1. Introduction
The incidence of type 2 diabetes (T2D) in youth (children and adolescents) has increased rapidly over the past 20 years and is expected to continue to rise in parallel with the global obesity epidemic as T2D is often a complication of obesity with over 85% of youth with T2D being overweight or obese at diagnosis (Pulgaron and Delamater 2014). In the United States, there are up to 5000 new cases of T2D in youth per year with economically disadvantaged racial/ethnic groups (African Americans, Hispanics, and Native American Indians) being most affected (Pettitt et al. 2014) and 1 of 5 adolescents (aged 12–18 years) have prediabetes (Andes et al. 2019). The data with respect to obesity are even more alarming: 35.1% of youth age 2–19 years are overweight (defined as BMI ≥ 85th percentile for age and sex); 19% have class I obesity (BMI ≥95th percentile); 6% have class II obesity (BMI ≥120% of the 95th percentile or BMI ≥35); and 2% have class III obesity (BMI ≥140% of the 95th percentile or BMI ≥40) per the National Health and Nutrition Examination Survey (NHANES), a nationally representative data source of over 3300 individuals (Skinner et al. 2018). Youth aged 12–19 are most affected by the disease, with a prevalence of overweight and obesity of 45% (Skinner et al. 2018). With no end in sight to reversing the obesity epidemic, it is likely that the number of new cases of T2D will continue to grow over the next several years. Thus, strategies to prevent and treat T2D in youth are of vital importance.
The diagnostic laboratory criteria for diabetes mellitus of any type in adults and youth is based on the presence of one or more of the following four determinants: fasting plasma glucose ≥126 mg/dL, random venous plasma glucose ≥200 mg/dL with classic symptoms of hyperglycemia such as polyuria and polydipsia, plasma glucose ≥200 mg/dL after 2-h oral glucose tolerance test, or glycated hemoglobin (A1C) ≥6.5% (American Diabetes A 2018a). Although there can be overlap of the clinical manifestations for Type 1 diabetes (T1D) and T2D, the diseases have different pathophysiology: T1D is characterized by autoimmune beta-cell failure leading to insulin deficiency whereas T2D is characterized by insulin resistance and gradual non-autoimmune beta-cell failure. Compared to T2D in adults, T2D in children is more virulent. There is a faster progressive decline in beta-cell function, more severe insulin resistance, and an accelerated development of diabetes complications in affected youth, such as microalbuminuria and retinopathy (American Diabetes A 2018b; Weiss et al. 2005; Gungor and Arslanian 2004). Moreover age of T2D onset is inversely associated with T2D complication risk and mortality, which further outlines why prevention and early intervention are crucial for children with T2D (Al-Saeed et al. 2016).
2. Current Management of T2D in Youth
Initial management of T2D consists of age-appropriate and culturally sensitive lifestyle modifications that focus on increasing daily physical activity and healthy eating. Lifestyle changes alone may reverse diabetes in a minority of patients, but the majority achieve only mild to moderate weight loss with reduction of BMI by −1 to −2 kg/m2, and unfortunately have difficulty implementing and sustaining their lifestyle changes (Reinehr 2013). Consequently many patients with T2D are treated with pharmacotherapy in addition to behavioral and dietary modification, with variable benefit.
Currently, the only FDA-approved drugs for youth-onset T2D are metformin, insulin, and liraglutide, a glucagon-like peptide-1 analogue (GLP-1A) (Tamborlane et al. 2019). Metformin is the first line drug in asymptomatic children with A1C of <8.5% and normal renal function, and should be gradually increased from 500 mg to 2 g daily to maximize the clinical response (American Diabetes A 2018b). Metformin’s mechanisms of action include: reduction of hepatic glucose production, opposition of glucagon action, and increase in insulin-mediated glucose uptake in peripheral tissues (Pernicova and Korbonits 2014). Its use is often limited by patient tolerance due to common side effects such as nausea, vomiting, diarrhea, and dyspepsia, which are frequently dose-limiting and may lead to the addition of liraglutide and/or insulin to the treatment regimen if A1C values are not well-controlled on Metformin alone. Liraglutide activates the GLP-1 receptor and increases insulin release from pancreatic beta cells in the presence of elevated glucose concentrations. Newer agents such as sodium-glucose cotransporter-2 (SGLT2) inhibitors and inhibitors of dipeptidyl peptidase 4 (DPP4 inhibitors) have also been used off-label in some youth in conjunction with metformin, but these drugs have not yet been FDA-approved for use in patients under the age of 18 (Tamborlane et al. 2019; Laffel et al. 2018; Tamborlane et al. 2018). SGLT2 inhibitors block the glucose reabsorption transporter in the proximal tubule of the kidney leading to increased glucosuria and decreased blood glucose. DPP4 inhibitors prevent the breakdown of endogenous GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) leading to increased glucose-mediated insulin secretion, suppressed glucagon secretion, and decreased gastric emptying.
