Abstract
With all the advances in tissue engineering for construction of fully functional skin tissue, complete regeneration of chronic wounds is an important challenge. Since immune reaction to the tissue damage is critical in regulating both the quality and duration of chronic wound healing cascade, strategies to modulate the immune system are of importance. Generally, in response to an injury macrophages switch from pro-inflammatory to an anti-inflammatory phenotype. Therefore, controlling macrophages’ polarization has become an appealing approach in regenerative medicine. Recently, hydrogels-based constructs, incorporated with various cellular and molecular signals, have been developed and utilized to adjust immune cell functions in various stages of wound healing. Here, we first discuss the current state of knowledge on immune cell functions during skin tissue regeneration. We then summarize recent advanced technologies used to design immunomodulatory hydrogels for controlling macrophages’ polarization. We particularly focus on rationally designed hydrogels to provide controlled immune stimulation via hydrogel chemistry and surface modification as well as incorporation of cell and molecules. In addition, the effects of hydrogels’ properties on immunogenic features and wound healing process are discussed. Finally, future directions and upcoming research strategies to control immune responses during chronic wound healing are highlighted.
Keywords: chronic wounds, immunomodulatory therapeutic, immune cell reprogramming, tissue engineering, hydrogel, biomaterials
Graphical Abstract

The quality and duration of healing process can be regulated via modulation of immune responses to tissue injury. Specifically, controlling pro-inflammatory phenotype– anti-inflammatory phenotype progression is a crucial step to ensure a conversion from the inflammatory to the healing stage. In this regard, immunomodulatory hydrogels with the specific chemistry and surface properties can influence immune cell polarization and chronic wound healing process.
1. Introduction
Skin is the body’s largest organ covering the interior organs and performs as a robust external barrier of protection. It initiates the first line of immunological defense mechanisms to tolerate numerous external stimuli consisting of mechanical, chemical, and pathogenic microorganisms. Structurally, skin consists of multi-histological layers comprising of a network of immune and non-immune cell populations. This includes epidermis, the outmost layer of the skin and the underlying dermis, a highly vascularized structure. In addition, there is a subcutaneous hypodermis that supports the upper layers and is primarily composed of fat and connective tissues.[1] While keratinocyte contained epidermis actively inhibits the infectious microorganisms and maintains the body hydration, dermis affords a structural toughness to the skin and offers necessary nutrients for the epidermal homoeostasis. Epidermis also consists of different types of cells with various functions. For instance, melanocytes support skin pigmentation, Merkel cells act as mechanoreceptors forming close contacts with sensory neurons, and Langerhans cells (LCs) are antigen-presenting dendritic cells.[2] Meanwhile, dermis layer is predominately rich in collagen protein and contains stromal cells such as fibroblasts along with human dermal microvascular endothelial cells (HDMECs) and pericytes. In addition, dermis layer is highly vascularized with both blood and lymph vessels and connects to epidermis via basal membrane.[3] Cell population of skin which are solely scattered in these three layers, delivers various structural and immunological functions at the same time. Interestingly, widespread crosstalk between these cells synchronizes the immune reactions in skin to provide effective protections. For example, non-immune cells, including keratinocytes, melanocytes, fibroblasts and endothelial cells, are well-known to control inflammation and organize immune reactions in addition to their contribution as essential structural units of the skin.[4]
Meanwhile, skin being the most exposed organ towards the outer surroundings, can easily disrupt due to trauma, damage, burn, ulceration, surgery, and chronic diseases, or inflammatory cutaneous reactions. Such destructions of the epithelium and connective organizations often hamper the basic skin functions, leading to the recovery through a process named wound healing.[5]
In general, wound healing is an orchestrated process compromising of various phases of i) hemostatic, ii) inflammatory, iii) granulation, and iv) maturation. The process begins with vasoconstriction of blood vessels and platelet aggregation to the sub-endothelium surface, which helps to stop bleeding from the injury site (hemostatic step). Consequently, the fibrin strands start to adhere and initiate thrombus formation. In the meantime, the process of wound healing follows rapid and nonspecific innate immune responses to detect both self and foreign signals including damaged cells, pathogens, and bacteria using white blood cells (inflammatory step). Further, the slower and specific adaptive immune response gets involve in the clearance of pathogens. Thus, the proliferative phase occurs by the formation of granulation tissue, re-epithelialization, and neovascularization in next several weeks. The wound area then contracts, leading to the formation of new tissues consisting of network of blood vessels to receive sufficient oxygen and nutrients. At the remodeling phase, which is considered to be the final stage of wound healing, collagen remodels from type III to type I and the wound closes completely. This stage facilitates the formation of a mature skin tissue with high mechanical strength.[6] Since the wound healing process requires a synchronized sequence of immune events, any interruption leads to a diversity of wound pathologies, such as non-functioning scar tissue formation and chronic wounds.[7] Mostly chronic wounds are severe damages, which delay healing and are frequently associated with infection and formation of microbial films. Chronic wounds may also lead to amputation, sepsis, and, even patient death if they do not treat in a timely manner.[8]
In the last decades, many approaches targeting various steps of wound healing have been investigated for the treatment of chronic wounds.[9] These includes different types of dressings, delivery of cytokines and growth factors, cell therapy, and applying electrical or mechanical stimulations.[10] Among all these techniques, use of artificial constructs that assist as a temporary substance and support the wound healing process, is considered to be the most effective strategy. In view of this, recently different classes of biomaterial-based wound dressings have been developed for simulating the skin microenvironment.[11] Among these biomaterials, hydrogels, with biomimetic structures and physical properties, have been widely utilized for wound healing applications.[12] High biocompatibility, ability to encapsulate various types of cells and bio-macromolecules followed by their controlled release under various external simulations have made these hydrogel materials a promising candidate for the wound healing application.[13]
Mostly, each phase of the wound healing process can be stimulated with specific components such as different types of nanomaterials, growth factors, cytokines, and hydrogels. In this regard, several review articles have discussed recent advances and current clinical strategies that promote wound healing and offer better treatment for patients with chronic wounds.[5, 14]. Recently, due to the crucial role of immune system during the chronic wound healing process, developing different immunomodulatory therapeutic strategies have attracted significant attention in current biomedical research. In short, immunomodulatory strategies control the immune responses after an injury. Then, in order to reduce tissue damage, it promotes an anti-inflammatory environment and accelerates the wound healing. Recently, several review articles have discussed significant roles of immunomodulation strategies for controlling tissue regeneration.[15] For instance, Julier et al. [15g] have highlighted different biomaterials for delivery of stem cells and drug molecules, which improve tissue regeneration and reduce fibrous tissue formation. In another review article, Larouche et al.[16] have summarized diverse approaches focused on the pathophysiology of acute and chronic wounds to control the immune system and accelerate the healing process. Despite extensive studies on hydrogel-based immunomodulatory approaches, according to our knowledge, the use of these immunomodulatory biomaterials for the wound healing have not been adequately explored.
In this review, we will summarize the cutting-edge strategies used for accelerating chronic wound healing based on utilization of different immunomodulatory hydrogels. First, various types of skin diseases and related immune features will be discussed. Hereafter, along with different strategies and recent advances in chronic wound healing, immunomodulation-based strategies will be presented as the next generation of chronic wound care system. Next, we will discuss recently developed immunomodulation strategies based on chemistry of hydrogel materials, surface properties and incorporated cells and molecules. Figure 1 schematically presents the overview of these technologies. Finally, future direction and advanced wound healing technologies with their potential clinical applications will be discussed.
Figure 1.
Schematic representation of various hydrogel-based immunomodulation strategies
2. Classification of skin diseases and the role of immune system during wound healing
According to the time frame of the healing process, skin damages can be classified into acute and chronic wounds.[17] In case of acute wounds, integrity of skin remains intact and healing occurs via normal stages of wound healing.[18] However, for chronic wounds, skin responses fail to proceed via the normal phases of wound healing due to the imbalance between pro- and anti-inflammatory signals.[19] In the United States alone, 2.4–4.5 million people per year suffer from various kinds of chronic wounds, consisting of diabetic wounds, venous stasis ulcers, and autoimmune diseases.[10] Meanwhile, chronic wounds, with persistent inflammatory responses, reduce inflammatory cell functions and change the concentrations of cytokines and growth factors significantly compared to the normal wound.[20] These changes are associated with increased levels of inflammation, insistent wound infections, hypoxia and poor nutrition transfer, and reduced vascularization and re-epithelization.[10, 21]
During chronic wound healing cascade, both innate and adaptive immunity responses remain activate. Innate immunity initiates the first steps of immune responses during wound healing. This includes the initial recognition of pathogenic signals and initiation of pro-inflammatory comeback. In the next stages, the adaptive immunity reduces the damaged cells and pathogens from the wound area to establish immunological memory against pathogens.[22] Generally, in these steps, different types of immune cells help to establish the adaptive immunity responses. Among many others, macrophages are known to contribute critically throughout the host immune responses in both healthy and diseased physiological situations, wound healing, and immune-regulation (Figure 2).[16, 23] Often, primary inflammation gets activated through the penetration of macrophages into the disturbed site, mediating wound debris via phagocytosis.[24] Macrophages also readily participate in auto-immune and inflammatory diseases,[25] infections,[26] and allergies.[27] They facilitate the innate immune progression and act several crucial functions throughout the wound healing process.[28] Meanwhile, innate immune process is supplemented with the release of numerous growth factors and cytokines such as Fibroblast growth factor (FGF), Transforming growth factor beta (TGF-β), and Vascular endothelial growth factor (VEGF), which encourage other cells (e.g. fibroblasts and endothelial cells) to support the skin regeneration process.[28-29] In addition, macrophages experience phenotypic changes during the healing process and switch from pro-inflammatory to a pro-resolution state. Classically, activated macrophages (pro-inflammatory phenotype) recognize the molecular patterns associated with pathogen, damages, peptidoglycan such as released intracellular proteins and nucleic acids. Pro-inflammatory macrophages exhibit antigen processing activity and enhance the secretion of pro-inflammatory cytokines (including Tumor necrosis factor-α (TNF-α), interleukin (IL)-1, IL-6 and IL-12) and oxidative metabolites (e.g., nitric oxide) upon tissue damages. Between these cytokines, TNF-α quickly releases and plays a crucial role in the pathogenesis and helps macrophages to promote phagocytosis and secret other pro-inflammatory cytokines and prostaglandin E2 (PGE2). It also acts as a chemoattractant for neutrophils and induces chemokine expression on endothelial cell lining to facilitate transendothelial migration of neutrophils. TNF-α synthesis occurs immediately after wound creation, enhances during the first hours, and reaches a maximum level on day 1 followed by a decrease to the basal level.[30] Meanwhile, pro-inflammatory macrophages are also known to promote host defense and removal of damaged tissues, and to participate in phagocytosis and angiogenesis. However, long-term presence of pro-inflammatory macrophages may cause chronic inflammation. In the meantime, alternatively-activated macrophages (anti-inflammatory phenotype) get ready for the declaration of the inflammatory phase by releasing anti-inflammatory cytokines such as IL-4, IL-10, Platelet-derived growth factor (PDGF), TGF-β, VEGF, FGF, and Epidermal growth factor (EGF) to promote wound healing.[29] Anti-inflammatory macrophages participate in re-establishing skin integrity, stimulation of ECM formation, remodeling, and angiogenesis.[31] In addition to the macrophages, regulatory T cells (Tregs) are also crucial to preserve skin homeostasis and support anti-inflammatory macrophage polarization by suppressing inflammatory responses via releasing anti-inflammatory cytokines (e.g. IL-10 and TGF-β1) and arginase. Moreover, Tregs in collaboration with Th2, secrete TGF-β1, IL-4, −5, −13, and −21 and contribute in the matrix formation.[32] Despite the specific role of innate immune cells to control wound healing, depending on the intensity and kind of injuries, other types of adaptive immune cells also get involved in the wound healing process. For instance, LCs, a subtype of dendritic cells (DCs), improve the healing of diabetic foot ulcers (DFUs).[33]
Figure 2. Schematic representation of macrophage polarization pathways.
Mature macrophages originated from blood monocytes are specific for wound healing. Two possible polarization states of macrophages are pro-inflammatory and pro-reparative phenotypes. Pro-inflammatory macrophages are defined based on the expression of various pro-inflammatory cytokines. In the reaction of interleukin (IL)-4 and −13, anti-inflammatory macrophages get activated, leading to the production of IL-10 and transforming growth factor (TGF-β). pro-inflammatory / pro-reparative phenotype polarization process depends on the physiological environment. Based on various stimulation such as using glucocorticoids, Lipopolysaccharides (LPS) and immune complexes, different kind of pro-reparative phenotype macrophages (M2a, M2b and M2c) are determined.
It is known that the intensity of chronic wounds and their recovery depend on the patient’s age[34] or primary comorbidities.[10] For example, in diabetic patients, limited response from endothelial cells hinders the release of cytokines, which delays angiogenesis. Moreover, low oxygen content decreases the immune cell capability to combat against pathogens, leading to acute wound ulcers.[35] In some cases, wound ulcers with reduced healing rate easily get infected with various environmental bacteria such as Staphylococcus aureus, resulting in bacteremia and sepsis.[36] Therefore, chronic wounds are the main reasons of limb eliminations. Accordingly, understanding the immune features of chronic wounds will help to control such diseases more effectively.
3. Immune features of skin diseases and wound
Chronic wounds are usually associated with bacterial infection, tissue hypoxia, local ischemia, and expression of a high level of inflammatory cytokines which produces a permanent inflammation which usually delay the re-epithelialization.[37] Therefore, identification and management of these events are critical for the treatment of chronic wounds. For example, since open skin wounds do not possess any protective defense, intense infection becomes the major and unavoidable obstruction towards the wound healing process. Generally, wound contamination readily occurs with typical skin microorganisms including exogenous bacterial, viral, and fungal.[38] These microorganisms easily diffuse to various tissues where optimal environmental conditions facilitate their colonization. Among various microorganisms, bacterial infection is one of the most conventional issues, encouraging via activation of leukocytes in the chronic wound sites. Activated leukocytes secrete an array of inflammatory mediators such as matrix metalloproteinases (MMPs), free radial oxygen radicals (ROS), and inflammatory moderators, boosting an imbalance between pathological native factors and integrity of immune defenses.[39] This supports colonization of different types of Gram-negative and Gram-positive bacteria including Staphylococcus aureus (S. aureus), Methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa.[40] While in the initial steps of chronic wound formation, presence of Gram-positive bacteria (typically S. aureus) is seen predominantly, in the progressive phases various Gram-negative bacteria (e.g., Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa)) are observed frequently. These bacteria interfere with the wound healing process and cause delay.[41] Bacterial colony also damages wound healing pathway via disturbing the tight junctions between the epithelial cells.[42] In addition, bacteria produces extracellular adherence protein (Eap) that interferes Leukocyte function-associated antigen-1 (LFA-1)/ Intercellular adhesion molecules-1(ICAM-1) interactions. This is essential for the attachment of neutrophils to endothelial cells, which hinders the phagocytosis and formation of fibrous capsule.[43]
Autoimmunity diseases are also known as another chronic skin wounds that target the immune system directly. Basically, autoimmunity is an abnormal immune response in which the immune system targets self-tissues and healthy cells, leading to serious damages and even organ failure. Interestingly, autoimmunity diseases rank as the third greatest widespread source of morbidity and mortality in the world.[44] Mostly, a close interaction between genetic factors and environmental triggers is responsible for the damage of immunological lenience and autoimmune diseases.[45] The autoimmunity mechanisms involve central tolerance via removal of T and B cells via CD25fl Tregs activity. Moreover, secretion of anti-inflammatory cytokines and down-modulation of pro-inflammatory cytokines readily facilitate the uptake of apoptotic cells in a physiologic environment[9d, 46]. Although different infectious agents, such as viruses, bacteria, and fungi, facilitate the autoimmune disorders through diverse mechanisms,[47] virus mediated infections are known to be the predominantly occurred autoimmune diseases. By definition, antibodies are produced in the body during an infection to create a crucial defense mechanism against viruses and bacteria. Even small alterations during the creation of these antibodies can create antibody-producing B cells which attack one’s own body. This can eventually lead to an autoimmune diseases, such as Type 1 diabetes mellitus (TIDM), inflammatory bowel disease, epidermolysis bullosa acquisita, lupus erythematosus, and bullous pemphigoid.[48] One of the most common autoimmune diseases is Type 1 TIDM. Type 1 diabetes results from the damage of β-islet cells by T cells and simultaneous secretion of numerous islet cell antigens.[49] In this case, antibodies secreted by B cells considerably participate in the disease pathogenesis. Meanwhile, long time activation of B cells, which usually supplemented with high level of antibody production, results in both activation and development of immune responses. Lastly, the combination of antibodies and immune complexes lead to the autoimmune disease.[47]
4. Current regenerative and therapeutic approaches for chronic skin wound healing
In general, chronic wounds are challenging to heal, cause insistent pain, and reduce patients’ quality of life. In addition, continuous release of exudates and free radicals stimulate the microbial infection and inflammatory responses, delaying the wound healing.[50] Recently, different therapeutic strategies have been developed for the treatment of skin chronic wounds, which include skin substitutes, cell delivery approaches, and biomaterial-based bandages, dressings and adhesives as well as various types of immunomodulatory pathways.[9d, 51] These strategies are briefly discussed in the following subsections.
4.1. Skin substitutes
Autologous skin grafting methods are well-known as a crucial approach for the chronic wound treatment, specifically for diabetic ulcers.[52] Here, differentiated epidermis is developed on the fibroblast-populated dermis with 3-mm punch biopsies, isolated from the patients. In this case, acellular allodermis was applied as a dermal.[52b] Broad injuries and chronic skin wounds lead to inadequate number of autografts, particularly in severe burn incidents. Skin allografts, such as cadaveric dermal allografts, have also been used as a promising strategy for the healing of numerous wounds including different types of chronic wounds and trauma injuries.[53] Although various immunosuppressive strategies have recently been used for allogeneic transplantation,[54] early rejection is still a major challenge for most of these allograft substitutions.[55]
4.2. Cell Delivery
Delivery of different types of cells with multiple differentiation potentials has also been proven as a promising strategy for skin regeneration. For example, recently, infusion of cadaver derived allogeneic pancreatic islet cells has been clinically employed for Type 1 diabetes. However, this process requires administration of immunosuppressive drugs that prevent the hyperactive immune system in patient’s body. Similarly, other multipotent stem cells consisting of mesenchymal stem cells (MSCs),[56] adipose stem cells (ASCs)[57] and human umbilical cord blood (hUCBs)[58] have revealed remarkable regenerative potential for the treatment of chronic wounds. In view of this, Bliley et al.[59] have found an improved messenger RNA (mRNA) expression ratio of type III to type I collagen, vascularity and collagen deposition, after ASCs transplantation in burn wounds. Therapeutic potential of implanted human amniotic epithelial cells (AMEs) also exhibited high engraftment rates and led to an accelerated wound healing.[60] However, direct use of cells for therapeutics have been restricted by various risk factors, including tumor formation, thrombosis, and unwanted immune responses.[61] In this regard, combination of autologous epidermal sheets with epidermal stem cells has showed complete functional re-establishment of epidermis and long-term regeneration.[62]
4.3. Biomaterials-based strategies
Mostly, conventional treatment strategies of chronic wounds involve daily wound supervision. This includes debridement to eliminate infected or necrotic tissues, and application of various bandages, adhesives and dressings to absorb wound exudate. Therefore, several types of cotton and wool bandages have been designed as primary protective layers to prevent the contamination and preserve a warm and moist environment while absorbing wound fluids.
To accelerate wound healing, favorable environments have been introduced through the application of functional materials that are biocompatible, hypoallergenic with reduced immune reactions, and semi-permeable to oxygen.[63] In this prospective, different synthetic, natural, and composite materials were developed with various shapes including thin films, microporous scaffolds, nanofibrous matrices, hydrocolloid and injectable hydrogels, and three-dimensional (3D) bioprinted grafts.[64] Today, various kind of wound dressings in different forms are commercially available and presented in Table 1. Among these, nanofibrous constructs were widely used as wound dressings. However, their utilization as dermal substitutions is limited due to the restricted cell migration within the constructs’ depth.[65] Therefore, use of hydrogel-based materials, supporting spontaneous cells migration and tissue ingrowth, are favorable and utilized frequently.[66]
Table 1.
