Abstract
Osteomyelitis refers to an inflammatory process causing bone destruction and necrosis. Managing such a persistent disease is complex, with a number of authors reporting different techniques. This scoping review aims to map and summarize the literature on treatment of chronic femoral and tibial osteomyelitis, in order to improve the reader’s understanding of potential treatments and identify areas of further research.
The methodological framework of the Joanna Briggs Institute was followed. A computer-based search was conducted in PubMed, EMBASE, MEDLINE, EMCARE and CINAHL, for articles reporting treatment of chronic tibial/femoral osteomyelitis. Two reviewers independently performed title/abstract and full-text screening according to pre-defined criteria.
A total of 1230 articles were identified, with 40 finally included. A range of treatments are reported, with the core principles being removal of infected tissue, dead-space management and antibiotic therapy. The majority (84.5%) of patients presented with stage III or IV disease according to the Cierny–Mader classification, and Staphylococcus aureus was the most commonly isolated organism. The proportion of patients achieving remission with no recurrence during follow-up varies from 67.7–100.0%.
The majority of studies report excellent outcomes in terms of infection remission and lack of recurrence. However, identifying specific patient or treatment-related factors which may affect outcomes is currently challenging due to the nature of the included studies and unclear reporting of treatment outcomes. It is now important to address this issue and identify such factors using further high-level research methods such as randomized controlled trials and comparative cohort studies.
Cite this article: EFORT Open Rev 2021;6:704-715. DOI: 10.1302/2058-5241.6.200136
Keywords: bone infection, femoral, tibial
Introduction
Osteomyelitis refers to a progressive inflammatory process causing destruction and necrosis of bone architecture.1 The disease may be classified, according to pathogenic mechanism, as either exogenous or haematogenous. The former describes direct contamination of bone due to trauma, surgical procedures/implants or local spread from contiguous sites of soft tissue infection or wounds.2,3 Haematogenous osteomyelitis refers to nidation of pathogens present in the systemic circulation into bone. This may occur secondary to a distal site of infection such as a minor skin abrasion or introduction of pathogens following a dental procedure or catheterization.2–4 Some previous studies also highlight a third, separate mechanism which involves vascular insufficiency secondary to diabetes mellitus.3
Chronic osteomyelitis is said to present six weeks after initial infection of the bone and is characterized by low-grade inflammation, the presence of a section of necrotic, dead bone (sequestrum) and fistulous tracts.2,3 The de-vascularized nature of the sequestrum may protect bacteria from the endogenous host immune response and limit the effectiveness of many antibiotics, although certain agents such as rifampicin and fosfomycin have demonstrated some ability to penetrate the sequestrum.5 This inhibition of pathogenic clearance may lead to formation of a nidus for a chronic and recurring infection.6
Managing such a persistent and recurrent disease is complex and resource-intensive, often requiring multiple surgical interventions and extended periods of antibiotic therapy. A number of authors have reported unique treatment strategies. For example, Kanakaris et al describe use of a reamer-irrigator-aspirator system and antibiotic cement rods, finding no recurrence in 96% of patients.7 Meanwhile, Huang et al utilized debridement and sequestrectomy with placement of an antibiotic-impregnated bone cement, reporting recurrence of infection in 50% of patients during the follow-up period.8 Other research also describes use of various local antibiotic delivery techniques such as implantable drug pumps and hyperbaric oxygen therapy.9,10
Systematic reviews investigating specific treatment strategies, such as bioactive glass implantation or single-stage treatment do exist.11,12 However, there is currently no systematic/scoping review investigating the full range of treatments reported for chronic osteomyelitis of the femur and tibia. This review aims to fill this gap in the literature by mapping the literature pertaining to treatment techniques whilst also identifying gaps in the current evidence. In doing so, we aim to improve the reader’s understanding of potential treatment strategies and guide clinical practice. Through reviewing the literature and comparing study outcomes, we also aim to identify the most beneficial treatment strategies in terms of infection eradication and lack of recurrence.
Methods
A number of scoping review frameworks have been outlined in the literature, such as those described by Arksey and O’Malley, Levac et al and Peters et al (as part of the Joanna Briggs Institute reviewer’s manual).13–15 These methodological frameworks outline five key stages involved in the development and completion of a scoping review, each of which is discussed below.
Identifying the research question
Following discussion amongst the reviewers, the following research question was developed: Which strategies are currently used in the treatment of chronic osteomyelitis of the femur and tibia, and which of these are most efficacious with regard to achieving infection remission and avoiding subsequent recurrence?
Identification of relevant studies
A thorough search strategy was developed as shown below (Table 1). Free-text and medical subject heading (MeSH) searches containing terms such as “osteomyelitis”, “chronic”, “femur” and “tibia” were used. Boolean operators (OR, AND) were used to combine search terms where relevant. English-language filters were applied on all search terms. The search strategy was designed in PubMed and adapted for and used in other electronic databases including Embase (1974 to June 2020), OVID MEDLINE® (1946 to June 2020), EMCARE and CINAHL. Manual reference-list checking of review-type articles was completed alongside searching of relevant orthopaedic journals such as The Bone & Joint Journal and Clinical Orthopaedics and Related Research, for articles published in press or ahead of print. Grey literature was also searched using a similar search strategy, for relevant thesis-type articles.
Table 1.
Search strategy used
| Search term |
|---|
| 1. Chronic osteomyelitis AND (tibia OR femur) |
| 2. Osteomyelitis [MeSH terms] AND chronic AND (femur OR tibia) |
| 3. 1 OR 2 |
Study selection
Studies identified using the above process were imported into Mendeley Reference Management software (Mendeley, London, UK) to facilitate screening, selection and record keeping. Two reviewers independently performed a two-stage title/abstract followed by full-text screening as guided by the a priori selection criteria outlined below. Differences in opinion concerning inclusion at either stage were resolved first by discussion between the two reviewers and then by consultation with a third reviewer.
Inclusion criteria
Study design: Original research including both observational and experimental studies consisting of ten or more patients, but excluding commentaries, abstracts and reviews.
Participants: Adult patients (> 18 years old) with chronic osteomyelitis of the femur (excluding femoral head) or tibia. No specific aetiologies of chronic osteomyelitis were excluded.
Intervention: Articles describing any treatment strategy including at least one surgical intervention are included. Those articles describing non-surgical treatment in a cohort of patients who have all previously received surgical intervention were excluded. Articles only reporting use of defect/dead-space management techniques are also excluded.
Comparators: No comparison criteria – articles are included regardless of a control/comparison group.
Outcome: The primary outcome investigated was treatment success rate. This is defined as the percentage of patients who achieve remission following initial treatment and show no further signs of recurrence/require no further therapy during the follow-up period.
Language: Only English-language articles are included. This decision was taken due to a lack of funding for the translation of articles published in other languages.
Date of publication: No publication-date restrictions are applied.
Articles describing treatment of chronic osteomyelitis in a series of different bones were included as long as the total number of patients with chronic osteomyelitis of the femur (excluding femoral head) or tibia totalled ten or more. Studies describing results of treatment for both chronic and acute osteomyelitis were only included if they provided a separate breakdown for results in these two groups and included at least ten patients with chronic disease.
Due to the recurring nature of chronic osteomyelitis, it was felt that the primary outcome, treatment success rate, could only be accurately assessed in studies using a sufficient follow-up period length. Therefore, studies with a mean follow-up period of less than 12 months were excluded. Those studies reporting both a mean and range were included provided the minimum mean follow-up was 12 months and the minimum shortest follow-up was at least 12 months. Articles reporting a follow-up range, rather than a mean, were included only if the minimum follow-up period was 12 months.
