Abstract
Background
Corticosteroids (CS) have been used extensively to induce remission in Crohn’s disease (CD); however, they are associated with severe side effects. We hypothesized that the administration of an exclusive enteral nutrition (EEN) formula to CS would lead to increased CD remission rates and to decreased CS-related adverse events. We proposed to undertake a pilot study comparing EEN and CS therapy to CS alone to assess decrease symptoms and inflammatory markers over 6 weeks.
Aim
The overall aim was to assess study feasibility based on recruitment rates and acceptability of treatment in arms involving EEN
Methods
The pilot study intended to recruit 100 adult patients with active CD who had been prescribed CS to induce remission as part of their care. The patients were randomized to one of three arms: (i) standard-dose CS; (ii) standard-dose CS plus EEN (Modulen 1.5 kcal); or (iii) short-course CS plus EEN.
Results
A total of 2009 CD patients attending gastroenterology clinics were screened from October 2018 to November 2019. Prednisone was prescribed to only 6.8% (27/399) of patients with active CD attending outpatient clinics. Of the remaining 372 patients with active CD, 34.8% (139/399) started or escalated immunosuppressant or biologics, 49.6% (198/399) underwent further investigation and 8.8% (35/399) were offered an alternative treatment (e.g., antibiotics, surgery or investigational agents in clinical trials). Only three patients were enrolled in the study (recruitment rate 11%; 3/27), and the study was terminated for poor recruitment.
Conclusion
The apparent decline in use of CS for treatment of CD has implications for CS use as an entry criterion for clinical trials.
Keywords: Change prescription, Inflammatory bowel disease, Nutrition, Pilot
Background
Crohn’s disease (CD) is characterized by gastrointestinal (GI) tract inflammation, leading to diarrhea, pain and rectal bleeding (1). The pathophysiology of CD is not well understood, but a dysregulated immune response to altered microbiota may play a key role (2). Treatment of CD requires a multi-disciplinary approach involving medical therapy, surgery and dietary changes (3,4). Corticosteroids (CS) are a first-line therapy to induce remission in CD patients with moderate or severe disease activity (5); however, they have significant adverse effects and 30% of patients may be steroid resistant or become steroid-dependent (6,7). Therefore, alternative therapies that can induce and maintain disease remission without short- and long-term side effects are needed.
Exclusive enteral nutrition (EEN) involves a liquid diet of elemental or polymeric formula (PF), given exclusively over a prolonged period (8,9). Elemental formula contains individual amino acids, while PF provides intact proteins (10). Although EEN is used in children as an alternative to CS to induce remission due to its excellent safety profile (10), and varies by region in routine use as more common in Japan (11) and Western Europe compared to North America (12). The lack of use in adults is mainly due to physicians’ concern regarding efficacy of EEN or PF in adults (8) and patients’ poor adherence on account of palatability (13).
The beneficial effects of EEN in CD were first documented over two decades ago, when O’Moráin et al. (14) reported on a controlled study in which an elemental diet was as effective as CS in achieving remission in adults with active CD. In particular, EEN was shown to induce the expression of growth factors, promote changes in intestinal permeability and stimulate mucosal healing (14,15). Since then, several studies aimed to investigate this area. Meta-analyses of EEN versus CS have found that, although CS are superior to EEN in inducing remission in adults, EEN may also have some efficacy in mucosal healing (16) and provides additional nutritional benefit (17,18).
Potential mechanisms by which EEN might act include relative bowel rest, reduced antigenic load, provision of trophic amino acids, local anti-inflammatory effects and modification of gut microbiota (17,18). However, there is limited evidence to fully support any one of these mechanisms. A recent meta-analysis (8) showed that the effects of the therapeutic formula on specific bacterial strains were variable and inconsistent among studies, possibly due to small sample sizes and methodological limitations. Our preliminary data showed that short-term dietary changes improve motility in patients with low-grade gut inflammation (19). However, the effects of EEN on GI transit in adults have not been yet investigated.
Evidence for the effect of EEN on quality of life, a key patient-reported outcome, is particularly scarce. Guo et al. (20) found an improvement of quality of life in 13 patients with CD receiving EEN for 6 weeks. However, it is currently unknown whether EEN is more effective than CS, or EEN in combination with CS provides incremental improvement in quality of life compared with CS alone.
The potentially synergistic effects of adding EEN to CS therapy have not been explored before. Previous studies evaluating the use of EEN and CS in adults experienced high dropout rates, suggesting low acceptability for these treatments and more randomized trial evidence is needed (8). Therefore, we designed a pilot randomized controlled trial to investigate the feasibility of a study assessing the effect of EEN in addition to different regimes of CS therapy (ECS) compared to CS alone (CS), for decreasing disease activity and adverse events in adult patients with CD. We anticipated that a study to prove efficacy of ECS over CS alone would add 10% to 20% benefit and would require a large population. A better understanding of recruitment rates and acceptability of treatment in arms involving EEN would provide information to plan a larger study.