Various types of commercially available wound dressings [230]
| Type of wound dressings | Trade name | Composition |
|---|---|---|
| Hydrocolloides | Debrisan Sorbex Granuflex Comfeel |
Dextran Cellulose sheets Sodium Carboxymethyl cellulose, gelatin Alginate |
| Hydrogels | TegaGel Intrasite Nu-gel Integra Hyalomatrix SilvaKollagen ScarX Prevascar |
Alginic acid Carboxymethyl cellulose polymer and propylene glycol Alginate Collagen, glycosaminoglycan (GAG), and a protective silicone membrane Hyaluronic acid Hydrolyzed collagen gel filler with silver oxide Nefopam incorporated hydrogel IL-10 incorporated hydrogel |
| Polymeric foam/ Semi-permeable film and foam dressings | Flexzan Biopatch Biatain Opsite Lyofoam DermaBlue GEMCORE360° |
A sterile, ultra-thin, highly conformable, semi-occlusive polyurethane foam A hydrophilic polyurethane absorptive foam with Chlorhexidine Gluconate Conformable polyurethane foam dressing with a semi-permeable, water- and bacteria proof top film and a soft silicone adhesive. Poly-urethane or silicone center with a semi- occlusive outer layer Polyurethane Foam Sterile Dressing Polyurethane/polyether foam consisting of methylene blue and silver sodium zirconium phosphate Antimicrobial dressing consisting of a high-performance foam impregnated with polyhexamethylene biguanide |
| Alginate dressing | Sorbsan Kaltostat Algisite |
Calcium salt of alginic acid, prepared as a textile fibrer 80% calcium alginate/20% sodium alginate dressing Calcium-alginate dressing which forms a soft, gel |
| Tissue engineered skin substitutes | Apligraf Alloderm Biobrane Hyalograft. |
FDA approved skin equivalent substitute consists of keratinocytes and fibroblast-seeded collagen for venous ulcers Normal human fibroblasts with all cellular materials removed A silicone membrane bonded to a nylon mesh to which peptides from dermal collagen have been bonded to nylon membrane Hyaluronan based scaffolds |
| Medicated dressings | DebridaceT | Ointment contains papain and urea |
| Fibrous membrane | Aquacel® Tegaderm™Nanofiber |
Non-woven sodium carboxymethyl cellulose hydrofibre integrated with ionic silver. Poly ɛ-caprolactone (PCL)/gelatin electrospun onto a polyurethane dressing |
4.3.1. Hydrogels-based strategies
Hydrogels with 3D porous structures easily swell with physiological fluids and show soft elastic properties. Hydrogel-based biomaterials are widely used as wound dressings and bioadhesives due to their wide applicability in the form of films, fibers, 3D scaffolds, injectable structures, and even as microneedle (MN)-based patches/adhesives. Compared to other types of dressings, hydrogel dressings reveal weak exudate absorptive capacity due to their high water content. In addition, weak mechanical properties of most hydrogels make them difficult to handle. On the other hand, hydrogels can actively provide water and moist environment for dry wounds. Soft and elastic properties of hydrogels can also support easy application and removal after wound healing without any damage. In addition, hydrogels are useful to reduce temperature of cutaneous wounds by providing a soothing and cooling effect.[9c] Depend on the component of the hydrogel materials, encapsulation of important molecules and cells are also feasible. This provides additional direction to the hydrogel based materials towards wound healing including integration to the wound bed, vascularization, immunocompatibility, and ability to support tissue remodeling.[67] For instance, recently, a 3D scaffold based skin graft, Integra®, is developed with collagen, glycosaminoglycan (GAG), and a protective silicone membrane that accelerates the effective wound healing in the clinical trials. In addition, Integra® has been proven to be favorably effective (success rate of 78–86%) in the organization of complicated wounds in harshly wounded military soldiers.[68] Hyalomatrix® is another biodegradable commercialized dermal matrix contact layer, which is developed with Hyaff (Medline), an esterified hyaluronic acid (HA). Upon application over full-thickness wounds,[69] Hyalomatrix® can improve the cellular function and extracellular matrix (ECM) construction. In contrast, Integra® has been observed to enhance the dermal regeneration with greater physical and mechanical properties.[70]
Up to date, a wide range of polymers (naturally derived or synthetic polymers) and crosslinking methods are implemented to design different hydrogels with variable characteristics including mechanical properties, swelling ratio, and degradation rate for wound healing applications, which are reviewed elsewhere.[71] Natural polymers, such as collagen, fibrin, collagen, elastin and HA, have high biocompatibility and cell affinity. On the other hand, synthetic polymers such as polyethylene glycol (PEG), poly(vinyl alcohol) (PVA) and others) provide controllable hydrolytic or enzymatic degradability and minimal inflammatory response. This depends on their structural design and flexibility. Meanwhile, synthetic hydrogels can bind with object proteins using molecularly imprinted polymers (MIPs) method for various therapeutic applications.[72] Mostly, chemical and physical interactions between the functional groups presented on polymers’ backbone control the crosslinking of the hydrogels, which eventually regulates the degradation rate and mechanical properties of biomaterials in biological environment (Figure 3A). For instance, covalent crosslinking is commonly used to develop 3D hydrogel networks suitable for the efficient encapsulation of cells and biomolecules such as proteins and growth factors.[73] Such hydrogels can be synthesized through a short ultra-violet or visible light radiation mediated in situ chemical crosslinking of unsaturated functional groups such as methacrylate or simple conjugate addition reactions between active functional groups.[64c, 74] Recently, injectable hydrogels have also been widely applied for delivery of cells, proteins, growth factors, and immunomodulatory agents.[71c] For instance, Zhao et al.[75] have fabricated human umbilical vein endothelial cells (HUVECs) incorporated gelatin methacryloyl (GelMA) hydrogel for the regeneration of full thickness cutaneous wounds.[75] Along with the efficient repair of the wound defect, GelMA-based hydrogel also provide a sustained release of exosomes. In this case, combination of chemically crosslinked hydrogels with other bonding interactions, namely, hydrogen and electrostatic interactions resulted in the formation of interpenetrating networks (IPNs) with reversible bonds and noticeable mechanical properties.[76] Similarly, Tavafoghi et al.[77] have developed a mechanically robust and injectable bioadhesive hydrogel that resist large strains for the sutureless sealing. Herein, the combination of GelMA with methacrylate alginate enabled ion-induced reversible crosslinking, which easily dissipate the energy under strain. Thereby, as-prepared IPN was observed to enhance the toughness (600%) of the material compared to GelMA hydrogels. This can have useful impact towards the soft and sensitive injury site. Meanwhile, self-assembled peptides have also proven to be another attractive class of hydrogels for the chronic wound dressing. Similar to the skin tissue ECM, peptide-based hydrogels were shown to act as a barrier against infection, maintained the tissue hydration, and facilitated autolytic debridement of necrotic eschar tissue in burn wounds.[78]
Figure 3. Hydrogels designed for the treatment of chronic wounds.
A) Synthesis of various types of hydrogels through chemical and physicals crosslinking mechanism. B) A hydrogel-based smart bandage for monitoring and healing of infected wounds: (i) the schematic of the smart bandage, made of pH sensors and heater, to activate thermo-responsive carriers compromising of antibiotics. The setup was also coupled with an electronic element to record the signals; (ii) the antibiotic encapsulated microparticles loaded in alginate matrix. Reproduced with permission from Ref.[87]. C) A nanocomposite hydrogel for chronic wound healing; the schematic representing the process of CuS/mSiO2-3-(trimethoxysilyl)propyl methacrylate(MPS)/ poly(N-isopropylacrylamide) (PNIPAM) hydrogel synthesis. Reproduced with permission from ref.[90].
Despite having a critical role towards the protection of the wounds, most of the hydrogel-based systems failed to accelerate the chronic wound healing process due to the lack of active ingredients. Therefore, incorporation of various active components into the hydrogel systems can dynamically contribute to the healing process. According to the nature of the material’s activity, hydrogel-based wound dressings can be categories in three ways: inert, bioactive, and interactive.[79] For instance, while gauzes are inert towards the interaction with wounds, soft nonwoven pads are known as bioactive dressings that absorb exudates, afford a moist environment in under layer wounds and reduce the risk of skin maceration.[80] Several alginate- and collagen-based hydrogels, scaffolds, and pads have been developed to provide bioactive dressings during the wound healing process.[81] For instance, while alginate-based hydrogels absorb large amounts of water and help in hemostasis[64e], collagen-based hydrogels stimulate the formation of collagen during wound healing. Moreover, being known as non-immunogenic, non-pyrogenic component, collagen is an appropriate material for wound healing process.[82] Hydrogel materials have also been introduced as interactive dressings that can modulate the wound microenvironment and interact with the skin tissue to promote healing. For example, GAG hydrogels such as HA is known as an interactive dressing. [83]
4.3.2. Bioactive and hybrid hydrogel based strategies
Bioactive and interactive dressings can be designed via incorporation of various active components, which provide a dynamic function in various stages of wound healing. The major disadvantages of hydrogel-based dressings are their gas and oxygen permeability, which restrains their use against various types of infections. To address this issue, active antimicrobial components such as antimicrobial peptides, antibiotic drugs, and antibacterial or antioxidant materials (e.g. silver in i.e. Aquacel® Ag) have been incorporated in hydrogels to promote antibacterial properties[84]. For instance, Alexandrino-Junior et al.[85] have developed a PVA-based hydrogel incorporated with Amphotericin B (Amb), an antifungal medication, for the treatment of chronic cutaneous leishmaniasis (CL) disease.[85] Hybrid hydrogel showed significant antifungal and leishmanicidal activity along with microbial impermeability and water vapor permeability, making it an appropriate candidate for CL disease treatment. In another study, Comotto et al.[86] have engineered an alginate-based breathable hydrogel dressing with two natural antioxidants: curcumin and t–resveratrol (t-Res) for the treatment of infected wounds. These antioxidants acted as bactericidal agents, while improved cell proliferation without affecting the hydrogel’s physical properties. Recently, smart bandages and dressings were also developed that simultaneously monitor and treat the infection. For instance, Mostafalu et al. [87] have fabricated a smart bandage for supervising and managing the infection in chronic wounds at the same time. This temperature and pH sensitive patch was assembled onto a flexible bandage to manage the wound status in real-time (Figure 3B(i)). Recently, a thermo-responsive hydrogel was synthesized with antibiotic loaded poly(N-isopropylacrylamide) (PNIPAM) microparticles and alginate for the stimuli-responsive drug release. In this case, the flexible heater combined with wound dressing controlled the drug release on-demand (Figure 3B(ii)). Antibacterial activity of the material was originated from the controlled release of antibiotic loaded inside the microparticles.
Despite having positive response towards the treatment of infected wounds, direct use of different bioactive molecules can raise some concerns due to their possible side effects. Besides, repetitive use of antibiotic may cause resistance. Therefore, bioactive components were incorporated within the hydrogels to develop hybrid networks as an alternative healing strategy for infected chronic wounds. Table 2 summarizes various types of bioactive components encapsulated in hydrogel matrices for chronic wound dressing applications. These components include various types of ceramic, metallic, polymeric, functional nanocomposite, carbon-based materials, and different natural materials such as honey as well as peptides molecules. These bioactive components provide considerable improvement in the antimicrobial activity and inhibit the bacterial colonization during the initial steps of wound treatment.[88] Besides, they have been applied to stimulate the proliferation and migration of different cells throughout the development of ECM in the remodeling stage of healing. Further, hybrid hydrogels incorporated with bioactive molecule can modulate the macrophage activity toward wound regeneration during the healing process.[89] Meanwhile, these bioactive components can also induce antioxidant property, angiogenesis, blood clotting ability and even enhance the mechanical properties of the wound dressings. Therefore, proper manipulation and combination of these components can be used to design multifunctional wound dressing materials with appropriate characteristics. For instance, recently a hybrid hydrogel was synthesized by using 3-(trimethoxysilyl)propyl methacrylate and mesoporous silica modified CuS nanoparticles via radical polymerization (Figure 3C).[90] This hybrid hydrogel revealed an antimicrobial activity, owing to the merged results of hyperthermia, ROS activity, and released copper ions upon near-infrared (NIR) irradiation. Moreover, the released ions promoted both proliferation of fibroblasts and angiogenesis, which resulted in antibacterial effect and skin tissue regeneration.
Table 2.
Various types of bioactive components used to form bioactive hydrogels for wound healing applications
| Components | Role | Reference | |
|---|---|---|---|
| Ceramic based NPs | Bioactive glass | Improve vascularization via stimulatory effects on gap junction communication between HUVECs and upregulated connexin43 expression. Prompt expression of VEGF and FGF in HUVECs | [231] |
| Titanium oxide | Improve antibacterial property through ROS generation. Improve mechanical and swelling properties along with hydrophilic characteristics. Accelerate healing of open excision wounds type | [232] | |
| Zinc oxide | Improve antibacterial property, antineoplastic, promote keratinocyte motility, angiogenesis and wound healing | [64a, 233] | |
| Copper oxide | Improve antibacterial property, increase gene and in situ up-regulation of proangiogenic factors. Increase blood vessel formation. Enhance wound closure | [234] | |
| Laponite | Improve antibacterial and hemostatic properties. Ability to encapsulate various types of drug like molecules | [64e, 74a, 208b] | |
| Carbon-based NPs | CNTs | Effective bactericidal activity against bacteria, cause RNA efflux, and disrupt cell membranes of bacteria | [208b, 235] |
| Carbon quantum dots | Intrinsic peroxidase-like activity. pH sensitivity. Antibacterial activity | [236] | |
| Graphene | Antimicrobial activity against microbes such as E. coli., S. aureus, P. aeruginosa and Candida albicans. Stimulate collagen synthesis. | [237] | |
| Nanodiamonds | Sustained release of the angiogenic growth factor. Improve mechanical properties of hydrogel, | [238] | |
| Metallic NPs | Au | Antimicrobial and antioxidant activity. Stimulate high expression of pro-angiogenic agents. Stability against enzymatic degradation of collagen | [239] |
| Silver | Avoid contamination and colonization. Anti-inflammatory and angiogenesis for wound treatment. Decrease secretion of VEGF and pro-inflammatory cytokines | [135, 240] | |
| Gallium | Antimicrobial activity. Increase capability to enhance thrombus generation | [241] | |
| Polymeric NPs | Chitosan | Assist blood coagulation by attaching to red blood cells. Promote inflammatory cell functions. | [242] |
| Lignin | Inherent antimicrobial and antioxidant capabilities | [243] | |
| PLGA | Control the release of various therapeutic agents | [93] | |
| Composite NPs | Mesoporous silica/ CuS | Antibacterial activity. Promote new tissue formation | [90] |
| Ag/graphene | Excellent antibacterial abilities. Accelerate the healing rate of artificial wounds | [244] | |
| Ag/chitosan NPs | Improve antibacterial activity. in vitro antioxidant activities and hemolytic behaviour. Enhance wound healing, Prevent the oxidative damage. Enhanced the wound re-epithelialization. | [245] | |
|
Essential Oils |
Melaleuca alternifolia, Hypericum perforatum | Improve antimicrobial properties, non-toxic to fibroblasts, antiviral, antioxidant, anti-inflammatory, anti-allergy | [246] |
| Honey | Inhibit microbial biofilm growth, granulation and angiogenesis stimulation, reduced inflammation, improve wound epithelialization | [247] | |
| Peptides | Antibacterial ability, anti-endotoxin, wound healing. | [91–92, 201, 248] | |
Recently, various synthetic and natural peptide molecules have also been used as bioactive components. For example, our group has engineered an elastic sprayable bioadhesive hydrogel with antibacterial activity for the management of infected wounds (Figure 4A).[91] In this study, various ECM-derived polymers including methacryloyl substituted recombinant human tropoelastin (MeTro) and GelMA were mixed with antimicrobial peptides (AMPs)-Tet213 to form an antimicrobial bioadhesive. Owing to several important properties including biocompatibility, adjustable biodegradation and antimicrobial activity, the developed hybrid hydrogel introduced an effective sutureless wound closure approach that could inhibit infection and facilitate wound regeneration. In another study, Yang et al.[92] have designed a therapeutic peptide-engineered nanosheet to treat diabetic wound ulcers (Figure 4B(i)).[92] Herein, a highly transparent and flexible silk fibroin nanosheet was modified with an integrin-binding pro-survival peptide (Figure 4B(ii)). As-synthesized material showed upregulation of angiogenesis-related markers (VEGF and CD31), leading to fast diabetic wound closure (Figure 4B(iii)). Recently, Chen et al.[93] have developed an injectable hydrogel through chemical interactions between aminated gelatin, adipic acid dihydrazide, and oxidized dextran. The developed hydrogel showed sequential release of chlorhexidine acetate (CHA), an antibacterial agent, and basic FGF (bFGF) during the wound healing (Figure 4C(i,ii)). Interestingly, the synthesized hydrogel showed excellent self-healing capability related to the dynamic bonds of imine and acylhydrazone (Figure 4C(iii)). Structurally, in this case, bFGF was embedded in poly(lactic-co-glycolic acid) (PLGA) microspheres, while CHA was loaded in the hydrogels for sequential release (Figure 4C(iv)). Because of the fast release of CHA, the hydrogel efficiently inhibited the infection at the early stages of in vivo studies, and consequently promoted the skin wound repair through the sustained release of bFGF.
Figure 4. Bioactive and interactive dressings for the treatment of chronic wounds:
A) An sprayable adhesive and elastic antimicrobial hydrogel for the chronic wound healing: (i) schematic illustration of the methacryloyl substituted recombinant human tropoelastin (MeTro)-gelatin methacryloyl (GelMA)-antimicrobial peptides (AMP) synthesis; (ii) the adhesive hydrogel was formed after spraying the pre-polymer solution and short visible light exposure; and (iii) Colony-forming unit (CFU) counting evaluation of MeTro/GelMA-AMP hydrogels compromising of various AMP contents (0, 0.1, 0.3 and 3 wt%) confirming the significant antibacterial activity of the adhesive hydrogel. Reproduced with permission from Ref.[91]. B) A peptide-designed nanolayer developed to manage diabetic wound ulcers: (i) A schematic showing QHREDGS peptide integrated into silk nanosheets. Polyvinyl alcohol (PVA) sacrificial layer was also applied which could be removed by facile water dissolution; ii) highly transparent bioactive adhesive sheet with sacrificial PVA supporting film; (iii) in vivo evaluation of diabetic wound treatment in db/db mice (α-SMA staining) on day 14. Reproduced with permission from Ref.[92]. C) A self-healable hydrogel for sequential release of antibacterial component and growth factors: (i) the preparation of the injectable and self-healing basic Fibroblast growth factor (bFGF)@ poly(lactic-co-glycolic acid) (PLGA)/chlorhexidine acetate(CHA)/hydrogel based on the reaction of aminated gelatin (NGel), oxidized dextran (ODex) and adipic acid dihydrazide (ADH); (ii) the synthesis of bFGF@PLGA microspheres; (iii) self-healing mechanism of NGel-ODex-ADH hydrogels; and (iv) sequential release of various agents from bFGF@PLGA/CHA/hydrogels, after injection in wound sites. Reproduced with permission from Ref.[93].
Having such impressive and important role in various phases of wound healing, different growth factors and cytokines have been introduced in wound dressing materials.[94] Mechanistically, these biomaterial-based growth factor delivery platforms enable local delivery. Therefore, they can reduce the total drug loading, which eventually decreases the distal effects and other associated side effects compared to the systemic infusion.[95] Meanwhile, among various types of growth factors, due to the significant role of FGF and EGF in re-epithelialization, these are widely applied for wound healing applications.[96] Moreover, the cooperation of pro-angiogenic molecules including Ang2 and VEGF encourages angiogenesis by accelerating the pericyte detachment and improving vascularization.[97] One of the main efforts to improve the stability and preserve the activity of the growth factors is proper molecular engineering and their controlled release by using various carriers, which is reviewed elsewhere.[98] However, these strategies have limited applications for clinical use, since the bioactive molecules within the matrices ultimately run out easily and further loading of molecules typically induces foreign body responses. To overcome this challenge, recently, the preferential localization of drug molecules in the epidermis of human skin has become a favorable method. In this regard, different smart hydrogels which response to various external stimulations, including temperature, pH and glucose-sensing moiety have been designed to control the drug release profile.[96b, 99] For example, Bagherifard et al.[100] have designed a dermal patch using thermosensitive drug microcarrier encapsulated in a alginate-based hydrogel layer which was combined with a flexible heater (Figure 5A(i, ii)). These thermos-responsive microparticles containing various active molecules, such as growth factors, were fabricated using a microfluidic device and then incorporated inside the hydrogel patch (Figure 5A(iii)). By regulating the temperature of the engineered patch electronically, release of the drug molecules was controlled over the wound area (Figure 5A(iv,v)). In another study, to provide adequate delivery of bioactive molecules for the management of exuding chronic wounds, a programmable platform was developed that readily regulates the release of multiple drugs (Figure 5B(i)).[101] Moreover, the platform was interfaced with a smartphone and an app that allow the physicians to control the wound condition remotely. Besides, the bandage was designed with an array of MNs in order to effectively release VEGF for the healing of a full thickness skin injury (Figure 5B(ii)). The VEGF released from the programmable platform revealed an accelerated wound closure, angiogenesis, and hair growth compared to standard relevant therapeutic transport (Figure 5B(iii)). In another interesting study, a glucose-responsive complex was designed from tert-butyl (2-acrylamidoethyl) carbamate (Boc-EDAA) and insulin for insulin delivery.[96b] In a hyperglycemic state, the glucose-responsive phenylboronic acid (PBA) could change the surface charge of polymer from positive to negative and provide insulin delivery. This was confirmed through the in vivo studies which demonstrated a fast response of the smart hydrogel, leading to a hyperglycemia-triggered insulin release.