According to the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study, metformin alone provided durable glycemic control (A1C less than or equal to 8% for 6 months) in only about half of the subjects enrolled (Group TS et al. 2012). Thus, given the challenges of managing diabetes with lifestyle changes and/or medications, an increasing number of youth with severe obesity and T2D are being treated with bariatric surgery with promising results.
3. Metabolic and Bariatric Surgery
The first bariatric surgical procedure was performed by Mason and Ito in an adult patient in 1967 and bariatric surgery has been performed in small cohorts of adolescents since the 1970s (Mason and Ito 1967). With the rapid rise in obesity, there has been an exponential increase in adolescent surgical cases since the early 2000s with 126 bariatric surgeries performed in 2012 and 220 cases performed in 2016 among patients aged greater than 16 years (Kyler et al. 2019).
In general, bariatric surgery mechanically restricts caloric intake by modifying the anatomy of the stomach and in some variants impairs nutrient absorption via alterations of the gastrointestinal tract. The three most commonly performed bariatric surgeries in adolescents are roux-en-Y gastric bypass (RYGB), vertical sleeve gastrectomy (VSG), and adjustable gastric banding (AGB) and they achieve weight loss by either restriction (AGB or VSG), or combined restriction and malabsorption (RYGB). Restrictive procedures limit intake by either creating a small gastric reservoir (VSG, RYGB) or provide a narrow outlet to delay emptying (AGB). Malabsorptive procedures (RYGB) bypass various portions of the small intestine where nutrient and calorie absorption takes place, and generally have a greater degree of weight loss when compared to restrictive procedures. Currently, the vast majority of bariatric procedures worldwide are performed laparoscopically because minimally invasive surgery is associated with shorter length of stay, lower overall complications, and decreased costs compared to open procedures. This approach is also used for adolescents and children.
National Surgical Quality Improvement Quality Program (NSQIP) data from 2005–2015 showed that the most commonly performed procedures were laparoscopic RYGB (43.4%), laparoscopic SG (32.5%) and laparoscopic AGB (18.7%) among 2,625 adolescents ages 18–21 with obesity (average BMI 48) in the United States (Arafat et al. 2019). However the AGB is not approved for patients under the age of 18, so younger adolescents are more often treated with VSG (U.S. Food and Drug Administration 2001).
4. Different Types of Bariatric Surgery
RYGB entails creating a small gastric pouch from the upper stomach that attaches to the esophagus at one end and a section of the small intestine at the other end. It has been the procedure that has been most studied, and is currently the gold standard bariatric surgery in adults in the United States (Elder and Wolfe 2007). However, it is now the second most common surgical procedure performed for weight loss in adults, mostly due to its surgical complication risk (Angrisani et al. 2015). Postoperative procedure-related complications such as anastomotic leak, strictures, bleeding, bowel obstructions, and wound infections are common with similar prevalence in adults and adolescents (Inge et al. 2014) and frequently lead to hospital readmissions. The procedure is also associated with nutritional complications and dumping syndrome long-term, and therefore, RYGB is often not the preferred primary surgical procedure for adolescents.
Vertical sleeve gastrectomy involves removal of most of the greater curvature of the stomach (about 80–90%) and has been shown to have lower morbidity and mortality compared to RYGB. Given its relative safety and simplicity, it is being increasingly used to treat adolescents with obesity. In 2010, 11.4% of cases were SG, 47.4% were laparoscopic RYGB and 30.9% were AGB and in 2015, 66.6% were SG, 28.6% were laparoscopic RYGB and 2.6% were AGB among adolescents aged 18–21 who underwent bariatric surgery in the United States (Kyler et al. 2019).
AGB involves placing an adjustable silicone band around the stomach below the gastroesophageal junction to create a small pouch with a narrow outlet and is the least invasive of the purely restrictive bariatric surgery procedures with a lower complication rate than other surgical options. However as stated above, it is only FDA approved for patients age 18 and older which limits its use in younger adolescents.
