Abstract
Osteoarthritis (OA) is a global health issue with myriad pathophysiological factors and is one of the most common causes of chronic disability in adults due to pain and altered joint function.
The end stage of OA develops from a destructive inflammatory cycle, driven by the pro-inflammatory cytokines interleukin-1β (IL-1β) and tumour necrosis factor alpha (TNFα).
Owing to the less predictable results of total knee arthroplasty (TKA) in younger patients presenting with knee OA, there has been a surge in research evaluating less invasive biological treatment options, one of which is autologous protein solution (APS).
APS is an autologous blood derivative obtained by using a proprietary device, made of APS separator, which isolates white blood cells (WBCs) and platelets in a small volume of plasma, and APS concentrator, which further concentrates platelets, WBCs and plasma proteins, resulting in a concentrated solution with high levels of growth factors including the anti-inflammatory mediators against IL-1β and TNFα.
A single intraarticular injection of APS appears to be a promising solution for treatment of early-stage OA from current evidence, the majority of which comes from preclinical studies.
More clinical studies are needed before APS can be widely accepted as a treatment modality for OA.
Cite this article: EFORT Open Rev 2021;6:716-726. DOI: 10.1302/2058-5241.6.200040
Keywords: APS, autologous protein solution, joint preservation, nSTRIDE, orthobiologics, osteoarthritis, regenerative medicine
Introduction
Osteoarthritis (OA) is a global disease with a variety of pathophysiological factors.1 These factors can be mechanical, biological or a combination of both. Cardinal features of OA include loss of joint space and cartilage, osteophyte formation, subchondral sclerosis and cyst formation.2 OA can either be primary with no known cause or secondary with a known cause such as injury. Primary OA is more common than secondary OA.3 Whatever the cause, literature supports that OA is linked to a destructive inflammatory cycle, driven by the pro-inflammatory cytokines interleukin-1β (IL-1β) and tumour necrosis factor alpha (TNFα).4,5 These cytokines play a critical role in the degradation of cartilage matrix by increasing chondrocyte production of matrix metalloproteinases (MMPs).4,5 This breakdown of cartilage matrix initiates the inflammatory response, promoting a positive feedback loop in which inflammatory cytokines induce tissue damage which then stimulates production of more inflammatory cytokines. This results in progressive cartilage degeneration, causing advanced OA.4,5 In the literature, the prevalence of OA ranges from 12.3% (self-reported in the ‘Disability-Health’ 2009 population-based survey in France)6 to 21.6% (physician-diagnosed OA in the United States (US) estimated by the 2003–2005 US National Health Interview Survey).7 In the United Kingdom (UK), Arthritis Research UK has reported that 18.2% of the population over the age of 45 years have knee OA. The morbidity burden of OA has been well documented. The trends in OA years lived with disability (YLD) showed a 75% increase, the third most rapidly rising condition associated with disability, just behind diabetes at 135% and dementia at 84%.8 The most recent update of the World Health Organization Global Burden of Disease estimated that 242 million people were living in the world with symptomatic and activity limiting OA, accounting for 13 million YLDs.8 OA also contributes to significant economic burden due to productivity costs, working days lost as well as treatment costs, of which the arthroplasty options are the most expensive. The cost of working days lost due to OA and rheumatoid arthritis (RA) was estimated at £2.58 billion in 2017 rising to £3.43 billion by 2020.9 Treating these two most common forms of arthritis is estimated to have cost the UK economy £10.2 billion in direct costs to the National Health Service and wider healthcare system in 2017. Cumulatively UK healthcare costs will reach £118.6 billion over the next decade.9 In searching for more a sustainable treatment model to effectively deal with the growing health problem of OA, there has been a recent focus on orthobiologic treatment options. There are many treatment modalities that now fit this overarching label. These include (in order of appearance over recent decades) whole blood therapy, traditional prolotherapy, platelet-rich plasma (PRP), bone marrow aspirate concentrate (BMAC), adipose biocellular autograft, mesenchymal stem cell (MSC) allograft cellular concentrates, amniotic cellular concentrates and cord-derived cellular concentrates. Most recently, autologous IL-1 receptor antagonist blood products (AILBPs), such as autologous protein solution (APS) and autologous conditioned serum (ACS), have been fabricated as emerging therapies for knee OA.10 In a systematic review by Ajrawat et al, it was concluded that AILBPs are a safe and tolerable injection therapy that may improve pain parameters and functionality for mild to moderate knee OA, despite the limited evidence.10 Today, nSTRIDE® (Biomet Biologics, Warsaw, IN, USA) is the only available APS for clinical use in patients with early to moderate OA. The aim of this review paper is to provide an overview of the disease burden and pathogenesis of OA, review the preclinical and clinical studies of APS as well as to discuss the potential role for APS in the clinical practice of orthopaedic surgery.
Osteoarthritis: the inflammatory cascade
OA has long been considered a ‘wear and tear’ disease leading to loss of cartilage. This paradigm was mainly fuelled by observations of chondrocytes, the only cell type present in articular cartilage. These cells have very low metabolic activity with no innate ability to repair damaged cartilage. Moreover, unlike all other tissues, articular cartilage, once damaged, cannot respond with a typical inflammatory response due to its lack of vascularity and innervation.11
Progress in molecular biology has deeply changed this paradigm. The discovery that cytokines and other chemokines can increase the production of MMPs by chondrocytes led to belief in an ‘inflammatory theory’ behind the pathogenesis of OA.11 These MMPs include MMP-1, MMP-3, MMP-8, MMP-13 and MMP-14.4 The current paradigm of OA is evolving from a purely mechanical disease towards a complex biological response connecting biomechanics, inflammation, and the immune system.