Figure 5. Smart hydrogel-based skin patches for the management of chronic wounds:
(A) Hydrogel-based smart bandage for monitoring and management of infection in chronic wounds: (i) Schematic representation of an engineered smart bandage; (ii) a representative image of the designed heater on the flexible substrate and the encapsulated thermo-responsive poly(N-isopropylacrylamide) (PNIPAM) microparticles with dextran; (iii) an optical image of the integrated wound dressing system; (iv) the ex vivo application of the smart patch over the human skin; (v) the release of dextran from the PNIPAM particles at various temperatures. Reproduced with permission from Ref.[100]. (B) A microneedle (MN)-based smart bandage for therapeutic delivery: (i) representation of various components of the bandage and its connection with the skin. Integrated wearable bandage connected to the controlling module to communicate with a smartphone; (ii) three-dimensional (3D)-printed MNs and its microscopic image; and (iii) Haemotoxylin and Eosin (H&E) staining for the evaluation of the neotissue in two groups. Noticeable wound closure (95%) was detected in contact with Vascular endothelial growth factor (VEGF)-released MNs group. Reproduced with permission from Ref.[101].
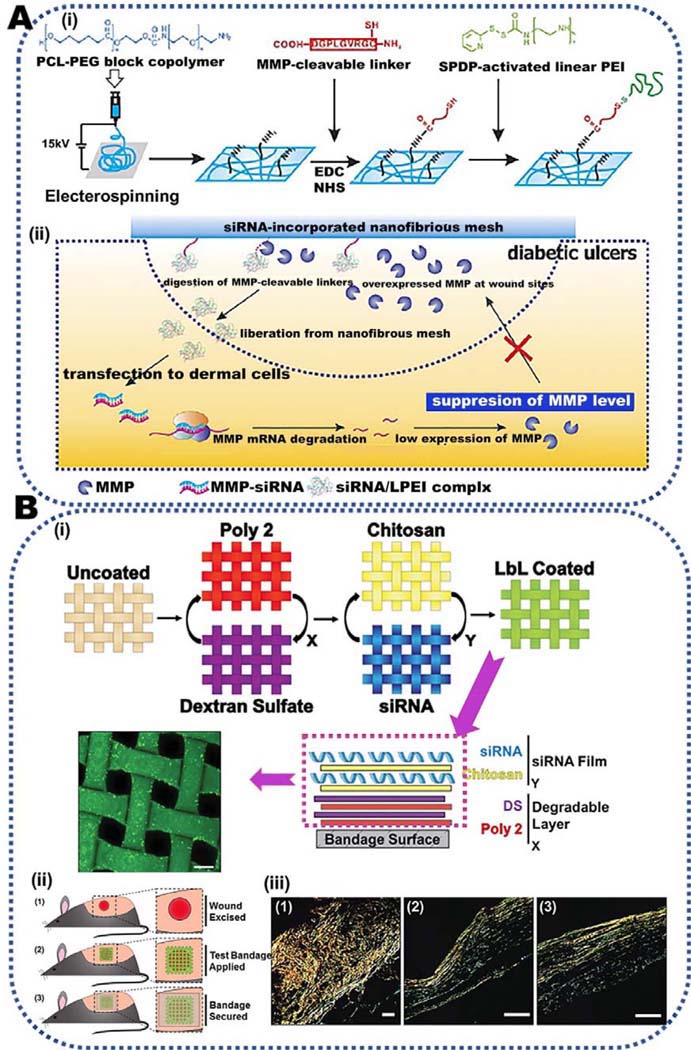
Another well-known strategy for the treatment of damaged wounds is modulation of immune responses via release of cytokines, protease inhibitors, growth factors, MicroRNAs (miRNAs) and small interfering RNA (siRNA). Since chronic wounds and scar formation result in extreme inflammation, monitoring immune reactions has become the current upward attention that can improve the immune-based treatments for chronic wounds and scar inhibition. Due to importance of this approach, it has been separately explained in the next section.
5. Immune regulation for chronic skin wound healing
Due to the effective role of immune cells in various stages of chronic wound healing, modulating immune response is a promising avenue to facilitate the wound healing process. Immune cell reprogramming has become the most common strategy to enhance, regulate, and/or suppress the immune responses.[102] This approach often includes macrophage polarization, promoting antigen-specific differentiation of T cells into T follicular helper cells, and encouraging DCs to express pro-inflammatory activation markers such as CD80 and CD86.[103]
Due to the importance of extreme inflammation in chronic wounds, macrophage attachment and control over their polarization to boost the inflammation resolution are considered to be the most common approach towards wound treatment (Figure 2). Various microenvironmental cues affecting macrophage polarization have recently been reviewed.[104] Macrophages shift the polarization status from pro-inflammatory to anti-inflammatory phenotype through the paracrine signaling mechanisms in response to different microenvironmental cues in the tissue, including cytokines, growth factors, and microorganism-associated molecular patterns. These signals direct a transcriptional response, which identities the phenotype and function of macrophages on the basis of the physiological or pathophysiological context. For instance, macrophages produce chemokine Interferon gamma (IFN-γ), IL-4 and IL-13 that support cell polarization. In addition, Bacterial lipopolysaccharide (LPS) stimulates the generation of pro-inflammatory macrophages.[104] LPS stimulates a strong pro-inflammatory phenotype in macrophages including the secretion of IFN-β and an antimicrobial components including nitric oxide.[105] While current data provide proof of concept that macrophages can experience dynamic transitions between different functional states, the stability of pro-inflammatory and anti-inflammatory phenotypes in a physiological condition requires further detailed investigation.[106]
Currently, controlling the pro-inflammatory / anti-inflammatory phenotype ratio during the wound healing is known to be an important parameter to modulate the inflammatory reactions and the switch from the inflammation to proliferation during the wound healing process. The treatment strategies that efficiently increase the initial inflammatory reactions and stimulate the macrophages’ polarization toward anti-reparative phenotype have extensively been investigated.[107] Recently, different hydrogel-based biomaterials have proven to control the immune responses through the enhancement, regulation, or inhabitation of immune responses. For example, hydrogels can be used to encapsulate various types of immune cells and prevent the cell death by delivering cytokines. They can eventually recruit host immune cells and induce a superior immune response. Specifically, the use of hydrogels is promising for immunotherapy of infections and autoimmune diseases.[108] In addition, hydrogels with interconnected pores can control the delivery of cytokines, adjuvants, and growth factors and efficiently enhance the immune cell responses.[109] Ability to release these bioactive molecules or cells locally down-regulates the immune responses via preventing from immune cell maturation, activation, and/or apoptosis to activate immune tolerance. Furthermore, hydrogels preserve the immune privileged characteristics of stem cells such as MSCs and can even promote their immunomodulatory ability.[110] Intrinsic physical and chemical properties of hydrogels are also known to modulate the immune responses. Accordingly, the immunomodulatory activity of hydrogels is explained in three main pathways namely hydrogels’ chemistry and properties and molecular-based approaches.
6. Immunomodulatory hydrogels for chronic skin wounds
6.1. Immunomodulation approaches based on hydrogel chemistry
In general, cells accept various signals from their microenvironment. For instance, biochemical signals (from their interfaces with other cells and ECM), biophysical signals (from externally applied mechanical and electrical forces) and different biomaterial mediated signals (from the cell-material interfaces).[111] Consequently, the average impact of these signals controls the cell behaviors. Therefore, selection of an appropriate immunomodulatory hydrogel is crucial in order to tune the anti-inflammatory / pro-inflammatory phenotype ratio that facilitates tissue remodeling. Since the last decades, various hydrogel materials have been developed for the immunomodulation and have shown to stimulate the inflammatory pathways. Sometimes these materials lack of any immunomodulatory signal. In this regard, different characteristics of biomaterials such as, degree of crosslinking, degradation rate in a physiological environment, mechanical properties, hydrophilicity, surface chemistry, energy and topography, size and shape, and the originality of hydrogel are important factors and need to be investigated.[112] Interestingly, various physicochemical characteristics of hydrogels are also shown to modulate the intensity and/or features of the immune responses. In addition, some of the biomaterials have identified to have danger-associated molecular patterns and pathogen-associated molecular patterns. For instance, human’s immune system usually responses to the cyclic patterns of hydrogel chains. The molecular features of polymers used to form the hydrogel can resemble the polysaccharide membranes of bacteria. Moreover, the specific class of micro and nanoparticles can possess distinctive dimensions of bacterial and viral pathogens.[113] In a recent study, DCs cultured on different naturally derived hydrogels were used for the differential expression of maturation markers such as CD40.[114] While alginate and agarose based hydrogel did not stimulate the DCs, treating these cells with chitosan supported the DCs maturation. Moreover, higher expression of pro-inflammatory markers including HLA-DQ, CD80, CD86, CD83 and CD44 and release of pro-inflammatory cytokines from the DCs were detected when cultured on chitosan-based hydrogel. According to another study by Cha et al.[115], interactions between biomaterials and macrophages mostly governed by the presence of integrin. While macrophages failed to recognize the cell attachment motifs on biomaterials, they were shown to produce pro-inflammatory markers. Whereas hydrogels consisting of specific attachment sites supported the expression of an anti-inflammatory like phenotype. Based on this behavior, naturally derived hydrogels formed by using high molecular weight polymers, such as HA and chitosan with ROS-scavenging characteristics, can effectively substitute the missing constituents of the ECM and reveal intrinsic anti-inflammatory characteristics.[116] In general, structural and biochemical diversity of HA macromolecules (e.g. having different high molecular weights) can significantly affect the immune cell responses. While high-molecular weight HA (High-MW HA) is immunosuppressive, low-molecular weight HA (Low-MW HA) enhances the inflammatory responses. Although all HA molecules consist of similar repeating disaccharide units, only Low-MW HA signals over Toll-like receptors (TLR2 or TLR4) and facilitates the pro-inflammatory effects through the interaction with these pattern recognition receptors (TLR2 or TLR4) (Figure 6A). Low-MW HA also acts as a pro-inflammatory danger-associated molecular patterns as it promotes the maturation of DCs. It also encourages the delivery of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12 by multiple cell types, and enhances the expression of chemokine and cell trafficking, and induce proliferation.[117] In contrary, high-MW HA hinders inflammation and reduces the secretion of inflammatory cytokines. This behavior is attributed to the interaction between high-MW HA and CD44. CD44 is the main HA-binding transmembrane glycoprotein in the cellular surfaces which translates the signals from the ECM. It affects cellular growth, activation, and differentiation as well as contributes immune homeostasis through the preservation of Th1 memory cells.[118] Meanwhile, crosslinking of HA also noticeably modulates the leukocyte function upon interaction with hyaladherins (HA-binding proteins).[119]
Figure 6. The immunomodulatory potential of hydrogels for chronic wound treatment:
A) Pro-inflammatory and anti-inflammatory responses of low-molecular weight (MW) hyaluronic acid (HA) and high-MW HA, respectively. While low-MW HA is a ligand of Toll-like receptor (TLR) signaling, high-MW HA negatively modulate pro-inflammatory TLR signaling at various steps. B) Photosensitive nanocomposite hydrogel based on HA for macrophage polarization: (i) Schematic for HA-based hydrogel fabrication using acrylated HA macromer (HA-AC) and alkoxylphenacyl-based polycarbonate (APP) nanocarrier encapsulated with Arg-Gly-Asp (RGD) peptide. After ultraviolet (UV) exposure, RGD covalently conjugated onto HA hydrogel to stimulate αvβ3 integrin macrophage expressions; (ii) conjugation kinetics of RGD peptide to HA-AC via various UV exposure times; and (iii) quantified αvβ3 integrin expression in nanocomposite hydrogel with increasing UV exposure time. Reproduced with permission from Ref.[120]. C) Controlled macrophage polarization in contact with GelMA and poly(ethylene glycol) diacrylate (PEGDA): proposed mechanism in which GelMA molecules could manage THP-1 cells into a pro-inflammatory or anti-inflammatory phenotypes. Reproduced with permission from Ref.[115]. D) Immunomodulatory properties of fibrin and fibrinogen hydrogel: (i) Schematic of experimental conditions evaluating the effect of fibrin and fibrinogen or mixture of these on macrophage activation, (ii) Tumor necrosis factor-α (TNF-α) and IL-10 secretion by macrophages which were stimulated using Lipopolysaccharides (LPS) and cytokine (IL-4/IL-13) and cultured on tissue culture plate (TCP, control) or 2 mg/ml fibrin gels, with and without 2 mg/ml fibrinogen (*p < 0.05). Reproduced with permission from Ref.[124].
In view of the significant contribution of high-MW HA in the wound skin treatment, it has been widely used to synthesize immunomodulatory hydrogel for the treatment of chronic wounds. This is recently reviewed by Zamboni et al.[119] In order to promote the dynamic macrophage immunomodulation, Wang et al.[120] have developed a photoresponsive nanocomposite hydrogel based on HA. In this study, photodegradative alkoxylphenacyl-based polycarbonate (APP) was conjugated to acrylated HA macromer (HA-AC) (Figure 6B(i)) to provide user-controlled RGD adhesive peptide release (Figure 6B(ii)). The conjugation of RGD peptide could activate αvβ3 integrin of macrophages (Figure 6B(iii)), leading to an increase in anti-inflammatory macrophage polarization.
Furthermore, collagen-based hydrogels have showed substantial influences on immune cells. For example, macrophage scavenger receptors can easily bind to the unprotected ligands on collagen and accelerate the conformation-specific effects. In addition, collagen-based hydrogels have shown to reduce the immunogenicity of the seeded allogeneic MSCs.[121] Therefore, denatured collagen (gelatin) has also been utilized to modulate cellular responses. For instance, monocytes cultured on GelMA hydrogels displayed lower inflammatory TNF-α gene expression after LPS stimulation, compared to the control (cells cultured on tissue culture plate).[122] It was also shown that GelMA “mops up” the released TNF-α by CD14+ monocytes. Therefore, it was hypothesized that TNF-α sequestration in GelMA decreased the accessibility of soluble TNF-α, which consequently resulted in prevention of TNF-α gene expression. Moreover, macrophages cultured on GelMA hydrogel showed greater gene expression of anti-inflammatory IL-10 and IL-1RA, lower inflammatory inducible iNOS and TNF-α compared to the cells cultured on PEG diacrylate (PEGDA) hydrogels.[115] This study concluded integrin α2β1 acted a crucial function in macrophage polarization (Figure 6C). Especially, IL-4 assimilated GelMA hydrogel could stimulate anti-inflammatory phenotype polymerization via integrin α2β1 attachment.
Recently, fibrin-based hydrogels are applied as another promising biomaterial for the skin wound healing. Fibrin and fibrinogen can be used as immunomodulatory components to control wound microenvironment. Fibrin acts as a temporary matrix and significantly promotes inflammatory and anti-inflammatory responses. Mostly, by the secretion of cytokines, fibrin increases initial inflammatory reactions and accelerates the evolution to the last proliferative and remodeling steps of wound healing process.[123] To evaluate the effect of fibrin versus fibrinogen, Hsieh et al.[124] have cultured macrophages on fibrin gels, stimulated with soluble fibrinogen, and investigated the cytokine secretion (Figure 6D(i)). According to Figure 6D(ii), fibrinogen stimulated both TNF-α and IL-10, while co-addition of IL-4/IL-13 or LPS/IL-4/IL-13 had no significant effects. In another word, macrophages cultured on fibrin revealed high levels of IL-10 and low levels of TNF-α regardless of further stimulation.
Recently, silk fibroin was also shown to promote the release of IL-10 from peripheral blood mononuclear cells (PBMCs).[125] Interestingly, silk fibroin possesses intrinsic ability to accelerate the wound healing via NF-κβ signaling. In view of this, extensive studies have been performed in various types of injures to manage the wound healing process using silk fibroin. This includes hypertrophic scars and diabetic burn where silk fibroin-based hydrogels triggered definite stages of the wound healing process.[126] Recently Chouhan et al.[127] have also demonstrated the treatment of third-degree burns or chronic wounds with an injectable silk fibroin hydrogel (Figure 7A). The designed hydrogel was observed to support the treatment of full-thickness wounds (Figure 7A(i)). Moreover, in vivo studies confirmed the effective role of silk fibrin-based hydrogel to accelerate the transition from inflammation to proliferation phase, according to the TNF-α expression. Noticeably, silk fibroin hydrogel significantly enhanced the collagen matrix remodeling as compared to the collagen hydrogel (Figure 7A(ii)). This study suggested the potential therapeutic impact of silk fibrin for the management of burn wounds.
Figure 7. Immunomodulation strategies based on hydrogel chemistry for chronic wound treatment:
A) In situ forming silk hydrogel for the treatment of a full-thickness burn wound: (i) Schematic showing the formation of silk hydrogel for wound healing; (ii) immunohistochemistry (IHC) of collagen type I in the wounds, representing scattering of collagen fibers in the regenerated tissues treated with SF and Col hydrogels. Reproduced with permission from Ref.[127]. B) Fabrication of immunomodulatory nanocomposite hydrogels: (i) Schematic showing the process of CS–MQD hydrogel synthesis; (ii) photographs presenting the self-healing of chitosan (CS)–MQD hydrogels. Reproduced with permission from Ref.[134]. C) Silver ions crosslinked chitosan hydrogel, loaded with Fibroblast growth factor (bFGF@CS–Ag) hydrogel for wound treatment: (i) Schematic showing the microscopic healing progression after bFGF@CS–Ag injection; (ii) representative micrographs of the control, bFGF, CS–Ag and bFGF@CS–Ag treated groups on day 7. Reproduced with permission from Ref.[135].
Chitosan is another attractive natural polysaccharide utilized for wound healing. This is related to its noticeable properties including biocompatibility, controllable degradation rate, bioactivity, non-antigenicity, bio-adhesiveness and antimicrobial property along with its significant hemostatic capacity.[128] Chitosan stimulates granulation of injured tissue and promotes wound healing via incorporation of inflammatory cells including leukocytes and macrophages to the wound site.[129] Recently, Shibata et al.[130] have showed that the chitin-based oligosaccharides, such as chitosan stimulated the expression of TNF-α and IL-12 in macrophages and exhibited a predominant pro-inflammatory phenotype response. Mechanism of chitosan-induced macrophage stimulation possesses mannose receptor-mediated phagocytosis. This mannose receptor favorably controls the macrophages and enhances the interaction with appropriate ligands including chitosan.[131] Chitosan-based hydrogels, synthesized with chemically modified chitosan molecules or additional components, were also shown to contribute in controlling inflammation and accelerating the wound closure process. For instance, Moura et al.[132] have used 5-methyl pyrrolidinone functionalized chitosan backbone for the treatment of diabetic wound, which accelerated the wound healing due to the decrease in the TNF-α levels, inflammatory cells, and MMP-9. Similarly, Ashouri et al.[133] have reported that the combination of chitosan with aloe vera could modulated pro-inflammatory and anti-inflammatory reactions, resulting in rapid wound healing. In this study, aloe vera-chitosan complexes led to an optimum wound repair by declining the expression of pro-inflammatory phenotype after 3 days and increasing anti-inflammatory phenotype expression after 14 days. In another study, immunomodulatory properties of chitosan was improved via incorporation of zero-dimensional auto-fluorescent Ti3C2 MXene quantum dots (MQDs).[134] Here, Ti3C2 MQDs was synthesized via hydrothermal process and consequently incorporated in chitosan-based hydrogel system (Figure 7B(i)). Along with the biocompatibility of the material, Ti3C2 MQDs reduced the proliferation of pro-inflammatory T cells. It also synchronously enhanced the CD4+ CD25+ FOXP3+ regulatory T-cell percentage. Apart from the immunomodulatory property of the hydrogel matrix, introduction of Ti3C2 MQDs enabled self-healing characteristics, which are critical for injectable scaffolds (Figure 7B(ii)). Meanwhile, silver ions crosslinked chitosan hydrogel, loaded with FGF (named bFGF@CS–Ag) was designed for infected wound treatment (Figure 7C(i)).[135] Following the application of the hydrogel, enhanced wound healing was obtained, which was due to the bacterial inhibition, improvement of collagen deposition, and neovascularization (Figure 7C(ii)). Moreover, introduction of bFGF@CS–Ag enhanced anti-inflammatory phenotype polarization, leading to reduced inflammatory reactions. Interestingly, incorporation of silver ions in bFGF@CS hydrogel endorsed controlled release of bFGF.