5. Effect of Bariatric Surgery on GI Physiology and Metabolism
Bariatric surgery effectively treats obesity and improves obesity associated comorbidities such as T2D. Improved glucose homeostasis is evident within days to weeks following bariatric surgery with acute calorie restriction, which suggests there is a weight loss-independent mechanism for VSG and RYGB. Proposed theories to explain this phenomenon that are under active investigation include: improved beta cell function due to reduced glucotoxicity, reduced islet inflammation, alteration of GI hormonal responses, and gut microbiota (Chen et al. 2019). The intravenous glucose tolerance test provides a way to assess beta cell function by measuring the insulin secretion response (also known as the disposition index) to a standardized dose of intravenous glucose regardless of the anatomical differences between surgical procedures (Hofso et al. 2019). At 1 year after VSG and RYGB, the disposition index increased by 6–8 times with no significant difference between groups in 109 patients supporting the theory that there is improved beta cell function following bariatric surgery; however, the mechanism of action is still not well understood (Hofso et al. 2019).
Both RYGB and VSG increase postprandial levels of distal GI hormones like GLP1 and peptide-YY via rapid nutrient delivery down the GI tract where enteroendocrine L-cells are located (Hutch and Sandoval 2017). It has been suggested that the rise in GLP-1 and peptide-YY subsequently leads to increased satiety, improved postprandial insulin secretion, and decreased hepatic glucose production, which may contribute to remission of T2D. In VSG, ghrelin levels also decrease with complete removal of the fundus of the stomach which is thought to suppress appetite (Chen et al. 2019). It is unclear whether RYGB or VSG leads to higher T2D remission with some studies suggesting no difference between the two (Hutch and Sandoval 2017) whereas others showing that RYGB is more effective (Schauer et al. 2017).
6. The Impact of Bariatric Surgery on Obesity and Type 2 Diabetes
The Teen-Longitudinal Assessment of Bariatric surgery (Teen-LABS) study, the largest prospective multi-center observational study to date of bariatric surgery in pediatric patients (≤ 19 years of age, mean BMI of 53) showed that teenagers achieve similar degrees of weight loss, diabetes remission, and improvement of cardio-metabolic risk factors such as dyslipidemia, elevated blood pressure, renal dysfunction, and prediabetes for at least 3 years after surgery compared to adults (Inge et al. 2016). Among their 242 participants who underwent any surgical intervention, 29 (13%) had T2D at baseline with a median HgbA1C of 6.3%, fasting glucose level of 110 mg/dL, and high fasting endogenous plasma insulin level of 43 IU/mL consistent with glucose intolerance. Of these 29 patients, 6 underwent vertical sleeve gastrectomy (VSG) and 23 underwent gastric bypass (RYGB). At 3 years following surgery, 19 of 20 participants with available data were in remission of type 2 diabetes with a median HgbA1C of 5.3%, fasting glucose level of 88 mg/dL, and fasting endogenous plasma insulin level of 12 IU/mL. Furthermore, remission of prediabetes occurred in 76% of participants who had the condition at baseline. The 5-year follow up data was recently published and compared only those adolescents who underwent RYGB with a similar cohort of adult patients 5 years after they underwent the same procedure (Inge et al. 2019). These data demonstrated that after 5 years, glycemic control was considerably better and T2D remission was significantly higher (86% vs. 53%) in the adolescent group when compared to the adult group. These data strongly suggest that early surgical intervention for patients with T2D is appropriate regardless of the patient’s age.
To date, there have been no randomized trials to compare the effectiveness and safety of surgery to those of conventional T2D treatment options in adolescents. However, comparison of data from adolescents with T2D from the Teen-LABS study to a matched cohort from the TODAY study revealed that surgical treatment of severely obese adolescents with T2D was associated with clinically significant weight reduction, remission of diabetes, and improvement in cardiovascular risk factors despite starting with a higher BMI (Inge et al. 2018) while those on medical treatment experienced modest weight gain, progression of T2D, and no improvement in cardiovascular risk factors in 2 years of follow-up. Randomized prospective studies have however been carried out in adults to compare the effectiveness of bariatric surgery to medications to treat T2D with data supporting the superiority of surgery. Five-year outcome data from the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial demonstrated that patients with T2DM and obesity with a mean BMI of 37 +/− 3.5 who underwent bariatric surgery with RYGB or VSG were significantly more likely to achieve and maintaingoodglycemiccontrol(HgbA1C<or to 6.0%) with or without medications, than were those who received intensive medical therapy alone. Furthermore, the surgically treated patients had superior glycemic control throughout the 5-year post-op period while having decreased diabetes medication use (Schauer et al. 2017). Thus, it is likely true that surgery provides better glycemic control in patients with severe obesity and T2D regardless of their age based on the results in adults and the findings from Teen-LABS. However, data from a prospective randomized controlled study in a strictly adolescent cohort is needed to confirm this hypothesis.