Charting the data
An iterative data charting process was employed, in accordance with the recommendations of Peters et al and Levac et al.14,15 An initial data extraction spreadsheet was created in Microsoft Excel using the following headings: (1) Author, (2) Year of publication, (3) Title, (4) Total number of participants, (5) Site of osteomyelitis (femur, tibia etc.), (6) Causative pathogens identified, (7) Treatments received, (8) Outcome, (9) Success rate, (10) Mean follow-up period. Two authors used this initial spreadsheet to independently extract information from the first ten included studies. Following this, discussion took place regarding the suitability of the spreadsheet. All reviewers agreed the spreadsheet was largely appropriate; however, it was suggested that the Cierny–Mader classification of osteomyelitis would be useful information to extract and so this heading was added to the spreadsheet. The final spreadsheet was then used to extract data from all included studies.
Data synthesis
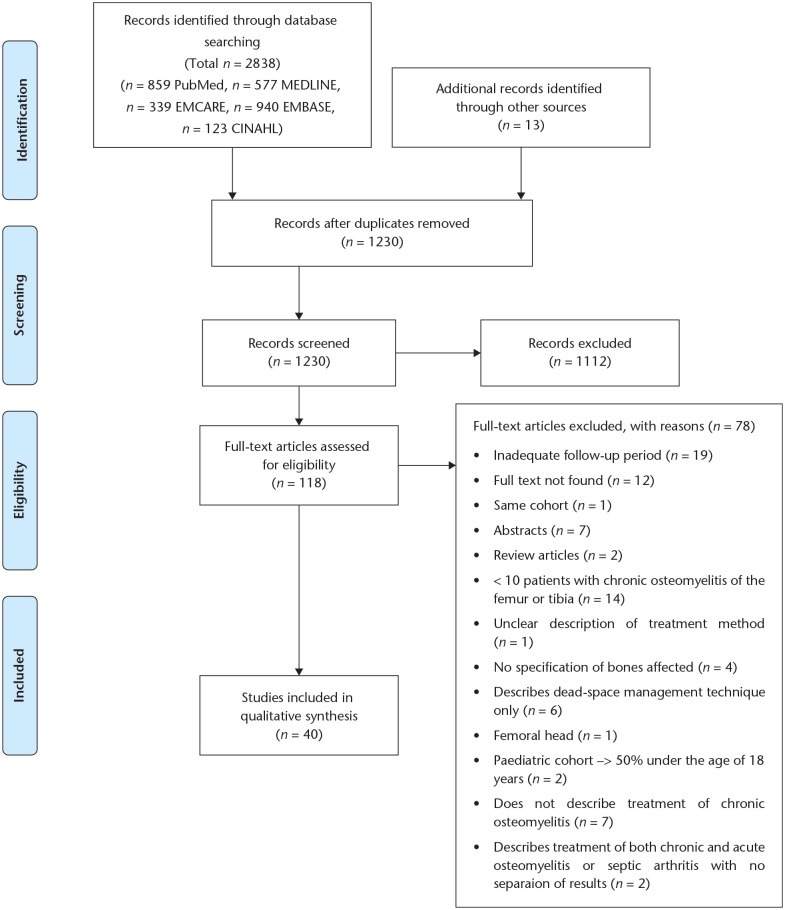
A PRISMA flowchart is displayed, showing results of the search and both screening processes (Fig. 1).16 A numerical summary of basic article characteristics such as year of publication, number of patients, number of cases of tibia/femur osteomyelitis and mean follow-up is given (Table 2). Quantitative data regarding the distribution of Cierny–Mader grades and variety of causative pathogens is also displayed (Table 3). A forest plot showing the treatment success rate of included studies was generated using R 4.0.0 software (R Foundation for Statistical Computing, Vienna, Austria) (Fig. 2).
Fig. 1.
PRISMA flow diagram demonstrating results of the search and screening process.
Table 2.
Numerical summary of included studies
| First author & year of publication | Number of patients | Femur | Tibia | Critical appraisal | Mean follow-up in months (range) |
|---|---|---|---|---|---|
| Beals 200518 | 30 | 0 | 30 | 6 | 72 |
| Chan 199819 | 36 | 0 | 36 | 8 | 44 (36–60) |
| Chan 200020 | 96 | 0 | 96 | 6 | 55.7 |
| Chang 200721 | 65 | 26 | 32 | 75 (36–334) | |
| Chen 200410 | 13 | 13 | 0 | 8 | 22 (12–42) |
| Emara 200222 | 20 | 0 | 20 | 6 | 34 |
| Euba 200923 | 50 | 17 | 16 | 8/13 | 106.8 (44.4–156) |
| Ferguson 201424 | 193 (195 cases) | 73 | 88 | 44.4 (15.6–85.2) | |
| Ferrando 201725 | 25 | 6 | 13 | 6 | 22.5 (16–33) |
| Fitzgerald 198526 | 42 | 10 | 24 | 10 | 32 (24–60+) |
| Hashmi 200427 | 17 | 9 | 8 | 75 (56–95) | |
| Huang 201828 | 80 | 0 | 80 | 5/13 | 37 (12–60) |
| Jiang 201529 | 394 | 96 | 154 | 10 | 12 |
| Kelly 199030 | 425 | 165 | 263 | 6 | Minimum 24 |
| Khan 201231 | 20 | 0 | 20 | 5 | 22.5 (19–36) |
| Kinik 200832 | 26 | 13 | 6 | 43.2 (24–72) | |
| Koval 199233 | 25 | 0 | 25 | 10 | 53.5 (20–95) |
| Lam 201934 | 67 | 0 | 34 | 8 | 46.8 (13.2–177.6) |
| Lê Thua 201535 | 29 | 4 | 22 | 7 | 12–120 |
| Li 201936 | 18 | 0 | 18 | 5 | 29.7 (24–36) |
| Lidgren 198037 | 17 patients (18 cases) | 9 | 9 | 6 | 28 (16–53) |
| Lindfors 201738 | 116 | 28 | 62 | 5 | Median 31 (12–95) |
| Marais 201639 | 28 | 8 | 15 | 8 | Minimum 12 |
| McNally 199340 | 37 | 9 | 25 | 6 | 49 (12–121) |
| McNally 201641 | 100 | 24 | 38 | 19.5 (12–34) | |
| Meissner 198942 | 60 | 13 | 43 | 8 | 37 |
| Pape 199543 | 32 (26 followed up) | 19 | 7 | 6 | 44.4 |
| Patzakis 199344 | 35 | 9 | 22 | 4 | Median 47 (24–68) |
| Romanò 201445 | 76 | 25 | 47 | 6 | 21.8 (12–36) |
| Sachs 198446 | 13 | 2 | 10 | 7 | 13–28 |
| Sen 201947 | 32 | 32 | 0 | 7 | 67.9 (24–240) |
| Shen 201548 | 14 | 0 | 11 | 7 | 38 (22–48) |
| Siegel 200049 | 46 | 0 | 46 | 5 | 61.2 (24–102) |
| Simpson 200150 | 50 | 17 | 18 | 6 | 26.2 (12–48) |
| Smith 200651 | 41 | 9 | 31 | 7 | Minimum 12 |
| Sun 201852 | 72 | 0 | 72 | 6/13 | 33 |
| Wang 201753 | 15 | 0 | 15 | 8 | 25 (24–28) |
| Yamashita 199854 | 18 | 4 | 10 | 52.7 (24–75) | |
| Zhou 202055 | 42 (43 cases) | 0 | 43 | 10 | 42.8 |
| Zweifel-Schlatter 200656 | 14 | 0 | 14 | 9 | 31.4 (12–52) |
Note. The critical appraisal score is out of a maximum of 10 points, except for randomized controlled trials which have a maximum score of 13 and are clearly labelled.