Among natural hydrogels, decellularized ECM, isolated from different tissues including porcine intestinal submucosa[136] and urinary bladder and brain-derived ECM[137], also showed significant immunomodulatory properties.[138] Initial study by Badylak et al.[136] have revealed that the decellularized ECM from urinary bladder matrix promoted anti-inflammatory phenotype polarization and decreased the fibrotic responses as compared to the cellular autografts. Recently, Brown et al.[139] have also found similar results via the autologous cell delivery in decellularized allografts. In addition, decellularized and dehydrated human amniotic membrane (DDHAM) was observed to enhance the wound perseverance through the secretion of numerous growth factors, cytokines, and proteases, consisting of PDGFs, TGF-a, FGF-2, EGF, IL-4, IL-10, and tissue inhibitors of metalloproteinases (TIMPs).[140] Therefore, in recent years, extensive research has been performed to explore the application of decellularized human amniotic membrane and umbilical cord (AM/UC) for the treatment of chronic wounds.[141] These grafts are Epifix, originated from human amnion matrix, and Neox, originated from human AM/UC tissue.[142] Interestingly, amniotic membranes were not rejected by the immune system owing to human leukocyte antigen (HLA) expression.[143]
Chemical crosslinking of hydrogels was also observed to modulate chronic foreign body reactions.[144] Generally, high degree of crosslinking influences inflammatory macrophage responses. For instance, crosslinking of collagen with 1-ethyl-3-(3 dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide (EDC/NHS) provided the lowest expression of inflammatory cytokine including TNF-α and C-C Motif Chemokine Ligand 22 (CCL22), compared to other types of crosslinked collagen.[145] In addition, glutaraldehyde crosslinked collagen has shown to reduce the anti-inflammatory / pro-inflammatory phenotype ratio and upregulate the proinflammatory cytokines and foreign body responses. [146] Moreover, crosslinking of decellularized pericardium with EDC resulted in reduced MMP-2 and MMP-9 secretion, whereas nominally altered the cytokine secretion by macrophage-like cells.[147] Cellular components, which may remain as a result of inadequate decellularization of naturally derived materials, can lead to harmful immune responses and scar formation.[148] Consequently, detailed investigation on the mechanisms by which these hydrogels modulating the inflammatory reactions is necessary.
In addition to the common natural polymers, various other biological ingredients were also found to provide excellent immunomodulatory property, such as Fucoidans and Carrageenan, two marine polysaccharides extracted from brown and red seaweeds, respectively. While Fucoidans show antiviral, anticoagulant and anti-inflammatory properties, [149] Carrageenan is known to stimulate IL-10 expression, prohibit cytotoxic T cell responses, and delay neutrophil activation.[150] Recently, Amin et al.[151] have found that both photopolymerizable carrageenan and fucoidan downregulated molecular response for adversative immune reactions. These polysaccharides significantly reduced nitric oxide production and established about 90% ROS scavenging, making them an alternative to IL-10 for immunomodulatory strategies.
Meanwhile, amino acid-based hydrogels have also demonstrated promising advancement towards their application in immune system modulation. Among various others, L-arginine (Arg) is one of the essential amino acids in the wound area that gets metabolized by nitric oxide synthase (NOS) to nitric oxide.[152] Therefore, decrease in the Arg concentration suppresses nitric oxide level and postpones the wound healing progression.[153] Besides, Arg also enhances fibroblast proliferation.[154] However, an elevated NO level decreases the myofibroblasts by hindering the replication which eventually decreases the collagen I expression of fibroblasts and myofibroblasts.[155] Recently, He et al. [156] have designed a new type of L-nitroarginine-based polyester amide (L-nitroarginine (NOArg)-Arg PEA) for affording a modulatory prevention of macrophage’s NOS pathway. Interestingly, in the presence of activated macrophages, the synthesized complexes decreased the nitric oxide production, while did not show any meaningful effect on TNF-α secretion. Application of the material over diabetic rat wounds cured the wound faster (40%–80%) as compared to control (Pluronic F-127 gel). Such significant impact on the wound healing made L-nitroarginine (NOArg)-Arg PEA hydrogel a favorable candidate for the chronic wound treatment. Recently, same research group [157] has described the development of a hybrid hydrogel based on unsaturated arginine-poly(ester amide) with glycidyl methacrylate chitosan. As-synthesized hybrid hydrogels stimulate the macrophages to express both TNF-α and NO, making it an appropriate wound healing accelerator. Similarly, Zhu et al.[158] have designed an antioxidant and shape-conforming dressing material based on 12-amino acid sequence in the α5 globular domain of laminin, named A5G81, for the treatment of diabetic wounds. A5G81 was encapsulated in a thermos-responsive hydrogel, which accelerated dermal and epidermal cell proliferation. It was also shown that the engineered biomaterials accelerated the tissue regeneration in diabetic wounds.
6.2. Immunomodulation approaches based on surface property of hydrogels
Immune responses, including macrophage polarization can also be controlled by tuning physio-chemical properties of hydrogels’ surfaces including topography, porosity, and hydrophilicity. Mechanistically, surface chemistry of the hydrogels plays the most important role in controlling immune responses. Although the surface topography of biomaterials is also known to be an essential parameter to control immune responses specifically macrophage polarization,[159] detailed mechanistic studies on these immunomodulatory responses have not been explored yet. In a recent study, the role of GelMA hydrogel with various micro topographies (micropatterns, micropillars and microgrooves) on the human macrophage functions was investigated based on the evaluation of conventional markers and the profile expression of genes (Figure 8A(i, ii)).[160] Induction of definite gene expression profiles in macrophages, seeded on the microgrooves and micropillars, was confirmed by the genes associated with the initial metabolic activities (Figure 8A(iii)). Nevertheless, the usual phenotyping approaches, related to the expression of surface markers, were identical between the diverse settings. In view of the role of surface chemistry on immune responses, in a recent study, it was found that neutrally charged hydrophilic surfaces showed less macrophage attachment as compared to hydrophobic and ionic surfaces. The attached macrophages on the hydrophobic surfaces showed higher secretion of cytokines than those attached to hydrophilic surfaces.[161] Meanwhile, PEG hydrogels are one of the most popular biomaterials due to the considerable capacity to change their compliance according to their molecular weight and crosslinking density. In a recent study, it was shown that although PEG/zwitterion phosphorylcholine (PC) hydrogels could stimulate chronic inflammation. Hydrophilic and zwitterionic nature also helped to regulate the immune cell adhesion.[162] In this study, a PEG-based hydrogel was functionalized with PC to develop a range of materials with different mechanical stiffness and hydrophilicity (Figure 8B(i)). Reducing the modulus (from 165 kPa to 3 kPa) and zwitterion PC content (from 20 wt% to 0 wt%) of the hydrogel eventually decrease the foreign body responses (Figure 8B(ii-iv)). Additionally, macrophages are also well known to change their surface protein expression according to the chemical perturbations applied through the surface composition of biomaterials.[163]
Figure 8. Immunomodulation strategies based on surface properties of hydrogels for chronic wound treatment:
A) Role of surface topography of various gelatin methacryloyl (GelMA) hydrogels on the immune cell behavior: (i) representative phase contrast images of macrophages on various patterned GelMA hydrogel and tissue culture plate (TCP) (G: microgrooves, P: micropillars and U: unpatterned) after 3 days (Scale bar=100 μm); (ii) cytokine secretion by macrophages on micropatterned GelMA; and (iii) heatmap of top 10 considerably changed genes from the microarray data. Reproduced with permission from Ref.[160]. B) The foreign body responses to polyethylene glycol (PEG) hydrogels with different mechanical stiffness and zwitterionic property: (i) A schematic showing the synthesis of zwitterionic hydrogel based on PEG diacrylate (PEGDA) and zwitterionic 2-methacryloxyloxethyl phosphorycholine (PC) group; (ii) the number of macrophages attached on the hydrogels; and release of (iii) Interleukin (IL)-10 and (iv) Tumor necrosis factor-α (TNF-α) from macrophages cultured on hydrogels with various stimulation factors (NS: no stimulation, LPS/IFN-γ: 1ng/mL Lipopolysaccharides (LPS) and IFN-γ, IL4/IL13: 20 ng/mL IL4 and IL13, LPS/IL4/IL13: 0.5ng/mL LPS and 20ng/mL IL4 and IL13). Reproduced with permission from Ref.[162].
In general, only a few types of hydrogels were observed to show intrinsic immunomodulatory property. Surface properties of hydrogel including surface topography and chemistry of hydrogels have also shown minimal effects on immunomodulation. Therefore, additional components or cells has been introduced inside the hydrogel materials as an effective strategy towards improvement their immunomodulatory performance.
6.3. Immunomodulation approaches based on delivery of stem cells
Immunomodulatory biomaterials are shown to reduce scar formation by delivering MSCs or ASCs to the wound area. MSCs have broad immunoregulatory capabilities, moderating both adaptive and innate immunity to provide therapeutic assistance through paracrine mechanisms.[164] MSCs secrete bioactive molecules including anti-inflammatory cytokines, chemokines, growth factors, hormones and extracellular vesicles (EVs, nanoparticles which usually comprise nucleic acids, lipids and proteins) with anti-apoptotic, immunomodulatory, and angiogenic functions.[165] For instance, soluble factors in MSC-conditioned media contain Prostaglandin E2 (PGE2), which prohibits pro-inflammatory cytokine release and enhances the secretion of anti-inflammatory cytokines and TGF-β1 release. In addition, PGE2 decreases the proliferation of T cells in the wound area. It is also known as a co-factor for the transition of Th1 to Th2 cells, supporting inflammation responses and promoting tissue regeneration.[166] These immunomodulatory properties of MSCs further demonstrate their utilization as an immunoregulatory tool for the autoimmune diseases and chronic wound treatment.[165a, 167] Recently, Blázquez et al.[168] have employed the immunomodulatory properties of MSCs to develop MSCs coated meshes similar to an ‘off the shelf’ product. The synthesized meshes showed limited immunomodulation of macrophages towards anti-inflammatory phenotype. Moreover, co-culture of MSCs and macrophages induced macrophages to turn out to be a regulatory phenotype. This was experimentally identified by a decrease in TNF-α and IL-12 levels along with an enhanced expression of IL-10.[166]
Ongoing studies revealed that the restorative prospective of implanted MSCs raise significant concerns related to the adhesion of microorganisms such as S. aureus and colony-forming capabilities of planktonic or biofilms from S. aureus.[169] In contrary, encapsulation of MSCs in a suitable hydrogel can meaningfully hinder the colony-forming abilities of planktonic S. aureus while preserving MSC multi-potency. Recently, a self-healing and shear-thinning hydrogel supplemented with adipose-derived mesenchymal stem cells exosomes (AMSCs-exo) was introduced for the treatment of damaged skin.[170] The hydrogel was chemically synthesized via a Schiff-based reaction between oxidative HA (OHA), and Poly-ε-L-lysine (EPL) in presence of Pluronic F127 (F127/OHA-EPL) (Figure 9A(i)). Easy injectability of the AMSCs-exo loaded hybrid hydrogel noticeably promoted the healing of diabetic full-thickness cutaneous wound (Figure 9A(ii, iii)). Similarly, another injectable hydrogel was synthesized via the interaction between hyper-branched multi-acrylated poly(ethylene glycol) macromers (HP-PEGs) and thiolated HA (HA-SH) for the effective adipose-derived stem cells (ADSCs) therapy.[171] The hydrogels displayed excellent mechanical characteristics and anti-fouling properties. Moreover, ADSCs loaded hydrogel revealed favorable regenerative capabilities specifically for the treatment of diabetic wounds.
Figure 9. Immunomodulation strategies based on stem cell delivery using hydrogels for chronic wound treatment:
A) Development of an injectable and thermal-responsive hydrogel based on Schiff based interaction between Poly-ε-L-lysine (EPL) and oxidative HA (OHA): (i) Schematic representing the synthesis process of the hybrid hydrogel; (ii) photographs showing the injectability of the hydrogel via the catheter; (iii) collagen type I expression in wounds preserved with adipose-derived mesenchymal stem cells exosomes (AMSCs-exo) loaded hybrid hydrogel (FHE@exo) compared to control. Reproduced with permission from Ref. [170]. B) Tethering of Interferon (IFN)–γ (cys–IFN–γ) onto the 4-armed polyethylene glycol macromere (PEG-4MAL) hydrogel as a carrier for human mesenchymal stem cells (hMSCs) to control immunomodulatory functions: (i) Schematic representation of tethering of cys–IFN–γ onto PEG-4MAL hydrogels. Cytokine functionalized with adhesive ligands, hMSC, and degradable crosslinker; (ii) encapsulation of hMSCs in cys–IFN–γ modified hydrogels promoted colonic wound treatment in an immunocompetent mouse. Reproduced with permission from Ref.[172]. C) HA based hydrogel for delivering adipose stem cells (ASCs) to promote regeneration of burn injury: (i) Schematic illustration of the ASCs loaded in the hydrogel made of hyper-branched poly(ethylene glycol) diacrylate (HB-PEGDA), thiolated HA (HA-SH) and RGD peptides; ii) schematic representing the role of RGD on the cellular function in the hydrogel environment; (iii) Haemotoxylin and Eosin (H&E) stained tissues after the treatment with ASC loaded PEG-HA-RGD hydrogel, showing its effective role on angiogenesis and re-epithelialization (scale bars ∼100 μm). Reproduced with permission from Ref.[173].
Recently, hydrogel-based co-delivery of cells and growth factors/cytokines has become a promising approach for the treatment of various types of chronic diseases and injures. For example, to overcome the challenges related to the stem cell delivery for the treatment of inflammatory bowel disorders, Garcia et al.[172] have designed an injectable hydrogel to promote immunomodulatory functions (Figure 9B(i)). This hydrogel was prepared from maleimide-functionalized 4-armed PEG macromer (PEG-4MAL) tethered with recombinant IFN-γ and hMSCs. Interestingly, the hybrid hydrogel exhibited enhanced cytokine secretion and accelerates the colonic mucosal wound healing in immunocompromised and immunocompetnent mice (Figure 9B(ii)). Such designed platform is considered to have significant potential towards the clinical translation and efficacy of hMSC-based therapies. In another recent study, ADSCs were loaded into a hydrogel made of HB-PEGDA, HA-SH, and RGD peptides (Figure 9C(i)).[173]. Incorporation of RGD peptide noticeably altered cellular function (Figure 9C(ii)) and enhanced the paracrine activity of angiogenesis. Besides, treatment with cell-laden hydrogels has improved the neovascularization, enhanced wound closure, and decreased scar formation (Figure 9C(iii)).
6.4. Immunomodulation approaches based on delivery of biological molecules
Recently, different molecular approaches are also adopted to control the immune reactions. In most of these cases, treatment of chronic wounds was achieved based on controlling the cytokine levels, which eventually modulate inflammation or promote the healing process. Similar to the hydrogel-based strategies described above, molecular approaches are often aimed to interfere with the inflammatory outcome of TNF-α[174] or stimulate the IL-10 release.[175] Herein, we have reviewed a range of controlled release strategies that influence different pathways of wound healing process, from inflammation to healing. This includes release of various kinds of bioactive components such as cytokines, growth factors, gene, antibodies and others. These strategies along with their outcomes are summarized in Table 3.
Table 3.
Various immunomodulation strategies based on the controlled release of biological molecules from hydrogels for the treatment of chronic wounds
| Biological molecules | Type | Composition | Results | Reference |
|---|---|---|---|---|
| Cytokines | IL-10 | Dextrin nanogel | Slow release of IL-10 reduced TNF-α synthesis and down-regulated class II major histocompatibility complex molecules expression on macrophages | [181a] |
| IL-10 | Collagen– silica hydrogel including PEI-DNA complexes | Decreased TNF-α and IL-1β gene expression confirmed successful inhibition of inflammation. | [181d] | |
| IL-10 | Nanocomposite hydrogel based on plasmid DNA-PEI-SiNP, collagen | Controlled release of IL-10 resulted in downregulation of TNF-α expression | [181e] | |
| IL-2 | An injectable hydrogel based on collagen, HA, and heparin | Sustained release of IL-2 in a two-week period decreased CD4+ and CD8+ T cells and increased FoxP3+ Treg in lymph nodes of mice. Increase immune tolerance in autoimmune diabetes | [249] | |
| SDF-1α | liposomes | Improve the granulation tissue formation and accelerate diabetic wound healing | [250] | |
| SDF-1α | liposome/GelMA | Effectively induce MSC migration. Controlled release of SDF-1α modulated intracellular cell signaling pathways in MSCs. | [251] | |
| IL-4 and MCP-1 | Nanofibrous peptide hydrogel | Controlled release of IL-4 and MCP-1 led to spatiotemporal activation of THP-1 macrophages and improvement of proresolution M2 | [183] | |
| Antibody | anti-TNF-α antibody | Chitosan | Slow release of anti-TNF-α antibody accelerated chronic wound treatment | [189a] |
| anti-TNF-α antibody | Shear-thinned glycosyl–nucleoside–lipid amphiphiles | Release of entrapped anti-TNF-α accelerated autoimmune disease treatment | [189b] | |
| anti-TNF-α antibody | HA hydrogel | Controlled release of anti-TNF-α antibody reduced inflammation in burns. | [189c] | |
| Anti-inflammatory factor | Resolvin D1 | Chitosan | Release of resolvin D1 decreased M1 cells and increased in M2 cells. Decrease pro-inflammatory cytokines expression leading to less inflammatory cells in implant sites. | [191] |
| Catechol | Chitosan and oxidized HA | Support hBMSCs against oxidative stress damages induced by ROS | [194] | |
| GM-CSF + BDC peptide | Alginate-Au NPs hydrogel | Induce Treg response via delivering peptide antigen to dendritic cells (DCs). The hydrogel was proposed for the treatment of autoimmune diseases such as type 1 diabetes mouse model. | [108] | |
| Peptides | L-12 peptides | Crosslinked polyanionic DNA nanostructure | Significant antimicrobial properties against S. aureus infections and noticeable anti-inflammatory response to accelerate the healing rates | [203] |
| LL-37 peptides | Chitosan | Significant antibacterial properties. Inhibit TNF-α release from macrophage, improve the density of newly-formed capillary with naked LL-37 and promote the expression of key macromolecules in the angiogenesis process. | [204] | |
| Pep4, Pep4M | HA hydrogel | No need for whole elastase inhibition in normal wound healing process | [252] | |
| Gene | PDRN a | An assembling nanofiber gel | Improve the new vessel formation and treatment of diabetic foot ulcers in a mouse model. | [253] |
| siRNA | LPEI hydrogel | Responsible for a high concentration of MMPs in diabetic ulcers leading to accelerated remodeling at injured site. | [212] | |
| siRNA | Star-shaped hydrogel | Controlled release of siRNA reduced the expression of MMP-9 and accelerated diabetic wound healing. | [254] | |
| siRNA | CD-(D3)7 a star-shaped hydrogel | Control the siRNA release from hydrogel leading to decreased expression of MMP-9 to accelerate diabetic wound healing. | [255] | |
| siRNA | A pH-sensitive nanogel | Local release of siRNA induced gene knockdown | [209a] | |
| miR-29b | Collagen | Regulate ECM remodeling and wound contraction in vivo | [256] | |
| Cell + miRNA delivery | Supramolecular hydrogel | Control miRNA release | [257] | |
| miRNA-132 | Neutral lipid emulsion mixed with pluronic F-127 gel | Improve re-epithelialization in damaged tissue | [223] | |
| miR-223* | GelMA- HA nanoparticles | Improve the expression of anti-inflammatory gene Arg-1 and decrease the proinflammatory markers | [74b] | |
| miR-302a and miR-155 | Light-activatable miRNA-loaded plasmonic gold nanocarriers | A sequentially release of miRNAs, using a NIR laser. Regulation of gene expression. | [258] | |
| Cytokine + growth factor | TGF-β1 and IL-10 | Functionalized PEG | Control dendritic cell maturation via reduced expression of IL-12 and MHCII. | [181b] |
| Cytokine +drug | IL-4, Dexamethasone | Self-assembled and injectable hydrogel | Successful anti-inflammatory macrophage polarization. Appropriate for the treatment of type 1 diabetes | [259] |
| Antibody +growth factor | anti-TNF-ɑ and HGF | Self-assembling peptide/heparin hydrogel | Effectively improve the tissue repairing process | [190] |
| Anti-inflammatory factor+ cytokine | FTY720+SDF-1α | Hep−N-functionalized PEG-DA hydrogel | Increase the recruitment of anti-inflammatory monocytes, improve early accumulation of the differentiated wound healing CD206+ macrophages, promote the vascularization. | [196] |
: A linear DNA sequence from human placenta, which can efficiently stimulate the adenosine A2A receptor
6.4.1. Controlled release of cytokines
Among various bioactive components, genes encoding growth factors and cytokines are the most suitable candidates to promote wound closure. Therefore, use of cytokines for the therapeutic development has become a novel strategy due to their crucial role during various stages of wound healing.[176] The most important and efficient cytokine-based strategy, which has recently been clinically applied for decreasing scar formation, is based on TGF-β3, M6P and IL-10 (trade name Prevascar), and nefopam (trade mane ScarX).[177] Meanwhile, among various cytokine-based strategies, manipulation of the local IL-10 concentration has been extensively used in chronic wound healing. This is due to the significant effect of IL-10 on various types of immune cells. IL-10 also simultaneously encourages Tregs and acts as an anti-inflammatory and stimulates macrophage polarization.[178] In addition, IL-10 has shown immunostimulatory effects on various cell types (e.g. T and B cells, and mast cells) and drives immunosuppressive role on a broad range of monocytes/macrophages.[179] Moreover, IL-10 inhibits the synthesis of various cytokines (e.g. IL-1, IL-2, IL-3, IL-6, IL-8, IL-12, TNF, and IFN) and downregulates CD86 expression, resulting in inhibition of antigen presentation.[180] Based on these durable immunoregulatory properties of IL-10, recent studies have showed its suppressor activity in controlling immune responses. Therefore, recently IL-10 has been proposed for clinical applications alone or in the combination with other types of biological macromolecules to modulate immune responses.[181] In addition to IL-10, other cytokines, such as IL-4, have also been investigated to develop immunomodulation therapies. It has been shown that IL-4 stimulates pro-inflammatory to pro-reparative polarization and promotes anti-inflammatory cell proliferation.[182] Kumar et al.[183] have developed a self-assembling peptide/heparin (SAP/Hep) hydrogel that provide a biphasic pattern of cytokine release of both IL-4 and monocyte chemotactic protein (MCP-1) (Figure 10A(i)). MCP-1 is a chemokine that releases from activate monocytes and plays an important role for pro-inflammatory stimuli by producing ROS/reactive nitrogen species (RNS). In this study, the active interactions between the fibrous hydrogel and macrophages (Figure 10A(ii)), and the biphasic pattern of cytokines resulted in activation of THP-1 monocytes and macrophages and enhanced anti-inflammatory phenotype polarization.