Perhaps the first step in understanding OA as an inflammatory disease is to acknowledge that inflammation is not exclusive to RA and the other classical inflammatory arthritides.12 In fact, it has been reported that levels of inflammation between OA and RA can be similar.13 In a study by Haraoui et al, histologic features of synovial biopsy samples revealed similar distribution patterns of the infiltrating inflammatory cells.13 Furthermore, fibrosis was markedly reduced in both groups post treatment with anti-inflammatory agents.13 Inflammation in OA is triggered by external mediators such as cytokines and proteases, as well as internal cellular mechanisms leading to increased production of inflammatory mediators.12 Local production of inflammatory mediators is well known to contribute to cartilage degradation and synovial cell activation. Many in vitro and in vivo investigations prove that fibroblast-like synoviocytes and chondrocytes can induce the production of cytokines and chemokines.14 However, inflammatory processes occurring within the joint could also be reflected outside the joint. Serum levels of inflammatory mediators have been found to be higher in patients with OA as compared to healthy individuals.15,16 Synovitis, stress and inflammatory factors as a biomechanical response, cell-matrix interactions, ageing and obesity can contribute to the overall inflammatory cascade that is seen in OA.
Mediators that play important roles in the progression of OA include IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-11, IL-13, IL-15, IL-17, leukocyte inhibitory factor (LIF), TNFα as well as IL-1 receptor antagonist (IL-1ra).17 These cytokines stimulate their own expression and activate chondrocytes, inducing them to synthesize MMPs, proteases, chemokines, nitric oxide (NO), and eicosanoids such as prostaglandins and leukotrienes, all of which lead to increased cartilage degradation.18 An increase in anti-inflammatory cytokines such as IL-4, IL-10 and IL-13 has also been observed in the synovial fluid of OA patients.19 Through complex mechanisms, these cytokines exert their anti-inflammatory effects following a reduction in the production of IL-1β, TNFα, MMPs and other inflammatory mediators.19 Amongst all, there is compelling evidence that IL-1β and TNFα are the most important pro-inflammatory mediators in the development and progression of OA (Fig. 1).20,21 IL-6, a pro-inflammatory cytokine enhanced by TNFα and IL-1β, has been known to inhibit type II collagen synthesis.22 A longitudinal study on women with knee OA through 15 years of follow-up reveals that higher levels of serum IL-6 is associated with an increased chance of diagnosis of OA.23 While IL-6 has been proposed as a potential marker for early diagnosis of OA, IL-4 has been found to correlate with radiographic severity of the disease.24
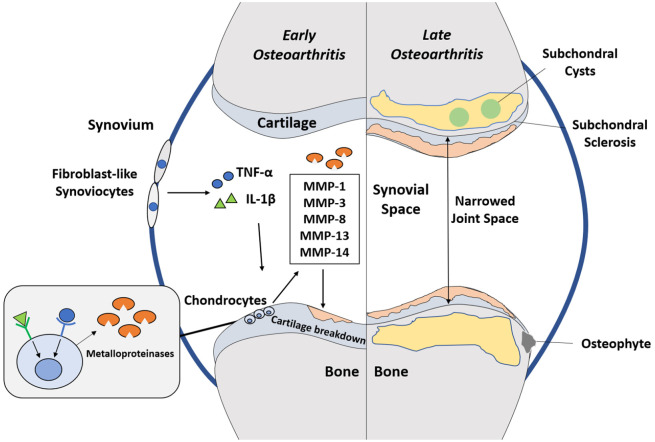
Fig. 1.
Pro-inflammatory mediators IL-1β and TNFα secreted by fibroblast-like synoviocytes bind to receptors on chondrocytes to promote synthesis of matrix metalloproteinases which then break down cartilage leading to progression of osteoarthritis, which is characterized by the cardinal features of narrowed joint space, osteophytosis, subchondral sclerosis and cyst formation.
Note. IL, interleukin; TNF, tumour necrosis factor; MMP, matrix metalloproteinases.
IL-1β has been shown to promote the synthesis of MMPs to inhibit the function of growth factors for extracellular matrix proteins, to enhance the expression of cell adhesion molecules, and to stimulate the synthesis of other pro-inflammatory cytokines such as TNFα and IL-6.25,26 TNFα exerts an effect similar to IL-1β and can act synergistically with this cytokine.27 Cartilage destruction was found to be more intense when IL-1 was associated with TNFα as opposed to intraarticular administration of IL-1 alone.28 It is now generally accepted that IL-1β and TNFα are the pivotal cytokines involved in OA pathophysiology.27 Therefore, the neutralization of these inflammatory mediators appears to be a logical development for OA therapy.
Current treatment modalities for osteoarthritis
The OA Research Society International (OARSI) has published global, evidence-based, consensus recommendations for the treatment of OA of the hip and knee.29–31 Of the 51 modalities of treatment addressed in the OARSI recommendations, 35 have been systematically reviewed, including a wide range of non-surgical methods. Initial treatment of knee OA needs to be conservative. Conservative treatment comprises physiotherapy, bracing, education, weight reduction, viscosupplementation, corticosteroid injections, analgesia and anti-inflammatory treatment.29–31 Orthobiologic injections are now also an emerging option.32 While there are no guidelines that have incorporated orthobiologic injections such as APS as part of a treatment algorithm for OA, we believe that orthobiologic injections are best placed as an intermediate option for symptomatic early to moderate OA or as a time-buying option for younger patients with severe OA, before consideration towards surgical treatment and after patients have exhausted other modalities of conservative treatment. While there are several phenotypes of OA, the most common pathophysiological response of the affected joint is an attempt to correct abnormal mechanical stresses and repair the underlying injury.33 If the mechanical environment continues to be abnormal, OA will progress despite conservative treatment. Therefore, if symptoms persist after the sustained and appropriate use of non-surgical treatment, then treatment should be escalated to utilize more invasive methods, including surgery.29–31 Realignment osteotomies have recently gained popularity for correcting mechanical overload of the knee joint when there are abnormalities in the native alignment of the femur and tibia.34 This is best performed in the early stages of OA to prevent the progression of mechanical disruption. Other surgical options include arthroscopic debridement, cartilage resurfacing surgery and unicompartmental or total knee arthroplasty.29–31
Is total knee arthroplasty the universal solution?