Table 3.
Numerical summary of the Cierny–Mader grade of patients in studies where this information was reported
| Cierny–Mader grade | Number of patients (%) |
|---|---|
| I A | 54 (4.89) |
| I B | 7 (0.63) |
| I C | 1 (0.09) |
| Grade I host status not specified | 37 (3.59) |
| Total grade I | 99 (8.97) |
| II A | 7 (0.63) |
| II B | 6 (0.54) |
| II C | 0 (0.00) |
| Grade II host status not specified | 27 (2.62) |
| Total grade II | 40 (3.62) |
| III A | 138 (12.50) |
| III B | 202 (18.30) |
| III C | 0 (0.00) |
| Grade III host status not specified | 190 (17.20) |
| Total grade III | 530 (48.00) |
| IV A | 141 (12.80) |
| IV B | 100 (9.05) |
| IV C | 4 (0.36) |
| Grade IV host status not specified | 158 (14.30) |
| Total grade IV | 403 (36.50) |
Fig. 2.

Forest plot displaying the treatment success rate of included studies.
Quality of evidence and risk of bias
The relevant Joanna Briggs Institute critical appraisal checklist was used to assess risk of bias in and methodological quality of all included studies.17
Statistical analysis
Where possible, treatment success rates were pooled according to various management categories. Study weighting was determined by the number of patients included in each individual study.
Results
A total of 1230 articles were identified following removal of duplicates (Fig. 1), with 40 articles involving 2529 patients finally included (Table 2). The majority of studies were case series type articles, with only three randomized controlled trials included. All studies were deemed to be of sufficient quality after critical appraisal using the relevant Joanna Briggs Institute checklist (see Appendix 1 (24.1KB, docx) for full details).
Bones affected
A total of 15 studies report treatment of chronic osteomyelitis of the tibia only, whereas two only report treatment of disease affecting the femur. The remaining 23 studies involve treatment of osteomyelitis affecting multiple different bones including the femur, tibia, humerus, calcaneus and metatarsals.
Cierny–Mader classification
The Cierny–Mader classification was used to assess severity of osteomyelitis in 21 studies involving 1104 cases (Table 3). Eight of these studies did not clearly report the host status element of the classification system and so are included in the ‘host status not specified’ rows. One study reports all 32 patients having stage IIIB or IVA chronic osteomyelitis but does not provide a more detailed numerical breakdown.47
Pathogens
The identification of pathogens in affected limbs was reported in 34 studies. However, a number of these studies did not clarify the number of patients showing growth of multiple pathogens, and what these pathogens were. It was therefore not feasible to provide an exact numerical breakdown of the number of patients showing growth of a specific organism. Instead, the number of times a particular organism was found to be amongst the top three most commonly identified species is displayed (Table 4), as well as the number of studies in which a specific organism was the most commonly identified pathogen (Table 5). One study commented that multiple organisms were seen in most patients, including Staphylococcus aureus, Proteus mirabilis, Bacteroides fragilis and Pseudomonas aeruginosa; however, a more detailed breakdown was not given and so this study was not included in the tables below.40
Table 4.
The number of times a particular organism was found to be amongst the top three most commonly identified species
| Organism | Number of times in the top three most common |
|---|---|
| Staphylococcus aureus | 34 |
| Pseudomonas aeruginosa | 19 |
| Staphylococcus epidermidis | 10 |
| Escherichia coli | 5 |
| Pseudomonas species | 5 |
| Coagulase negative Staphylococcus | 7 |
| Enterococcus faecalis | 5 |
| Enterobacter species | 3 |
| Klebsiella species | 3 |
| Streptococcus faecalis | 2 |
| Proteus species | 2 |
| Serratia species | 2 |
| Proteus mirabilis | 2 |
| Acidaminococcus fermentans | 1 |
| Acinetobacter lwoffii | 1 |
| Aeromonas hydrophila | 1 |
| B-haemolytic streptococcus | 1 |
| Corynebacterium species | 1 |
| Diphtheroids | 1 |
| Finegoldia magna | 1 |
| Klebsiella pneumoniae | 1 |
| Micrococcus luteus | 1 |
| Pantoea species | 1 |
| Pseudomonas putida | 1 |
| Staphylococcus hominis | 1 |
| Stenotrophomonas maltophilia | 1 |
| Streptococcus agalactiae | 1 |
| Streptococcus infantarius | 1 |
| Streptococcus viridans | 1 |
Table 5.
The number of times a particular organism was the most commonly identified organism in each individual study
| Organism | Number of times most common |
|---|---|
| Staphylococcus aureus | 28 |
| Colon bacillus | 1 |
| Escherichia coli | 1 |
| Pseudomonas aeruginosa | 1 |
| Staphylococcus epidermidis | 1 |
| Staphylococcus species | 1 |
Treatment
A wide range of treatment strategies are reported in the included literature (Table 6), with some articles describing the use of multiple slightly different techniques within the same study cohort. All techniques described involve at least two of the seven following basic principles: (1) removal of infected tissue, (2) removal of metalwork, (3) irrigation, (4) local antibiotic delivery, (5) dead-space management, (6) identification of pathogen and (7) targeted or empirical systemic intravenous/oral antibiotic therapy. Techniques used to remove infected tissue include radical or conservative debridement, sequestrectomy and intramedullary reaming, for example using the reamer-irrigator-aspirator system. A variety of local antibiotic delivery/dead-space management systems are also used, such as the insertion of antibiotic-coated poly(methyl methacrylate) beads/chains, Septopal® (Merck, Darmstadt, Germany) beads, calcium sulphate antibiotic carriers, Osteoset®-T pellets (Wright Medical Technology Inc. Arlington, Tennessee, USA) and antibiotic-loaded cement. Other dead-space management strategies reported include bone grafting, muscle transfer and bioactive glass. One study used debridement with subsequent osteotomy and bone transport using an Iliazarov frame.
Table 6.
