Abstract
Combined angiography-CT (angio-CT) systems, which combine traditional angiographic imaging with cross-sectional imaging, are a valuable tool for interventional radiology. Although cone-beam CT (CBCT) technology from flat-panel angiography systems has been established as an adjunct cross-sectional imaging tool during interventional procedures, the intrinsic advantages of angio-CT systems concerning superior soft-tissue imaging and contrast resolution, along with operational ease, have sparked renewed interest in their use in interventional oncology procedures. Owing to increases in affordability and usability due to an improved workflow, angio-CT systems have become a viable alternative to stand-alone flat-panel angiographic systems equipped with CBCT. This review aims to provide a comprehensive technical and clinical guide for the use of angio-CT systems in interventional oncology. The basic concepts related to the use of angio-CT systems, including concepts related to workflow setup, imaging characteristics, and acquisition parameters, will be discussed. Additionally, an overview on the clinical applications and the benefits of angio-CT systems in routine therapeutic and palliative interventional oncology procedures will be reviewed.
Keywords: Ablation Techniques, CT-Angiography, Interventional-Body, Interventional-MSK, Chemoembolization, Embolization, Radiation Therapy/Oncology, Abdomen/GI, Skeletal-Axial
Supplemental material is available for this article.
© RSNA, 2021
Keywords: Ablation Techniques, CT-Angiography, Interventional-Body, Interventional-MSK, Chemoembolization, Embolization, Radiation Therapy/Oncology, Abdomen/GI, Skeletal-Axial
Summary
The combination of cross-sectional imaging from the CT scanner and fluoroscopic imaging from the angiography system allows for more precise planning of oncologic interventions, combining multiple therapies in the same setting and monitoring treatment responses intraprocedurally, which both minimizes complications and improves therapeutic outcomes.
Essentials
■ Cross-sectional imaging plays a critical role in interventional oncology as it provides useful three-dimensional soft-tissue information for tumor identification and monitoring of therapy response.
■ Combined angiography-CT (angio-CT) systems are equipped with a helical sliding gantry CT unit on rails and a fully functioning angiographic unit.
■ Angio-CT systems outperform diagnostic intravenous CT for hepatocellular carcinoma detection and are also superior to cone-beam CT (CBCT) hepatic angiography for transarterial chemoembolization planning.
■ Radiation dose exposure to the patient is similar for angio-CT and CBCT.
Introduction
Cross-sectional imaging plays a critical role in interventional oncology as it provides useful three-dimensional soft-tissue information for tumor identification and monitoring of therapy response. Cross-sectional imaging during fluoroscopically guided procedures is typically performed with angiography systems equipped with a flat-panel detector equipped with cone-beam CT (CBCT) technology or with a CT scanner integrated with the angiography system, also known as a combined angio-CT system (1–4). Although angio-CT systems were introduced earlier than CBCT technology, their adoption was limited due to the added cost of installing a CT scanner in the standard angiography room. Additionally, there were challenges in multimodality imaging integration, prompting the use of CBCT as the cross-sectional modality of choice in clinical practice. The routine adoption of cross-sectional imaging during fluoroscopically guided procedures has led to advances in tumor visualization, treatment guidance, and response assessment (5,6). Moreover, the ability to use two imaging modalities interchangeably in an angio-CT system simplifies the procedure workflow for procedures that require both cross-sectional imaging and fluoroscopy imaging, which improves the patient experience and outcomes (1,3,5–11).
Recent improvements in the integration of combined angio-CT systems, along with their improved affordability, have renewed the interest in use of this modality as an alternative to flat-panel angiographic systems equipped with CBCT for interventional oncology procedures. However, given the widespread experience with CBCT technology and the historical prohibitive costs of angio-CT systems, there is currently very limited literature on the clinical use of angio-CT systems in a comprehensive manner in interventional oncology (2).
In this review, we aim to discuss the status of angio-CT systems in interventional oncology. First, we discuss basic concepts related to room setup, patient positioning, angio-CT imaging characteristics, and acquisition parameters. Additionally, we review current clinical applications of angio-CT systems for different therapeutic and palliative oncologic procedures performed at our tertiary cancer center.
Technical Background and Room Setup
Brief History
As the number and complexity of interventional procedures have increased over the last 4 decades, a number of attempts were made to combine cross-sectional radiography-based imaging and fluoroscopy to facilitate treatment guidance and response assessment (12). Several approaches were investigated for combining these two pieces of medical equipment in the same procedure room, including integrating a portable C-arm fluoroscope into a CT room (13–15), integrating a portable CT scanner into an angiography suite (16), or installing a permanently mounted CT scanner and a C-arm fluoroscope in the same room (17,18).
Among those, the first combined angio-CT system was developed in 1992 by Yasuaki Arai (19) at the Aichi Cancer Center in Japan. Combined angio-CT systems are equipped with a helical sliding gantry CT unit on rails and a fully functioning angiographic unit. A single patient table is used for both CT and angiography, obviating patient transportation between CT and fluoroscopy. The CT scanner is installed at the head end of the table and moves on rails for positioning during imaging. This solution not only provides efficient cross-sectional imaging but also decreases the risks of catheter dislodgement and infections due to patient transportation. Earlier installation of these systems had very basic integration between the two modalities, almost operating independently of each other, which required the operator to fuse the imaging information. In recent years, several technological improvements in angiography and CT systems, as well as better integration of imaging information between the modalities, facilitated procedure workflow. For instance, CT imaging–generated information can be analyzed and processed with the angiography workstation and overlaid on fluoroscopic images without any need for external imaging registration. Additionally, advanced imaging, such as CT perfusion, subtraction, and dual-energy CT, can be performed during the intervention. Such incremental advancements have made angio-CT systems increasingly desirable in the interventional oncology community.
Room Installation Planning
Combined angio-CT systems comprise three distinct moving parts: the patient table, the C-arm fluoroscope, and the sliding gantry CT scanner, with the two imaging systems sharing the same patient table (Fig 1). The incorporation of two imaging systems at the same location and the flexibility to use either system by itself or both systems at the same time creates additional operational complexity with respect to safety hazards, as well as personnel and equipment positioning.
Figure 1:

Panoramic view of a combined angiography-CT system. The system includes (A) a sliding gantry CT scanner, (B) a C-arm fluoroscope, and (C) a patient table. Note the anesthesia equipment located at the end of the patient table, allowing the free space between the CT scanner and the patient table (highlighted floor in red). Inset: Sliding gantry CT scanner positioned for liver CT imaging acquisition. Note the limited space between the CT scanner and the C-arm fluoroscope (arrow), which precludes placement of anesthesia equipment in this location.
To minimize operational complexities, attention must be paid toward room planning prior to installation of an angio-CT system. Three elements are helpful: adequate room size, use of a site-specific real-size mock-up, and involvement of the team that will use the angio-CT system. It is suggested that the minimal acceptable room size for accommodating an angio-CT system is at least 50 m2, which is 66% larger than a traditional angiography room (20). This extra space is required so that the sliding gantry CT scanner can have a stationary location in the room. However, different vendors offer custom-fit angio-CT systems with different space requirements, and it is imperative to understand the space that will be required for the particular vendor’s system. The use of site-specific real-size mock-ups, scale models, or virtual reality simulations can help planners understand the actual interactions of the angio-CT system with other pieces of equipment in the room and with personnel. Finally, during room planning, it is essential to involve a multiprofessional and multidisciplinary team that includes key staff who will work in the environment to seamlessly integrate the other procedural equipment and components such as anesthesia gas outlets, electrocardiography cables, and intravenous poles with the angio-CT system.