In recent times, utilization rates of knee arthroplasty have increased exponentially.35 In the United Kingdom, the inpatient cases of TKA per 100,000 total population have increased from 111 in 2005 to 140 in 2011.35 In a study by Losina et al, OA diagnosis peaked in a younger age group, consistent with current trends where use of TKA occurs earlier in life with 40% of TKA recipients being younger than 65 years of age.36 While TKA may be the ‘easiest’ surgical option in patients presenting with advanced knee OA, the outcomes of TKA in younger patients have not been predictable. In a retrospective registry study by Lange et al, 529 younger patients aged 18 to 55 years and 2001 older patients aged 65 to 75 years were propensity score matched and compared with regard to satisfaction after TKA.37 There was significantly higher dissatisfaction (14%) in the young patients compared to their older counterparts (9%). In another study by McCalden et al, 6275 consecutive TKA patients were divided into three groups based on their age: < 55, 55–70, and > 70 years. While the difference in the change in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score and Knee Society Score (KSS) favoured the younger populations, the revision rate was higher in the group < 55 years with a Kaplan–Meier survivorship of 95.5% at five years and 92.2% at 10 years.38 This higher revision rate in younger patients undergoing TKA was also reported by Bayliss et al.39 In their study, 54,276 patients who had undergone TKA with a maximum follow-up of 20 years were evaluated. Those who had surgery younger than 70 years had a significantly higher lifetime risk of revision as compared to those who were older than 70 years (35% vs. 5%). With these findings of inferior outcomes in younger patients, there is a need for alternative options in this patient population with severe OA. Perhaps APS could be the option that could buy them some time until reaching an appropriate age for TKA.
Autologous protein solution: a promising solution?
Autologous protein solution (APS) is a novel orthobiologic approach created with the aim of treating OA by targeting the inflammatory pathways mediated by IL-1β and TNFα (Fig. 2).40–43 Its objective is to reduce pain as well as to mitigate OA progression via a disease-modifying injection into the joint site, without systemic complications.44 Previous analysis of APS had identified high concentrations of anti-inflammatory and anabolic cytokines, along with low levels of catabolic cytokines.44 This cytokine cocktail is also accompanied by high concentrations of white blood cells (WBC) and platelets.41,43 Critically, it was found that APS produced from the whole blood of osteoarthritic patients still contains a high WBC, platelet, and good cytokine profile, indicating that this is a practical therapeutic option.42
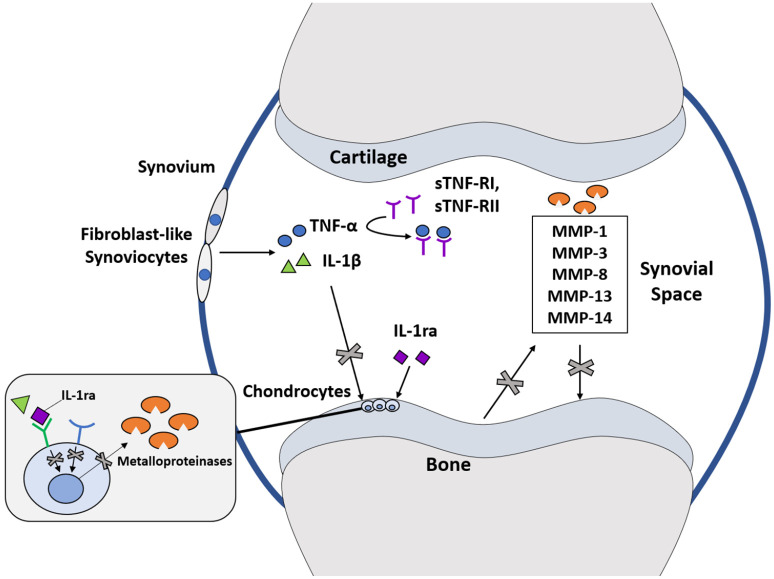
Fig. 2.
Autologous protein solution (APS) contains a number of anti-inflammatory cytokines including IL-1 receptor antagonist (IL-1ra) and soluble receptors I and II against TNFα (sTNF-RI, and sTNF-RII), IL-1ra blocks the action of IL-1β by preferentially binding to the receptor on chondrocytes while sTNF-R1 and sTNF-RII bind directly to TNFα. Via these mechanisms, APS inhibits production of matrix metalloproteinases and thus prevents progression of osteoarthritis.
Note. IL, interleukin; TNF, tumour necrosis factor; MMP, matrix metalloproteinases.
APS is produced by passing whole blood through an APS kit in a two-stage process as described in the paper by Kon et al.41 Firstly, cellular components including white blood cells and platelets including cytokines are separated from the whole blood with an APS separator. Secondly, the resultant solution is further concentrated via an APS concentrator containing polyacrylamide beads. These serve as a dehydrating agent to increase the concentration of cytokines that are found in APS to 2–3 times that found in blood plasma.41,43
APS contains a number of anti-inflammatory cytokines, including IL-1 receptor antagonist (IL-1ra), soluble receptors I and II against TNFα (sTNF-RI, and sTNF-RII), which are antagonistic to the inflammatory cytokines IL-1β and TNFα that are largely responsible for driving cartilage degeneration in OA.42 The majority of in vitro work into APS has thus focused on its ability to interfere with the pathways of these two cytokines. Previous anti-cytokine research into methods of halting OA progression explored IL-1β and TNFα antagonists as a solution. While useful in treating RA,45,46 they were unable to influence OA.47,48 However, Zwerina et al demonstrated that while TNFα and IL-1β monotherapies could not fully treat OA in a TNFα-driven model, combined therapy brought it to near complete remission.49 Hence, the composition of APS makes it a prime contender for disease modification in OA. The rest of this review article will qualitatively summarize the main findings of in vitro, preclinical (Table 1) as well as clinical studies undertaken thus far to evaluate the effects of APS in OA.