METHODS
We initiated a pilot open randomized controlled trial to assess the additional value of EEN to CS. The study was approved by the Hamilton Integrated Research Ethics Board and all participants signed informed consent. The study was registered in ClinicalTrials.gov Identifier: NCT03833596. Subjects were recruited from McMaster University Medical Centre (MUMC) Digestive Diseases Clinic, St. Joseph’s Healthcare Centre, the GI Health Centre (Burlington, ON) and the Brampton Endoscopic Centre (Brampton, ON), and by recruitment flyers posted at McMaster University Medical Centre, Juravinski Hospital and St. Joseph’s Healthcare Hamilton (all in Hamilton, ON).
Participants
Adult patients were eligible for enrolment if they had a diagnosis of CD with clinical evidence (Crohn’s Disease Activity Index [CDAI] (21) > 220 or Harvey–Bradshaw Index [HBI] (22) > 6), endoscopic evidence or biochemical evidence (C-reactive protein [CRP] > 5 mg/L and/or fecal calprotectin > 250 mg/L) of disease activity sufficient to require prednisone to induce remission. Exclusion criteria included treatment with EEN, evidence of intestinal obstruction, perforation, toxic megacolon, massive GI bleeding, abdominal abscess, stricturing disease, short bowel (remnant small bowel <180 cm), treatment with prednisone, probiotics or antibiotics in the last 30 days, a new treatment or change in doses of azathioprine, 6-mercaptopurine, cyclosporine, other immunosuppressant or biologics in the last 90 days, a new start or change in dose of 5-aminosalicylic acid in the last 30 days, pregnancy or lactation or other criteria considered by the investigator to preclude the use of EEN.
Aims
The primary aim was to assess study feasibility based on recruitment rates and acceptability of treatment in arms involving EEN. The primary efficacy aim was to explore the efficacy of 6 weeks of ECS in inducing remission (CDAI < 150) compared to treatment with CS alone in adult patients with active CD. Additional, exploratory secondary aims were to determine the beneficial effect of 6 weeks of ECS as compared to CS in inducing: (i) clinical improvement (decrease in CDAI >70 at two consecutive visits) (23); (ii) improvement in quality of life (increase in short Inflammatory Bowel Disease Questionnaire [IBDQ] scores); (iii) biochemical remission (normalization of either serum CRP and/or fecal calprotectin); (iv) changes in microbiota composition (16S sequencing Illumina); (v) normalization of colonic transit (SHAPE 5 or less markers) among those with altered transit at baseline; and (vi) decrease in anxiety and/or depression scores (decrease >2 points Hospital Anxiety and Depression [HAD]-A and/or HAD-D scores).
Study Design
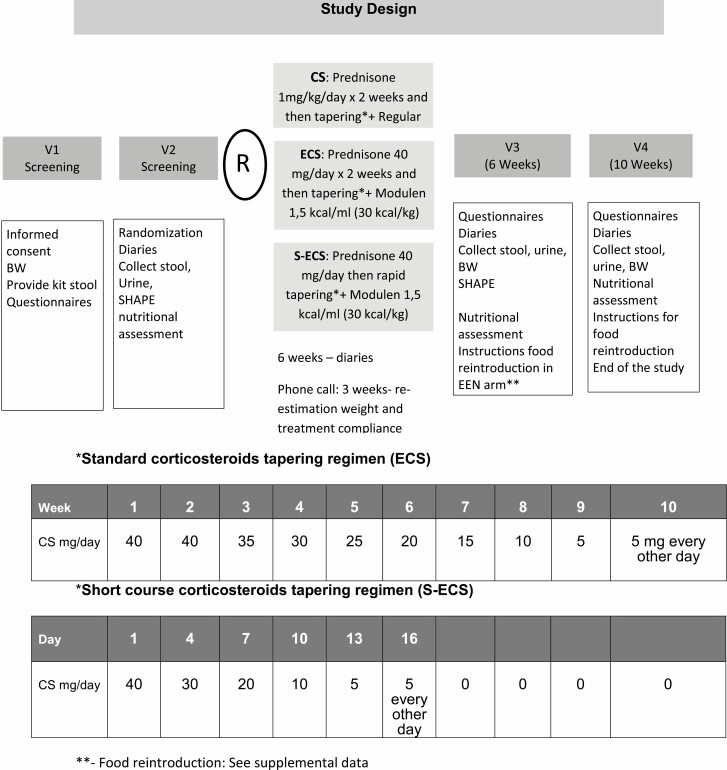
The study involved four visits to the hospital in a period of 10 weeks (Figure 1). Patients were recruited from different centres affiliated to the co-investigators, and visits occurred at the McMaster University Medical Centre. At the screening visit, clinical history and symptoms were assessed and physical examination and complete bloodwork were performed. Participants were provided with kits to collect stool and Sitzmark capsules and instructions for SHAPE study (24). At baseline, patients provided a stool sample for fecal calprotectin and microbiota analysis and were randomized to one of the following treatments: (i) standard-dose CS (CS; taper from 40 mg/day prednisone over 8 weeks) + regular diet; (ii) standard-dose CS plus EEN using Modulen 1.5 kcal (ECS); or (iii) short-course CS (taper over 16 days) + EEN (S-ECS) (Figure 1). Six weeks later (visit 3), patients completed questionnaires to assess symptoms and quality of life. Blood and stool samples were collected and a SHAPE study was performed at this visit after treatment. Patients on EEN in the ECS and S-ECS arms were instructed to gradually decrease the EEN and reintroduce foods (Supplemental Figure 1) after visit 3. In the last visit (visit 4; 4 weeks after end of treatment), we collected stool samples and information on symptoms.