Description of the treatment strategies reported by all included studies
| Study | Treatment |
|---|---|
| Beals 200518 | 4 treatment patterns: 8 patients received local debridement with or without soft tissue coverage, 3 radical debridement and bone transport using circular frame, 8 treated by Papineau grafting technique post debridement, 11 received debridement and circular frame fixation. All patients received 6 months targeted IV antibiotics. |
| Chan 199819 | Stage 1: debridement, sequestrectomy and removal of metalwork with implantation of targeted antibiotic-impregnated poly(methyl methacrylate) bead chains. Stage 2: 2 – 10 weeks later, bead removal and targeted antibiotic-coated cancellous bone graft from anterior iliac crest. All patients received one week of post-operative intravenous antibiotic therapy. |
| Chan 2000 20 | Staged antibiotic-impregnated autogenous cancellous bone graft (46 patients) or pure autogenous cancellous bone graft (50 patients). |
| Chang 200721 | Group 1: debridement and targeted systemic antibiotic therapy only (40 patients). Group 2: debridement with insertion of tobramycin OSTEOSET- T® pellets and targeted systemic antibiotic therapy. |
| Chen 200410 | Debridement followed by a course of hyperbaric oxygen therapy. The compression chamber used 100% oxygen delivered via a mask system with 2.5 atmospheres absolute for 2-hour duration with intermittent schedule of 25 minutes of 100% oxygen breathing and 5 minutes air breathing, 1 session per day, 5 days per week. Additional debridement followed if necessary; 11 patients also received cancellous bone grafting. All patients received 2 weeks targeted IV antibiotic, followed by 2–4 weeks oral antibiotic therapy. |
| Emara 200222 | Debridement followed by hemi-corticotomy. Ilizarov external fixation frame then applied; Orthofix limb reconstruction external fixation system used. All patients given two weeks targeted antibiotic therapy post-operatively. |
| Euba 200923 | Group 1: debridement with 6 weeks IV (2 g/4 h) then 2 weeks oral (500 mg/6 h) cloxacillin (22 patients). Group 2: debridement with rifampicin-cotrimoxazole (600 mg rifampicin/24 h + 7/8 mg/kg body weight/day cotrimoxazole) (28 patients). Surgery included extensive debridement of bone and soft tissue, removal of foreign material. Closed suction irrigation or muscular flap complemented debridement when indicated. |
| Ferguson 201424 | Debridement with insertion of tobramycin OSTEOSET-T® pellets and targeted systemic antibiotic therapy. |
| Ferrando 201725 | Group 1: surgical debridement, systemic antibiotics and local application of bioactive glass S53P4 (12 patients). Group 2: surgical debridement, systemic antibiotics and local application of calcium sulphate antibiotic beads with a combination of vancomycin and gentamycin (13 patients). All patients received 6–12 weeks of antibiotic therapy. |
| Fitzgerald 198526 | Debridement/sequestrectomy and application of a local muscle flap. A soleus or gastrocnemius muscle flap was most frequently utilized to achieve closure. In 5 patients, combination of 2 muscle flaps was utilized. Multiple debridements performed in 28/42 patients. All patients received targeted IV antibiotic therapy, for a mean duration of 24 days following surgery. |
| Hashmi 200427 | Lautenbach procedure – radical debridement and reaming with insertion of double lumen local antibiotic system with targeted systemic antibiotics. |
| Huang 201828 | Autoplastic transplantation of ilium to eliminate sequestrum and inflammatory granuloma (all patients). Control group (40 patients): targeted antibiotic therapy, no treatment length recorded. Observation group (40 patients): antibiotic therapy and Chinese medicinal wuwei xiadou drink. |
| Jiang 201529 | Radical debridement followed by IV antibiotic therapy and reconstruction of bone/soft tissue if necessary. Median duration of IV antibiotic treatment was 14 days. |
| Kelly 199030 | Stage 1: soft tissue and bone debridement until bleeding tissue encountered. Chronically infected skin, soft tissue and muscle also excised. Stage 2: wound closure and coverage of saucerized osseous cavity by delayed closure muscle flap, free-tissue transfer and skin graft with suction drainage/irrigation. Bone grafts used in cases of nonunion. At least 4 weeks targeted IV antimicrobial therapy. |
| Khan 201231 | Radical debridement and stabilization of fracture with open reduction and internal fixation (5 patients) or external fixation with Hoffman frame/hybrid ring/Braun frame (15 patients). Local flap skin grafts for soft tissue cover, taken from radial forearm. Detail regarding any antibiotic therapy not available. |
| Kinik 200832 | Debridement with saline irrigation and insertion of vancomycin poly(methyl methacrylate) beads and targeted systemic antibiotic therapy. |
| Koval 199233 | All patients received debridement and osseous stabilization if needed. Soft tissue management was divided into 3 groups: Group 1 (15): free or rotational muscle flap coverage. Group 2 (11): suction irrigation primary closure. Group 3 (5): Papineau technique of open cancellous bone grafting. All patients received antibiotic therapy for an average of 39.2 days (range 5–84 days). |
| Lam 201934 | Stage 1: debridement – single (21 patients), multiple 46. 14 patients received local antibiotic-loaded beads and 10 patients received poly(methyl methacrylate)-coated intramedullary nails. Stage 2: limb salvage and soft tissue coverage using free tissue transfer. Stage 3: IV/PO antibiotics for 6 weeks in all patients, and average of 2.7 months of additional daily antibiotics for infected nonunion cases (54 patients). |
| Lê Thua 201535 | Drainage, extensive debridement, reduction of dead space, soft tissue coverage and antibiotic therapy for 4-6 weeks. When the clinical absence of infection was confirmed by microbiological testing, open bone was irrigated with saline solution for 2 weeks, followed by free muscle transfer. Muscle flaps included latissimus dorsi, gracilis and rectus abdominis muscles depending on the location, size and length of dead bone. |
| Li 201936 | Debridement with external fixation of the tibia and vacuum seal drainage. Antibiotic bone cement consisting of vancomycin-loaded poly(methyl methacrylate) inserted into defect. Post-operative antibiotic treatment for 6 weeks. |
| Lidgren 198037 | Intramedullary reaming in all. Closed intramedullary suction irrigation drainage daily for 5 days (9 patients). Gentamycin-poly(methyl methacrylate) beads were implanted into marrow cavity (5 patients). Post-operative targeted antibiotics were administered perorally for average of 10 months, according to pattern of resistance. |
| Lindfors 2017 38 | Group 1 (98 patients) – 1-stage procedure: debridement followed by filling of cavitary defect with bag-S53P4. Group 2 (18 patients) – 2-stage procedure: debridement with implantation of Septopal antibiotic beads. Second stage performed 1–4 months later, at which time the beads were removed, and defect filled with bag-S53P4. Muscles flaps used in 15 patients, and skin transplantation performed in 3 patients. All patients received systemic antibiotics (unknown period). |
| Marais 201639 | Marginal or wide resection, dead-space management, provision of bony stability, soft tissue reconstruction, and/or skeletal reconstruction. Post-operatively, all patients were treated with IV cefazolin and imipenem until microscopy culture and sensitivity results became available. Following this, patients received targeted oral antibiotic therapy for 6 weeks. |
| McNally 199340 | Belfast technique: Stage 1 – radical debridement with dead-space elimination using either muscle flap transfer, direct skin closure with implantation of gentamycin loaded poly(methyl methacrylate) beads or composite flaps from deep circumflex iliac artery. At least 2 antibiotics were given before surgery, these antibiotics were continued with oral preparations of the chosen drug. Stage 2 – autogenous bone grafting, carried out between 3–6 weeks after stage 1 when soft tissues had adequately healed. 5 patients did not have 2nd stage to their treatment. Antibiotics were continued until there was radiological and clinical evidence of union. |
| McNally 201641 | Debridement with insertion of gentamycin-infused CERAMENT G® (Bonesupport, Lund, Switzerland) and targeted systemic antibiotic therapy. |