Anesthesia Personnel and Equipment Considerations
Understanding the implications of different possible room setups is critical not only to perform procedures safely, but also to ensure that anesthesia personnel and equipment are positioned adequately. In a standard angiography room, anesthesia personnel and equipment are positioned adjacent to the patient’s head, at the head end of the patient table away from the C-arm. Similarly, in a traditional CT room, anesthesia personnel and equipment are usually positioned adjacent to the patient’s head, either away from the CT gantry (patient positioned with feet closer to the gantry) or behind the CT gantry (patient positioned with head closer to the gantry). In a combined angio-CT room, locating the anesthesia personnel and equipment adjacent to the patient’s head would obstruct the movement of the sliding gantry CT scanner toward the patient. Therefore, it is recommended that anesthesia personnel and equipment be positioned close to the patient’s feet, away from the moving parts of the angio-CT system (Fig 1). Although this is the preferred setup at our center, other centers position anesthesia equipment on the head side and route all the anesthesia cables through the CT gantry. However, with such a setup, extension cables are necessary, and extreme care must be taken while the gantry is moved to avoid cable disconnection and entangling.
Given the flexibility and versatility of angio-CT systems, they can be used for angiography-only or CT-only procedures. In such scenarios, it is still advantageous to set up the anesthesia as if the procedure was going to use both imaging modalities, as reverting room setup for a single imaging modality may limit the use of the other imaging system. For example, a good practice is to keep the rails clear of any equipment so that the CT gantry can be rolled in as needed, even if not anticipated. Finally, prompt and unobstructed access to the patient’s head by anesthesia personnel should be considered of utmost importance during room setup for urgent patient rescue, if required.
Patient Positioning
For procedures where angiography will be used in isolation or in combination with CT, the patient should be positioned with head close to the CT gantry, similarly to patient positioning in stand-alone angiography rooms. Positioning the patient with feet close to the CT gantry will greatly limit the scan range to the patient’s upper midsection, as the patient table base will preclude the range of imaging acquisition. For CT guided–only procedures, it is advised to position the patient with the target organ as close as possible to the CT gantry. For instance, for pelvic interventions, a patient should be positioned with the feet close to the CT gantry, whereas for lung intervention, a patient should be positioned with the head close to the CT gantry. Such positioning strategies allow for minimizing the sliding gantry CT movement, as well to keep the target organ away from the base of the patient table, which would limit the use of fluoroscopy in case needed.
During flat-panel CBCT, a patient’s arms are positioned above the head and outside the field of view to reduce beam-hardening artifacts and mitigate the risk of collision between the patient and the moving parts of the angiography system. For combined angio-CT systems it is recommended, but not required, to move the patient’s arms away from the field of view given that postprocessing CT reconstruction algorithms can substantially reduce artifacts associated with beam hardening (21). Moreover, as the moving parts of the CT system are enclosed within the CT gantry, there is no risk that moving parts will hit the patient. However, for superior image quality, it is still recommended that the patient’s arms be folded on the chest as opposed to all the way on the head. Finally, care must be taken during radial access procedures during CT. The accessory arm board must be either removed or tucked inside close to the patient such that the CT gantry can move freely toward and around the patient without any collisions.
Imaging Performance of Angio-CT Systems
Comparison with Traditional Intravenous CT
Prior to the development of angio-CT systems, the combination of CT during hepatic arteriography (CTHA) and CT during arterial portography (CTAP) for diagnosis of hepatocellular carcinoma (HCC) was not widely used because of its invasiveness and the complexity of transporting patients between the angiography and CT rooms (12). The early literature on the use of combined angio-CT systems for detection of HCC demonstrated limited use of this method when compared with traditional triphasic intravenous contrast–enhanced CT and MRI (22–24). Nevertheless, combined CTHA and CTAP can outperform diagnostic CT (25,26) and affect choice of treatment, especially in those patients presenting with HCC recurrence (25) (Fig 2). The use of CTHA and CTAP during transarterial hepatic therapy procedures has been demonstrated to be a highly sensitive method not only to identify liver tumors and their hepatic feeding arteries but also to detect important extrahepatic feeding arteries (27,28) and hepatic arterial variations (Fig 3). Ozaki et al analyzed the visualization of extrahepatic feeding arteries at digital subtraction angiography (DSA) and CTHA; they reported that use of CTHA resulted in higher frequencies of detection of extrahepatic arterial branches than DSA (98.0% vs 40.6% for the same 943 patients) (29).
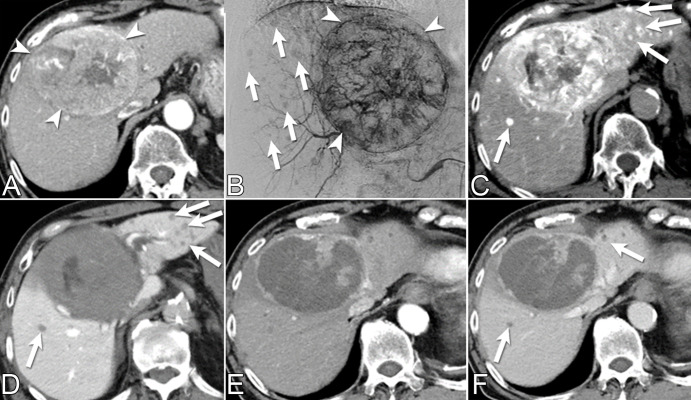
Figure 2:
Multiple incidental small hepatocellular carcinomas (HCCs) detected with CT during hepatic arteriography (CTHA) and CT arterial portography (CTAP) during transarterial chemoembolization (TACE) for a patient presenting with a recurrent HCC. (A) Diagnostic quadriphasic contrast-enhanced CT performed 3 weeks before TACE shows solitary large central HCC (arrowheads). (B) Pre-TACE digital subtraction angiographic image from the common hepatic artery shows large hypervascular tumor (arrowheads) and small hypervascular nodules of unclear clinical significance (arrows). (C, D) CTHA and CTAP demonstrate multiple small hypervascular tumors with portal venous washout (arrows), compatible with incidental HCCs. (E, F) One month post-TACE follow-up contrast-enhanced CT arterial phase and portal venous phase scans help confirm treated large HCC and satellite hypovascular nodules (arrows in F). The ability to obtain arterial and portovenous images with combined angio-CT systems allows prompt differentiation between true HCCs and other regenerative and dysplastic liver nodules.
Figure 3:
Extrahepatic tumor supply detected with CT during hepatic arteriography (CTHA) in a patient with multifocal hepatocellular carcinoma. (A) Image from CT during arterial portography shows hypoattenuating tumors in segments 7, 5, and 6 (arrows). (B) Image from CTHA performed from common hepatic artery demonstrates partial tumor enhancement (arrow). (C) Digital subtraction angiography (DSA) image of A7 shows tumor blushes (arrows) and vague enhancement corresponding to S7 tumor (arrowhead). (D) Left: DSA of the right inferior phrenic artery. Middle and right: CTHA and DSA images, respectively, from the distal right inferior phrenic artery demonstrate extrahepatic arterial supply to the S7 tumor arising from the right inferior phrenic artery (arrow and circle). (E) Image from noncontrast CT after conventional transarterial chemoembolization performed via A7 and the right inferior phrenic artery shows adequate lipiodol deposition within the entire extent of the S7 tumor (arrow).
Comparison with CBCT
Although both CTHA and CBCT during hepatic arteriography (CBCTHA) can provide adequate information for treatment planning, fundamental differences exist between these two modalities with respect to imaging acquisition, reconstruction algorithm, and noise-reducing ability. In a comparative analysis of 181 transarterial chemoembolization (TACE) procedures where CBCTHA (n = 111) or CTHA (n = 70) was used for TACE planning, the image quality of CTHA was superior to that of CBCTHA for TACE planning in respect to tumor visualization, tumor arterial feeder identification, streaking artifact reduction, field of view encompassing the whole liver, and overall subjective image quality; there were fewer breathing motion artifacts with CTHA (21). Even though the spatial resolution of CBCT is superior to that of CT (30), CTHA still provides adequate spatial and contrast resolution to identify the tumor and its arterial feeder vessels.