Table 1.
Summary of key findings from in vitro and preclinical studies
| In vitro | |||
|---|---|---|---|
| Authors | Methodology | Assessed parameters | Key findings |
| Woodell-May et al44 | Human knee chondrocytes were pre-incubated with APS, sTNF-RI, or IL-1Ra. These chondrocytes were then challenged with IL-1β or TNFα. Culture media was centrifuged, and supernatant was tested for MMP-13 by EILSA assay. | - MMP-13 concentration | APS successfully inhibited MMP-13 synthesis from chondrocytes following IL-1β or TNFα challenge. When IL-1ra and sTNF-RI were used individually, a much higher concentration than that seen in APS was needed to inhibit MMP-13 synthesis to a similar extent. This suggests that a combined cytokine interaction is altering MMP-13 expression. |
| Matuska et al54 | Bovine cartilage explants were pre-incubated with APS for 1 hour before the addition of IL-1α or TNFα. Incubation proceeded for 3–21 days prior to testing of explants. | - GAG release from explants - Collagen release from explants - Safranin-O staining |
APS treatment significantly reduced the amount of GAG and collagen released from cartilage explants. Safranin-O staining was significantly higher when explants were incubated in the presence of APS, signalling a preservation of cartilage. |
| Preclinical | |||
| Authors | Methodology | Assessed parameters | Key findings |
| Bertone et al56 | 40 horses with OA were divided into control or intervention groups to received intraarticular saline or APS injection, respectively. Horses were assessed for 14 days post injection by researchers, and questionnaire feedback was collected from clients at 12 and 52 weeks post APS treatment. | Experimentally assessed parameters: - Lameness grades - Kinetic gait analysis - Joint pain on flexion and swelling - Synovial fluid analysis - Blood analysis - Radiography Client-assessed parameters: - Lameness - Comfort at rest - Comfort at turnout - General attitude - Appetite - Body condition - Hair condition |
Experimentally assessed parameters: Lameness in APS-treated horses improved significantly within 7 days, while kinetic gait analysis revealed improvement in gait symmetry compared at day 7, and improvement in vertical peak force at day 14. Although the range joint flexion without pain improved in the APS group, joint swelling did not differ from control. Among the variables tested in synovial fluid analysis, the only significant finding was an increase in total protein concentration in the control group on day 14 relative to baseline, while there was no change in the APS group. Blood analysis was normal for all horses, and there was no significant difference between the two groups. No radiographic changes were observed in either group at day 14. Client-assessed parameters: Following APS injection, lameness, comfort at rest, and comfort at turnout improved significantly at 12 weeks and 52 weeks. However, general attitude, appetite, body condition, and hair condition were not affected. No adverse events due to APS injection were reported by clients. |
| Wanstrath et al57 | 20 canines with OA of the elbow or stifle joint were divided into control or intervention groups to receive intraarticular saline or APS injection, respectively. Canines were assessed at 2 and 12 weeks post treatment by researchers, veterinarians, and clients. | Experimentally assessed parameters: - Blood analysis - Kinetic gait analysis - Radiography Client-assessed parameters: - Pain - Lameness |
Experimentally assessed parameters: Blood analysis revealed normal values in both groups. Kinetic gait analysis found significant increase in peak vertical force at week 12 compared to baseline, while no change was seen in the control group. Except for one dog in each group, radiographic scoring remained unchanged at week 12 relative to baseline. Client-assessed parameters: Pain as assessed using the CBPI index improved significantly in the APS group at week 12 compared to baseline and control. In addition, lameness as assessed by HVAS index significantly improved in the APS group compared to baseline at week 12, but there was no identifiable difference from control. |
| King et al40 | OA was induced in 30 rat knees using a meniscal-tear-induced OA model. 7 days post operation, rats were divided into either control or intervention groups to receive intraarticular saline or APS injection, respectively. Weight and gait were tracked for 28 days, after which the rats were euthanized for histological analysis. | - Gait analysis - Weight changes - Changes in bone - Changes in synovium - Collagen degeneration - Cartilage degeneration |
No differences in terms of gait or weight changes were observed between APS and control groups. In addition, histological analysis found no differences between osteophyte scores, synovitis scores, bone sclerosis scores, and bone damage scores between both groups. However, rats which were treated with APS had reduced collagen degeneration and preserved cartilage matrix integrity compared to control. Nevertheless, an overall assessment of joint parameters showed that APS was unable to fully prevent the structural OA changes that occurred in the rat knees. |
Note. APS, TNF, IL, MMP, EILSA, OA, GAG, CBPI, HVAS, .
In vitro studies
Type II collagen and aggrecan are molecules responsible for maintaining the structural integrity of articular cartilage.50 Aggrecan normally serves to protect collagen from degradation, but in the OA state, aggrecanase and MMPs are released by chondrocytes.50 This leads to breakdown of the protective aggrecan and subsequent glycosaminoglycans (GAG) release by aggrecanase, as well as underlying articular cartilage breakdown by MMPs.51,52 Woodell-May et al found that the synthesis of MMP-13, one of the IL-1β and TNFα-induced proteases found in OA, could be inhibited by pre-incubating chondrocytes with APS two hours prior to human recombinant IL-1β or TNFα addition.44 Notably, while there was upregulated MMP-13 release due to the synergistic effects of IL-1 and TNFα, its production was still inhibited by APS. This can be attributed to its multi-cytokine composition that allows it to address cytokine redundancy.43,53 In addition, while APS concentration of IL-1ra and sTNF-RI was much lower than the concentration at which recombinant inhibitors exerted their inhibitory effects in vitro, MMP-13 production was blocked nonetheless.44 Matuska et al performed a study to determine the chondroprotective effect of APS on IL-1α- or TNFα-challenged bovine articular cartilage explants.54 They found that APS was more effective than recombinant antagonists in preventing cartilage matrix degradation. In addition, histological examination of the effects of human recombinant IL-1α and TNFα on explants found better preserved Safranin-O staining intensity when samples were also exposed to APS. A previous study had identified a relationship between loss of Safranin-O staining intensity and chondrocyte apoptosis in osteoarthritic cartilage. Based on these findings, APS might have a chondroprotective function.54,55 However, there are no mechanistic studies to date that have evaluated the underlying mechanisms that underpin the therapeutic effects of APS besides the putative IL-1α and TNFα.