7. Criteria for Use of Bariatric Surgery in Youth
In 2018, the American Society for Metabolic and Bariatric Surgery (ASMBS) Pediatric Committee released an update of their evidence-based guidelines on pediatric metabolic surgery with the following recommended indications for MBS in youth between the ages of 10 and 19: BMI >35 kg/m2 and major comorbidities of obesity such as T2D, sleep apnea, or hepatic steatosis with fibrosis; or BMI > 40 kg/mg2 with mild comorbidities such as hypertension or dyslipidemia, extreme obesity, or those who fail to improve comorbidities even after a formal trial of lifestyle modifications, with or without pharmacotherapy (Pratt et al. 2018). In general, previous guidelines have suggested that pediatric patients should attain Tanner 4–5 pubertal development and be at final or near-final adult height prior to undergoing bariatric surgery, but there is no evidence to suggest that a patient’s linear growth is adversely affected by MBS. Thus, the ASMBS Pediatric Committee recommends that Tanner staging, bone age or height no longer be used to determine eligibility for adolescent MBS. In addition, they suggest that cognitive disabilities, a history of mental illness, or eating disorders that are treated should no longer serve as contraindications given the potential benefit of surgery.
Other organizations such as the American Academy of Pediatrics (AAP) and American Diabetes Association (ADA) also acknowledge the benefit of metabolic surgery in their clinical practice guidelines. The AAP recommends that pediatricians identify patients with class 2 and class 3 obesity who meet criteria for metabolic and bariatric surgery and provide timely referrals to comprehensive, multidisciplinary, pediatric-focused metabolic and bariatric surgery programs (Armstrong et al. 2019). The ADA recommends that metabolic surgery be considered for treatment of adolescents with type 2 diabetes who are markedly obese (BMI >35 kg/m2) and who have uncontrolled glycemia and/or serious comorbidities despite lifestyle and pharmacologic intervention (American Diabetes A 2019).
8. Potential Complications of Bariatric Surgery
While increasing data support the use of bariatric surgery to treat youth with severe obesity, all surgery is associated with possible post-operative complications that patients and their providers must consider in order to be best informed of the risks associated with the procedures. However, as the adolescent bariatric field has moved away from RYGB and toward VSG, the risks are less likely to be procedurally related for pediatric patients, at least in the short-term.
8.1. Need for Repeat Surgery
In the Teen-LABS study, 13% of patients had repeat abdominal surgeries for a total of 47 procedures with 24% of procedures occurring in the first postoperative year and 55% occurring in the second postoperative year (Inge et al. 2019). These repeat operations were performed due to various conditions such as gastric outlet/bowel obstruction, gastrointestinal leak, wound infection, gastrointestinal bleeding, abdominal pain, and gastroesophageal reflux. The majority of the reoperations resulted from complications of their original RYGB, whereas GERD was more often seen in patients after VSG. Similarly, the Swedish Adolescent Morbid Obesity Study which included only patients undergoing RYGB procedures showed a 15% reoperation rate due to a variety of reasons related to their procedure such as internal hernias and post-operative adhesions, and the rest of the operations were from symptomatic cholelithiasis which can result from rapid weight loss from any of the bariatric surgery procedures (Olbers et al. 2017). One study noted that among 309 patients who underwent laparoscopic SG (LSG), 18 patients (6%) underwent cholecystectomy between 4 weeks and 29 months after LSG (Tashiro et al. 2019).