Comparison of radiation dose between CT and CBCT is challenging. Different dose indexes are reported for CT compared with CBCT: dose-length product (DLP) for CT and kerma-area product (PKA, also referred to as dose-area product) for CBCT. DLP and PKA are not directly comparable, and a typical approach toward comparison has been to estimate effective dose from DLP and PKA using body region–specific conversion factors (31). In CBCT, these factors are also procedure (ie, projection) specific (31). According to a recent study, the radiation dose of angio-CT is less than the radiation dose of CBCT when automatic exposure control is used (32). If noise levels are matched between angio-CT and CBCT, the radiation dose is approximately the same for angio-CT and CBCT. Two recent clinical studies substantiated such findings. In an analysis of 22 patients undergoing transarterial radioembolization who underwent 178 individual scans, the mean effective dose between individual CT and CBCT scans was found to be comparable (33). In another study of 114 TACE procedures, authors demonstrated that total effective dose was 2.5 times lower at angio-CT when compared with CBCT (32).
Imaging Acquisition Parameters for CT during Angiography
The imaging parameters and injection protocols used in our center for intra-arterial CT angiography of different organs and clinical applications are described in Figure 4. These protocols take into consideration the need for optimal contrast media presence at the target organ as the gantry is translated over the patient. This differs from CBCT injection protocols given the fact that the region of interest is continuously sampled during the entire C-arm rotation for CBCT imaging acquisition. In addition, as CT acquisition is fast and easily repeatable, multiphasic angiographic images can be acquired as well, which provides additional information about tumor characteristics.
Figure 4:
Imaging and contrast media injection parameters for intra-arterial CT angiography according to distinct clinical applications. For clinical situations not depicted, digital subtraction angiography with catheter located at the site of desired contrast material injection for CT image acquisition might be helpful to provide guidance for optimal injection and acquisition parameters. CTA = CT during arteriography, CTAP = CT during arterial portography, CTHA = CT during hepatic arteriography, CTRA = CT during renal arteriography, Fr. = French, ROI = region of interest, SMA = superior mesenteric artery. * = need for noncontrast CT phase at the operating physicians’ discretion. † = If clinically indicated for “washout” HCC detection.
Clinical Applications
Treatment of Hepatic Malignancies
Transarterial therapies.— Angio-CT has been demonstrated to be an accurate modality for detecting HCC for TACE (23,25). Tumor arterial and portal blood supplies can be independently evaluated with CTHA and CTAP (26). The improved sensitivity of CTHA and CTAP in the detection of HCC can be attributed to the contrast material being administered selectively to tumors with CTHA or to hepatic parenchyma with CTAP (25). It has been reported that the sensitivity of combined CTHA and CTAP for HCC detection is 89%–95% (34,35).
Similar to CBCTHA, CTHA can be used to identify tumor feeder vessels and hepaticoenteric collaterals (29) that cannot be routinely depicted with intravenous multidetector CT and MR angiography (36). Also, the high contrast resolution and faster scan speed acquisition of CTHA reduces the frequency of streaking artifacts around arterial tumor feeder vessels that is associated with CBCTHA (37), improving overall imaging quality (2).
New generation angiography systems are equipped with CBCT-based navigational guidance packages such as embolization guidance software that identifies arterial feeder supply to the lesion or needle guidance software for percutaneous needle guidance procedures (2). Recent advancements in angio-CT setup allow the use of such software packages available on the angiography system to be used with the CT scans acquired during the procedure, thus allowing seamless information exchange between the two modalities (38). For example, the use of embolization guidance software to assist with catheter navigation in angio-CT systems also allows selection of the intended target vessel(s) and performance of focused CTHA to confirm proper vessel selection (Fig 5).
Figure 5:
Images from CT during hepatic arteriography (CTHA)–based embolization guidance software in a patient with an angiographically occult hepatocellular carcinoma (HCC). (A) Image from pretransarterial chemoembolization (preTACE) MRI shows a 1.5-cm HCC on segment IV (arrow). (B) CTHA image depicts the target HCC (arrow). (C) Left: Software-aided catheterization via fluoroscopy guidance of the feeder artery (*). Right: Selective digital subtraction angiography (arrowhead) of the target artery did not show tumor hypervascularity. (D) Image from CTHA performed with the microcatheter at the same location shows tumor blushing (arrow). On the basis of that information, TACE was carried out uneventfully. Intravenous follow-up CT performed 30 days after TACE demonstrated complete response of the treated HCC according to modified Response Evaluation Criteria in Solid Tumors (not shown).
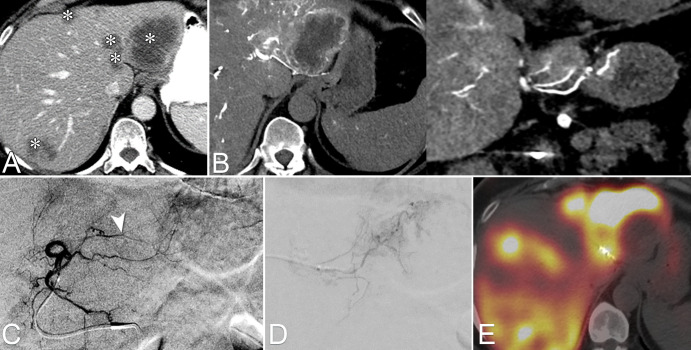
In patients undergoing yttrium 90 preparation with administration of technetium 99m macroaggregated albumin (99mTc-MAA), CTHA can be used to identify hepaticoenteric collaterals not normally seen on DSA images (Fig 6). Because the larger field of view in conventional CT always encompasses the whole liver, CTHA can be used to compute liver, lobar, and segmental volumes for dosimetry calculations. More recently, CTHA-based relative enhancement was also shown to be an accurate surrogate for 99mTc-MAA deposition–based tumor to normal liver tissue ratio, indicating that CTHA might become a useful alternative to SPECT/CT 99mTc-MAA for partition and voxel dosimetry methods (39).
Figure 6:
Images from CT during hepatic arteriography (CTHA) to identify hepaticoenteric collaterals not seen on digital subtraction angiography (DSA) images. (A) Multifocal colorectal liver metastasis to both hepatic lobes (*). (B) Axial and coronal CTHA reconstructions demonstrate an accessory right gastric artery arising from segment II arterial branch. (C) Pre-embolization DSA from the left hepatic artery. On the basis of CTHA images, the arterial branch consisting of the accessory right gastric artery was identified (arrowhead). (D) Selective DSA image depicts the right gastric artery, which was selected and embolized with coils. (E) Image from SPECT-CT with technetium 99m macroaggregated albumin (99mTc-MAA) obtained after embolization of the accessory right gastric artery shows lack of gastric 99mTc-MAA deposition.
Ablative therapies.— Proper identification of hepatic tumors for accurate ablation probe placement is the initial step in ablation procedures. In patients with primary liver cancer, liver parenchyma heterogeneity related to cirrhotic background might make it difficult to accurately identify the target tumor(s) with US. Chemotherapy-related steatohepatitis and sinusoidal obstruction could cause similar issues for tumor identification in patients with secondary liver cancer. Therefore, the ability to identify and target the tumors under US imaging guidance during ablation procedures can be negatively impacted in these patients. In such scenarios, use of CT imaging with intravenous contrast medium injection greatly facilitates lesion identification and proper planning of probe placement (40).