| Meissner 198942 | Debridement with adjuvant IV Fosfomycin therapy. Fosfomycin therapy was withdrawn around 3 days after confirmed reduction in inflammatory parameters. |
| Pape 199543 | Reaming of intramedullary canal in all. 13/32 patients received poly(methyl methacrylate) chains after reaming, 3 received instillation drainage. No data regarding antibiotic therapy recorded. |
| Patzakis 199344 | Randomized Septopal-gentamicin group (17 patients) of which 12 in Septopal arm, 5 in antibiotic arm. Remainder (16) in non-randomized group. Septopal patients (12 patients): systemic antibiotic therapy. Antibiotic arm (5 patients): 4–6 weeks of IV antibiotic therapy followed by 3 months of oral antibiotic therapy. Non-randomized group (16): 3–4 weeks of IV antibiotic therapy followed by oral antibiotic therapy. Randomized group received primary closure of wounds or soft tissue muscle transfer at time of debridement. Non-randomized group received initial debridement with muscle transfer 3–9 days later. |
| Romanò 201445 | Group 1: debridement and application of S53P4 bioactive glass, no local antibiotics (27 patients). Group 2: debridement and targeted antibiotic-loaded hydroxyapatite and calcium sulphate (27 patients). Group 3: debridement and teicoplanin-loaded demineralized bone matrix (22 patients). All groups received 4–12 weeks teicoplanin. Debridement involved removal of all foreign materials, bone substitutes and macroscopically infected/necrotic tissues and debridement of medullary canal followed by repeated lavage with saline. Post-operatively, all groups received 4–12 weeks of systemic antibiotic therapy, determined by prior consultation with infectious diseases specialist and microbiology department. A combination of targeted 2 antibiotics usually administered or if cultures were negative, vancomycin or teicoplanin and meropenem for 14 days followed by oral levofloxacin and rifampicin. |
| Sachs 198446 | 3-stage Papineau protocol. Oral antibiotics were continued until each wound had complete skin coverage. |
| Sen 201947 | Stage 1: radical bone resection and soft tissue debridement, with implantation of antibiotic-coated beads and targeted intravenous antibiotic therapy. Stage 2: acute shortening and re-lengthening osteotomy (17 patients) or segmental bone transport with a 4-ring Ilizarov frame (15 patients). In all cases, targeted IV antibiotics were administrated for a minimum of 6 weeks, or until CRP and ESR levels had returned to normal. |
| Shen 201548 | Debridement and vacuum sealing drainage after which culture and sensitivity testing performed. 2nd debridement with internal fixation device removed if not contributing to stability of bone. External fixation added if unstable fracture present. Defect packed with calcium sulphate beads impregnated with vancomycin 1 g. If debridement cavity was large and autograft was used to fill the residual space. Systemic targeted antibiotics administered for 2–4 weeks. |
| Siegel 2000 49 | Limb salvage, including extensive debridement, autogenous bone grafting, and soft tissue reconstruction and flap coverage (30 free flaps, 16 rotational flaps). All patients were treated with local and systemic antibiotics under the supervision of an infectious-disease specialist. Duration of treatment not provided. |
| Simpson 200150 | Group 1: wide excision > 5 mm margin (15 patients). All necrotic and infected bone was excised, remaining bone was clearly viable with good punctate bleeding. Group 2: marginal excision < 5 mm margin (29 patients). All necrotic and infected bone was excised but with smaller margin of clearance. Group 3: intralesional biopsy with pus drainage, lavage and debulking (6 patients). Broad-spectrum IV antibiotics administered to all patients, later modified after results from cultures and sensitivity assessment were obtained and continued for 6 weeks. Patients then switched to 6 weeks oral antibiotic therapy. |
| Smith 200651 | Wide excision of affected soft and hard tissue. Involucrum fenestrated and necrotic bone sequestra excised. Suction drain placed in remaining gutter. Dead space obliterated with free muscle transfer. IV ciprofloxacin given at time of surgery and then orally for 6 weeks post-operatively. If microbiology culture and sensitivity suggested another more appropriate antibiotic, targeted antibiotics were given. |
| Sun 201852 | Experimental group (36 patients): debridement with insertion of gentamycin-impregnated beads, drainage and wound closure. IV antibiotics administered to all patients (unknown period). Control group (36): removal of necrotic and infected tissue with lavage/drainage. Daily local rinsing with gentamycin dissolved in saline. All patients received targeted IV antibiotic therapy. |
| Wang 201753 | Induced membrane 2-stage surgical technique, Stage 1: radical debridement followed by insertion of antibiotic-loaded poly(methyl methacrylate). Targeted IV antibiotics administered for 2 weeks post-operatively. Stage 2 occurred 6–8 weeks later: bone graft implantation to repair defects after 6–8 weeks, prophylactic antibiotics administered for 24 hours and suction drainage applied for 10–12 days. |
| Yamashita 199854 | Debridement with insertion of gentamycin/imipenem calcium hydroxyapatite implants and systemic targeted antibiotic therapy. |
| Zhou 202055 | Fenestration and debridement with placement of vancomycin/gentamycin-loaded calcium sulphate. Broad-spectrum antibiotics administered post-operatively, later switched to targeted therapy for no more than 2 weeks. |
| Zweifel-Schlatter 200656 | Radical bone excision and, if necessary, further stabilization of the tibia carried out followed by microvascular transfer of fasciocutaneous flap after 7–10 days. Targeted antibiotic treatment started from time of definitive coverage with free fasciocutaneous flap and continued for 3–6 months post-operatively. |
Note. IV, intravenous; PO, per os (by mouth) ; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Outcomes
The reporting of treatment outcomes in included studies is highly heterogenous. Our primary outcome of interest was proportion of patients achieving remission with no subsequent recurrence during the follow-up period. This was clearly reported by 29 studies (Fig. 2), with success rates of 67.7–100.0 %. A pooled estimate of treatment success rate according to various treatment categories is shown in Table 7.
Table 7.
Breakdown of treatment success rate according to various different surgical management strategies
| Treatment method | Study | Success rate (%) | Total number of patients |
|---|---|---|---|
| Debridement + Exfix/ORIF + systemic antibiotic therapy | Khan 2012 | 100.0 | 20 |
| Emara 2002 | 95.0 | 20 | |
| Total | 2 studies | 97.5 | 40 |
| Debridement + muscle flap/fasciocutaneous flap + systemic antibiotic therapy | Zweiffel-Schlatter 200656 | 100.0 | 14 |
| Lê Thua 201535 | 96.6 | 29 | |
| Smith 200651 | 95.00 | 41 | |
| Fitzgerald 198526 | 92.9 | 42 | |
| Total | 4 studies | 95.2 | 126 |
| Debridement + bone graft + systemic antibiotic therapy | Huang 201828 | 95.0 | 80 |
| Chan 200020 | 82.0 | 50 | |
| Total | 2 studies | 90.0 | 130 |
| Debridement + local antibiotic delivery + systemic antibiotic therapy | Chan 199819 | 94.4 | 36 |
| Li 201936 | 94.4 | 18 | |
| Ferrando 201725 | 92.3 | 13 | |
| Romanò 201445 | 87.7 | 49 | |
| Lindfors 201738 | 72.0 | 18 | |
| Lidgren 198037 | 88.9 | 18 | |
| Chan 200020 | 95.7 | 46 | |
| Zhou 202055 | 88.4 | 43 | |
| Wang 201753 | 86.7 | 15 | |
| Pape 199543 | 84.4 | 26 | |
| Chang 200721 | 80.0 | 25 | |
| Kinik 200832 | 88.5 | 26 | |
| McNally 201641 | 96.0 | 100 | |
| Ferguson 201424 | 90.8 | 195 | |
| Hashmi 200427 | 94.1 | 17 | |
| Yamashita 199854 | 100.0 | 18 | |
| McNally 199340 | 91.9 | 37 | |
| Total | 17 studies | 90.9 | 700 |
| Debridement + hyperbaric oxygen therapy + systemic antibiotic therapy | Chen 200410 | 92.3 | 13 |
| Total | 1 study | 92.3 | 13 |
| Debridement + bioactive glass + systemic antibiotic therapy | Romanò 201445 | 92.6 | 27 |
| Lindfors 201738 | 95.9 | 98 | |
| Ferrando 201725 | 91.7 | 12 | |
| Total | 3 studies | 94.9 | 137 |
| Debridement+ systemic antibiotic therapy | Euba 200923 | 90.0 | 50 |
| Meissner 198942 | 76.7 | 60 | |
| Chang 200721 | 60.0 | 40 | |
| Simpson 200150 | 72.0 | 50 | |
| Jiang 201529 | 77.4 | 394 | |
| Total | 5 studies | 76.8 | 594 |
Note. Exfix, external fixation; ORIF, open reduction and internal fixation.