In adults, late weight regain has been found to occur in up to 20% of patients, especially with extremely elevated BMI of >50 kg/m2 prior to RYGB due to maladaptive eating patterns, gradual enlargement of the gastric pouch, dilatation of the surgical anastomosis, and development of a gastrogastric fistula (Kalarchian et al. 2002; Spaulding 2003; Thompson et al. 2006). Patients with gastrogastric fistulae may require endoscopic procedures to reduce the pouch size, tighten the stoma, or close the fistula if feasible. While revision or reoperation may be needed after any bariatric surgery, the rate of revision is most common following laparoscopic adjustable gastric banding and estimated to be 26% versus 4.9% for RYGB and 9.8% for VSG (Altieri et al. 2018). The need for revisional bariatric surgery for weight regain in pediatric patients remains unknown given absence of long-term data following bariatric surgery in youth. However since VSG has become the mainstay of most adolescent bariatric surgical programs, and one adult series show at least a 20% revision rate at 7 years post VSG (Clapp et al. 2018), adolescent patients must be followed to ensure that those who may be regaining weight are recognized early and additional support and/or interventions offered in order to prevent the need for additional surgical procedures.
8.2. Vitamin and Mineral Deficiencies
Bariatric surgery, especially RYGB is associated with multiple nutritional deficiencies and can occur in more than 30% of patients after 5 years from surgery (Lupoli et al. 2017). For example, patients can develop anemia secondary to iron and vitamin B12 deficiency likely due to a combination of factors including restricted nutritional intake, malabsorption, decreased gastric acid, decreased intrinsic factor, and food intolerance with dumping. Other deficiencies that are seen post-surgery include: thiamin, vitamin D, vitamin A, folic acid, iron, copper, and zinc deficiency (Desai et al. 2016). Therefore, pre-op screening, lifelong nutritional monitoring, and start of supplementation are recommended to prevent vitamin/mineral insufficiency or deficiency. For example, standard post-RYGB and VSG supplementation in some adolescent MBS centers includes a multivitamin, calcium, vitamin D, elemental iron, and vitamin B12, and nearly all centers recommend a multivitamin at a minimum (Nogueira and Hrovat 2014).
8.3. Bone Disease
In adults, dramatic and rapid weight loss following RYGB has been associated with loss of bone mass and increased bone fracture risk likely due to decreased vitamin D and calcium intake, decreased calcium absorption, and bone marrow fat changes (Gagnon and Schafer 2018). Similarly, adolescents in the first year after RYGB were found to have Vitamin D deficiency and inadequate calcium levels (Xanthakos 2009). When compared to VSG, RYGB is associated with greater femoral bone loss in adults but similar declines in lumbar spine BMD (Bredella et al. 2017). Whether VSG results in a similar degree of bone mass loss and vitamin D deficiency in adults is unknown. Correction of nutritional deficiencies pre-operatively may be useful in preventing early post-operative micronutrient deficiencies, but serum values still need to be monitored after surgery. Thus studies are needed in adolescents who undergo MBS to determine whether MBS has an adverse acute and long-term impact on their bone health and other nutritional indices, especially if the procedure was a RYGB.
8.4. Psychological Function
The majority of adolescents after RYGB were found to have improvements in mental health and self-esteem mainly during the first post-op year (Jarvholm et al. 2016). However, despite improvements in mental health a year following RGYB, one study showed that 14% had suicidal ideation and 20% had poor mental health so long-term mental health monitoring is recommended (Jarvholm et al. 2016). In the Swedish Adolescent Morbid Obesity Study, 7% had psychological sequelae such as suicidal attempt, suicidal ideation, depression, and anxiety in patients with pre-surgical psychiatric histories (Olbers et al. 2017). Consequently, adolescents with ongoing substance abuse or untreated psychiatric illness are generally not considered candidates for bariatric surgery but rather need those issues addressed first. However, the presence or number of psychiatric illnesses did not predict outcome in our cohort and once patients are properly managed from a mental health perspective, they can undergo bariatric surgery and expect weight loss success (Mackey et al. 2018). Furthermore, there are studies that suggest bariatric surgery may improve neurocognitive functioning within a year following MBS. Improvements in executive function and reward anticipation help guide healthy decision-making about food and physical activity and may consequently help sustain weight loss (Thiara et al. 2017; Pearce et al. 2017). These improvements have been found after VSG as well as RYGB (Thiara et al. 2017; Pearce et al. 2017). Thus, while mental health issues should be identified and treated prior to MBS, the brain may actually function better after MBS in patients who suffer from obesity.