Figure 10. Immunomodulation strategies based on release of cytokines and antibody components.
A) Synthesis of a multi-domain peptide for immunomodulation: (i) Engineering and (ii) immunologic assessment of multi-domain peptides. Reproduced with permission from Ref. [183]. B) Development of immunomodulatory hydrogel based on KLD2R/heparin (Hep) γhydrogel for dual-drug delivery of anti- Tumor necrosis factor-α(anti-TNF-α) and Hepatocyte growth factor (HGF): (i) Schematic illustration of hydrogel for co-delivery of KLD2R/Hep. The hydrogel was formed based on the electrostatic interaction between the cationic KLD2R peptide and anionic Hep. Heparin also showed great affinity to HGF; and (ii) representative images of CD68 staining, with and without release of anti-TNF-α and HGF. Reproduced with permission from Ref.[190]. C) Low molecular weight glycosylnucleoside-lipid (GNL) amphiphiles for sustained release of TNF-α: (i) transmission electron microcopy (TEM) image of oleoylamide GNL nanofibrous structure, (ii) schematic of the mechanical shear stimulation for controlled release of anti-TNFα. The captured macromolecules (“Y”) diffused out into the water because of GNL hydrogel formation (blue lines); and (iii) the anti-human TNF-α IgG release from GNL which gets enhanced after mechanical shear stimulation using 10 Pa shear stress. Reproduced with permission from Ref.[189b].
Furthermore, in situ gelation of hydrogel provides capability to preserve cytokines, which was released upon different cellular immune responses. In addition to the above mentioned clinically acceptable cytokines, other potent cytokines, such as IL-1β and stromal cell derived factor-1α (SDF-1α), are still under investigation for the chronic wound treatment. For instance, IL-1β is a segment of a pro-inflammatory positive response circle, which withstands a persistent pro-inflammatory wound macrophage phenotype, contributing to the healing of diabetic wounds. A recent study showed a substantial enhancement in wound closure in a diabetic mice model by using an antibody that hinders IL-1β.[184]
6.4.2. Controlled release of antibodies
The main goal towards the controlled release of antibodies is to interfere with TNF-α. It is well-demonstrated that in diabetic diseases having high glucose environment supports pro-inflammatory phenotype macrophages. Pro-inflammatory phenotype produces noticeable amount of TNF-α which is harmful for keratinocyte migration.[185] Therefore, neutralization of TNF-α at the injured site can considerably stimulate the chronic wound healing by reducing the inflammatory cell infiltration and suppressing pro-inflammatory macrophage activation.[186] A detailed study in a mouse model showed excessive inflammation upon anti-TNF-α antibody injection, which altered the pro-inflammatory to anti-inflammatory phenotype balance and switched the macrophage phenotype to anti-inflammatory phenotype.[187] In this regards, different healing formulations has been developed and are commercially available to prohibit the TNF-α release, including infliximab, and golimumab which have been reviewed elsewhere.[188] However, anti-TNF-α neutralizing antibody injection may also have harmful side effects. According to our knowledge, limited studies have been focused on the controlled release of anti-TNF-α.[189] Moreover, such anti-TNF-α controlled release systems were commonly studied in the environment of inflammatory diseases and were not assessed in modulating macrophage pro-inflammatory / anti-reparative polarization balance. In a recent study, a self-assembled KLD2R peptide/heparin hydrogel was developed for co-delivery of anti-TNF-ɑ and Hepatocyte growth factor (HGF) with desirable release kinetics for the treatment of Ischemia-reperfusion (I/R)-induced organ injury (Figure 10B(i)).[190] This biomaterial was designed to accomplish fast release of anti-TNF-ɑ along with slow release of HGF. The designed nanoscale delivery platform successfully promoted the tissue repair via directing inflammation in mice with renal I/R (Figure 10B(ii)). In another study, Kaplan et al.[189b] have synthesized low molecular weight glycosylnucleoside-lipid (GNL) amphiphiles nanofibers (Figure 10C(i)) that showed excellent shear-thinning properties. By using the mechanical shear stimulation, engineered hydrogels control the release of anti-TNFα for the treatment of autoimmune diseases (Figure 10C(ii, iii)). However, the efficient role of anti-TNF-α on the immune cell behavior was not investigated in detail. Although significant efforts have been made in the recent year toward the development of antibody delivery systems for immunomodulation, there is still need of further improvement and study for the clinical applications.
6.4.3. Controlled release of anti-inflammatory molecules
In addition to numerous biological components, several immunomodulatory molecules based on small molecules including resolvin D1, lipoxin A4 (pro-resolution lipid mediators),[191] catechol,[192] and Res[193] have been reported for wound healing. These molecules affect various stages of wound healing process including the inflammatory reactions via anti-inflammatory phenotype polarization, enhancement in the antigen-specific CD4+T cells and downregulation of the IL-1β. For instance, in a study, catechol, a crucial molecule which is responsible for mussel adhesion, was used to develop an IPN hydrogels. In this case, along with the self-covalent crosslinking between chitosan and oxidized HA, hydrogel material was further secondarily crosslinked with Fe+3 ions (Figure 11A(i)).[194] In addition to the strong adhesion to the wet tissues (Figure 11A(ii)), the engineered hybrid hydrogel also down-regulated the pro-inflammatory cytokine (e.g. IL-1β) release (Figure 11A(iii)). Moreover, in vivo experiments showed fast vascularization upon application of the IPN hydrogels.[194] Combination of IPN hydrogels with growth factors was also used to control the immune responses. For example, co-delivery of granulocyte-macrophage colony-stimulating factor (GM-CSF) and BDC peptide using a pore-forming injectable gel was investigated by Verbeke et al.[108] BDC peptide is a peptide antigen mimotope which can be detected by T cells in a mouse model of type 1 diabetes.[195] In this study, an alginate-based hydrogel was used for the encapsulation of GM-CSF conjugated AuNPs and peptide-encapsulated PLGA (Figure 11B(i)). Subcutaneous administration of the hybrid hydrogel resulted in a concentrated antigen-specific T cell expression in the lymph nodes. Consequently, antigen-specific CD4+ T cells enhanced the cognate antigen accumulation (Figure 11B(ii)) and expressed markers of Tregs, leading to a decrease in the acceleration of disease progression in NOD mice.[108] However, co-delivery of various agents with different hydrophobic and hydrophilic properties is not feasible by using a single hydrogel network.
Figure 11. Immunomodulation strategies based on the release of inflammatory components:
A) Catechol based hydrogels for chronic wound healing: (i) Scheme of the interpenetrating network (IPN) formation based on chitosan (Ch) and oxidized HA (HAox), crosslinked using terpolymer (T) and metal ion (Fe3+) ligand complexation of the catechol groups; (ii) IPN hydrogel was attached to the porcine skin for the lap shear test; (iii) Interleukin (IL)-1β expression of the H2O2 treated (positive control), hydrogel treated, and non-treated (negative control) samples measured using ELISA kit ((*p < 0.05) and (#p < 0.05)). Reproduced with permission from Ref.[194]. B) Development of immunomodulatory hydrogel based on alginate: (i) schematic representing of a matrix metalloproteinases (MMPs)-cleavable BDC peptide conjugated alginate for controlled release of granulocyte-macrophage colony-stimulating factor (GM-CSF) and BDC peptide; and (ii) delivery of peptide-loaded alginate led to peptide presentation of tetramer+ CD4+T cells. Proliferation of T cells after interaction with BDC peptide or BDC peptide-loaded alginate, following co-culture with bone marrow-derived dendritic cells (BMDCs). Reproduced with permission from Ref.[108]. C) Development of immunomodulatory hydrogel based on poly(ethylene glycol) (PEG): (i) schematic illustration of the synthesis process of Hep−N-PEGDA hydrogel for the release of SDF-1α and FTY720; and (ii) confocal images of the hydrogel implant, showing that tissue around stromal cell derived factor-1α (SDF-1α) hydrogel included rounded CX3CR1-GFP+ cells neighboring with the vasculature, while tissue around the SDF-1α + FTY720 hydrogel revealed an elongated morphology. Reproduced with permission from Ref.[196].
To overcome the issues of co-delivery of both hydrophilic and hydrophobic molecules, a dual affinity hydrogel was developed using PEGDA, which gain the growth factor affinity of a heparin derivative (Hep−N) and lipid chaperone activity of albumin (Figure 11C(i)).[196] Two bioactive molecules, SDF-1α and sphingosine-1-phosphate receptor 3 (S1PR3), were effectively encapsulated and released from the functionalized PEGDA hydrogels, while preserving their functionality. Injection of the hydrogel into a skin wound injury exhibited accelerated macrophage polarization and neovascularization, which promoted rapid tissue repair (Figure 11C(ii)).
6.4.4. Controlled release of Antimicrobial peptides
Antimicrobial peptides (AMPs) are another group of molecules which could easily modulate to immune system. AMPs are natural or synthetic polypeptide molecules with amphipathic structure.[197] These molecules are naturally constructed by various microorganisms such as bacteria, insects and mammals.[198]
There are numerous kinds of AMPs that can be categorized in four main subdivisions, depending on their structural assortment: α-helix, β-sheet, extended, and loop.[199] Due to the rise of antibiotic-resistant infection agents, AMPs have been loaded in different biomaterials to be used as wound dressings, and reviewed recently.[200] While the important aspect of using AMPs is related to their ability to control the microbial proliferation and colonization of pathogens, they also assist in both controlling the immune responses and regeneration of the wounds. Mechanistically, AMPs reveal anti-infective properties and manipulate various immune cell functions. These types of peptides are named as host defense peptides (HDPs) and contain positively charged and hydrophobic residues. Immunomodulatory activity of these peptides is at the level of innate immunity and, hereafter, they are referred to innate defense regulator (IDR) peptides.[201] The immunomodulatory properties of IDR peptides consist of activation of leukocyte, differentiation of macrophage and leukocyte, modulation of ROS expression, decrease the level of pro-inflammatory cytokines, and finally increase in the angiogenesis and wound healing.[197, 202]
Among various others, recently, AMPs consisting of L12 peptides[203] and LL-37[204] were encapsulated in hydrogels for immunomodulation. Generally, these bioactive molecules, beside their significant antimicrobial activity, interact with TLRs, hinder TLR signaling pathways, inhibit TNF-α release from macrophage, and finally accelerate wound healing.[205] For instance, Yang et al.[204] have developed LL-37 encapsulated chitosan hydrogels that showed controlled release of LL-37 in addition to the significant antibacterial activity against the growth of Staphylococcus aureus. LL-37 encapsulated chitosan hydrogel hindered the TNF-α release from macrophage, improved the newly formed capillary formation, and promoted the expression of key macromolecules in the angiogenesis process, consisting of hypoxia inducible factor-1α (HIF-1α) and VEGF-A. Having such interesting properties, as-prepared LL-37 encapsulated chitosan hydrogel was suggested as a promising therapeutic component to decrease the pressure ulcers area in a mice model.[204] Besides such considerable progress towards the application of AMPs for immunomodulation strategies, certain limitations including high cost of production, toxicity, and low stability in vivo and their unidentified mechanisms of action raise significant concerns, which require more investigation in the future.
6.5. Immunomodulation approaches based on gene delivery
Gene delivery is known to be a multi-purpose alternative strategy, specifically for the direct delivery of immunomodulatory factors in the wound area. The main goal of gene delivery mediated immunomodulation strategy is to modulate the macrophage phenotype in order to increase the IL-10 and IL-4 expression, reduce the pro-inflammatory cytokine secretion, and increase the recruitment of Tregs to hinder the inflammation.[206] Till today, most of the platforms based on controlled release of nucleic acids have been designed for the treatment of various cancer diseases.[207] Only limited studies have focused to modulate inflammation that support wound healing.
In general, vectors for gene delivery including DNA and RNA are lipids, proteins or biomaterials that effectively overcome the in vivo barriers to transfer the gene efficiently.[208] RNA delivery approaches are often based on their internalization into dividing cells. Meanwhile, macrophages secrete various types of degradative enzymes. Therefore, the carriage vehicles, which effectively target the macrophages, should protect the RNA against enzymatic degradation of macrophages. During the past few years, delivery of siRNA and miRNAs has obtained great attention for reducing the expression of a target gene.[209] Mostly, siRNA and miRNAs are noncoding RNAs with crucial functions in the management of various types of genes. They employ gene silencing effects at the post-transcriptional level by directing mRNA.[210]
siRNA is a short molecule (~21–25 nucleotides length encoding) which can especially silent the projected genes via knockdown of mRNA target. siRNAs have been studied against different receptors in chronic wound healing including TGF-β1, Prolyl hydroxylase domain protein 2 (PHD2), MMP9, and connective TGF (CTGF).[211] For instance, expression of CTGF was reported for the siRNA encapsulated skin sutures applied in a 3rd degree burn stimulated scar animal model.[211c] Generally, due to the anionic nature of siRNA, the cationic complexes are often applied as the carriers for their controlled release in the damaged area (Table 3). Recently, Kim et al.[212] have developed a matrix for MMP-responsive release of siRNA. They applied a polyethyleneimine hydrogel conjugated to a polycaprolactone (PCL)-PEG matrix using a MMP-cleavable linker, while siRNA was electrostatically loaded in it (Figure 12A(i)). In this study, siRNA-incorporated construct remarkably improved MMP-2 gene-silencing properties of siRNA and accelerated the wound remodeling at the injured site (Figure 12A(ii)). In addition, the recovery rate of diabetic ulcer wound was significantly enhanced. In another study, a layer by layer (LBL) technology was applied for the controlled release of siRNA from the coating on a commercial nylon bandage (Figure 12B(i)).[211c] In this study, the authors aimed to locally knockdown the MMP-9 expression in the proteolytic wound using controlled release of siRNA that enhanced the ECM accumulation. Chemically, this immunomodulatory coating (Figure 12B(i)) was made of poly(β-aminoester)2 (Poly2) and dextran sulfate organized in a hierarchical model. On the top of this coating (referred as X), a LBL film (referred as Y), accumulated from chitosan and siRNA, was placed for controlled release of siRNA ranging from hours to weeks. in vivo studies in a genetically diabetic mouse model (Figure 12B(ii)) revealed that siMMP-9 incorporated bandages significantly improved re-epithelialization as compared to control. In addition, the wounds treated with MMP-9 siRNA revealed more than five times of collagen deposition as compared to the control (Figure 12B(iii)).
Figure 12. Immunomodulation strategies based on Small interfering RNA (siRNA) delivery.
A) siRNA-decorated nanofibrous meshes for treatment of diabetic ulcers: (i) Formation of the matrix metalloproteinases (MMPs)-responsive nanofibrous meshes; (ii) the engineered strategy for the treatment of diabetic ulcers using the siRNA-incorporated nanofibrous membrane. Reproduced with permission from Ref.[212]. B) A layer-by-layer (LBL) wound dressing for diabetic wound healing: (i) A schematic representation of the LbL coating on a nylon bandage for the sustained release of siRNA. The first (X) LBL layer was a degradable coating, the second (Y) layer comprised the siRNA; (ii) schematic of the bandages applied to the full-thickness wounds on the mice: (1) a circle wound was removed from the mouse back, (2) The designed bandage was placed on the wound site and (3) the bandage was preserved using an adhesive bandage; (iii) the collagen stained images via crossed-polarizers. While collagen type I was the large orange-red fibers, collagen type was the thin green fibers. Reproduced with permission from Ref.[211c].
Meanwhile, miRNAs are short molecules (22-nucleotides length noncoding RNAs) which have been used as a crucial gene regulator. miRNAs can bind to 3’ untranslated region of targeted mRNA, leading to translational suppression or degradation of the same.[213] Compared to siRNA which is highly specific with unique mRNA target, miRNAs have several targets.[210] Therefore, miRNAs have obtained substantial attention for the treatment of various diseases in particular wound healing.[214] To date, numerous miRNAs have been identified for controlling various steps of wound healing process and reviewed recently.[215] For example, miR-21 promotes fibroblast migration, differentiation, and contraction by moderating the TGF-β signaling, which often decreased in diabetic wounds.[209a] In addition, miR-21 acts as a moderator of pro-inflammatory / pro-reparative phenotype and modulates the switch from pro-inflammatory to anti-inflammatory states [216]. miR-132 also supports the alteration from inflammatory to proliferation phase of wound healing by declining chemokine secretion and suppressing the NF-jB pathway, so diminishing leukocytes recruitment.[217] Furthermore, miR-223 acts as a controller for inflammatory responses that motivates the polarization of macrophages to a healing phenotype[218]. Moreover, the expression of miR-223 gets enhanced during the differentiation of monocytes[219] and granulocytes [220], while decreases during the differentiation of macrophages.[221]
Despite the pivotal role of miRNA in regulating chronic wounds, gene therapy approach has not been broadly investigated due to the absence of ideal carriage systems. This could be also related to the specific properties of miRNAs including fast degradation rate using RNases, their negative charge, and a limited half-life (10 min in plasma).[222] In a recent study, our group developed a GelMA-based adhesive hydrogel comprising of miR-223 5p mimic (miR-223*) encapsulated HA nanoparticles in order to modulate macrophages’ polarization in vitro and in vivo (Figure 13A(i)).[74b] While inclusion of nanoparticles did not change the adhesive strength of the hydrogels (Figure 13A(ii)), upregulation of miR-223* in macrophages improved the anti-inflammatory gene expression and reduced the proinflammatory markers (Figure 13A(iii)). In vivo studies also revealed that controlled release of miR-223* significantly promoted the vascularization at injury site. Meanwhile, Li et al. [223] have also evaluated the local release of miR-132 in a wound of leptin receptor-deficient diabetic mice (Figure 13B(i)). In this study, local release of miR-132 accelerated the wound closure, which was accompanied by enhanced proliferation of keratinocytes at the wound edge (Figure 13B(ii)).[223] This group also applied a liposome-formulated miR-132 in the combination with a pluronic F-127 hydrogel on a skin wound model and showed a significant increase in re-epithelialization upon application of the biomaterial (Figure 13B(iii)). Overall, both miRNAs and siRNAs have revealed promising potential to control the immune system and promote the wound healing process, while none have reached clinical trials yet. Therefore, further advances in drug delivery techniques are necessary for their usage as therapeutics in the clinic.
Figure 13. Immunomodulation strategies based on microRNA (miRNA) delivery.
