;28(1):230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang F, Lu X, Peng K, et al. Antidiuretic action of collecting duct (Pro)Renin receptor downstream of vasopressin and PGE2 receptor EP4. J Am Soc Nephrol. 2016;27(10):3022-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramkumar N, Stuart D, Mironova E, et al. Collecting duct principal, but not intercalated, cell prorenin receptor regulates renal sodium and water excretion. Am J Physiol Renal Physiol. 2018;315(3):F607-F617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu X, Wang F, Liu M, et al. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol. 2016;310(11):F1243-F1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takaori K, Nakamura J, Yamamoto S, et al. Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol. 2016;27(8):2393-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Izquierdo-Lahuerta A, Martínez-García C, Medina-Gómez G. Lipotoxicity as a trigger factor of renal disease. J Nephrol. 2016;29(5):603-610. [DOI] [PubMed] [Google Scholar]
- 38. Simons M. The benefits of tubular proteinuria: an evolutionary perspective. J Am Soc Nephrol. 2018;29(3):710-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuwahara S, Hosojima M, Kaneko R, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol. 2016;27(7):1996-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xie S, Su J, Lu A, et al. Soluble (pro)renin receptor promotes the fibrotic response in renal proximal tubule epithelial cells in vitro via the Akt/β-catenin/Snail signaling pathway. Am J Physiol Renal Physiol. 2020;319(5):F941-F953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.