9. Conclusion
The incidence of T2D among youth in the United States is increasing at an alarming rate as a consequence of the obesity epidemic. Lifestyle modifications and medications such as metformin and insulin are the first line of therapy, but may not be sufficient to treat youth with severe obesity and T2D. Studies thus far suggest that bariatric surgery in youth is safe and effective in decreasing weight and improving the comorbidities of obesity including T2D. Questions that still need to be explored include: whether early surgical intervention can prevent T2D and metabolic disease in youth with severe obesity and pre-diabetes, the duration of diabetes remission following MBS, the potential role of weight loss medications in youth post-MBS to sustain weight loss and remission of comorbidities including T2D, the heterogeneity of obesity pathophysiology and the variability of patient response to MBS. Further understanding of these areas will hopefully enable us to predict which patients would benefit the most from MBS, including those on the insulin-resistance to T2D spectrum (Fig. 1).
Fig. 1.
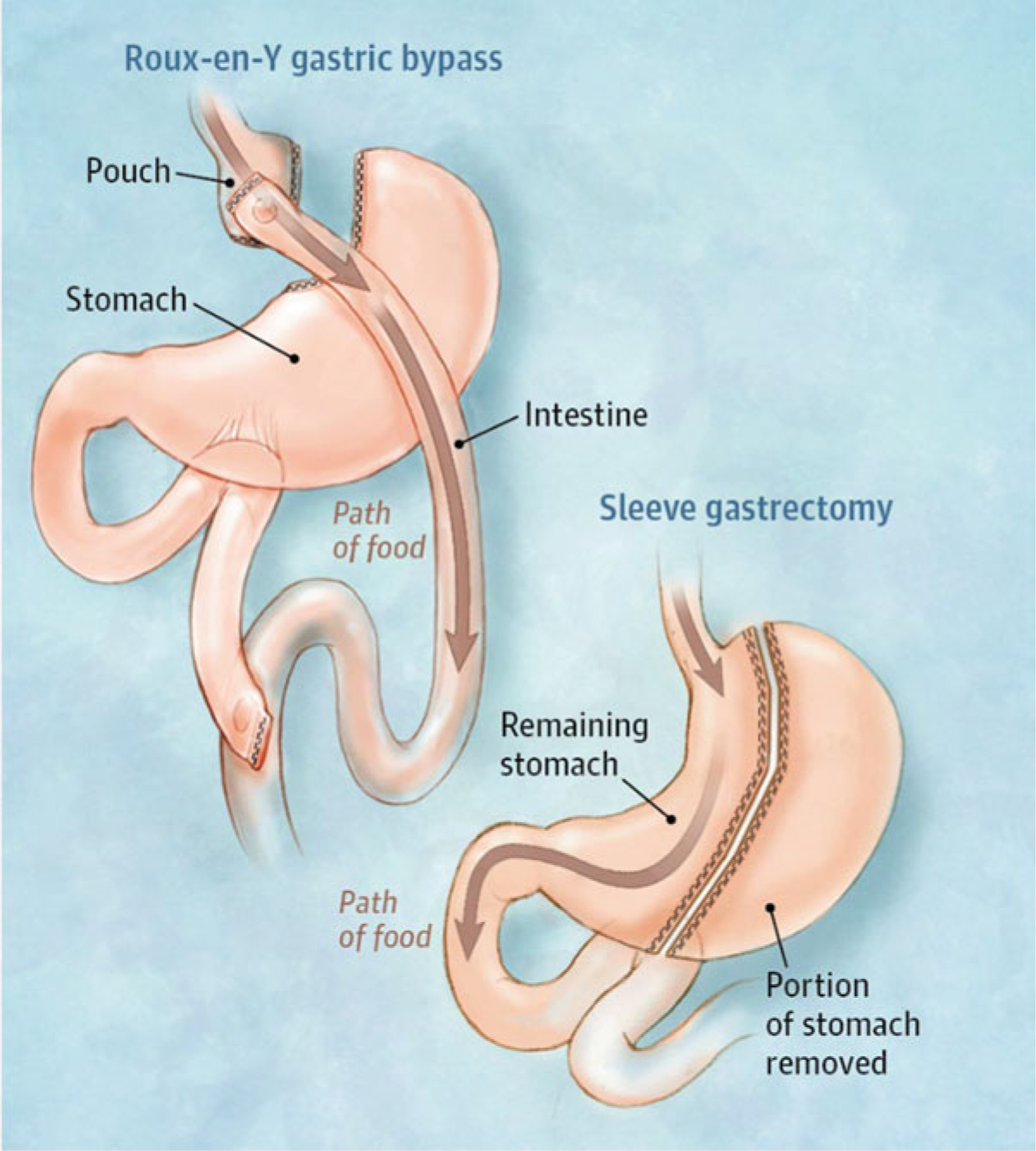
Roux-en-Y gastric bypass and sleeve gastrectomy. (From Slomski Slomski 2017)
Footnotes
Conflict-of-Interest Statement No potential conflicts of interest. No financial support.