More recently, several studies have demonstrated the importance of achieving adequate ablation margins to improve outcomes after treatment of primary and secondary liver cancers (41–43). Considering those findings, the improved contrast resolution of CT is a major advantage point to monitor ablation margins in a three-dimensional fashion, therefore potentially increasing the local tumor control rates. With this aim, investigators have been using CTHA for intraprocedure tumor identification (10) and ablation margin monitoring (Movie 1). The main advantage of this approach is the reduced amount of contrast medium required to obtain contrast-enhanced CT images through intra-arterial injection of contrast medium (16 mL to 20 mL) into the hepatic artery as opposed to the systemic intravenous injection (10,44). This approach also facilitates repeated imaging throughout the procedure with minimal contrast load and facilitates the ability to monitor the size and shape of the ablation margins in near real time, helping the operator achieve adequate tumor coverage with desired margins while avoiding thermal damage to adjacent nontarget structures. Puijk et al compared their experience with this approach in 108 patients with colorectal liver metastasis who underwent 156 percutaneous ablation procedures (114 ablation procedures performed under CT hepatic arteriography guidance vs 42 ablation procedures performed under CT fluoroscopy guidance) (10,44). No complications related to transarterial catheter procedure were reported. Two-year local tumor progression-free survival (LTPFS) was significantly improved toward CT hepatic arteriography versus CT fluoroscopy (18 of 202 [8.9%] vs 19 of 58 [32.8%], P < .01, respectively). Use of CT hepatic arteriography was also considered a positive predictor for longer LTPFS, as also demonstrated on multivariate analysis (hazard ratio = 0.41; 95% CI: 0.19, 0.90; P = .025) (10,44). Despite such positive results, the added complexity and potential additional risks related to transarterial access for hepatic angiography should be factored. In this scenario, combined angio-CT systems have a unique advantage as providing arterial access without having to move the patient physically from a fluoroscopy room to a CT room.
Movie 1:
Use of a combined angio-CT system for performing percutaneous liver ablation on a patient with prostate cancer metastasis. Preablation diagnostic CT scan shows hypoattenuating tumors at segment VIII. Intraprocedural CTHA scan demonstrates hypervascular tumors. Microwave antenna percutaneous placement was performed via fluoroscopy imaging guidance aided by needle-guidance software with fusion of CT and fluoroscopy data sets. Intra- and postablation CTHA shows adequate ablation coverage of the target tumors. CTHA = CT during hepatic arteriography.
One of the key contributors to suboptimal ablation margins is the presence of heat-sink effect due to vascular structures adjacent to the target tumor or the hypervascularity commonly associated with some types of cancer. Angio-CT can be a useful tool to perform temporary occlusion of major hepatic vessels adjacent to the tumor (Movie 2) or tumor embolization with the aim of vascular deprivation prior to the ablation procedure (40).
Movie 2:
Percutaneous liver ablation of an epithelioid hemangioendothelioma tumor assisted by intermittent balloon occlusion of the adjacent right hepatic vein. (A) Digital subtraction venogram of the right hepatic vein after percutaneous US-guided transhepatic access. Note the proximity of the microwave antenna placed within the tumor (*). (B) Intermittent balloon occlusion of the right hepatic vein during ablation of the target tumor. (C) Postablation intravenous CT scan shows ablation zone and deflated balloon catheter at the right hepatic vein. Primary efficacy was achieved. The patient remains without local tumor progression 16 months after the procedure.
Combination therapies.— The combination of transarterial embolization (TAE) or TACE with ablation is used to treat both primary and secondary liver cancers (45–47). For primary liver cancers, TAE or TACE is typically performed first, with the intent of achieving tumor devascularization and reduction of heat-sink effect; for secondary liver cancers, ablation is performed first, followed by TACE, with the intent of taking advantage of the area of hyperemia around the ablation margins to optimize chemotherapy delivery. Regardless of the treatment sequence, these combined therapies are usually performed within 24 hours of each other. The lag time between procedures is not based on biologic considerations but rather is mainly dictated by availability of the angiography and CT rooms. With the incorporation of angio-CT systems, both procedures can be performed during the same intervention, optimizing procedural workflow and minimizing patient length of stay (Fig 7). Additionally, the combined approach provides opportunities to investigate timing for performing transarterial and ablative therapies for optimal ablation zones and drug delivery (48).
Figure 7:
Images from combination of transarterial chemoembolization (TACE) and ablation using an angio-CT system in a 58-year-old woman presenting with a 2.7-cm solitary hepatocellular carcinoma on the left hepatic lobe. (A) Image from digital subtraction angiography performed at the level of A4 artery demonstrates tumor blush (arrow). (B) CT scan during hepatic arteriography at the level of the common hepatic artery demonstrates hypervascular tumor at the segment IV (arrow). (C) Image from percutaneous ablation under CT guidance performed with two microwave antennas. Following the ablation, TACE was carried out to address any potential residual tumor areas due to heat-sink effect adjacent to the paraumbilical vein. (D) Immediate intraprocedure intravenous CT scan shows successful chemoembolization and ablation of the target tumor (arrow). Note patent paraumbilical vein (*). (E) CT scan 6 months after the ablation helps confirm successful procedure with lacelike arterial enhancement at the treated tumor (circle).
Treatment of Musculoskeletal Malignancies
Minimally invasive treatment of musculoskeletal metastases can achieve local disease control to improve quality of life with minimal disruption of osseous structures. Depending on the disease manifestation, several different minimally invasive procedures can be considered, including embolization, sclerosis, percutaneous thermal ablation, vertebral augmentation, cementoplasty, and percutaneous screw fixation (49,50). A single imaging modality may not be sufficient because of the high variability in musculoskeletal tumor biology, location, size, and vascularity (38).
Safer and more effective approaches may require a combination of treatments under multimodality imaging guidance. Integrated multimodality imaging equipment within the same procedure room, such as angio-CT, can facilitate sequential procedures and protective measures, thus enabling a safe, diversified, and individualized therapy. For example, in a patient with a vascular lytic bone tumor, embolization may be deemed critical before ablation and consolidative stabilization to reduce bleeding risk and augment the outcome of ablation through devascularization (51–53). The embolization is performed under fluoroscopy guidance, while the ablation and stabilization are performed under CT guidance. With a combined angio-CT unit, both procedures can be completed in the same procedural setting (Fig 8).
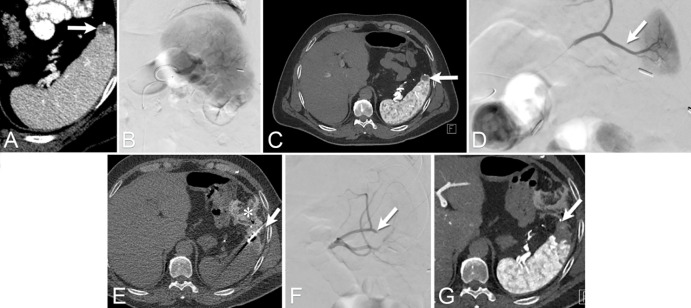
Figure 8:
Images from CT fluoroscopy for embolization followed by ablation and stabilization of lytic bone tumors in a 66-year-old man with thyroid cancer metastatic to the left glenoid bone. (A, B) PET/CT scan shows the metastasis (arrow) resulting in both resting and mechanical pain that severely impaired the quality of life. Given the large size, comparative hypervascularity to surrounding muscle, and location adjacent to the shoulder joint, a comprehensive combination treatment using angio-CT was pursued. (C) Image from fluoroscopically guided embolization performed via left radial artery access shows pre-embolization tumor blush (arrow). (D) Successful embolization of the tumor with minimal residual tumor blush (arrow). After the embolization, the patient was immediately repositioned prone. (E, F) CT scans show the cryoablation of the tumor and the therapeutic ice ball (arrow in E), which was followed by percutaneous injection of polymethyl methacrylate for stabilization, and demonstrate cementoplasty results filling tumor void in the glenoid bone (arrow in F). The patient regained range of motion in the shoulder, with ability to hyperextend the arm above the head. (G, H) Follow-up CT scans 18.5 months after the procedure demonstrate stability of the treatment area, without progression of fracture or tumor.
Angio-CT can also enable complex treatments that would not be possible with a single imaging modality. For example, large tumors in the pelvis may be difficult to treat because of aggressive underlying tumor biology and higher likelihood of abutting adjacent critical structures, and a combination of polymethyl methacrylate cement injection and percutaneous screw placement may be needed to achieve meaningful stabilization (54,55). To address such challenges, multiple imaging modalities can be used to optimally delineate the lesion, permit more aggressive therapy through a combination of minimally invasive treatments, and monitor for potential complications (Fig 9).