A) An adhesive gelatin methacryloyl (GelMA) hydrogel encapsulated with miRNA-loaded nanoparticles (GelMA/NP/miR-223*) for local immunomodulation: (i) A Schematic illustration of the GelMA/NP/miR-223* synthesis as an adhesive hydrogel; (ii) adhesive properties of GelMA/NP/miR-223*; and (iii) quantitative polymerase chain reaction (qPCR) analysis of anti-inflammatory results of miR-223* transfection in J774A.1 macrophages, after incubation with GelMA/NP, before and after miR-223* encapsulation. Reproduced with permission from Ref.[74b]. B) miRNA-132 loaded hydrogel for the chronic wound healing: (i) a wound edge biopsy was obtained from day 7 of diabetic foot ulcer (DFU); (ii) representative images of Haemotoxylin and Eosin (H&E)-stained wound tissues. Dashed lines show the new epithelial tongue; (iii) immunostaining of Ki-67 on day 5. Reproduced with permission from Ref. [223].
7. Conclusions and Future Perspective
Wound healing is a complex process considered by organization of adjusting molecules, cells, signaling pathways and finally, matrix synthesis. Slow rate of healing in chronic wounds, such as diabetic and trauma wounds, is one of the serious problems, threatening the life quality of millions of people around the world. To overcome this challenge, extensive research on engineering functionalized hydrogels, incorporated with different types of bioactive molecules including growth factors or cytokines, has resulted in the development of smart wound dressings. Recently, the application of smart hydrogel-based biomaterials for the management of a few types of autoimmune diseases has been reviewed[9d]. Ongoing improvement in the bioengineering of skin, by using cutting-edge tissue engineering strategies and knowledge of molecular signaling pathways, has made further advancement in management of wound healing.
Recent findings have increasingly shown a great potential of directing the immune system for improved chronic wound healing. In this regard, various strategies are presented for the spatiotemporal control over immune responses. The success of immunoregulatory constructs spurs the development of novel ways for scarless tissue regeneration. This review discussed the design of advanced hydrogels with controlled characteristics for modulating immune responses and promoting chronic wound healing. Studies have shown that the hydrogel properties can regulate either innate or adaptive immune cell responses in various stages of wound healing. Ongoing research has been focused on the new hydrogel synthesis methods to modulate the adaptive immune responses. Therefore, investigating the interactions between immune cells and various types of natural and synthetic hydrogels may afford critical mechanistic insight. However, clinical translation of these structures requires more analysis of in vivo responses.
Moreover, hydrogels can be designed for immunomodulatory therapy of chronic wounds via delivery of bioactive molecules, including antimicrobial molecules, immunomodulatory components, growth factors and gene as well as cell delivery (Figure 1). Various types of natural and synthetic hydrogels have been served as a delivery system by protecting bioactive molecules from protease degradation. However, control over the release of bioactive molecules to tailor immunotherapy is one of the main challenges. This challenge can be addressed via designing new types of stimuli-responsive hydrogels and employment of numerous strategies to engineer micro- and nanoscale platforms for the treatment of chronic wounds.[9e, 101, 208b, 224] For example, a GelMA-based MN patch has recently been applied for local and controlled delivery of plasmid DNA (pDNA). In this research, intracellular delivery of the nucleic acid cargo was provided using poly(β-amino ester) (PBAE) nanoparticles.[208b] In addition, a dissolvable poly-γ-glutamate (γ-PGA) MN patch with immunomodulatory effects has recently been developed. The patch provided the controlled release of γ-PGA into the DC–rich dermis to interact with DCs for immunomodulatory effects. In this study, immune responses were modulated via changing the molecular-weight of γ-PGA.[225] In addition, a detachable MN patch based on GelMA was developed for MSCs delivery.[226] Such innovative cell delivery technology revealed great promise for improved treatment of skin wounds. Meanwhile, simultaneous and/or gradient delivery of two or more immunomodulatory agents using MN patches is another interesting direction for the future studies on chronic skin wound healing. Currently, this strategy has been applied for cancer immunotherapy[224e, 224j] and its application for skin regeneration has not been investigated yet.
Hydrogels have also been combined with other advanced manufacturing techniques such as 3D printing technology for immunomodulatory purpose and/or skin regeneration.[227] Moreover, designing smart and flexible wound dressings, interfacing biological tissues with flexible electronics, is another direction which may significantly affect the chronic wounds treatment.[87, 228] In this regard, hydrogel based bioelectronics which can simultaneously monitor wound status in real time and provide controlled release of bioactive molecule will interesting. Thus, developing such stimuli-responsive hydrogels has become the center of the growing field of smart drug systems. [87, 229] For instance, a conductive hydrogel made of polydopamine functionalized silver nanoparticles, polyaniline, and PVA with tunable mechanical and electrochemical properties, has recently been developed for epidermal sensors and diabetic foot wound dressing.[229a] However, lack of knowledge on the current hydrogel based bioelectronics that can control the release of immunomodulatory agents for chronic skin wound healing, is the main challenge of these promising smart integrations.
Acknowledgements
N.A. acknowledges the support from the National Institutes of Health (NIH) (R01EB023052 and R01HL140618).
Biography
Dr. Annabi is an Assistant Professor in the Department of Chemical and Biomolecular Engineering at UCLA. She has published over 130 articles on the design and engineering of advanced biomaterials for a wide range of biomedical applications. Her interdisciplinary research has been recognized by several awards such as the 2020 NSEF Young Investigator Award of American Institute of Chemical Engineers (AIChE) and the 2021 Young Investigator Award from the Society for Biomaterials (SFB).

Contributor Information
Dr. Mahshid Kharaziha, Department of Materials Engineering, Isfahan University of Technology, Isfahan 84156-83111, Iran.
Dr. Avijit Baidya, Chemical and Biomolecular Engineering, University of California - Los Angeles, Los Angeles, CA, USA
Prof. Nasim Annabi, Chemical and Biomolecular Engineering, University of California - Los Angeles, Los Angeles, CA, USA
Reference
- [1].Nylén S, Eidsmo L, Parasite immunology 2012, 34, 551. [DOI] [PubMed] [Google Scholar]
- [2].Kolarsick PA, Kolarsick MA, Goodwin C, Journal of the Dermatology Nurses’ Association 2011, 3, 203. [Google Scholar]
- [3].Blanpain C, Nature 2010, 464, 686. [DOI] [PubMed] [Google Scholar]
- [4].Girolomoni G, Tessari G, Bos JD, Handbook of Systemic Autoimmune Diseases 2006, 5, 3. [Google Scholar]
- [5].Eming SA, Martin P, Tomic-Canic M, Science translational medicine 2014, 6, 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gurtner GC, Werner S, Barrandon Y, Longaker MT, Nature 2008, 453, 314. [DOI] [PubMed] [Google Scholar]
- [7].Richmond JM, Harris JE, Cold Spring Harbor perspectives in medicine 2014, 4, a015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ellis S, Lin EJ, Tartar D, Current dermatology reports 2018, 7, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang L, Yang J, Nguyen KT, Acta biomaterialia 2013, 9, 9351. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nair A, Thevenot P, Dey J, Shen J, Sun MW, Yang J, Tang L, Tissue Engineering Part C: Methods 2010, 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) da Silva LP, Reis RL, Correlo VM, Marques AP, Annual review of biomedical engineering 2019, 21. [DOI] [PubMed] [Google Scholar]; d) Shodeinde AB, Murphy AC, Oldenkamp HF, Potdar AS, Ludolph CM, Peppas NA, Advanced Functional Materials 2020, 1909556. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wang Z, Wang J, Li H, Yu J, Chen G, Kahkoska AR, Wu V, Zeng Y, Wen D, Miedema JR, Buse JB, Gu Z, Proceedings of the National Academy of Sciences 2020, 117, 29512. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Zhou X, Luo Z, Baidya A, Kim HJ, Wang C, Jiang X, Qu M, Zhu J, Ren L, Vajhadin F, Tebon P, Advanced Healthcare Materials 2020, 2000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frykberg RG, Banks J, Advances in wound care 2015, 4, 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhardwaj N, Chouhan D, Mandal B, Current pharmaceutical design 2017, 23, 3455. [DOI] [PubMed] [Google Scholar]
- [12].Xie Z, Aphale NV, Kadapure TD, Wadajkar AS, Orr S, Gyawali D, Qian G, Nguyen KT, Yang J, Journal of Biomedical Materials Research Part A 2015, 103, 3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13] a).Kim GB, Aragon-Sanabria V, Randolph L, Jiang H, Reynolds JA, Webb BS, Madhankumar A, Lian X, Connor JR, Yang J, Dong C, Bioactive Materials 2020, 5, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F, Journal of Materials Science: Materials in Medicine 2019, 30, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HK, Yang J, Biomaterials 2010, 31, 9092. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Su Y, Xie Z, Kim GB, Dong C, Yang J, ACS biomaterials science & engineering 2015, 1, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Tarafder S, Gulko J, Sim KH, Yang J, Cook JL, Lee CH, Scientific reports 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Teixeira SP, Domingues RM, Shevchuk M, Gomes ME, Peppas NA, Reis RL, Advanced Functional Materials 2020, 1909011 [Google Scholar]; g) Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z, Advanced Materials 2020, 32, 1902004. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Xie Z, Su Y, Kim GB, Selvi E, Ma C, Aragon-Sanabria V, Hsieh JT, Dong C, Yang J, Small 2017, 13, 1603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14] a).Chouhan D, Dey N, Bhardwaj N, Mandal BB, Biomaterials 2019, 119267. [DOI] [PubMed] [Google Scholar]; b) Gianino E, Miller C, Gilmore J, Bioengineering 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ceelen W, Pattyn P, Mareel M, Critical reviews in oncology/hematology 2014, 89, 16. [DOI] [PubMed] [Google Scholar]
- [15] a).Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H, Advanced healthcare materials 2019, 8, 1801106. [DOI] [PubMed] [Google Scholar]; b) Leach DG, Young S, Hartgerink JD, Acta biomaterialia. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Materials Today 2015, 18, 313 [Google Scholar]; d) Singh A, Peppas NA, Advanced Materials 2014, 26, 6530. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Wei W, Zhang Q, Zhou W, Liu Z, Wang Y, Alakpa EV, Ouyang H, Liu H, Applied Materials Today 2019, 14, 126 [Google Scholar]; f) Hotaling NA, Tang L, Irvine DJ, Babensee JE, Annual review of biomedical engineering 2015, 17, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Julier Z, Park AJ, Briquez PS, Martino MM, Acta biomaterialia 2017, 53, 13. [DOI] [PubMed] [Google Scholar]; h) Dziki JL, Huleihel L, Scarritt ME, Badylak SF, Tissue Engineering Part A 2017, 23, 1152. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Trends in immunology 2018, 39, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Zhang X, Kim TH, Thauland TJ, Li H, Majedi FS, Ly C, Gu Z, Butte MJ, Rowat AC, Li S, Current Opinion in Biotechnology 2020, 66, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Larouche J, Sheoran S, Maruyama K, Martino MM, Advances in wound care 2018, 7, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robson MC, Steed DL, Franz MG, Current problems in surgery 2001, 2, 72. [DOI] [PubMed] [Google Scholar]
- [18].Hogle W, in Principles of skin care and the oncology patient. (Eds: Haas M, L., Moore-Higgs GJ), Oncology Nursing Society; 2010. [Google Scholar]
- [19].Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J, Systematic reviews 2016, 5, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Falanga V, The Lancet 2005, 366, 1736. [DOI] [PubMed] [Google Scholar]
- [21].Zhao R, Liang H, Clarke E, Jackson C, Xue M, International journal of molecular sciences 2016, 17, 2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yao S, Zhu Y, Chen L, Nature reviews Drug discovery 2013, 12, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chávez-Galán L, Olleros ML, Vesin D, Garcia I, Frontiers in immunology 2015, 6, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Opdenakker G, Van Damme J, Vranckx JJ, Trends in immunology 2018, 39, 341. [DOI] [PubMed] [Google Scholar]
- [25].Navegantes KC, de Souza Gomes R, Pereira PAT, Czaikoski PG, Azevedo CHM, Monteiro MC, Journal of translational medicine 2017, 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26] a).Giudice A, Vendrame C, Bezerra C, Carvalho LP, Delavechia T, Carvalho EM, Bacellar O, BMC infectious diseases 2012, 12, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM, AIDS (London, England) 2014, 28, 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumar S, Dwivedi PD, Das M, Tripathi A, Molecular immunology 2013, 56, 612. [DOI] [PubMed] [Google Scholar]
- [28].Koh TJ, DiPietro LA, Expert reviews in molecular medicine 2011, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kwon MJ, An S, Choi S, Nam K, Jung HS, Yoon CS, Ko JH, Jun HJ, Kim TK, Jung SJ, Park JH, The journal of gene medicine 2012, 14, 272. [DOI] [PubMed] [Google Scholar]
- [30].Ritsu M, Kawakami K, Kanno E, Tanno H, Ishii K, Imai Y, Maruyama R, Tachi M, Journal of Dermatology & Dermatologic Surgery 2017, 12, 14. [Google Scholar]
- [31] a).Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S, Journal of Investigative Dermatology 2000, 115, 245. [DOI] [PubMed] [Google Scholar]; b) Wynn TA, Vannella KM, Immunity 2016, 44, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL, Microcirculation 2016, 23, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Delavary BM, van der Veer WM, van Egmond M, Niessen FB, Beelen RH, Immunobiology 2011, 216, 753. [DOI] [PubMed] [Google Scholar]
- [33].Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M, Immunologic research 2013, 57, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gould L, Abadir P, Brem H, Carter M, Conner-Kerr T, Davidson J, DiPietro L, Falanga V, Fife C, Gardner S, Grice E, Wound Repair and Regeneration 2015, 23, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rübsam A, Parikh S, Fort PE, International journal of molecular sciences, 2018, 19, 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36] a).Bowler PG, Duerden BI, Armstrong DG, Clinical microbiology reviews 2001, 14, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, Costerton JW, Stewart PS, Wound Repair and regeneration 2008, 16, 37. [DOI] [PubMed] [Google Scholar]
- [37] a).Harding KG, Morris HL, Patel GK, Br. Med. J. 2002, 324, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mekkes JR, Loots MA, Van Der Wal AC, Bos JD, Br. J. Dermatol. 2003, 148, 388. [DOI] [PubMed] [Google Scholar]
- [38].Breitkreutz D, Koxholt I, Thiemann K, Nischt R, Biomed. Res. Int. 2013, 2013, 179784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Serra R, Grande R, Buffone G, Molinari V, Perri P, Perri A, Amato B, Colosimo M, de Franciscis S, International Wound Journal 2016, 13, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matsukawa M, Greenberg EP, Journal of bacteriology 2004, 186, 4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bowler PG, Duerden BI, Armstrong DG, Clinical microbiology reviews 2001, 142, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, Miller C, Khanna S, Bergdall VK, Powell HM, Cook CH, J Pathol 2014, 233, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Haggar A, Ehrnfelt C, Holgersson J, Flock JI, Infection and immunity 2004, 72, 6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vojdani A, Autoimmune diseases, 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu F, Fu Y, Sun TY, Jiang Z, Miao Z, Shuai M, Gou W, Ling CW, Yang J, Wang J, Chen YM, Microbiome 2020, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wu J, Xie A, Chen W, Burns & trauma 2014, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y, Trends in Immunology, 2009, 30, 409. [DOI] [PubMed] [Google Scholar]
- [48] a).Botta D, Fuller MJ, Marquez-Lago TT, Bachus H, Bradley JE, Weinmann AS, Zajac AJ, Randall TD, Lund FE, León B, Ballesteros-Tato A, Nature Immunology 2017, 18, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang X, Kang Y, Wang J, Yan J, Chen Q, Cheng H, Huang P, Gu Z, Advanced Materials 2020, 1907692. [DOI] [PubMed] [Google Scholar]
- [49].Oldstone MBA, Nerenberg M, Southern P, Price J, Lewicki H, Cell 1991, 65, 319. [DOI] [PubMed] [Google Scholar]
- [50].Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M, Advances in wound care 2014, 3, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Augustine R, Kalarikkal N, Thomas S, Progress in biomaterials 2014, 3, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52] a).Cervelli V, Lucarini L, Cerretani C, Spallone D, Palla L, Brinci L, De Angelis B, International wound journal 2010, 7, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gibbs S, Van Den Hoogenband HM, Kirtschig G, Richters CD, Spiekstra SW, Breetveld M, Scheper RJ, De Boer EM, British Journal of Dermatology 2006, 155, 267. [DOI] [PubMed] [Google Scholar]
- [53].Towler MA, Rush EW, Richardson MK, Williams CL, Clinics in podiatric medicine and surgery 2018, 35, 357. [DOI] [PubMed] [Google Scholar]
- [54].Uehara M, Li X, Sheikhi A, Zandi N, Walker B, Saleh B, Banouni N, Jiang L, Ordikhani F, Dai L, Yonar M, Scientific reports 2019, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dixit S, Baganizi DR, Sahu R, Dosunmu E, Chaudhari A, Vig K, Pillai SR, Singh SR, Dennis VA, Journal of biological engineering 2017, 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56] a).Motegi SI, Ishikawa O, Journal of dermatological science, 2017, 82, 83. [DOI] [PubMed] [Google Scholar]; b) Porada CD, Zanjani ED, Almeida-Porada G, Current stem cell research & therapy 2006, 1, 365. [DOI] [PubMed] [Google Scholar]
- [57].Strong AL, Neumeister MW, Levi B, Clinics in plastic surgery 2017, 44, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Verina T, Fatemi A, Johnston MV, Comi AM, Pediatric neurology 2013, 48, 346. [DOI] [PubMed] [Google Scholar]
- [59].Bliley JM, Argenta A, Satish L, McLaughlin MM, Dees A, Tompkins-Rhoades C, Marra KG, Rubin JP, Burns 2016, 42, 1212. [DOI] [PubMed] [Google Scholar]
- [60].Jin E, Kim TH, Han S, Kim SW, Journal of tissue engineering and regenerative medicine 2016, 10, 613. [DOI] [PubMed] [Google Scholar]