Contributor Information
Anna Zenno, Division of Endocrinology, Children’s National Health System, Washington, DC, USA.
Evan P. Nadler, Division of Pediatric Surgery, Children’s National Health System, Washington, DC, USA; The George Washington University School of Medicine & Health Sciences, Washington, DC, USA.
References
- Al-Saeed AH, Constantino MI, Molyneaux L, D’Souza M, Limacher-Gisler F, Luo C et al. (2016) An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 39(5):823–829 [DOI] [PubMed] [Google Scholar]
- Altieri MS, Yang J, Nie L, Blackstone R, Spaniolas K, Pryor A (2018) Rate of revisions or conversion after bariatric surgery over 10 years in the state of New York. Surg Obes Relat Dis 14(4):500–507 [DOI] [PubMed] [Google Scholar]
- American Diabetes A (2018a) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 41(Suppl 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- American Diabetes A (2018b) 12. Children and adolescents: standards of medical care in diabetes-2018. Diabetes Care 41(Suppl 1):S126–SS36 [DOI] [PubMed] [Google Scholar]
- American Diabetes A (2019) 13. Children and adolescents: standards of medical care in diabetes-2019. Diabetes Care 42(Suppl 1):S148–S164 [DOI] [PubMed] [Google Scholar]
- Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G (2019) Prevalence of prediabetes among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatr 174:e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N (2015) Bariatric surgery worldwide 2013. Obes Surg 25(10):1822–1832 [DOI] [PubMed] [Google Scholar]
- Arafat M, Norain A, Burjonrappa S (2019) Characterizing bariatric surgery utilization and complication rates in the adolescent population. J Pediatr Surg 54 (2):288–292 [DOI] [PubMed] [Google Scholar]
- Armstrong SC, Bolling CF, Michalsky MP, Reichard KW et al. (2019) Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics 144 (6):1–6 [DOI] [PubMed] [Google Scholar]
- Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW (2017) Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone 95:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang J, Zhou Z (2019) Targeting islets: metabolic surgery is more than a bariatric surgery. Obes Surg 29(9):3001–3009 [DOI] [PubMed] [Google Scholar]
- Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A (2018) Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis 14(6):741–747 [DOI] [PubMed] [Google Scholar]
- Desai NK, Wulkan ML, Inge TH (2016) Update on adolescent bariatric surgery. Endocrinol Metab Clin N Am 45(3):667–676 [DOI] [PubMed] [Google Scholar]
- Elder KA, Wolfe BM (2007) Bariatric surgery: a review of procedures and outcomes. Gastroenterology 132 (6):2253–2271 [DOI] [PubMed] [Google Scholar]
- Gagnon C, Schafer AL (2018) Bone health after bariatric surgery. JBMR Plus 2(3):121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group TS, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K et al. (2012) A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 366(24):2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor N, Arslanian S (2004) Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr 144 (5):656–659 [DOI] [PubMed] [Google Scholar]
- Hofso D, Fatima F, Borgeraas H, Birkeland KI, Gulseth HL, Hertel JK et al. (2019) Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol 7(12):912–924 [DOI] [PubMed] [Google Scholar]
- Hutch CR, Sandoval D (2017) The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology 158(12):4139–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP et al. (2014) Perioperative outcomes of adolescents undergoing bariatric surgery: the teen-longitudinal assessment of bariatric surgery (Teen-LABS) study. JAMA Pediatr 168(1):47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML et al. (2016) Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 374(2):113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML et al. (2018) Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 172(5):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Brandt ML, Xanthakos SA et al. (2019) Five-year outcomes of gastric bypass in adolescents as compared with adults. N Engl J Med 380(22):2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvholm K, Karlsson J, Olbers T, Peltonen M, Marcus C, Dahlgren J et al. (2016) Characteristics of adolescents with poor mental health after bariatric surgery. Surg Obes Relat Dis 12(4):882–890 [DOI] [PubMed] [Google Scholar]
- Kalarchian MA, Marcus MD, Wilson GT, Labouvie EW, Brolin RE, LaMarca LB (2002) Binge eating among gastric bypass patients at long-term follow-up. Obes Surg 12(2):270–275 [DOI] [PubMed] [Google Scholar]