Figure 9:
Images from integrated fluoroscopy and CT for palliative treatment in an 87-year-old woman with cholangiocarcinoma metastatic to the iliac bone. (A, B) Images from contrast-enhanced CT and MRI, respectively, show the metastasis (arrow) resulting in both resting and mechanical pain that severely impaired quality of life. Given the large tumor size and instability from the osseous defect, a comprehensive combination treatment with local-regional control by cryoablation followed by fixation by internal cemented screw procedure was pursued. (C) Image from CT-guided cryoablation performed via a posterior approach shows the cryoprobe placement (arrow). After completion of cryoablation, (D) fixation and injection of polymethyl methacrylate (PMMA) cement was performed under fluoroscopic guidance. Image shows screws and cement needle (arrow). (E, F) Oblique sagittal and axial postprocedure CT images confirm screws and PMMA (arrows).
Treatment of Genitourinary Malignancies
Renal cell carcinoma (RCC) comprises a diverse group of malignancies arising from renal tubular epithelial cells and is one of the 10 most common cancers worldwide. Clear cell RCC is the most common subtype, accounting for 75% of all RCC cases (56). Despite recent advances in systemic therapies, the mortality rate for patients with metastatic RCC is high (57). Most patients with RCC present with localized disease, which provides an opportunity for aggressive local control to prevent systemic spread. For such patients, standard treatment options include surgical resection and thermal ablation. Large series have demonstrated that percutaneous ablation has oncologic outcomes similar to those of surgery for patients with stage T1a (≤4 cm) RCC tumors (58). However, for larger tumors such as T1b tumors (4.1–7 cm), rates of primary efficacy and local tumor control following RCC ablation have been reported to be lower when radiofrequency ablation or microwave ablation are applied (59,60). There are several reasons why achieving complete response in larger RCCs is challenging. Incomplete coverage of the target lesion may occur because of poor visualization of the full extent of the lesion at procedural imaging. Thermal sink effects from larger blood vessels nourishing the target tumor can diminish the size of the adjacent ablation zone (61). Some margins of large lesions may be adjacent to critical structures such as the renal pelvis or the ureter, precluding comprehensive coverage with an ablation zone. Furthermore, larger lesions require a greater number of ablation needles, potentially increasing the risk for procedural bleeding. A potential solution to the challenges posed by large RCC lesions is to combine thermal ablation with TAE (62–64). TAE is a well-established curative therapy for benign renal tumors such as angiomyolipoma and has also been used successfully to palliate symptoms from RCC such as bleeding or mass effect (65–67). From our experience, when performed in combination with thermal ablation using angio-CT technology, TAE provides several distinct benefits. The contrast enhancement of the target RCC lesion following TAE provides ideal visualization of the tumor margins to ensure that the subsequently performed ablation covers the full extent of the lesion. Embolization of intratumoral vasculature from TAE ensures that target temperatures can be achieved within the ablation zone without loss of energy due to heat-sink effect. This also adds the potential to reduce the risk of hemorrhage and bleeding complications. For areas of the target lesion that are high risk for ablation because of the proximity to critical structures, TAE can provide complementary oncologic effect by embolization (68) (Fig 10).
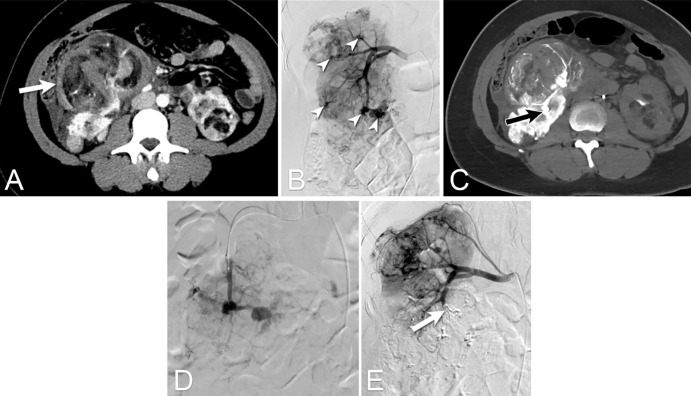
Figure 10:
Images from combination transarterial embolization (TAE) and cryoablation for renal cell carcinoma (RCC) using angio-CT in a 90-year-old man presenting with symptoms suggestive of RCC. (A) Contrast-enhanced CT scan shows a 4.3-cm solid enhancing mass in the upper pole of the left kidney, suggestive of RCC. Given the lesion’s size, a combination TAE and cryoablation procedure was performed. (B) TAE using a lipiodol-ethanol mixture was performed under fluoroscopic guidance followed immediately by (C, D) percutaneous cryoablation. Note that the residual contrast staining from the embolization allowed for clear visualization of the target lesion during the ablation.
Treatment of Other Malignancies
The ability to have real-time imaging provided by fluoroscopy in combination with three-dimensional thin-section soft-tissue imaging provided with CT in an angio-CT room can also be used to perform some nonroutine high-risk oncologic procedures where safety is the main concern. For instance, splenic interventions are known to be high risk associated with an increased chance of bleeding because of the prominent vascular supply of this organ. Due to such risks, ablation of splenic metastasis has been only sporadically reported in the literature, with varying degrees of success (69,70). In such circumstances, splenic angiography and transarterial embolization might be employed during percutaneous ablation for immediate assessment and treatment of bleeding-related complications (Fig 11).
Figure 11:
Images from spleen tumor ablation in a 58-year-old man with prior history of colorectal cancer at the splenic flexure with pericolonic adipose tissue invasion, liver metastasis, and enlarging splenic lesion concerning for a spleen metastasis. (A) Diagnostic CT scan shows the spleen metastasis (arrow). (B) Digital subtraction angiography (DSA) image of the splenic artery. (C) CT angiographic image of splenic artery depicts the target lesion (arrow). Note metallic surgical clip anterior to it. (D) DSA image shows microcatheter selection of the polar branch (arrow) supplying the spleen parenchyma adjacent to the lesion. Embolization of this branch was performed with 900-μ beads. (E) CT scan shows microwave antenna (arrow) placement at the target tumor after successful hydrodissection with the displacement of the adjacent colon (*). Immediately after ablation completion and microwave antenna probe removal, the patient became hypotensive and bradycardic. Using the microcatheter located at the splenic artery, (F) DSA image demonstrates successful obliteration of the arterial branch (arrow) and absence of contrast extravasation. The patient became normotensive, and the symptoms were attributed to a self-limited vasovagal event. (G) Postablation CT angiographic image of splenic artery depicts ablation defect at the spleen. The patient remains without local tumor progression at the spleen 3 years after the procedure.
Other Applications
While the most common oncologic applications of angio-CT systems are in transarterial hepatic embolization and hepatic tumor ablation, a small number of case reports have demonstrated the value of angio-CT systems in other procedures, such as challenging abscess drainage, extrahepatic tumor embolization or ablation, and percutaneous nephrostomy (2,71,72).
A valuable application of angio-CT systems is in the trauma environment, where these systems are used to obtain timely diagnostic CT angiographic images and, if warranted, guide therapeutic intervention without the need to transport patients to a different room. This combination of diagnostic and intervention capabilities in the same physical environment reduces time between diagnosis and definitive therapy and is thought to be associated with improved outcomes. Similarly, in interventional oncology, angio-CT systems can be used to perform traditional diagnostic CT angiography in the interventional radiology room for hemodynamically stable patients suspected of having acute bleeding, which minimizes the time between diagnostic CT and any required intervention.
Alternatively, in patients with active bleeding episodes in whom TAE is anticipated or who are clinically unstable, diagnostic intra-arterial CT angiography can serve as a complementary method to DSA to help elucidate the bleeding source. Likewise, in patients with an established diagnosis of bleeding but with multiple potential sources that are not actively bleeding at the time of the diagnostic CT or DSA, intra-arterial CT angiography can provide valuable information about the relationship between the hematoma and bleeding source and the adjacent structures (Fig 12).