Preclinical studies
Three preclinical studies testing the viability of APS against normal saline control have been performed on rats, canines, and horses. These studies assessed the safety of a single dose of intraarticular APS injection and found no evident association with adverse events.40,56,57 Radiographic scoring in canines and horses with naturally occurring OA found no significant change relative to baseline, although it should be noted that the timescales for radiography were different in both cases. While the canine study performed radiography at weeks 0 and 12, horses were scanned on day 14 post injection.56,57 In vitro work has identified that the proliferative effects of APS on cartilage continue to at least day 28.54 Therefore, radiography performed prior to that may have been too premature to visualize the effects of APS. Although no radiographic changes were seen, there was improvement in clinical outcomes following APS administration.56,57 Horses were assessed for joint pain, joint swelling, lameness, and gait; canines were assessed for pain, lameness, and gait. In all assessed parameters, both species improved relative to baseline. Gait was measured via kinetic gait analysis in both studies. This involved measuring vertical peak force, and in canines, it also looked at vertical impulse. Both parameters are justifiably accurate in reflecting lameness and gait abnormalities in these animals.58–60 In addition, during the long-term follow-up of horses at 12 and 52 weeks, it was found that three parameters – lameness grade, comfort at rest, and comfort at turnout – had improved compared to pre treatment. However, the improvement seen at 12 weeks was much higher than that seen at 52 weeks, suggesting that the treatment effects plateaus with time. On the other hand, no improvement was seen in the gait analysis of rats as observed by Matuska et al after APS administration, although it should be noted that the assessment was performed on the same day as APS injection, as well as the day after, to compare with pre-injection data.54 This may have been a limiting factor in performing an accurate gait assessment as the duration offered for APS to exert a therapeutic effect may not have been sufficient. Histology of OA-induced rat knees by medial meniscus tear found that administration of APS 21 days prior to sacrifice reduced the level of collagen degradation, and correlated with better cartilage matrix integrity.40 This correlation was seen in both the medial tibial (p = 0.04) and medial femoral (p = 0.05) cartilage, with APS treatment reducing the level of degeneration observed.40 However, while APS treatment was correlated with less cartilage breakdown, the OA phenotype was still identified. The mechanism through which APS impaired breakdown in this study could not be elucidated as it was unclear whether the degenerative OA pathway itself was impaired by APS, or whether regeneration of cartilage was initiated by APS after degeneration had already occurred. It is also interesting to note that the treatment effect of APS seen in these preclinical studies tends to plateau with time, based on a much stronger improvement in the early phase of treatment. However, we will see in the next section that this is completely the opposite in clinical studies, where we see improvement in patient-reported outcome measures in the later phases following APS injection. The authors believe that this discordance could be due to the phenotypic difference in the OA that is induced in the in vivo models as compared to primary OA in humans.
Clinical studies
There have been several clinical studies evaluating the therapeutic effects of APS in patients with OA.41,61–63 In all these studies, the nSTRIDE® APS kit (Biomet Biologics, Warsaw, IN, USA) was used. This kit contains two blood processing devices and a 30 mL vial of Anticoagulant Citrate Dextrose Solution Formula A (ACD-A). The first of the two devices is the nSTRIDE Cell Separator. It is a plastic tube containing a tuned-density buoy, which separates cellular and platelet components of whole blood to form a cell solution. This output is then further processes by the second device, the nSTRIDE Concentrator. This second device is a plastic tube containing polyacrylamide absorbent beads to concentrate the cell solution and produce an injectable output, the APS. In all four studies, approximately 2 to 3 millilitres of APS was produced from 50 to 60 millilitres of peripheral blood. The APS was injected intraarticularly into the affected knee joint as a one-time single-dose injection. These studies also reported the concentration of the cytokines in their APS (Table 2). The safety and efficacy results, as well as clinical outcomes from these studies, are described below.
Table 2.
Concentrations, pg/mL (Mean ± SD) of cytokines in autologous protein solution
| Authors | IL-1ra | sIL-1RII | IL-1β | sTNF-RII | TNFα |
|---|---|---|---|---|---|
| Kon et al41 | 33,482 ± 16,103 | 27,874 ± 12,087 | 23.4 ± 28.6 | 6052 ± 1643 | 0.6 ± 1.3 |
| Hix et al61 | 63,740 ± 23,556 | 26,217 ± 6126 | 16.5 ± 25.5 | 6348 ± 1425 | Below the range of the ELISA assay |
| King et al62 | 57,511 ± 24,272 | 20,121 ± 6654 | 20.3 ± 34.2 | 5,520 ± 1,174 | 2.6 ± 2.9 |
Note. IL, interleukin; TNF, tumour necrosis factor; ELISA, .
Safety and efficacy
In clinical studies, only minor adverse events (AEs) were associated with APS administration, although some severe unrelated AEs were seen. Minor AEs reported were largely musculoskeletal in nature such as arthralgia, joint effusion and joint stiffness. Less common AEs included injection site pain and discomfort, and procedure-associated nausea.41,61–63 However, these AEs resolved soon after presentation either spontaneously or after symptomatic treatment. Serious AEs following APS injection comprised diverticulitis, bladder cancer, and kidney stones, but these were justifiably deemed to be unrelated to APS administration.41,61 Imaging of the OA knee was assessed in one study which scored radiographic changes on magnetic resonance imaging (MRI) using the MRI Osteoarthritis Knee Score (MOAKS).61 Scoring showed significant effects only on the size of lateral femoral condyle bone marrow lesions (p = 0.041) and osteophytes (p = 0.032) in a study of sample size 46 (31 patients in the intervention group and 15 in a saline control group).41 The lesions had worsened in the saline control patients 12 months post injection, while they had remained unchanged in APS-treated patients. However, similar effects were not seen in other parts of the knee. In addition, other MOAKS parameters remained unchanged.