It was not possible to perform a meta-analysis comparing the success rates achieved using the above treatment methods due to the low-level study design and the inherent heterogeneity they provide. Furthermore, the included studies are highly heterogenous with factors such as Cierny–Mader grade, type and number of causative organisms, patient demographics and bones affected differing between studies. This heterogeneity renders comparison between different treatment groups challenging.
It is important to note that, although articles with short mean follow-up periods of less than 12 months have been excluded from this review, there is still a large degree of variation in the follow-up period of included studies (Table 2). Mean follow-up ranges from 106.8 months in the study by Euba et al to just 12 months in the study by Jiang et al.23,29 Therefore, data concerning the percentage of patients achieving remission with no recurrence during follow-up should be interpreted with caution as these figures are, by their definition, likely to vary according to the length of follow-up period used in each study. It is also important to be aware of this effect within studies. Some articles also describe a large variation of follow-up within the same patient cohort, for example, the study Euba et al reports a follow-up range of 44.4–156 months. 23
Of the studies reporting success rate, six directly compare the efficacy of two or more treatment strategies. Chan et al 2000 report a higher success rate of 95.6% in those receiving antibiotic-impregnated cancellous bone graft compared to 82% in those receiving a pure cancellous bone graft.10 Simpson et al compare the effect of radical (> 5 mm margin) and conservative (< 5 mm margin) debridement, finding a higher success rate of 100% in the former, compared to 72% in the latter group.50 Euba et al compare two post-debridement antibiotic regimens: six weeks intravenous, followed by two weeks oral cloxacillin or eight weeks oral rifampicin/cotrimoxazole. 23 Similar success rates of 90.5% and 88.9% were reported in the two groups respectively; however, the rifampicin group showed a significantly lower mean hospital stay of 31 days compared to 51 in the cloxacillin group. Romanò et al and Ferrando et al both compare the efficacy of S53P4 bioactive glass with various local antibiotic delivery methods such as calcium sulphate beads or teicoplanin-loaded demineralized bone matrix. 25,45 Romanò et al reports a higher success rate in those receiving bioactive glass, whilst the opposite is seen in Ferrando et al (Table 7). Lindfors et al divide patients into those receiving one-stage debridement and immediate insertion of S53P4 bioactive glass with those given a two-stage procedure involving debridement and insertion of Septopal® antibiotic beads, followed by bead removal and insertion of S53P4 bioactive glass, 1–4 months later. 38 A higher success rate of 95% was reported in the one-stage group, compared to 72% in the two-stage group.
Of the 18 recurrences in the study by Ferguson et al, 11 were seen in the 144 patients with Cierny–Mader grade 3 (recurrence rate of 7.6%), whilst a much higher recurrence rate of 7/38 (18.4%) was reported in those with stage 4 disease.24 No recurrence occurred in those with stage 1 or 2 osteomyelitis. Similarly, all four recurrences in McNally et al 2014 occurred in those with stage 3 disease.41
Discussion
This scoping review aimed to map and summarize current literature pertaining to treatment options for chronic femoral and tibial osteomyelitis, whilst also identifying the most beneficial treatment strategies in terms of infection control and relapse prevention. There currently exists a lack of high-quality research on this topic, with the majority of included articles being level 4 case series type studies.
A wide variety of unique treatment strategies are described in the literature, with the key principles being removal of infected tissue, dead-space management along with systemic and/or local antibiotic therapy. These key principles are often achieved using different techniques. For example, McNally et al, Siegel et al and Beals et al describe the use of radical or extensive debridement, whilst Kanakaris et al report use of a reamer-irrigator-aspirator system.7,18,40,57 A number of studies describe the use of local antibiotic delivery systems such as antibiotic-coated beads or cement whilst others employ dead-space management techniques involving muscle transfer or bone grafting.7,37,48,58 The use of adjunctive antibiotic therapy also varies widely between included studies. For example, some authors describe techniques involving both systemic oral/intravenous antibiotics and local antibiotic coated beads/cement, whilst others use only one of these techniques. Furthermore, the length of time for which antibiotic treatment is administered is highly variable, with some studies giving patients antibiotics until ‘normal’ C-reactive protein (CRP)/erythrocyte sedimentation rate (ESR) levels are seen and others halting therapy after a certain length of time.
Despite this large variation in exact treatment strategy employed, the majority of included studies report highly favourable outcomes. Of the 29 studies clearly reporting the percentage of patients achieving infection eradication with no subsequent recurrence during follow-up, 18 (62.1%) report a figure of at least 90%, whilst 25 (86.2%) report the equivalent in at least 80% of patients.
However, identifying the most beneficial treatment method is challenging, with authors often reporting different outcomes, despite the use of similar techniques. For example, Jiang et al describe a technique involving radical debridement followed by a median of two weeks of intravenous antibiotic therapy, achieving a treatment success rate of 77.40%, whilst Euba et al use a similar technique, achieving a much higher success rate of 96.60%.23,29.
Pooling study results (Table 7) does reveal some differences in success rate between different treatment strategies. For example, debridement followed by systemic antibiotic therapy shows a lower pooled success rate of 76.8% compared to techniques involving local antibiotic delivery and insertion of bioactive glass, with pooled success rates of 90.8 and 94.3% respectively. However, a formal meta-analysis comparing these treatments could not be conducted due to low-level study design and between study heterogeneity. It is not clear whether these differences in treatment success reflect superiority of a particular management strategy, or instead arise due to between-study differences in patient and treatment-related factors. Such factors may include follow-up period, patient demographics (age, sex), co-morbidities, Cierny–Mader grade, range and type of causative pathogens and bones affected.
It would be useful for clinicians to have an understanding of how individual patients may respond to particular treatment strategies. This could include, for example, the effect of a specific patient’s Cierny–Mader grade, number of causative organisms, age/sex, specific bones affected, and co-morbidities on response to surgical treatment and which treatment regime may be best for individual patients with differences in these factors. Again, it is difficult to answer these questions using the current evidence as, although studies often do report patient factors such as Cierny–Mader grade and number/type of causative organisms, they fail to separate treatment results according to these factors. Future research should endeavour to provide a more detailed breakdown of results, separating outcomes according to some of the factors discussed above in order to provide clinicians with a better understanding of the role of these factors in deciding treatment strategy.
The use of more rigorous, higher level research methodology such as randomized controlled trials or cohort studies may help to control for these potential factors and allow us to better understand their impact on treatment outcomes. Such research techniques would also allow the identification of specific parts of treatment strategies which may influence patient outcomes. For example, the case series of Zhou et al describes a technique involving debridement, placement of antibiotic-coated beads followed by systemic antibiotic therapy, achieving a success rate of 88.40%.55 Meanwhile, the technique of Kanakaris et al, involving antibiotic cement rods and six weeks of antibiotic therapy, demonstrates a higher success rate of 95.80%.7 It may therefore be argued that the use of antibiotic-loaded cement rods as opposed to beads and/or an extended period of antibiotic administration may be responsible for the higher success rate seen in the latter study. However, given the possible confounding factors described above, it is difficult to accurately identify the effect that such differences in treatment technique may have on patient outcome using the current low-quality literature. For example, Kanakaris et al was removed from this review due to a minimum follow-up period of less than 12 months, which may have influenced the higher success rate described. Clearly, high-level randomized controlled trials are required to allow us to adequately control for any potential confounding factors and directly compare the effect of specific aspects of treatment strategy such as radical versus conservative debridement, six weeks versus two weeks of antibiotic administration or use of antibiotic-loaded cement versus beads. Such comparative research would allow the identification of the most beneficial treatment characteristics in order to build the most optimal overall strategy.