- [61].Krishna M, Nadler SG, Frontiers in immunology 2016, 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jackson CJ, Tønseth KA, Utheim TP, Stem cell research & therapy 2017, 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Negut I, Grumezescu V, Grumezescu AM, Molecules 2018, 23, 2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64] a).Tavakoli S, Mokhtari H, Kharaziha M, Kermanpour A, Talebi A, Moshtaghian J, Materials Science and Engineering: C 2020, 110837. [DOI] [PubMed] [Google Scholar]; b) Goudarzi ZM, Behzad T, Ghasemi-Mobarakeh L, Kharaziha M, Enayati MS, Polymer Bulletin 2020, 77, 717 [Google Scholar]; c) Tavakoli S, Kharaziha M, Kermanpur A, Mokhtari H, International journal of biological macromolecules, 2019, 138, 590. [DOI] [PubMed] [Google Scholar]; d) Shamirzaei Jeshvaghani E, Ghasemi-Mobarakeh L, Mansurnezhad R, Ajalloueian F, Kharaziha M, Dinari M, Sami Jokandan M, Chronakis IS, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018, 106, 2371. [DOI] [PubMed] [Google Scholar]; e) Golafshan N, Rezahasani R, Esfahani MT, Kharaziha M, Khorasani SN, Carbohydrate polymers 2017, 176, 392. [DOI] [PubMed] [Google Scholar]; f) Boateng J, Catanzano O, Journal of pharmaceutical sciences 2015, 104, 3653. [DOI] [PubMed] [Google Scholar]
- [65].Venugopal JR, Zhang Y, Ramakrishna S, Artificial organs 2006, 30, 440. [DOI] [PubMed] [Google Scholar]
- [66].Nicholas MN, Jeschke MG, Amini-Nik S, Cellular and molecular life sciences 2016, 73, 3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Greaves NS, Iqbal SA, Baguneid M, Bayat A, Wound repair and regeneration 2013, 21, 194. [DOI] [PubMed] [Google Scholar]
- [68].Seavey JG, Masters ZA, Balazs GC, Tintle SM, Sabino J, Fleming ME, Valerio IL, Regenerative medicine 2016, 11, 81. [DOI] [PubMed] [Google Scholar]
- [69].Simman R, Mari W, Younes S, Wilson M, Eplasty 2018, 18, 82. [PMC free article] [PubMed] [Google Scholar]
- [70].Nicoletti G, Brenta F, Bleve M, Pellegatta T, Malovini A, Faga A, Perugini P, Journal of tissue engineering and regenerative medicine 2015, 9, 460. [DOI] [PubMed] [Google Scholar]
- [71] a).Lin CC, Metters AT, Advanced drug delivery reviews 2006, 58, 1379. [DOI] [PubMed] [Google Scholar]; b) Saunders L, Ma PX, Macromolecular bioscience 2019, 19, 1800313. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Peppas NA, Hilt JZ, Khademhosseini A, Langer R, Advanced materials 2006, 18, 1345. [Google Scholar]
- [72] a).Venkataraman AK, Clegg JR, Peppas NA, Journal of Materials Chemistry B 2020, 8, 7685. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Byrne ME, Park K, Peppas NA, Advanced drug delivery reviews 2002, 54, 149. [DOI] [PubMed] [Google Scholar]
- [73].Seliktar D, Science 2012, 336, 124. [DOI] [PubMed] [Google Scholar]
- [74] a).Rajabi N, Kharaziha M, Emadi R, Zarrabi A, Mokhtari H, Salehi S, Journal of Colloid and Interface Science 2020, 564, 155. [DOI] [PubMed] [Google Scholar]; b) Saleh B, Dhaliwal HK, Portillo-Lara R, Shirzaei Sani E, Abdi R, Amiji MM, Annabi N, Small 2019, 15, 1902232. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C, Camci-Unal G, Dokmeci MR, Peppas NA, Khademhosseini A, Advanced materials 2014, 26, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D, Journal of molecular histology. 2020. [Google Scholar]
- [76].Uman S, Dhand A, Burdick JA, Journal of Applied Polymer Science 2019, 48668. [Google Scholar]
- [77].Tavafoghi M, Sheikhi A, Tutar R, Jahangiry J, Baidya A, Haghniaz R, Khademhosseini A, Advanced Healthcare Materials 2020, 1901722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Loo Y, Wong YC, Cai EZ, Ang CH, Raju A, Lakshmanan A, Koh AG, Zhou HJ, Lim TC, Moochhala SM, Hauser CA, Biomaterials 2014, 35, 4805. [DOI] [PubMed] [Google Scholar]
- [79].Kujath P, Michelsen A, Deutsches Ärzteblatt International 2008, 105, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80] a).Sood A, Granick MS, Tomaselli NL, Advances in wound care 2014, 3, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Weller CD, Team V, Sussman G, Frontiers in Pharmacology, 2020, 11, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81] a).Boateng J, Catanzano O, Journal of pharmaceutical sciences 2015, 104, 3653. [DOI] [PubMed] [Google Scholar]; b) Moura LI, Dias AM, Carvalho E, de Sousa HC, Acta biomaterialia 2013, 9, 7093. [DOI] [PubMed] [Google Scholar]
- [82] a).Chattopadhyay S, Raines RT, Biopolymers 2014, 101, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Koehler J, Brandl FP, Goepferich AM, European Polymer Journal 2018, 100, 1 [Google Scholar]; c) Sweeney IR, Miraftab M, Collyer G, International wound journal 2012, 9, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83] <b/>a).Singh TRR, Laverty G, Donnelly R, Hydrogels: design, synthesis and application in drug delivery and regenerative medicine., CRC Press., 2018 [Google Scholar]; b) Francesko A, Petkova P, Tzanov T, Current medicinal chemistry 2018, 25, 5782. [DOI] [PubMed] [Google Scholar]
- [84].Barnea Y, Weiss J, Gur E, Therapeutics and clinical risk management 2010, 6, 21. [PMC free article] [PubMed] [Google Scholar]
- [85].Alexandrino-Junior F, Freire MCLC, Lione VDOF, Cardoso EA, Marcelino HR, Genre J, Oliveira AGD, Egito ESTD, Pharmaceutics 2019, 11, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Comotto M, Saghazadeh S, Bagherifard S, Aliakbarian B, Kazemzadeh-Narbat M, Sharifi F, Mousavi Shaegh SA, Arab-Tehrany E, Annabi N, Perego P, Khademhosseini A, Journal of biomaterials applications 2019, 33, 1265. [DOI] [PubMed] [Google Scholar]
- [87].Mostafalu P, Tamayol A, Rahimi R, Ochoa M, Khalilpour A, Kiaee G, Yazdi IK, Bagherifard S, Dokmeci MR, Ziaie B, Sonkusale SR, Small 2018, 14, 1703509. [DOI] [PubMed] [Google Scholar]
- [88].Wang L, Hu C, Shao L, International journal of nanomedicine 2017, 12, 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mihai MM, Dima MB, Dima B, Holban AM, Materials 2019, 12, 2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li M, Liu X, Tan L, Cui Z, Yang X, Li Z, Zheng Y, Yeung KWK, Chu PK, Wu S, Biomaterials science 2018, 6, 2110. [DOI] [PubMed] [Google Scholar]
- [91].Annabi N, Rana D, Sani ES, Portillo-Lara R, Gifford JL, Fares MM, Mithieux SM, Weiss AS, Biomaterials 2017, 139, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yang H, Lai C, Xuan C, Chai M, Liu X, Chen Y, Shi X, Chemical Engineering Journal 2020, 125617. [Google Scholar]
- [93].Chen M, Tian J, Liu Y, Cao H, Li R, Wang J, Wu J, Zhang Q, Chemical Engineering Journal 2019, 373, 413. [Google Scholar]
- [94].Rousselle P, Braye F, Dayan G, Advanced Drug Delivery Reviews 2019, 146, 344. [DOI] [PubMed] [Google Scholar]
- [95].Yamakawa S, Hayashida K, Burns & trauma 2019, 7, s41038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96] a).Park JW, Hwang SR, Yoon IS, Molecules 2017, 22, 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Niu Y, Li Q, Ding Y, Dong L, Wang C, Advanced drug delivery reviews, 2019, 146, 190. [DOI] [PubMed] [Google Scholar]
- [97].Brudno Y, Ennett-Shepard AB, Chen RR, Aizenberg M, Mooney DJ, Biomaterials 2013, 34, 9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S, Npg Asia Materials 2017, 9, e435. [Google Scholar]
- [99] a).Cinay GE, Erkoc P, Alipour M, Hashimoto Y, Sasaki Y, Akiyoshi K, Kizilel S, ACS Biomaterials Science & Engineering 2017, 3, 370. [DOI] [PubMed] [Google Scholar]; b) Koetting MC, Peters JT, Steichen SD, Peppas NA, Materials Science and Engineering: R: Reports 2015, 93, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yu S, Wang C, Yu J, Wang J, Lu Y, Zhang Y, Zhang X, Hu Q, Sun W, He C, Chen X, Advanced Materials 2018, 30, 1801527. [DOI] [PubMed] [Google Scholar]; d) Zhang Y, Wang J, Yu J, Wen D, Kahkoska AR, Lu Y, Zhang X, use JB, Gu Z, Small 2018, 14, 1704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bagherifard S, Tamayol A, Mostafalu P, Akbari M, Comotto M, Annabi N, Ghaderi M, Sonkusale S, Dokmeci MR, Khademhosseini A, Advanced healthcare materials 2016, 5, 175. [DOI] [PubMed] [Google Scholar]
- [101].Derakhshandeh H, Aghabaglou F, McCarthy A, Mostafavi A, Wiseman C, Bonick Z, Ghanavati I, Harris S, Kreikemeier-Bower C, Basri SMM, Rosenbohm J, Yang R, Mostafalu P, Orgill D, Tamayol A, Advanced Functional Materials 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bygd HC, Forsmark KD, Bratlie KM, Biomaterials 2015, 56, 187. [DOI] [PubMed] [Google Scholar]
- [103].Knopf-Marques H, Pravda M, Wolfova L, Velebny V, Schaaf P, Vrana NE, Lavalle P, Advanced healthcare materials 2016, 5, 2841. [DOI] [PubMed] [Google Scholar]
- [104].Mosser DM, Edwards JP, Nature Reviews Immunology, 2008, 8, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, Williams BR, Major J, Hamilton TA, Fenton MJ, Vogel SN, Nature immunology 2002, 3, 392. [DOI] [PubMed] [Google Scholar]
- [106].Lawrence T, Natoli G, Nature reviews immunology 2011, 11, 750. [DOI] [PubMed] [Google Scholar]
- [107].Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Immunity 2014, 40, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Verbeke CS, Gordo S, Schubert DA, Lewin SA, Desai RM, Dobbins J, Wucherpfennig KW, Mooney DJ, Advanced healthcare materials 2017, 6, 1600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nochi T, Yuki Y, Takahashi H, Sawada SI, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Nature materials 2010, 9, 572. [DOI] [PubMed] [Google Scholar]
- [110].Dhingra S, Li P, Huang XP, Guo J, Wu J, Mihic A, Li SH, Zang WF, Shen D, Weisel RD, Singal PK, Circulation 2013, 128, S69. [DOI] [PubMed] [Google Scholar]
- [111].Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG, Biomaterials 2014, 35, 3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A, Trends in biotechnology 2016, 34, 470. [DOI] [PubMed] [Google Scholar]
- [113] a).Shizuo A, Takeda K, Nat Rev Immunol 2004, 4, 499. [DOI] [PubMed] [Google Scholar]; b) Zakrzewski JL, van den Brink MRM, Hubbell JA, Nat Biotechnol. 32, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Babensee JE, Paranjpe A, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2005, 74, 503. [DOI] [PubMed] [Google Scholar]
- [115].Cha BH, Shin SR, Leijten J, Li YC, Singh S, Liu JC, Annabi N, Abdi R, Dokmeci MR, Vrana NE, Ghaemmaghami AM, Advanced healthcare materials 2017, 6, 1700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116] a).Franz S, Rammelt S, Scharnweber D, Simon JC, Biomaterials 2011, 32, 6692. [DOI] [PubMed] [Google Scholar]; b) Hubbell JA, Thomas SN, Swartz MA, Nature 2009, 462, 449. [DOI] [PubMed] [Google Scholar]
- [117].Rayahin JE, Buhrman JS, Zhang Y, Koh TJ, Gemeinhart RA, ACS Biomater Sci Eng 2015, 1, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ruppert SM, Hawn TR, Arrigoni A, Wight TN, Bollyky PL, Immunologic research 2014, 58, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zamboni F, Vieira S, Reis RL, Oliveira JM, Collins MN, Progress in Materials Science 2018, 97, 97. [Google Scholar]
- [120].Wang H, Morales RTT, Cui X, Huang J, Qian W, Tong J, Chen W, Advanced healthcare materials 2019, 8, 1801234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121] a).Yuan T, Zhang L, Feng L, Fan HS, Zhang XD, Biotechnol Prog 2010, 26, 1749. [DOI] [PubMed] [Google Scholar]; b) Yuan T, Li K, Guo L, Fan H, Zhang X, J Biomed Mater Res A 2011, 98, 332. [DOI] [PubMed] [Google Scholar]
- [122].Donaldson AR, Tanase CE, Awuah D, Vasanthi Bathrinarayanan P, Hall L, Nikkhah M, Khademhosseini A, Rose F, Alexander C, Ghaemmaghami AM, Frontiers in bioengineering and biotechnology 2018, 6, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123] a).Mokarram N, Bellamkonda RV, Annals of biomedical engineering 2014, 42, 338. [DOI] [PubMed] [Google Scholar]; b) Eming SA, Krieg T, Davidson JM, Journal of Investigative Dermatology 2007, 217, 514. [DOI] [PubMed] [Google Scholar]; c) Hashimoto T, Kojima K, Tamada Y, Materialia 2020, 9, 100519. [Google Scholar]
- [124].Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF, Acta biomaterialia 2017, 47, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chancheewa B, Buranapraditkun S, Laomeephol C, Rerknimitr P, Kanokpanont S, Damrongsakkul S, Klaewsongkram J, Materials Today Communications 2020, 101044. [Google Scholar]
- [126] a).Park YR, Sultan MT, Park HJ, Lee JM, Ju HW, Lee OJ, Lee DJ, Kaplan DL, Park CH, Acta biomaterialia 2018, 67, 183. [DOI] [PubMed] [Google Scholar]; b) Sen S, Basak P, Sinha BP, Maurye P, Jaiswal KK, Das P, Mandal TK, International journal of biological macromolecules 2020, 143, 1009. [DOI] [PubMed] [Google Scholar]; c) Li Z, Song J, Zhang J, Hao K, Liu L, Wu B, Zheng X, Xiao B, Tong X, Dai F, Colloids and Surfaces B: Biointerfaces 2020, 186, 110735. [DOI] [PubMed] [Google Scholar]
- [127].Chouhan D, Lohe TU, Samudrala PK, Mandal BB, Advanced healthcare materials 2018, 7, 1801092. [DOI] [PubMed] [Google Scholar]
- [128].Dai T, Tanaka M, Huang YY, Hamblin MR, Expert review of anti-infective therapy 2011, 9, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Takei T, Nakahara H, Ijima H, Kawakami K, Acta Biomaterialia 2012, 8, 686. [DOI] [PubMed] [Google Scholar]
- [130].Shibata Y, Foster LA, Metzger WJ, Myrvik QN, Infect Immun 65, 1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Porporatto C, Bianco ID, Riera CM, Correa SG, Biochem Biophys Res Commun 2003, 304, 266. [DOI] [PubMed] [Google Scholar]
- [132].Moura LI, Dias AM, Leal EC, Carvalho L, de Sousa HC, Carvalho E, Acta biomaterialia 2014, 10, 843. [DOI] [PubMed] [Google Scholar]
- [133].Ashouri F, Beyranvand F, Boroujeni NB, Tavafi M, Sheikhian A, Varzi AM, Shahrokhi S, Drug delivery and translational research 2019, 9, 1027. [DOI] [PubMed] [Google Scholar]
- [134].Rafieerad A, Yan W, Sequiera GL, Sareen N, Abu-El-Rub E, Moudgil M, Dhingra S, Advanced healthcare materials 2019, 8, 1900569. [DOI] [PubMed] [Google Scholar]
- [135].Xuan X, Zhou Y, Chen A, Zheng S, An Y, He H, Huang W, Chen Y, Yang Y, Li S, Xuan T, Journal of Materials Chemistry B 2020. [DOI] [PubMed] [Google Scholar]
- [136].Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM, Tissue Engineering Part A 2008, 14, 1835. [DOI] [PubMed] [Google Scholar]
- [137].Meng FW, Slivka PF, Dearth CL, Badylak SF, Biomaterials 2015, 46, 131. [DOI] [PubMed] [Google Scholar]
- [138].Chakraborty J, Roy S, Ghosh S, Biomaterials Science 2020. [DOI] [PubMed] [Google Scholar]
- [139].Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF, Biomaterials 2009, 30, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Koob TJ, Rennert R, Zabek N, e. al, Int Wound J. 2013, 10, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141] a).Huddleston HP, Cohn MR, Haunschild ED, Wong SE, Farr J, Yanke AB, Current Reviews in Musculoskeletal Medicine 2020, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Oesman I, Hutami WD, International Journal of Surgery Case Reports 2020, 66, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Villamil Ballesteros AC, Segura Puello HR, Lopez-Garcia JA, Bernal-Ballen A, Nieto Mosquera DL, Muñoz Forero DM, Segura Charry JS, Neira Bejarano YA, Polymers 2020, 12, 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142] a).Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, Kashefsky H, Owings TM, Nadarajah J, International wound journal 2014, 11, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Caputo WJ, Vaquero C, Monterosa A, Monterosa P, Johnson E, Beggs D, Fahoury GJ, Wound Repair and Regeneration 2016, 24, 885. [DOI] [PubMed] [Google Scholar]
- [143].Pourmoussa A, Gardner DJ, Johnson MB, Wong AK, Annals of translational medicine 2016, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sridharan R, Ryan EJ, Kearney CJ, Kelly DJ, O’Brien FJ, ACS Biomaterials Science & Engineering 2018, 5, 544. [DOI] [PubMed] [Google Scholar]
- [145].Witherel CE, Graney PL, Freytes DO, Weingarten MS, Spiller KL, Wound Repair and Regeneration 2016, 24, 514. [DOI] [PubMed] [Google Scholar]
- [146].Ye Q, Harmsen MC, van Luyn MJ, Bank RA, Biomaterials 2010, 31, 9192. [DOI] [PubMed] [Google Scholar]
- [147].McDade JK, Brennan-Pierce EP, Ariganello MB, Labow RS, Lee JM, Acta biomaterialia 2013, 9, 7191. [DOI] [PubMed] [Google Scholar]
- [148].Roy S, Sen CK, Physiological genomics 2011, 43, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149] a).Fitton JH, Stringer DN, Karpiniec SS, Marine drugs 2015, 13, 5920. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wijesekara I, Pangestuti R, Kim SK, Carbohydrate polymers 2011, 84, 14. [Google Scholar]
- [150] a).Kalitnik AA, Anastyuk SD, Sokolova EV, Kravchenko AO, Khasina EI, Yermak IM, Journal of applied phycology 2016, 28, 545 [Google Scholar]; b) Huang Y, Zhang L, Song R, Mao X, Tang S, Journal of Biomedical Materials Research Part A. 2020. [DOI] [PubMed] [Google Scholar]
- [151].Amin ML, Mawad D, Dokos S, Koshy P, Martens PJ, Sorrell CC, Carbohydrate Polymers 2020, 230, 115691. [DOI] [PubMed] [Google Scholar]
- [152].Bronte V, Zanovello P, Nature Reviews Immunology, 2005, 5, 641. [DOI] [PubMed] [Google Scholar]
- [153].Shi HP, Efron DT, Most D, Tantry US, Barbul A, Surgery 2000, 128, 374. [DOI] [PubMed] [Google Scholar]
- [154].Fujiwara T, Kanazawa S, Ichibori R, Tanigawa T, Magome T, Shingaki K, Miyata S, Tohyama M, Hosokawa K, Plos one 2014, 9, e92168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Vernet D, Ferrini MG, Valente EG, Magee TR, Bou-Gharios G, Rajfer J, Gonzalez-Cadavid NF, Nitric Oxide 2002, 7, 262. [DOI] [PubMed] [Google Scholar]
- [156].He M, Sun L, Fu X, McDonough SP, Chu CC, Acta biomaterialia 2019, 84, 114. [DOI] [PubMed] [Google Scholar]
- [157].He M, Potuck A, Zhang Y, Chu CC, Acta biomaterialia 2014, 10, 2482. [DOI] [PubMed] [Google Scholar]
- [158].Zhu Y, Cankova Z, Iwanaszko M, Lichtor S, Mrksich M, Ameer GA, Proceedings of the National Academy of Sciences 2018, 112, 6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159] a).Curtis A, Wilkinson C, Biomaterials 1997, 18, 1573. [DOI] [PubMed] [Google Scholar]; b) Vassey MJ, Figueredo GP, Scurr DJ, Vasilevich AS, Vermeulen S, Carlier A, Luckett J, Beijer NR, Williams P, Winkler DA, de Boer J, Advanced Science 2020, 7, 1903392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Singh S, Awuah D, Rostam HM, Emes RD, Kandola NK, Onion D, Htwe SS, Rajchagool B, Cha BH, Kim D, Tighe PJ, ACS Biomaterials Science & Engineering 2017, 3, 969. [DOI] [PubMed] [Google Scholar]
- [161] a).Wilgus TA, Pharmacological research 2008, 58, 112. [DOI] [PubMed] [Google Scholar]; b) Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2007, 83, 585. [DOI] [PubMed] [Google Scholar]
- [162].Jansen LE, Amer LD, Chen EYT, Nguyen TV, Saleh LS, Emrick T, Liu WF, Bryant SJ, Peyton SR, Biomacromolecules 2018, 19, 2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163] a).Dinnes DL, Marcal H, Mahler SM, Santerre J, P., Labow RS, J Biomed Mater Res A 2007, 80, 895. [DOI] [PubMed] [Google Scholar]; b) Blatt SE, Lurier EB, Risser GE, Spiller KL, Tissue Engineering Part C: Methods 2020, 26, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ, Nature medicine 2005, 11, 367. [DOI] [PubMed] [Google Scholar]