Patient-reported outcome measures (PROMs)
Patient-reported outcome measures (PROMs) that have been used to assess the effect of APS on OA include the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC),64 Knee injury and Osteoarthritis Outcome Score (KOOS),65 Numeric Rating Scale (NRS) pain,66 Visual Analog Scale (VAS) pain,66 and Patient Global Impression of Change (PGI-C).67 WOMAC was the common measure for pain across all human studies. This is a scale which measures ‘pain’, ‘stiffness’, and ‘function’ subscales, and tallies these to a total composite WOMAC score. Single-arm studies found significant improvements in total WOMAC score as soon as two weeks after injection (p < 0.01).63 WOMAC subscales and composite score continued to improve until 6 months, and at 12 months post injection.61–63 However, these were non-randomized studies with small patient numbers. They were designed to test safety and are not powered enough to study the long-term therapeutic effects of APS.
In a recent double-blinded randomized controlled trial (RCT) of 46 individuals by Kon et al, WOMAC pain improvement in APS-treated patients relative to saline control was not seen until 12 months post injection, though stiffness and function subscales remained indifferent throughout the experiment duration.41 Nevertheless, it should be noted that the APS intervention group and saline control group both reported improvements in WOMAC pain score of 65% and 41% respectively at 12 months. Furthermore, at this time, stiffness improved by 41% and 38% in the APS intervention and saline control groups respectively, while function improved by 55% and 45%. The improving scores for both groups could have reduced the significance of difference at earlier time points for pain, and at all points for stiffness and function. That being said, the longevity of the placebo effect is a critical point to address, especially in a knee injection study, where the placebo effect is even greater as patients perceive that they are receiving a ‘regenerative medicine’ therapy.68 This effect has been known to affect scores differently, and this is likely because these scoring systems measure different aspects of the patient outcomes in OA.69
KOOS is another measure for pain that was assessed in two previous studies. The KOOS is a similar scoring system to the WOMAC, but measures ‘sports function’ and ‘quality of life’ while also building upon the ‘pain’, ‘function’, and ‘stiffness’ subscales used in the WOMAC. However, it is considered to be more responsive in younger, more active individuals in assessing OA.70 There were discordant KOOS results seen in the two studies. Hix et al reported that KOOS significantly improved relative to baseline for the subscales of pain, symptom, stiffness, daily function, and sport function in their non-comparative study.61 The KOOS pain score improved approximately by 50% at one-week and 115% at one-year post injection. However, Kon et al did not observe differences compared against a placebo in their RCT at any time point over 12 months.41 Nevertheless, it should be noted that, similar to the WOMAC scores seen in this RCT, all subscales improved in both APS intervention groups and saline control groups relative to baseline. In addition, the deviating trend between the six-month and 12-month time points between APS and saline groups was again identified, with the APS group seeing improved scores across all subscales, while the saline group saw worsened scores. These results were neither statistically not clinically different and took place over too short a period of follow-up to enable definitive conclusions to be made. However, when reviewed in the context of the positive radiological findings of the RCT at 12 months post injection of unchanged bone lesions and osteophytes in the APS group as opposed to the worsened state seen in the saline control group,41 these results suggest that APS might only begin expressing effects after at least six months, instead of the shorter durations previously suggested. While non-comparative studies found improved PGI-C after APS was administered,61,63 no differences were found throughout the experiment duration in an RCT.41 In addition, of the eight subscales, SF-36 assessment in the RCT only improved for ‘bodily pain’ and ‘role emotional health’ after 12 months, but remained unchanged for the other subscales at all time points.41
Clinician-reported outcome measures
The Clinical Global Impression of Change (CGI-C) is the clinician-reported counterpart to the PGI-C. While there are inconsistent data with regard to the PGI-C, the CGI-C has been shown to significantly improve in patients following treatment with APS.41,61 However, it must be taken into consideration that global impression of change scales, while easily understood and applied in different cases,71–73 are non-specific measures of OA, unlike WOMAC and KOOS. In all clinical trials, the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) criteria were used to assess the responsiveness to treatment.41,61–63 Good response to the treatment was found in all trials relative to baseline. Moreover, response rate continued to increase over time, with Drumpt et al finding five of six subjects being ‘high pain responders’ at 18 months post injection, the longest duration post injection across all studies.63 However, Kon et al found that when compared to a placebo, there was no difference in OMERACT-OARSI response rate at all time points, three, six, and 12 months following treatment.41 Nevertheless, they identified a continued increase in response rate throughout follow-up in patients administered APS, while saline administered patients reached a peak response rate at six months, before falling at 12 months. Unfortunately, the lack of further data collection impairs the ability to recognize a deviating trend between both groups. King et al investigated the relationship between APS composition and OMERACT-OARSI response criteria. They found that subjects with IL-1ra:IL-1B ratios > 1000 were more likely to respond at all time points one week, two weeks, four weeks, three months, and six months, post injection. A similarly improved response rate was also observed for subjects whose WBC concentration in APS was > 30 k/μL.62
Summary
The clinical studies involving APS to date show that there has been improvement in pain and function in a heterogenous population of patients with knee OA, not different from placebo. The bias of placebo effect in these studies has to be considered and there has been no imaging confirmation of cartilage growth. It is also important to note that these studies were not free from conflicts of interest, either due to direct research funding support by the industry that produces and markets APS or due to the research being conducted in industry-owned laboratories. Another potential issue that might affect interpretation of the results from these clinical trials is the fact all studies utilized a single product, the nSTRIDE® APS (Biomet Biologics, Warsaw, IN, USA). There are currently no other brands of APS that are being marketed. Another product, Orthokin® (Orthogen AG, Dusseldorf, Germany) is marketed as an autologous conditioned serum (ACS). However, Orthokin® solely contains IL-1ra, without the soluble receptors I and II against TNFα. As such, we did not consider studies evaluating ACS in our review. The authors have no conflicts of interest to declare with regard to nSTRIDE® APS.