It is important to acknowledge that this article is not without its limitations. This study was originally designed as a systematic review and registered in the international prospective register of systematic reviews (PROSPERO) (CRD42020194178). However, following an initial literature search it was decided to alter the format to a scoping review. Whilst systematic reviews are typically used to answer a specific research question such as those pertaining to the efficacy of a particular treatment, scoping reviews have a broader remit in mapping and summarizing the full breadth of literature surrounding a topic. It was therefore felt that a scoping review would better allow us to achieve the described aims of the study.
Furthermore, as chronic osteomyelitis is a complex, often recurring disease, it was decided to exclude all articles with a mean follow-up period of less than 12 months. This led to the exclusion of 20 potentially relevant articles at the full-text screening stage (see Appendix 2 (46.4KB, docx) ). However, even so, some included studies do have a short follow-up period, with the mean follow-up ranging from 12–106.8 months in included studies. This is important to be aware of as described study outcomes, particularly our primary outcome, the proportion of patients achieving remission with no follow-up recurrence, are likely to be affected by the follow-up period used.
Conclusions
In conclusion, a wide variety of surgical treatment strategies for chronic osteomyelitis of the femur and tibia are described in the current literature, with the majority of these showing excellent results in terms of infection, eradication, recurrence and prevention. These studies do provide clinicians with a good understanding of different treatment strategies and their benefits. It is important to note that some included studies have a short mean follow-up period, which may affect treatment outcomes described. It is now important to go one step further and clarify the role of both specific patient-related factors such as co-morbidities, Cierny–Mader grade and causative organisms as well as treatment-related factors such as length of antibiotic therapy, width of bone resection and dead-space management technique on treatment outcomes. As discussed, understanding the effects of the above factors using the current literature is challenging. There is therefore a need for more detailed reporting of treatment results and high-level comparative research in order to give clinicians a better understanding of what treatment strategy may be best for the specific patient presenting to them. We recommend the use of a minimum data collection set for future studies (see Appendix 3 (17.6KB, docx) ), to ensure adequate and clear reporting of patient-related factors and treatment results.
Footnotes
ICMJE Conflict of interest statement: The authors declare no conflict of interest relevant to this work.
Permissions: Zaki Arshad https://www.linkedin.com/in/zaki-arshad-6b149812b; Edward Jun-Shing Lau https://www.linkedin.com/in/edward-lau-134565168; Aiman Aslam https://www.linkedin.com/in/aiman-aslam-4a105616a; Azeem Thahir https://www.linkedin.com/in/azeem-thahir-a628b741; Matija Krkovic https://www.linkedin.com/in/matijakrkovic
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Supplemental Material: Supplemental material is available for this paper at https://online.boneandjoint.org.uk/doi/suppl/10.1302/2058-5241.6.200136
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Birt MC, Anderson DW, Bruce Toby E, Wang J. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 2017;14:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panteli M, Giannoudis PV. Chronic osteomyelitis: what the surgeon needs to know. EFORT Open Rev 2016;1:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lew PDP, Waldvogel PFA. Osteomyelitis. Lancet 2004;364:369–379. [DOI] [PubMed] [Google Scholar]
- 4. Macnicol M, Watts AC. Haematogenous osteomyelitis. Surg 2005;23:25–30. [Google Scholar]
- 5. Kim BN, Kim ES, Oh MD. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J Antimicrob Chemother 2014;69:309–322. [DOI] [PubMed] [Google Scholar]
- 6. Maffulli N, Papalia R, Zampogna B, Torre G, Albo E, Denaro V. The management of osteomyelitis in the adult. Surgeon 2016;14:345–360. [DOI] [PubMed] [Google Scholar]
- 7. Kanakaris N, Gudipati S, Tosounidis T, Harwood P, Britten S, Giannoudis PV. The treatment of intramedullary osteomyelitis of the femur and tibia using the Reamer-Irrigator-Aspirator system and antibiotic cement rods. J Bone Joint Surg [Br] 2014;96-B:783–788. [DOI] [PubMed] [Google Scholar]
- 8. Huang P-Y, Wu P-K, Chen C-F, et al. Osteomyelitis of the femur mimicking bone tumors: a review of 10 cases. World J Surg Oncol 2013;11:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perry CR, Ritterbusch JK, Rice SH, Davenport K, Burdge RE. Antibiotics delivered by an implantable drug pump: a new application for treating osteomyelitis. Am J Med 1986;80:222–227. [DOI] [PubMed] [Google Scholar]
- 10. Chen C-E, Ko J-Y, Fu T-H, Wang CJ. Results of chronic osteomyelitis of the femur treated with hyperbaric oxygen: a preliminary report. Chang Gung Med J 2004;27:91–97. [PubMed] [Google Scholar]
- 11. Pincher B, Fenton C, Jeyapalan R, Barlow G, Sharma HK. A systematic review of the single-stage treatment of chronic osteomyelitis. J Orthop Surg Res 2019;14:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanwar YS, Ferreira N. The role of bioactive glass in the management of chronic osteomyelitis: a systematic review of literature and current evidence. Infectious Diseases 2020;52:219–226. [DOI] [PubMed] [Google Scholar]
- 13. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. [Google Scholar]
- 14. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z. eds. JBI manual for evidence synthesis. JBI, 2020. https://synthesismanual.jbi.global (Date last Accessed 02 March 2021).
- 15. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z. eds. JBI manual for evidence synthesis. JBI, 2020. https://synthesismanual.jbi.global (Date last Accessed 01 March 2021).