Figure 12:
Intra-arterial CT angiographic images in a 33-year-old woman with a family history of tuberous sclerosis who presented with acute abdominal pain. (A) Single-phase (venous) intravenous contrast-enhanced CT scan demonstrates a right perirenal hematoma (arrow) and multiple bilateral renal lesions consistent with angiomyolipoma (AML). (B) Images from urgent digital subtraction angiography (DSA) of the right renal artery shows multiple pseudoaneurysms (arrowheads). There was no active contrast extravasation. (C) CT during renal arteriography was performed to evaluate the relationship between renal artery branches and the perirenal hematoma. Image shows that the branches adjacent to the perirenal hematoma originated from the inferior segmental artery (arrow). (D) Selective DSA of the right inferior segmental artery was performed with a microcatheter, followed by embolization with glue-lipiodol solution. (E) Postembolization DSA image demonstrates successful exclusion of the right inferior segmental renal artery (arrow). In this patient with potential multiple sources of bleeding due to multiple AML, the three-dimensional soft-tissue capabilities of CT were critical to depict the relationship of the perirenal hematoma with the renal artery branches, therefore allowing precise treatment of the culprit vessel while preserving normal renal parenchyma.
Conclusions
In conclusion, angio-CT is a valuable tool for performing a variety of routine and complex interventional oncologic procedures safely and effectively. The new generation of combined angio-CT systems, with their advanced imaging and integration capabilities, provide seamless workflow for accurate disease staging, tumor target visualization, and therapeutic guidance, while maintaining radiation levels within acceptable limits. Finally, the combination of cross-sectional imaging from the CT scanner and fluoroscopic imaging from the angiography system allows more precise performance of oncologic interventions, combining therapies in the same setting, and intraprocedural therapy response monitoring, thus minimizing complications and improving therapeutic outcomes.
Acknowledgments
Acknowledgment
We thank Stephanie Deming, ELS, Research Medical Library, MD Anderson Cancer Center, for editing the article.
Authors declared no funding for this work.
Disclosures of Conflicts of Interest: R.T. disclosed no relevant relationships. E.Y.L. disclosed no relevant relationships. Y.M.L. disclosed no relevant relationships. S.Y. disclosed no relevant relationships. R.A. disclosed no relevant relationships. R.A.S. received consulting fee or honorarium from Siemens. J.R.R. disclosed no relevant relationships. A.K.J. disclosed no relevant relationships. G.C. is a full-time employee of Siemens Medical Solutions USA. H.N. disclosed no relevant relationships. T.T. disclosed no relevant relationships. K.K. disclosed no relevant relationships. S.G. is employed by University of Texas MD Anderson Cancer Center; author’s department received grant money for research from Siemens and Cook. B.C.O. received consultancy fees from Siemens Healthineers for an advisory meeting regarding imaging-guided surgery and IR; author has research grant/grants pending from Siemens Healthineers; author received speaker honoraria from Siemens Healthineers.
Abbreviations:
- angio-CT
- combined angiography and CT systems
- CBCT
- cone-beam CT
- CBCTHA
- CBCT during hepatic angiography
- CTAP
- CT during arterial portography
- CTHA
- CT during hepatic arteriography
- DSA
- digital subtraction angiography
- DLP
- dose-length product
- HCC
- hepatocellular carcinoma
- LTPFS
- local tumor progression-free survival
- MAA
- macroaggregated albumin
- PKA
- kerma-area product
- RCC
- renal cell carcinoma
- TACE
- transarterial chemoembolization
- TAE
- transarterial embolization
References
- 1. Toyoda H , Kumada T , Sone Y . Impact of a unified CT angiography system on outcome of patients with hepatocellular carcinoma . AJR Am J Roentgenol 2009. ; 192 ( 3 ): 766 – 774 . [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T , Arai Y , Inaba Y , et al . Current role of hybrid CT/angiography system compared with C-arm cone beam CT for interventional oncology . Br J Radiol 2014. ; 87 ( 1041 ): 20140126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tacher V , Radaelli A , Lin M , Geschwind JF . How I do it: Cone-beam CT during transarterial chemoembolization for liver cancer . Radiology 2015. ; 274 ( 2 ): 320 – 334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pung L , Ahmad M , Mueller K , et al . The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: A systematic review and meta-analysis . J Vasc Interv Radiol 2017. ; 28 ( 3 ): 334 – 341 . [DOI] [PubMed] [Google Scholar]
- 5. Schernthaner RE , Lin M , Duran R , Chapiro J , Wang Z , Geschwind JF . Delayed-phase cone-beam CT improves detectability of intrahepatic cholangiocarcinoma during conventional transarterial chemoembolization . Cardiovasc Intervent Radiol 2015. ; 38 ( 4 ): 929 – 936 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golfieri R , Cappelli A , Cucchetti A , et al . Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas . Hepatology 2011. ; 53 ( 5 ): 1580 – 1589 . [DOI] [PubMed] [Google Scholar]
- 7. Schernthaner RE , Haroun RR , Duran R , et al . Improved visibility of metastatic disease in the liver during intra-arterial therapy using delayed arterial phase cone-beam CT . Cardiovasc Intervent Radiol 2016. ; 39 ( 10 ): 1429 – 1437 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornelis FH , Borgheresi A , Petre EN , Santos E , Solomon SB , Brown K . Hepatic arterial embolization using cone beam CT with tumor feeding vessel detection software: Impact on hepatocellular carcinoma response . Cardiovasc Intervent Radiol 2018. ; 41 ( 1 ): 104 – 111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takayasu K , Muramatsu Y , Maeda T , et al . Targeted transarterial oily chemoembolization for small foci of hepatocellular carcinoma using a unified helical CT and angiography system: analysis of factors affecting local recurrence and survival rates . AJR Am J Roentgenol 2001. ; 176 ( 3 ): 681 – 688 . [DOI] [PubMed] [Google Scholar]
- 10. van Tilborg AA , Scheffer HJ , Nielsen K , et al . Transcatheter CT arterial portography and CT hepatic arteriography for liver tumor visualization during percutaneous ablation . J Vasc Interv Radiol 2014. ; 25 ( 7 ): 1101 – 1111.e4 . [DOI] [PubMed] [Google Scholar]
- 11. Feinberg N , Funaki B , Hieromnimon M , et al . Improved utilization following conversion of a fluoroscopy suite to hybrid CT/angiography system . J Vasc Interv Radiol 2020. ; 31 ( 11 ): 1857 – 1863 . [DOI] [PubMed] [Google Scholar]
- 12. Prando A , Wallace S , Bernardino ME , Lindell MM Jr . Computed tomographic arteriography of the liver . Radiology 1979. ; 130 ( 3 ): 697 – 701 . [DOI] [PubMed] [Google Scholar]
- 13. Dixon GD . Combined CT and fluoroscopic guidance for liver abscess drainage . AJR Am J Roentgenol 1980. ; 135 ( 2 ): 397 – 399 . [DOI] [PubMed] [Google Scholar]
- 14. Seibel RM , Grönemeyer DH , Sörensen RA . Percutaneous nucleotomy with CT and fluoroscopic guidance . J Vasc Interv Radiol 1992. ; 3 ( 3 ): 571 – 576 . [DOI] [PubMed] [Google Scholar]