What does the future hold?
While APS is still a fairly new treatment modality, there is promising evidence that its administration is able to influence the inflammatory cascade and improve the disease state. Although TKA is an easy surgical intervention for tackling advanced OA, the high percentage of knee OA patients requiring TKA, inconsistent satisfaction rate, and likely revision incidence of TKAs mandate that a disease-modifying, orthobiologic intervention is necessary to adequately tackle this global disease. While the studies so far are suggestive that an earlier, minimally invasive intraarticular APS injection may be able to tackle intraarticular inflammation, one cannot overlook the mechanical factors that drive the progression of OA. As our understanding of APS grows, there is the possibility that it could become a standard-of-care for treatment of early OA, together with realignment surgery where appropriate.
Conclusion
A single intraarticular injection of APS appears to be a promising solution for treatment of early-stage OA from current evidence, of which the majority is made up of preclinical studies. Therefore, more clinical studies are needed before APS can be widely accepted as a treatment modality for OA.
Footnotes
ICMJE Conflict of interest statement: The authors declare no conflict of interest relevant to this work.
Social media: Twitter: @hamidrazak85
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.Center for Disease Control and Prevention. Osteoarthritis fact sheet. https://www.cdc.gov/arthritis/basics/osteoarthritis.htm [Date last accessed 1 April 2020].
- 2.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965–973. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F, Wallace IJ, Lieberman DE, Felson DT. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2018;14:674–681. [DOI] [PubMed] [Google Scholar]
- 4.Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis 2008;67:S75–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB. Anticytokine therapy for osteoarthritis. Expert Opin Biol Ther 2001;1:817–829. [DOI] [PubMed] [Google Scholar]
- 6.Palazzo C, Ravaud J-F, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLoS One 2014;9:e90633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmick CG, Felson DT, Lawrence RC, et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- 8.Vos T, Barber RM, Bell B, et al. ; Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The cost of arthritis: calculation conducted on behalf of Arthritis Research UK (unpublished). York Health Economics, 2017.
- 10.Ajrawat P, Dwyer T, Chahal J. Autologous interleukin 1 receptor antagonist blood-derived products for knee osteoarthritis: a systematic review. Arthroscopy 2019;35:2211–2221. [DOI] [PubMed] [Google Scholar]
- 11.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 2013;21:16–21. [DOI] [PubMed] [Google Scholar]
- 12.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 2013;5:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haraoui B, Pelletier JP, Cloutier JM, Faure MP, Martel-Pelletier J. Synovial membrane histology and immunopathology in rheumatoid arthritis and osteoarthritis: in vivo effects of antirheumatic drugs. Arthritis Rheum 1991;34:153–163. [DOI] [PubMed] [Google Scholar]
- 14.Rahmati M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016;85:81–90. [DOI] [PubMed] [Google Scholar]
- 15.Attur M, Statnikov A, Aliferis CF, et al. Inflammatory genomic and plasma biomarkers predict progression of symptomatic knee OA (SKOA). Osteoarthritis Cartilage 2012;20:S34–S35. [Google Scholar]
- 16.Fernández-Puente P, Mateos J, Fernández-Costa C, et al. Identification of a panel of novel serum osteoarthritis biomarkers. J Proteome Res 2011;10:5095–5101. [DOI] [PubMed] [Google Scholar]
- 17.Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci 1999;4:D694–D703. [DOI] [PubMed] [Google Scholar]
- 18.Katona G. Osteoarthritis – an inflammatory disease? Int J Tissue React 1984;6:453–461. [PubMed] [Google Scholar]
- 19.Sutton S, Clutterbuck A, Harris P, et al. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet J 2009;179:10–24. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- 21.Westacott CI, Barakat AF, Wood L, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthritis Cartilage 2000;8:213–221. [DOI] [PubMed] [Google Scholar]
- 22.Porée B, Kypriotou M, Chadjichristos C, et al. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem 2008;283:4850–4865. [DOI] [PubMed] [Google Scholar]
- 23.Livshits G, Zhai G, Hart DJ, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum 2009;60:2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabey T, Honsawek S, Tanavalee A, Yuktanandana P, Wilairatana V, Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers 2016;21:639–644. [DOI] [PubMed] [Google Scholar]
- 25.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006;8:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlaak JF, Schwarting A, Knolle P, Meyer zum Büschenfelde KH, Mayet W. Effects of Th1 and Th2 cytokines on cytokine production and ICAM-1 expression on synovial fibroblasts. Ann Rheum Dis 1995;54:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaventura P, Lamboux A, Albarède F, Miossec P. Differential effects of TNF-α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PloS One 2018;13:e0196285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furman BD, Mangiapani DS, Zeitler E, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther 2014;16:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage 2007;15:981–1000. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–162. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–499. [DOI] [PubMed] [Google Scholar]
- 32.Gobbi A, Espregueira-Mendes J, Lane JG, Karahan M. Bio-orthopaedics: a new approach. Berlin/ Heidelberg: Springer, 2017. [Google Scholar]
- 33.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am 2009;93:1–24. [DOI] [PubMed] [Google Scholar]
- 34.Vaquero J, Arnal J, Perez-Mañanes R, Calvo-Haro J, Chana F. 3D patient-specific surgical printing cutting blocks guides and spacers for open-wedge high tibial osteotomy (HTO): do it yourself. Revue de Chirurgie Orthopédique et Traumatologique 2016;102.S131. [Google Scholar]