- 18. Beals RK, Bryant RE. The treatment of chronic open osteomyelitis of the tibia in adults. Clin Orthop Relat Res 2005;433:212–217. [DOI] [PubMed] [Google Scholar]
- 19. Chan YS, Ueng SW, Wang CJ, et al. Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. J Trauma 1998;45:758–764. [DOI] [PubMed] [Google Scholar]
- 20. Chan Y, Ueng S, Wang C, et al. Antibiotic-impregnated autogenic cancellous bone grafting is an effective and safe method for the management of small infected tibial defects: A comparison study. J Trauma 2000;48:246–255. [DOI] [PubMed] [Google Scholar]
- 21. Chang W, Colangeli M, Colangeli S, Di Bella C, Gozzi E, Donati D. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop Belg 2007;73:238–243. [PubMed] [Google Scholar]
- 22. Emara KM. Hemi-corticotomy in the management of chronic osteomyelitis of the tibia. Int Orthop 2002;26:310–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Euba G, Murillo O, Fernández-Sabé N, et al. Long-term follow-up trial of oral rifampin-cotrimoxazole combination versus intravenous cloxacillin in treatment of chronic staphylococcal osteomyelitis. Antimicrob Agents Chemother 2009;53:2672–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferguson JY, Dudareva M, Riley ND, et al. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: a series of 195 cases. J Bone Joint Surg [Br] 2014;96-B:829–836. [DOI] [PubMed] [Google Scholar]
- 25. Ferrando A, Part J, Baeza J. Treatment of cavitary bone defects in chronic osteomyelitis: biogactive glass S53P4 vs. calcium sulphate antibiotic beads. J Bone Jt Infect 2017;2:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitzgerald RHJ, Jr, Ruttle PE, Arnold PG, Kelly PJ, Irons GB. Local muscle flaps in the treatment of chronic osteomyelitis. J Bone Joint Surg [Am] 1985;67-A:175–185. [PubMed] [Google Scholar]
- 27. Hashmi MA, Norman P, Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg [Br] 2004;86-B:269–275. [DOI] [PubMed] [Google Scholar]
- 28. Huang K, Lin B, Guo Q, et al. Research on the clinical efficacy of the combination of Chinese traditional medicine and Western medicine on the chronic traumatic tibial osteomyelitis. Pak J Pharm Sci 2018;31:2841–2845. [PubMed] [Google Scholar]
- 29. Jiang N, Ma Y-F, Jiang Y, et al. Clinical characteristics and treatment of extremity chronic osteomyelitis in southern China: a retrospective analysis of 394 consecutive patients. Medicine (Baltimore) 2015;94:e1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly PJ, Fitzgerald RHJ, Jr, Cabanela ME, et al. Results of treatment of tibial and femoral osteomyelitis in adults. Clin Orthop Relat Res 1990;259:295–303. [PubMed] [Google Scholar]
- 31. Khan MAA, Jose RM, Taylor C, Ahmed W, Prinsloo D. Free radial forearm fasciocutaneous flap in the treatment of distal third tibial osteomyelitis. Ann Plast Surg 2012;68:58–61. [DOI] [PubMed] [Google Scholar]
- 32. Kinik H, Karaduman M. Cierny–Mader Type III chronic osteomyelitis: the results of patients treated with debridement, irrigation, vancomycin beads and systemic antibiotics. Int Orthop 2008;32:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koval KJ, Meadows SE, Rosen H, Silver L, Zuckerman JD. Posttraumatic tibial osteomyelitis: a comparison of three treatment approaches. Orthopedics 1992;15:455–460. [DOI] [PubMed] [Google Scholar]
- 34. Lam A, Richardson SS, Buksbaum J, et al. Chronic osteomyelitis of the tibia and ankle treated with limb salvage reconstruction. J Bone Jt Infect 2019;4:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lê Thua T-H, Boeckx WD, Zirak C, De Mey A. Free intra-osseous muscle transfer for treatment of chronic osteomyelitis. J Plast Surg Hand Surg 2015;49:306–310. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Zhang H, Qi B, Pan Z. Outcomes of vacuum sealing drainage treatment combined with skin flap transplantation and antibiotic bone cement on chronic tibia osteomyelitis: a case series study. Med Sci Monit 2019;25:5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lidgren L, Törholm C. Intramedullary reaming in chronic diaphyseal osteomyelitis: a preliminary report. Clin Orthop Relat Res 1980;151:215–221. [PubMed] [Google Scholar]
- 38. Lindfors N, Geurts J, Drago L, et al. Antibacterial bioactive glass S53P4 for chronic bone infections: a multinational study. Adv Exp Med Biol 2017;971:81–92. [DOI] [PubMed] [Google Scholar]
- 39. Marais LC, Ferreira N, Aldous C, et al. The outcome of treatment of chronic osteomyelitis according to an integrated approach. Strat Traum Limb Recon 2016;11:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McNally MA, Small JO, Tofighi HG, Mollan RA. Two-stage management of chronic osteomyelitis of the long bones: the Belfast technique. J Bone Joint Surg [Br] 1993;75-B:375–380. [DOI] [PubMed] [Google Scholar]
- 41. McNally MA, Ferguson JY, Lau ACK, et al. Single-stage treatment of chronic osteomyelitis with a new absorbable, gentamicin-loaded, calcium sulphate/hydroxyapatite biocomposite: a prospective series of 100 cases. J Bone Joint Surg [Br] 2016;98-B:1289–1296. [DOI] [PubMed] [Google Scholar]
- 42. Meissner A, Haag R, Rahmanzadeh R. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection 1989;17:146–151. [DOI] [PubMed] [Google Scholar]
- 43. Pape HC, Zwipp H, Regel G, Maschek H, Tscherne H. Chronic diaphyseal osteomyelitis of long bones refractory to conventional therapy: benefits and risks of reaming of the femoral medullary cavity. Eur J Orthop Surg Traumatol 1995;5:53–58. [DOI] [PubMed] [Google Scholar]
- 44. Patzakis MJ, Mazur K, Wilkins J, Sherman R, Holtom P. Septopal beads and autogenous bone grafting for bone defects in patients with chronic osteomyelitis. Clin Orthop Relat Res 1993;295:112–118. [PubMed] [Google Scholar]
- 45. Romanò CL, Logoluso N, Meani E, et al. A comparative study of the use of bioactive glass S53P4 and antibiotic-loaded calcium-based bone substitutes in the treatment of chronic osteomyelitis: a retrospective comparative study. J Bone Joint Surg [Br] 2014;96:845–850. [DOI] [PubMed] [Google Scholar]
- 46. Sachs BL, Shaffer JW. A staged Papineau protocol for chronic osteomyelitis. Clin Orthop Relat Res 1984;184:256–263. [PubMed] [Google Scholar]
- 47. Sen C, Demirel M, Sağlam Y, Balcı HI, Eralp L, Kocaoğlu M. Acute shortening versus bone transport for the treatment of infected femur non-unions with bone defects. Injury 2019;50:2075–2083. [DOI] [PubMed] [Google Scholar]
- 48. Shen L, Dong Y, Zhang C, et al. Chronic osteomyelitis treatment: a clinical and pharmaco-kinetic study of vancomycin impregnated calcium sulphate. J Med Imaging Heal Informatics 2015;5:36–42. [Google Scholar]
- 49. Siegel HJ, Patzakis MJ, Holtom PD, Sherman R, Shepherd L. Limb salvage for chronic tibial osteomyelitis: an outcomes study. J Trauma 2000;48:484–489. [DOI] [PubMed] [Google Scholar]
- 50. Simpson AH, Deakin M, Latham J. Chronic osteomyelitis: the effect of the extent of surgical resection on infection-free survival. J Bone Joint Surg [Br] 2001;83-B:403–407. [DOI] [PubMed] [Google Scholar]
- 51. Smith IM, Austin OMB, Batchelor AG. The treatment of chronic osteomyelitis: a 10 year audit. J Plast Reconstr Aesthet Surg 2006;59:11–15. [DOI] [PubMed] [Google Scholar]
- 52. Sun P-Q, Ma Y, Zhang Y-C, Cheng MG. Application of antibiotic impregnated beads on the patients with tibial chronic osteomyelitis. Pak J Pharm Sci 2018;31:2783–2786. [PubMed] [Google Scholar]
- 53. Wang X, Wang Z, Fu J, Huang K, Xie Z. Induced membrane technique for the treatment of chronic hematogenous tibia osteomyelitis. BMC Musculoskelet Disord 2017;18:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamashita Y, Uchida A, Yamakawa T, Shinto Y, Araki N, Kato K. Treatment of chronic osteomyelitis using calcium hydroxyapatite ceramic implants impregnated with antibiotic. Int Orthop 1998;22:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou C-H, Ren Y, Ali A, et al. Single-stage treatment of chronic localized tibial osteomyelitis with local debridement and antibiotic-loaded calcium sulfate implantation: a retrospective study of 42 patients. J Orthop Surg Res 2020;15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zweifel-Schlatter M, Haug M, Schaefer DJ, Wolfinger E, Ochsner P, Pierer G. Free fasciocutaneous flaps in the treatment of chronic osteomyelitis of the tibia: a retrospective study. J Reconstr Microsurg 2006;22:41–47. [DOI] [PubMed] [Google Scholar]
- 57. Siegel HJ, Patzakis MJ, Holtom PD, Sherman R, Shepherd L. Limb salvage for chronic tibial osteomyelitis: an outcomes study. J Trauma 2000;48:484–489. [DOI] [PubMed] [Google Scholar]
- 58. Ma X, Han S, Ma J, et al. Epidemiology, microbiology and therapeutic consequences of chronic osteomyelitis in northern China: a retrospective analysis of 255 Patients. Sci Rep 2018;8:14895. [DOI] [PMC free article] [PubMed] [Google Scholar]