- Kyler KE, Bettenhausen JL, Hall M, Fraser JD, Sweeney B (2019) Trends in volume and utilization outcomes in adolescent metabolic and bariatric surgery at children’s hospitals. J Adolesc Health 65(3):331–336 [DOI] [PubMed] [Google Scholar]
- Laffel LMB, Tamborlane WV, Yver A, Simons G, Wu J, Nock V et al. (2018) Pharmacokinetic and pharmaco-dynamic profile of the sodium-glucose co-transporter-2 inhibitor empagliflozin in young people with type 2 diabetes: a randomized trial. Diabet Med 35 (8):1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupoli R, Lembo E, Saldalamacchia G, Avola CK, Angrisani L, Capaldo B (2017) Bariatric surgery and long-term nutritional issues. World J Diabetes 8 (11):464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey ER, Wang J, Harrington C, Nadler EP (2018) Psychiatric diagnoses and weight loss among adolescents receiving sleeve gastrectomy. Pediatrics 142(1):e20173432. [DOI] [PubMed] [Google Scholar]
- Mason EE, Ito C (1967) Gastric bypass in obesity. Surg Clin North Am 47(6):1345–1351 [DOI] [PubMed] [Google Scholar]
- Nogueira I, Hrovat K (2014) Adolescent bariatric surgery: review on nutrition considerations. Nutr Clin Pract 29 (6):740–746 [DOI] [PubMed] [Google Scholar]
- Olbers T, Beamish AJ, Gronowitz E, Flodmark CE, Dahlgren J, Bruze G et al. (2017) Laparoscopic Rouxen-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol 5(3):174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AL, Mackey E, Cherry JBC, Olson A, You X, Magge SN et al. (2017) Effect of adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity (Silver Spring) 25(11):1852–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernicova I, Korbonits M (2014) Metformin – mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 10(3):143–156 [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM et al. (2014) Prevalence of diabetes in U.S. Youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care 37(2):402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JSA, Browne A, Browne NT, Bruzoni M, Cohen M, Desai A et al. (2018) ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg Obes Relat Dis 14(7):882–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulgaron ER, Delamater AM (2014) Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep 14(8):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T (2013) Lifestyle intervention in childhood obesity: changes and challenges. Nat Rev Endocrinol 9 (10):607–614 [DOI] [PubMed] [Google Scholar]
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA et al. (2017) Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 376(7):641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC (2018) Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics 141(3): e20173459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomski A (2017) Bariatric surgery has durable effects in controlling diabetes. JAMA 317(16):1615. [DOI] [PubMed] [Google Scholar]
- Spaulding L (2003) Treatment of dilated gastroje-junostomy with sclerotherapy. Obes Surg 13 (2):254–257 [DOI] [PubMed] [Google Scholar]
- Tamborlane WV, Laffel LM, Weill J, Gordat M, Neubacher D, Retlich S et al. (2018) Randomized, double-blind, placebo-controlled dose-finding study of the dipeptidyl peptidase-4 inhibitor linagliptin in pediatric patients with type 2 diabetes. Pediatr Diabetes 19(4):640–648 [DOI] [PubMed] [Google Scholar]
- Tamborlane WV, Barrientos-Perez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM et al. (2019) Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 381(7):637–646 [DOI] [PubMed] [Google Scholar]
- Tashiro J, Thenappan AA, Nadler EP (2019) Pattern of biliary disease following laparoscopic sleeve gastrectomy in adolescents. Obesity (Silver Spring) 27 (11):1750–1753 [DOI] [PubMed] [Google Scholar]
- Thiara G, Cigliobianco M, Muravsky A, Paoli RA, Mansur R, Hawa R et al. (2017) Evidence for neurocognitive improvement after bariatric surgery: a systematic review. Psychosomatics 58(3):217–227 [DOI] [PubMed] [Google Scholar]
- Thompson CC, Slattery J, Bundga ME, Lautz DB (2006) Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc 20(11):1744–1748 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (2001) The lap-band adjustable gastric banding system summary of safety and effectiveness data. PMA no: P000008. FDA, United States, 25 p [Google Scholar]
- Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S (2005) Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 28(4):902–909 [DOI] [PubMed] [Google Scholar]
- Xanthakos SA (2009) Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin N Am 56 (5):1105–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]