- [165] a).Brennan MÁ, Layrolle P, Mooney DJ, Advanced Functional Materials, 2020, 1909125. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Montemurro T, Vigano M, Ragni E, Barilani M, Parazzi V, Boldrin V, Lavazza C, Montelatici E, Banfi F, Lauri E, Giovanelli S, European journal of cell biology 2016, 95, 228. [DOI] [PubMed] [Google Scholar]
- [166].Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR, PloS one 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167] a).Aurora AB, Olson EN, Cell stem cell 2014, 15, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ben-Ami E, Berrih-Aknin S, Miller A, Autoimmunity reviews 2011, 10, 410. [DOI] [PubMed] [Google Scholar]
- [168].Blázquez R, Sánchez-Margallo FM, Álvarez V, Usón A, Casado JG, Acta biomaterialia 2016, 31, 221. [DOI] [PubMed] [Google Scholar]
- [169] a).Guerra AD, Rose WE, Hematti P, Kao WJ, Acta biomaterialia 2017, 51, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Guerra AD, Cantu DA, Vecchi JT, Rose WE, Hematti P, Kao WJ, The AAPS journal 2015, 17, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C, Theranostics 2019, 9, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Xu Q, Sigen A, Gao Y, Guo L, Creagh-Flynn J, Zhou D, Greiser U, Dong Y, Wang F, Tai H, Liu W, Acta biomaterialia 2018, 75, 63. [DOI] [PubMed] [Google Scholar]
- [172].García JR, Quirós M, Han WM, O’Leary MN, Cox GN, Nusrat A, García AJ, Biomaterials 2019, 220, 119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Dong Y, Cui M, Qu J, Wang X, Kwon SH, Barrera J, Elvassore N, Gurtner GC, Acta Biomaterialia 2020. [DOI] [PubMed] [Google Scholar]
- [174].Lima MSR, de Lima VCO, Piuvezam G, de Azevedo KPM, Maciel BLL, de Araújo Morais AH, Medicine 2019, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Iyer SS, Cheng G, Critical Reviews™ in Immunology 2012, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Singh A, Talekar M, Raikar A, Amiji M, Journal of controlled release 2014, 190, 515. [DOI] [PubMed] [Google Scholar]
- [177].Frangogiannis NG, Journal of thoracic disease 2017, 9(Suppl 1), S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Dennis KL, Blatner NR, Gounari F, Khazaie K, Current opinion in oncology 2013, 25, 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179] a).Bogdan C, Vodovotz Y, Nathan C, Journal of experimental medicine 1991, 174, 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gazzinelli RT, Oswald IP, James SL, Sher A, The Journal of Immunology 1992, 148, 1792. [PubMed] [Google Scholar]
- [180].Nova-Lamperti E, Fanelli G, Becker PD, Chana P, Elgueta R, Dodd PC, Lord GM, Lombardi G, Hernandez-Fuentes MP, Scientific reports 2016, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181] a).Carvalho V, Castanheira P, Faria TQ, Gonçalves C, Madureira P, Faro C, Domingues L, Brito RM, Vilanova M, Gama M, International journal of pharmaceutics 2010, 400, 234. [DOI] [PubMed] [Google Scholar]; b) Hume PS, He J, Haskins K, Anseth KS, Biomaterials 2012, 33, 3615. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shamskhou EA, Kratochvil MJ, Orcholski ME, Nagy N, Kaber G, Steen E, Balaji S, Yuan K, Keswani S, Danielson B, Gao M, Biomaterials 2019, 203, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang X, Coradin T, Hélary C, Biomaterials science 2018, 6, 398. [DOI] [PubMed] [Google Scholar]; e) Wang X, Coradin T, Helary C, Front. Bioeng. Biotechnol. 2016. [Google Scholar]
- [182].Murray PJ, Wynn TA, Nature Reviews Immunology, 2011, 11, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D’Souza RN, Hartgerink JD, Biomaterials 2015, 52, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Mirza RE, Fang MM, Ennis WJ, Koh TJ, Diabetes care 2013, 62, 2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Huang SM, Wu CS, Chiu MH, Wu CH, Chang YT, Chen GS, Lan CCE, Journal of dermatological science 2019, 96, 159. [DOI] [PubMed] [Google Scholar]
- [186].Horiuchi T, Mitoma H, Harashima SI, Tsukamoto H, Shimoda T, Rheumatology 2010, 49, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Wu X, Xu W, Feng X, He Y, Liu X, Gao Y, Yang S, Shao Z, Yang C, Ye Z, International journal of immunopathology and pharmacology 2015, 28, 351. [DOI] [PubMed] [Google Scholar]
- [188].Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha BH, Vishwakarma A, Ghaemmaghami AM, Khademhosseini A, Journal of Controlled Release 2016, 240, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189] a).Shamji MF, Hwang P, Bullock RW, Adams SB, Nettles DL, Setton LA, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2009, 90, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kaplan JA, Barthélémy P, Grinstaff MW, Chemical Communications 2016, 52, 5860. [DOI] [PubMed] [Google Scholar]; c) Friedrich EE, Sun LT, Natesan S, Zamora DO, Christy RJ, Washburn NR, Journal of biomedical materials research Part A 2014, 102, 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Liu S, Zhao M, Zhou Y, Li L, Wang C, Yuan Y, Li L, Liao G, Bresette W, Chen Y, Cheng J, Acta Biomaterialia 2020, 103, 102. [DOI] [PubMed] [Google Scholar]
- [191].Vasconcelos DP, Costa M, Amaral IF, Barbosa MA, Águas AP, Barbosa JN, Biomaterials 2015, 37, 116. [DOI] [PubMed] [Google Scholar]
- [192].Puertas-Bartolomé M, Fernández-Gutiérrez M, García-Fernández L, Vázquez-Lasa B, San Román J, European Polymer Journal 2018, 98, 47. [Google Scholar]
- [193].Wang P, Huang S, Hu Z, Yang W, Lan Y, Zhu J, Hancharou A, Guo R, Tang B, Acta biomaterialia 2019, 100, 191. [DOI] [PubMed] [Google Scholar]
- [194].Puertas-Bartolomé M, Benito-Garzón L, Fung S, Kohn J, Vázquez-Lasa B, San Román J, Materials Science and Engineering: C 2019, 105, 110040. [DOI] [PubMed] [Google Scholar]
- [195].Jang MH, Seth NP, Wucherpfennig KW, The Journal of Immunology 2003, 171, 4175. [DOI] [PubMed] [Google Scholar]
- [196].Ogle ME, Krieger JR, Tellier LE, McFaline-Figueroa J, Temenoff JS, Botchwey EA, ACS biomaterials science & engineering 2017, 4, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Lai Y, Gallo RL, Trends in immunology 2009, 30, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Reddy KVR, Yedery RD, Aranha C, International journal of antimicrobial agents 2004, 24, 536. [DOI] [PubMed] [Google Scholar]
- [199].Hancock RE, Sahl HG, Nature biotechnology 2006, 24, 1551. [DOI] [PubMed] [Google Scholar]
- [200].Felgueiras HP, Amorim MTP, Colloids and Surfaces B: Biointerfaces 2017, 156, 133. [DOI] [PubMed] [Google Scholar]
- [201].Hilchie AL, Wuerth K, Hancock RE, Nature chemical biology 2013, 9, 761. [DOI] [PubMed] [Google Scholar]
- [202].Hancock RE, Nijnik A, Philpott DJ, Nature Reviews Microbiology 2012, 10, 243. [DOI] [PubMed] [Google Scholar]
- [203].Obuobi S, Tay HKL, Tram NDT, Selvarajan V, Khara JS, Wang Y, Ee PLR, Journal of Controlled Release 2019, 313, 120. [DOI] [PubMed] [Google Scholar]
- [204].Yang X, Guo JL, Han J, Si RJ, Liu PP, Zhang ZR, Wang AM, Zhang J, Military Medical Research 2020, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Agier J, Brzezińska-Błaszczyk E, Żelechowska P, Wiktorska M, Pietrzak J, Różalska S, Journal of immunology research 2016, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206] a).Goudy K, Song S, Wasserfall C, Zhang YC, Kapturczak M, Muir A, Powers M, Scott-Jorgensen M, Campbell-Thompson M, Crawford JM, Ellis TM, Proceedings of the National Academy of Sciences 2001, 98, 13913. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gower RM, Boehler RM, Azarin SM, Ricci CF, Leonard JN, Shea LD, Biomaterials 2014, 35, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Li J, Wang Y, Zhu Y, Oupický D, Journal of Controlled Release 2013, 172, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208] a).Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Nature reviews genetics, 2014, 15, 541. [DOI] [PubMed] [Google Scholar]; b) Qu M, Kim HJ, Zhou X, Wang C, Jiang X, Zhu J, Xue Y, Tebon P, Sarabi SA, Ahadian S, Dokmeci MR, Nanoscale 2020, 12, 16724. [DOI] [PubMed] [Google Scholar]
- [209] a).Liechty WB, Scheuerle RL, Ramirez JEV, Peppas NA, International journal of pharmaceutics 2019, 562, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Knipe JM, Strong LE, Peppas NA, Biomacromolecules 2016, 17, 788. [DOI] [PubMed] [Google Scholar]
- [210].Lam JK, Chow MY, Zhang Y, Leung SW, Molecular Therapy-Nucleic Acids 2015, 4, e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211] a).Chouhan D, Dey N, Bhardwaj N, Mandal BB, Biomaterials 2019, 119267. [DOI] [PubMed] [Google Scholar]; b) Wang YW, Liou NH, Cherng JH, Chang SJ, Ma KH, Fu E, Liu JC, Dai NT, Journal of Investigative Dermatology 2014, 134, 2016. [DOI] [PubMed] [Google Scholar]; c) Castleberry SA, Almquist BD, Li W, Reis T, Chow J, Mayner S, Hammond PT, Advanced Materials 2016, 28, 1809. [DOI] [PubMed] [Google Scholar]
- [212].Kim HS, Yoo HS, Gene therapy, 2013, 20, 378. [DOI] [PubMed] [Google Scholar]
- [213].Jonas S, Izaurralde E, Nature reviews genetics, 2015, 16, 421. [DOI] [PubMed] [Google Scholar]
- [214].Aunin E, Broadley D, Ahmed MI, Mardaryev AN, Botchkareva NV, Scientific reports 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mulholland EJ, Dunne N, McCarthy HO, Molecular Therapy-Nucleic Acids 2017, 8, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Sheedy FJ, Frontiers in immunology 2015, 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Li D, Wang A, Liu X, Meisgen F, Grünler J, Botusan IR, Narayanan S, Erikci E, Li X, Blomqvist L, Du L, The Journal of clinical investigation 2015, 125, 3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Circulation 2012, 125, 2892. [DOI] [PubMed] [Google Scholar]
- [219].Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O’Neill LA, Masters SL, The Journal of Immunology 2012, 189, 3795. [DOI] [PubMed] [Google Scholar]
- [220].Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD, Nature 2008, 451, 1125. [DOI] [PubMed] [Google Scholar]
- [221].Haneklaus M, Gerlic M, O’Neill LA, Masters SL, Journal of internal medicine 2013, 274, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Ben-Shushan D, Markovsky E, Gibori H, Tiram G, Scomparin A, Satchi-Fainaro R, Drug delivery and translational research 2014, 4, 38. [DOI] [PubMed] [Google Scholar]
- [223].Li X, Li D, Wang A, Chu T, Lohcharoenkal W, Zheng X, Grünler J, Narayanan S, Eliasson S, Herter EK, Wang Y, Journal of Investigative Dermatology, 2017, 137, 2630. [DOI] [PubMed] [Google Scholar]
- [224] a).Angkawinitwong U, Courtenay AJ, Rodgers AM, Larrañeta E, McCarthy HO, Brocchini S, Donnelly RF, Williams GR, ACS Applied Materials & Interfaces 2020, 12, 12478. [DOI] [PubMed] [Google Scholar]; b) Zhang T, Qin XY, Cao X, Li WH, Gong T, Zhang ZR, Acta Pharmacologica Sinica 2019, 40, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Leone M, Romeijn S, Slütter B, O’Mahony C, Kersten G, Bouwstra JA, European Journal of Pharmaceutical Sciences 2020, 146, 105269. [DOI] [PubMed] [Google Scholar]; d) Zhou X, Brown BA, Siegel AP, El Masry MS, Zeng X, Song W, Das A, Khandelwal P, Clark A, Singh K, Guda PR, ACS nano 2020, 14, 12732. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Frydman GH, Olaleye D, Annamalai D, Layne K, Yang I, Kaafarani HM, Fox JG, Scientific Reports 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Lopez-Ramirez MA, Soto F, Wang C, Rueda R, Shukla S, Silva-Lopez C, Kupor D, McBride DA, Pokorski JK, Nourhani A, Steinmetz NF, Advanced Materials 2020, 32, 1905740. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Koh KJ, Liu Y, Lim SH, Loh XJ, Kang L, Lim CY, Phua KK, Scientific reports 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Wechsler ME, Stephenson RE, Murphy AC, Oldenkamp HF, Singh A, Peppas NA, Biomedical microdevices 2019, 21, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Zeng Y, Wang J, Wang Z, Chen G, Yu J, Li S, Li Q, Li H, Wen D, Gu Z, Gu Z, Nano Today 2020, 35, 100984 [Google Scholar]; j) Jamaledin R, Yiu CK, Zare EN, Niu LN, Vecchione R, Chen G, Gu Z, Tay FR, Makvandi P, Advanced Materials 2020, 32, 2002129. [DOI] [PubMed] [Google Scholar]; k) Zhu J, Zhou X, Kim HJ, Qu M, Jiang X, Lee K, Ren L, Wu Q, Wang C, Zhu X, Tebon P, Small 2020, 16, 1905910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Chen MC, Chen CS, Wu YW, Yang YY, Acta Biomaterialia 2020, 114, 183. [DOI] [PubMed] [Google Scholar]
- [226].Lee K, Xue Y, Lee J, Kim HJ, Liu Y, Tebon P, Sarikhani E, Sun W, Zhang S, Haghniaz R, Çelebi-Saltik B, Advanced Functional Materials 2020, 2000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227] a).Sun X, Ma Z, Zhao X, Jin W, Zhang C, Ma J, Qiang L, Wang W, Deng Q, Yang H, Zhao J, Bioactive materials 2020, 6, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S, Stem cells translational medicine 2012, 1, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Clegg JR, Wagner AM, Shin SR, Hassan S, Khademhosseini A, Peppas NA, Progress in Materials Science 2019, 106, 100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229] a).Zhao Y, Li Z, Song S, Yang K, Liu H, Yang Z, Wang J, Yang B, Lin Q, Advanced Functional Materials 2019, 29, 1901474 [Google Scholar]; b) Cai Y, Shen J, Yang CW, Wan Y, Tang HL, Aljarb AA, Chen C, Fu JH, Wei X, Huang KW, Han Y, Science advances 2020, 6, eabb5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230] a).Boateng JS, Matthews KH, Stevens HN, Eccleston GM, Journal of pharmaceutical science 2008, 97, 2892. [DOI] [PubMed] [Google Scholar]; b) Thomas S, Loveless P, Hay NP, Pharm J 1988, 240, 785 [Google Scholar]; c) Ramos-e-Silva M, de Castro MCR, Clinics in dermatology 2002, 20, 715.12490366 [Google Scholar]
- [231] a).Zhang Y, Chen F, Zhang Y, Du C, Tribology International 2020, 146, 106135 [Google Scholar]; b) Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y, Biomaterials 2013, 34, 422. [DOI] [PubMed] [Google Scholar]
- [232] a).Archana D, Singh BK, Dutta J, Dutta PK, Carbohydrate polymers 2013, 95, 530. [DOI] [PubMed] [Google Scholar]; b) Seisenbaeva GA, Fromell K, Vinogradov VV, Terekhov AN, Pakhomov AV, Nilsson B, Ekdahl KN, Vinogradov VV, Kessler VG, Scientific reports 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233] a).Raguvaran R, Manuja A, Manuja BK, Immunome Research 2015, 11, 1 [Google Scholar]; b) Sudheesh Kumar PT, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, Nair SV, Jayakumar R, ACS applied materials & interfaces 2012, 4, 2618. [DOI] [PubMed] [Google Scholar]
- [234].Borkow G, Gabbay J, Dardik R, Eidelman AI, Lavie Y, Grunfeld Y, Ikher S, Huszar M, Zatcoff RC, Marikovsky M, Wound repair and regeneration, 2010, 18, 266. [DOI] [PubMed] [Google Scholar]
- [235].Zhao X, Guo B, Wu H, Liang Y, Ma PX, Nature communications 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236] a).Omidi M, Yadegari A, Tayebi L, RSC advances 2017, 7, 10638 [Google Scholar]; b) Sun H, Gao N, Dong K, Ren J, Qu X, ACS nano 2014, 8, 6202. [DOI] [PubMed] [Google Scholar]
- [237] a).Ji H, Sun H, Qu X, Advanced drug delivery reviews 2016, 105, 176. [DOI] [PubMed] [Google Scholar]; b) Thangavel P, Kannan R, Ramachandran B, Moorthy G, Suguna L, Muthuvijayan V, Journal of colloid and interface science 2018, 517, 251. [DOI] [PubMed] [Google Scholar]
- [238].Pacelli S, Acosta F, Chakravarti AR, Samanta SG, Whitlow J, Modaresi S, Ahmed RP, Rajasingh J, Paul A, Acta biomaterialia 2017, 58, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239] a).Lee J, Kim J, Go J, Lee JH, Han DW, Hwang D, Lee J, Colloids and Surfaces B: Biointerfaces 2015, 135, 166. [DOI] [PubMed] [Google Scholar]; b) Akturk O, Kismet K, Yasti AC, Kuru S, Duymus ME, Kaya F, Caydere M, Hucumenoglu S, Keskin D, Journal of biomaterials applications 2016, 31, 283. [DOI] [PubMed] [Google Scholar]; c) Arafa MG, El-Kased RF, Elmazar MM, Scientific reports 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Kalantari K, Mostafavi E, Afifi AM, Izadiyan Z, Jahangirian H, Rafiee-Moghaddam R, Webster TJ, Nanoscale 2020, 12, 2268. [DOI] [PubMed] [Google Scholar]
- [241].Pourshahrestani S, Zeimaran E, Kadri NA, Gargiulo N, Jindal HM, Naveen SV, Sekaran SD, Kamarul T, Towler MR, ACS applied materials & interfaces 2017, 9, 31381. [DOI] [PubMed] [Google Scholar]
- [242].Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Hamblin P, Biomaterials 2006, 27, 4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243] a).Yang W, Owczarek JS, Fortunati E, Kozanecki M, Mazzaglia A, Balestra GM, Kenny JM, Torre L, Puglia D, Industrial crops and products 2016, 94, 800 [Google Scholar]; b)Kavoosi G, Dadfar SMM, Purfard AM, Journal of Food Science 2013, 78, E244. [DOI] [PubMed] [Google Scholar]
- [244].Fan Z, Liu B, Wang J, Zhang S, Lin Q, Gong P, Ma L, Yang S, Advanced Functional Materials, 2014, 24, 3933. [Google Scholar]
- [245].Hajji S, Khedir SB, Hamza-Mnif I, Hamdi M, Jedidi I, Kallel R, Boufi S, Nasri M, Biochimica et Biophysica Acta (BBA)-General Subjects, 2019, 1863, 241. [DOI] [PubMed] [Google Scholar]
- [246] a).Saddiqe Z, Naeem I, Maimoona A, J. Ethnopharmacol. 2010, 131, 511. [DOI] [PubMed] [Google Scholar]; b) Evandri MG, Battinelli L, Daniele C, Mastrangelo S, Bolle P, Mazzanti G, Food Chem. Toxicol. 2005, 43, 1381. [DOI] [PubMed] [Google Scholar]
- [247].Kwakman PH, e Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA, FASEB 2010, 24, 2576. [DOI] [PubMed] [Google Scholar]
- [248] a).Mangoni ML, McDermott AM, Zasloff M, Experimental dermatology 2016, 25, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li X, Fan R, Tong A, Yang M, Deng J, Zhou L, Zhang X, Guo G, International journal of pharmaceutics 2015, 495, 560. [DOI] [PubMed] [Google Scholar]
- [249].Nagy N, Kaber G, Kratochvil M, Kuipers H, Ruppert S, Yadava K, Yang J, Heilshorn S, Long A, Pugliese A, Bollyky P, bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Olekson MAP, Faulknor R, Bandekar A, Sempkowski M, Hsia HC, Berthiaume F, Wound Repair and Regeneration 2015, 23, 711. [DOI] [PubMed] [Google Scholar]
- [251].Justine RY, Janssen M, Liang BJ, Huang HC, Fisher JP, Acta Biomaterialia. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Barros SC, Martins JA, Marcos JC, Cavaco-Paulo A, Peptide Science 2012, 98, 576. [DOI] [PubMed] [Google Scholar]
- [253].Chen X, Zhou W, Zha K, Liu G, Yang S, Ye S, Liu Y, Xiong Y, Wu Y, Cao F, American journal of translational research 2016, 8, 3067. [PMC free article] [PubMed] [Google Scholar]
- [254].Li N, Luo HC, Yang C, Deng JJ, Ren M, Xie XY, Lin DZ, Yan L, Zhang LM, International journal of nanomedicine 2014, 9, 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Li N, Luo HC, Ren M, Zhang LM, Wang W, Pan CL, Yang LQ, Lao GJ, Deng JJ, Mai KJ, Sun K, ACS applied materials & interfaces, 2017, 90, 17417. [DOI] [PubMed] [Google Scholar]
- [256].Monaghan M, Browne S, Schenke-Layland K, Pandit A, Molecular Therapy 2014, 22, 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Li J, Kooger R, He M, Xiao X, Zheng L, Zhang Y, Chemical Communications 2014, 50, 3722. [DOI] [PubMed] [Google Scholar]
- [258].Lino MM, Simões S, Vilaça A, Antunes H, Zonari A, Ferreira L, ACS nano 2018, 12, 5207. [DOI] [PubMed] [Google Scholar]
- [259].Kumar M, Gupta P, Bhattacharjee S, Nandi SK, Mandal BB, Biomaterials 2018, 187, 1. [DOI] [PubMed] [Google Scholar]