- 35.Pabinger C, Lothaller H, Geissler A. Utilization rates of knee-arthroplasty in OECD countries. Osteoarthritis Cartilage 2015;23:1664–1673. [DOI] [PubMed] [Google Scholar]
- 36.Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken) 2013;65:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange JK, Lee YY, Spiro SK, Haas SB. Satisfaction rates and quality of life changes following total knee arthroplasty in age-differentiated cohorts. J Arthroplasty 2018;33:1373–1378. [DOI] [PubMed] [Google Scholar]
- 38.McCalden RW, Robert CE, Howard JL, Naudie DD, McAuley JP, MacDonald SJ. Comparison of outcomes and survivorship between patients of different age groups following TKA. J Arthroplasty 2013;28:83–86. [DOI] [PubMed] [Google Scholar]
- 39.Bayliss LE, Culliford D, Monk AP, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet 2017;389:1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King W, Bendele A, Marohl T, Woodell-May J. Human blood-based anti-inflammatory solution inhibits osteoarthritis progression in a meniscal-tear rat study. J Orthop Res 2017;35:2260–2268. [DOI] [PubMed] [Google Scholar]
- 41.Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: a 1-year pilot double-blinded randomized controlled trial. Am J Sports Med 2018;46:171–180. [DOI] [PubMed] [Google Scholar]
- 42.O’Shaughnessey K, Matuska A, Hoeppner J, et al. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J Orthop Res 2014;32:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Shaughnessey KM, Panitch A, Woodell-May JE. Blood-derived anti-inflammatory protein solution blocks the effect of IL-1β on human macrophages in vitro. Inflamm Res 2011;60:929–936. [DOI] [PubMed] [Google Scholar]
- 44.Woodell-May J, Matuska A, Oyster M, Welch Z, O’Shaughnessey K, Hoeppner J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J Orthop Res 2011;29:1320–1326. [DOI] [PubMed] [Google Scholar]
- 45.Criscione LG, St Clair EW. Tumor necrosis factor-α antagonists for the treatment of rheumatic diseases. Curr Opin Rheumatol 2002;14:204–211. [DOI] [PubMed] [Google Scholar]
- 46.Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane Database Syst Rev 2009. doi: 10.1002/14651858.CD005121.pub3 [DOI] [PubMed] [Google Scholar]
- 47.Magnano MD, Chakravarty EF, Broudy C, et al. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol 2007;34:1323–1327. [PubMed] [Google Scholar]
- 48.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2009;61:344–352. [DOI] [PubMed] [Google Scholar]
- 49.Zwerina J, Hayer S, Tohidast-Akrad M, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum 2004;50:277–290. [DOI] [PubMed] [Google Scholar]
- 50.Pratta MA, Yao W, Decicco C, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem 2003;278:45539–45545. [DOI] [PubMed] [Google Scholar]
- 51.Dejica VM, Mort JS, Laverty S, et al. Increased type II collagen cleavage by cathepsin K and collagenase activities with aging and osteoarthritis in human articular cartilage. Arthritis Res Ther 2012;14:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 2001;3:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today 1999;20:254–257. [DOI] [PubMed] [Google Scholar]
- 54.Matuska A, O’shaughnessey K, King W, Woodell-May J. Autologous solution protects bovine cartilage explants from IL-1α- and TNFα-induced cartilage degradation. J Orthop Res 2013;31:1929–1935. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998;41:1632–1638. [DOI] [PubMed] [Google Scholar]
- 56.Bertone AL, Ishihara A, Zekas LJ, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res 2014;75:141–151. [DOI] [PubMed] [Google Scholar]
- 57.Wanstrath AW, Hettlich BF, Su L, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in a canine population. Vet Surg 2016;45:764–774. [DOI] [PubMed] [Google Scholar]
- 58.Ishihara A, Bertone AL, Rajala-Schultz PJ. Association between subjective lameness grade and kinetic gait parameters in horses with experimentally induced forelimb lameness. Am J Vet Res 2005;66:1805–1815. [DOI] [PubMed] [Google Scholar]
- 59.Oosterlinck M, Pille F, Huppes T, Gasthuys F, Back W. Comparison of pressure plate and force plate gait kinetics in sound Warmbloods at walk and trot. Vet J 2010;186:347–351. [DOI] [PubMed] [Google Scholar]
- 60.Oosterlinck M, Bosmans T, Gasthuys F, et al. Accuracy of pressure plate kinetic asymmetry indices and their correlation with visual gait assessment scores in lame and nonlame dogs. Am J Vet Res 2011;72:820–825. [DOI] [PubMed] [Google Scholar]
- 61.Hix J, Klaassen M, Foreman R, et al. An autologous anti-inflammatory protein solution yielded a favorable safety profile and significant pain relief in an open-label pilot study of patients with osteoarthritis. Biores Open Access 2017;6:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King W, van der Weegen W, Van Drumpt R, Soons H, Toler K, Woodell-May J. White blood cell concentration correlates with increased concentrations of IL-1ra and improvement in WOMAC pain scores in an open-label safety study of autologous protein solution. J Exp Orthop 2016;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Drumpt RA, van der Weegen W, King W, Toler K, Macenski MM. Safety and treatment effectiveness of a single autologous protein solution injection in patients with knee osteoarthritis. Biores Open Access 2016;5:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840. [PubMed] [Google Scholar]
- 65.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005;14:798–804. [DOI] [PubMed] [Google Scholar]
- 67.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 68.Filardo G, Kon E. PRP: product rich in placebo? Knee Surg Sports Traumatol Arthrosc 2016;24:3702–3703. [DOI] [PubMed] [Google Scholar]
- 69.Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J Rheumatol 1999;26:1785–1792. [PubMed] [Google Scholar]
- 70.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS): validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical global impression of change in physical frailty: development of a measure based on clinical judgment. J Am Geriatr Soc 2004;52:1560–1566. [DOI] [PubMed] [Google Scholar]
- 72.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 73.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Manual Manip Ther 2009;17:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]