- 15. Costello P , Gaa J . Clinical assessment of an interventional CT table . Radiology 1993. ; 189 ( 1 ): 284 – 285 . [DOI] [PubMed] [Google Scholar]
- 16. Dowd MT , Hathaway PB , Fontaine AB , Borsa JJ , Nelsen M , Hoffer E . Combined use of portable CT and fluoroscopy in the angiography suite . AJR Am J Roentgenol 1999. ; 172 ( 2 ): 497 – 498 . [DOI] [PubMed] [Google Scholar]
- 17. Capasso P , Trotteur G , Flandroy P , Dondelinger RF . A combined CT and angiography suite with a pivoting table . Radiology 1996. ; 199 ( 2 ): 561 – 563 . [DOI] [PubMed] [Google Scholar]
- 18. Barbaric ZL , Hall T , Cochran ST , et al . Percutaneous nephrostomy: placement under CT and fluoroscopy guidance . AJR Am J Roentgenol 1997. ; 169 ( 1 ): 151 – 155 . [DOI] [PubMed] [Google Scholar]
- 19. Inaba Y , Arai Y , Takeuchi Y . Clinical effectiveness of a newly developed interventional-CT system . J Jpn Soc Angiogr Interv Radiol 1996. ; 11 ( 1 ): 43 – 49 . https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=200902150551428412 . [Google Scholar]
- 20. Komemushi A , Suzuki S , Sano A , et al . Radiation dose of nurses during IR procedures: a controlled trial evaluating operator alerts before nursing tasks . J Vasc Interv Radiol 2014. ; 25 ( 8 ): 1195 – 1199 . [DOI] [PubMed] [Google Scholar]
- 21. Lin EY , Jones AK , Chintalapani G , Jeng ZS , Ensor J , Odisio BC . Comparative analysis of intra-arterial cone-beam versus conventional computed tomography during hepatic arteriography for Transarterial chemoembolization planning . Cardiovasc Intervent Radiol 2019. ; 42 ( 4 ): 591 – 600 . [DOI] [PubMed] [Google Scholar]
- 22. Kondo H , Kanematsu M , Hoshi H , et al . Preoperative detection of malignant hepatic tumors: comparison of combined methods of MR imaging with combined methods of CT . AJR Am J Roentgenol 2000. ; 174 ( 4 ): 947 – 954 . [DOI] [PubMed] [Google Scholar]
- 23. Choi D , Kim S , Lim J , et al . Preoperative detection of hepatocellular carcinoma: ferumoxides-enhanced mr imaging versus combined helical CT during arterial portography and CT hepatic arteriography . AJR Am J Roentgenol 2001. ; 176 ( 2 ): 475 – 482 . [DOI] [PubMed] [Google Scholar]
- 24. Jang HJ , Lim JH , Lee SJ , Park CK , Park HS , Do YS . Hepatocellular carcinoma: Are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology 2000. ; 215 ( 2 ): 373 – 380 . [DOI] [PubMed] [Google Scholar]
- 25. Fujishima T , Yoshida H , Obi S , et al . Analysis of factors influencing hepatocellular carcinoma detection: efficient use of computed tomography during arterial portography and during hepatic arteriography . J Gastroenterol 2005. ; 40 ( 3 ): 266 – 273 . [DOI] [PubMed] [Google Scholar]
- 26. Ohki T , Tateishi R , Akahane M , et al . Characteristics of hepatocellular carcinoma nodules newly detected by computed tomography during arteriography and arterial portography: preliminary report of a randomized controlled trial . Hepatol Int 2012. ; 6 ( 3 ): 639 – 645 . [DOI] [PubMed] [Google Scholar]
- 27. Takada K , Ito T , Kumada T , et al . Extra-hepatic feeding arteries of hepatocellular carcinoma: An investigation based on intra-arterial CT aortography images using an angio-MDCT system . Eur J Radiol 2016. ; 85 ( 8 ): 1400 – 1406 . [DOI] [PubMed] [Google Scholar]
- 28. Minamiguchi H , Kawai N , Sato M , et al . Hepatoma feeding arteriogram created by CT during aortography using IVR 64-multidetector-row CT for catheterization in transcatheter arterial chemoembolization for hepatocellular carcinoma . Jpn J Radiol 2013. ; 31 ( 6 ): 428 – 436 . [DOI] [PubMed] [Google Scholar]
- 29. Ozaki K , Kobayashi S , Matsui O , Minami T , Koda W , Gabata T . Extrahepatic arteries originating from hepatic arteries: Analysis using CT during hepatic arteriography and visualization on digital subtraction angiography . Cardiovasc Intervent Radiol 2017. ; 40 ( 6 ): 822 – 830 . [DOI] [PubMed] [Google Scholar]
- 30. Jones AK . Abstract 1017: An apples to apples comparison of radiation dose and image quality between flat panel CT and multidetect CT . Society of Interventional Radiology Annual Meeting , Los Angeles, CA , 2018 . [Google Scholar]
- 31. Steuwe A , Geisbüsch P , Schulz CJ , Böckler D , Kauczor HU , Stiller W . Comparison of radiation exposure associated with intraoperative cone-beam computed tomography and follow-up multidetector computed tomography angiography for evaluating endovascular aneurysm repairs . J Endovasc Ther 2016. ; 23 ( 4 ): 583 – 592 . [DOI] [PubMed] [Google Scholar]
- 32. Piron L , Le Roy J , Cassinotto C , et al . Radiation exposure during Transarterial chemoembolization: Angio-CT versus cone-beam CT . Cardiovasc Intervent Radiol 2019. ; 42 ( 11 ): 1609 – 1618 . [DOI] [PubMed] [Google Scholar]
- 33. Marshall EL , Guajardo S , Sellers E , et al . Radiation dose during Transarterial radioembolization: A dosimetric comparison of cone-beam CT and angio-CT technologies . J Vasc Interv Radiol 2021. ; 32 ( 3 ): 429 – 438 . [DOI] [PubMed] [Google Scholar]
- 34. Kanematsu M , Hoshi H , Imaeda T , et al . Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography . AJR Am J Roentgenol 1997. ; 168 ( 5 ): 1193 – 1198 . [DOI] [PubMed] [Google Scholar]
- 35. Murakami T , Oi H , Hori M , et al . Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma . AJR Am J Roentgenol 1997. ; 169 ( 1 ): 131 – 135 . [DOI] [PubMed] [Google Scholar]
- 36. Sahani D , Mehta A , Blake M , Prasad S , Harris G , Saini S . Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery . RadioGraphics 2004. ; 24 ( 5 ): 1367 – 1380 . [DOI] [PubMed] [Google Scholar]
- 37. Miyayama S , Matsui O , Yamashiro M , et al . Detection of hepatocellular carcinoma by CT during arterial portography using a cone-beam CT technology: comparison with conventional CTAP . Abdom Imaging 2009. ; 34 ( 4 ): 502 – 506 . [DOI] [PubMed] [Google Scholar]
- 38. Yevich S , Odisio BC , Sheth R , Tselikas L , de Baère T , Deschamps F . Integrated CT-fluoroscopy equipment: Improving the interventional radiology approach and patient experience for treatment of musculoskeletal malignancies . Semin Intervent Radiol 2018. ; 35 ( 4 ): 229 – 237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. CIRSE 2019 Abstracts . Cardiovasc Intervent Radiol 2019. ; 42 ( Suppl 3 ): 65 – 549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamasaki T , Kurokawa F , Shirahashi H , Kusano N , Hironaka K , Okita K . Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma . Cancer 2001. ; 91 ( 7 ): 1342 – 1348 . [DOI] [PubMed] [Google Scholar]
- 41. Boulkhrif H , Luu HM , van Walsum T , Moelker A . Accuracy of semi-automated versus manual localisation of liver tumours in CT-guided ablation procedures . Eur Radiol 2018. ; 28 ( 12 ): 4978 – 4984 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luu HM , Klink C , Niessen W , Moelker A , Walsum T . Non-rigid registration of liver CT images for CT-guided ablation of liver tumors . PLoS One 2016. ; 11 ( 9 ): e0161600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elhawary H , Oguro S , Tuncali K , et al . Multimodality non-rigid image registration for planning, targeting and monitoring during CT-guided percutaneous liver tumor cryoablation . Acad Radiol 2010. ; 17 ( 11 ): 1334 – 1344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puijk RS , Nieuwenhuizen S , van den Bemd BAT , et al . Transcatheter CT hepatic arteriography compared with conventional CT fluoroscopy guidance in percutaneous thermal ablation to treat colorectal liver metastases: A single-center comparative analysis of 2 historical cohorts . J Vasc Interv Radiol 2020. ; 31 ( 11 ): 1772 – 1783 . [DOI] [PubMed] [Google Scholar]
- 45. Morimoto M , Numata K , Kondou M , Nozaki A , Morita S , Tanaka K . Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization . Cancer 2010. ; 116 ( 23 ): 5452 – 5460 . [DOI] [PubMed] [Google Scholar]
- 46. Zhao M , Wang JP , Pan CC , et al . CT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvement . Eur J Radiol 2012. ; 81 ( 10 ): 2717 – 2725 . [DOI] [PubMed] [Google Scholar]
- 47. Alexander ES , Mick R , Nadolski GJ , Mondschein JI , Stavropoulos SW , Soulen MC . Combined chemoembolization and thermal ablation for the treatment of metastases to the liver . Abdom Radiol (NY) 2018. ; 43 ( 10 ): 2859 – 2867 . [DOI] [PubMed] [Google Scholar]
- 48. Yuan H , Li X , Tian X , Ji K , Liu F . Comparison of angio-CT and cone-beam CT-guided immediate radiofrequency ablation after transcatheter arterial chemoembolization for large hepatocellular carcinoma . Abdom Radiol (NY) 2020. ; 45 ( 8 ): 2585 – 2592 . [DOI] [PubMed] [Google Scholar]
- 49. Kurup AN , Callstrom MR . Expanding role of percutaneous ablative and consolidative treatments for musculoskeletal tumours . Clin Radiol 2017. ; 72 ( 8 ): 645 – 656 . [DOI] [PubMed] [Google Scholar]
- 50. Yevich S , Tselikas L , Kelekis A , Filippiadis D , de Baere T , Deschamps F . Percutaneous management of metastatic osseous disease . Chin Clin Oncol 2019. ; 8 ( 6 ): 62 . [DOI] [PubMed] [Google Scholar]
- 51. Kobayashi K , Ozkan E , Tam A , Ensor J , Wallace MJ , Gupta S . Preoperative embolization of spinal tumors: variables affecting intraoperative blood loss after embolization . Acta Radiol 2012. ; 53 ( 8 ): 935 – 942 . [DOI] [PubMed] [Google Scholar]
- 52. Owen RJ . Embolization of musculoskeletal bone tumors . Semin Intervent Radiol 2010. ; 27 ( 2 ): 111 – 123 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rilling WS , Chen GW . Preoperative embolization . Semin Intervent Radiol 2004. ; 21 ( 1 ): 3 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roux C , Tselikas L , Yevich S , et al . Fluoroscopy and cone-beam CT-guided fixation by internal cemented screw for pathologic pelvic fractures . Radiology 2019. ; 290 ( 2 ): 418 – 425 . [DOI] [PubMed] [Google Scholar]
- 55. Deschamps F , Yevich S , Gravel G , et al . Percutaneous fixation by internal cemented screw for the treatment of unstable osseous disease in cancer patients . Semin Intervent Radiol 2018. ; 35 ( 4 ): 238 – 247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hsieh JJ , Purdue MP , Signoretti S , et al . Renal cell carcinoma . Nat Rev Dis Primers 2017. ; 3 ( 1 ): 17009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kalra S , Atkinson BJ , Matrana MR , et al . Prognosis of patients with metastatic renal cell carcinoma and pancreatic metastases . BJU Int 2016. ; 117 ( 5 ): 761 – 765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xing M , Kokabi N , Zhang D , Ludwig JM , Kim HS . Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: A surveillance, epidemiology, and end results (SEER)-Medicare-linked population study . Radiology 2018. ; 288 ( 1 ): 81 – 90 . [DOI] [PubMed] [Google Scholar]
- 59. Takaki H , Soga N , Kanda H , et al . Radiofrequency ablation versus radical nephrectomy: clinical outcomes for stage T1b renal cell carcinoma . Radiology 2014. ; 270 ( 1 ): 292 – 299 . [DOI] [PubMed] [Google Scholar]
- 60. Aarts BM , Prevoo W , Meier MAJ , et al . Percutaneous microwave ablation of histologically proven T1 renal cell carcinoma . Cardiovasc Intervent Radiol 2020. ; 43 ( 7 ): 1025 – 1033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goldberg SN , Hahn PF , Tanabe KK , et al . Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol 1998. ; 9 ( 1 Pt 1 ): 101 – 111 . [DOI] [PubMed] [Google Scholar]
- 62. Arima K , Yamakado K , Kinbara H , Nakatsuka A , Takeda K , Sugimura Y . Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up . Int J Urol 2007. ; 14 ( 7 ): 585 – 590 ; discussion 590 . [DOI] [PubMed] [Google Scholar]
- 63. Yamakado K , Nakatsuka A , Kobayashi S , et al . Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: initial experience . Cardiovasc Intervent Radiol 2006. ; 29 ( 3 ): 389 – 394 . [DOI] [PubMed] [Google Scholar]
- 64. Gunn AJ , Mullenbach BJ , Poundstone MM , Gordetsky JB , Underwood ES , Rais-Bahrami S . Trans-arterial embolization of renal cell carcinoma prior to percutaneous ablation: Technical aspects, institutional experience, and brief review of the literature . Curr Urol 2018. ; 12 ( 1 ): 43 – 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sommer CM , Pallwein-Prettner L , Vollherbst DF , et al . Transarterial embolization (TAE) as add-on to percutaneous radiofrequency ablation (RFA) for the treatment of renal tumors: Review of the literature, overview of state-of-the-art embolization materials and further perspective of advanced image-guided tumor ablation . Eur J Radiol 2017. ; 86 ( 143 ): 162 . [DOI] [PubMed] [Google Scholar]
- 66. Sawada Y , Shimohira M , Hashizume T , et al . Transcatheter arterial embolization for renal angiomyolipoma using a micro-balloon catheter and a mixture of ethanol and lipiodol . Cardiovasc Intervent Radiol 2017. ; 40 ( 12 ): 1933 – 1939 . [DOI] [PubMed] [Google Scholar]
- 67. Nakasone Y , Kawanaka K , Ikeda O , Tamura Y , Yamashita Y . Sequential combination treatment (arterial embolization and percutaneous radiofrequency ablation) of inoperable renal cell carcinoma: single-center pilot study . Acta Radiol 2012. ; 53 ( 4 ): 410 – 414 . [DOI] [PubMed] [Google Scholar]
- 68. Winokur RS , Pua BB , Madoff DC . Role of combined embolization and ablation in management of renal masses . Semin Intervent Radiol 2014. ; 31 ( 1 ): 82 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marangio A , Prati U , Luinetti O , Brunetti E , Fìlice C . Radiofrequency ablation of colorectal splenic metastasis . AJR Am J Roentgenol 2002. ; 178 ( 6 ): 1481 – 1482 . [DOI] [PubMed] [Google Scholar]
- 70. Lardière-Deguelte S , de Mestier L , Amroun KL , et al . Laparoscopic thermal ablation of splenic metastases initial experience and present aspects . J Visc Surg 2013. ; 150 ( 5 ): 355 – 358 . [DOI] [PubMed] [Google Scholar]
- 71. Tanaka T , Inaba Y , Arai Y , Matsueda K , Aramaki T , Dendo S . Mediastinal abscess successfully treated by percutaneous drainage using a unified CT and fluoroscopy system . Br J Radiol 2002. ; 75 ( 893 ): 470 – 473 . [DOI] [PubMed] [Google Scholar]
- 72. Inaba Y , Arai Y , Matsueda K , Aramaki T , Kodera Y . Intractable massive ascites following radical gastrectomy, treatment with local intraperitoneal administration of OK-432 using a unified CT and fluoroscopy system . Australas Radiol 2003. ; 47 ( 4 ): 465 – 467 . [DOI] [PubMed] [Google Scholar]