Figure 1.
Study design and description of corticosteroids regimes involved in the study.
Randomization Process
The sequence of the treatments was randomly generated using a computer-based pseudo-random number generator (RStudio: Integrated Development for R. RStudio, Inc., Boston, MA). A research coordinator not involved in the study performed the randomization process treatment allocation concealment was maintained from participants and study staff. Treatment was not blinded for patients or study staff.
Intervention
Patients randomized to the ECS and S-ECS arms received hypercaloric PF (Modulen 1.5 kcal/mL; Nestlé (25,26)) as sole source nutrition for 6 weeks. To increase palatability, patients were allowed to add the ‘Nestlé Nutrition Flavour Mix’. Daily volumes of formula were prescribed based upon the patient’s estimated energy requirement, calculated using the Harris-Benedict equation (27) (calculator available at https://manytools.org/handy/bmr-calculator), which estimates basal metabolic rate from weight and height and then applies an activity factor (AF) which ranges from 1.2 to 2.4 (sedentary AF 1.2; mild activity 1.375, moderate activity AF 1.55, heavy activity AF 1.7, extreme AF 1.9; see Supplementary Table 1). Actual body weight or ideal body weight for height were used in the equation depending on whether the patient was of an appropriate weight or underweight for height. Oral administration was encouraged wherever possible, with formula volume taken at set meal and snack times throughout the day. Volume taken per meal and distribution during the day were recorded in the case report form. Only clear fluids were allowed during the study period. Vitamins and supplements were allowed if doses have been stable before entering in the study, and doses are not changed during the study.
Patients in regular CS arms received oral prednisone 40 mg × day for 2 weeks; with subsequent taper of daily dose by 5 mg per week. Patients randomized to S-ECS arm received oral prednisone 40 mg daily for 3 days and tapered over 16 days (Figure 1).
Following the completion of the period of EEN feeding, patients receiving EEN were instructed to gradually reintroduce foods by adding one individual item every 2 days as shown in Supplementary Figure 1 (28,29) with instructions for food introduction with coincident reduction of formula volumes under the supervision of a clinician certified in nutrition support.
The subjects were asked to keep the empty EEN cans and prednisone blister packs during the entire treatment period and bring them back to the investigator at the end of that period. Adherence was recorded in the case review form (CRF). Patients consuming <80% of the prescribed treatment dose throughout the study, or those who interrupted therapy for more than three consecutive days were considered to be non-adherent.
Measurements
Clinical data regarding demographics, disease location, social life and habits, past and current clinical medical background, previous surgeries, nutritional status (malnourishment, weight/ height) were collected in the CRF.
Blood was collected in the central laboratory to assess for CRP, erythrocyte sedimentation rate, as well as complete blood count, platelets, albumin and micronutrients (vitamins A, B12 and D, zinc, copper, chromium).
Disease activity was calculated based on CDAI (21) score and biochemical activity was estimated based on fecal calprotectin (30) and CRP.
Quality of life was assessed using the short form of the IBDQ (SIBDQ) (20), anxiety and depression using the HAD score (31) and Beck Depression Inventory (32) and nutrition using the Subjective Global Assessment (33).
Gastrointestinal transit (colonic transit: SHAPE study and orocecal transit (24,34)) for which the patient took one capsule containing 24 radiopaque markers, and had an x-ray on day 5, following standard protocol (34), where six or more markers indicate delayed transit, therefore less than or equal to five was considered normalization of colonic transit.
Microbiota analysis was performed using Illumina sequencing of the V3 region of 16S ribosomal RNA (rRNA) gene as described previously (17).
All adverse events occurring during the study were reported and recorded whether or not they were considered to be non-serious, serious and/or related to the treatment (Supplementary Table 2).
Statistical Analysis
There is no previous estimate of the effect of ECS compared to CS alone in inducing symptomatic improvement and associated changes in gut microbiota composition. Therefore, for this pilot study, a sample size of 30 patients per arm (35,36) was considered sufficient to estimate the results and allow us to plan for a future larger study; we planned to recruit 10 additional patients to account for drop-outs. We intended to perform analysis as intention-to-treat (for primary efficacy outcome) and per protocol (excluding patients non-compliant with EN). The results were expressed as n (%), mean (SD) or median (IQR) as appropriate. ANOVA using IBM-SPSS (v21, Chicago, IL) was planned to assess differences between groups.
RESULTS
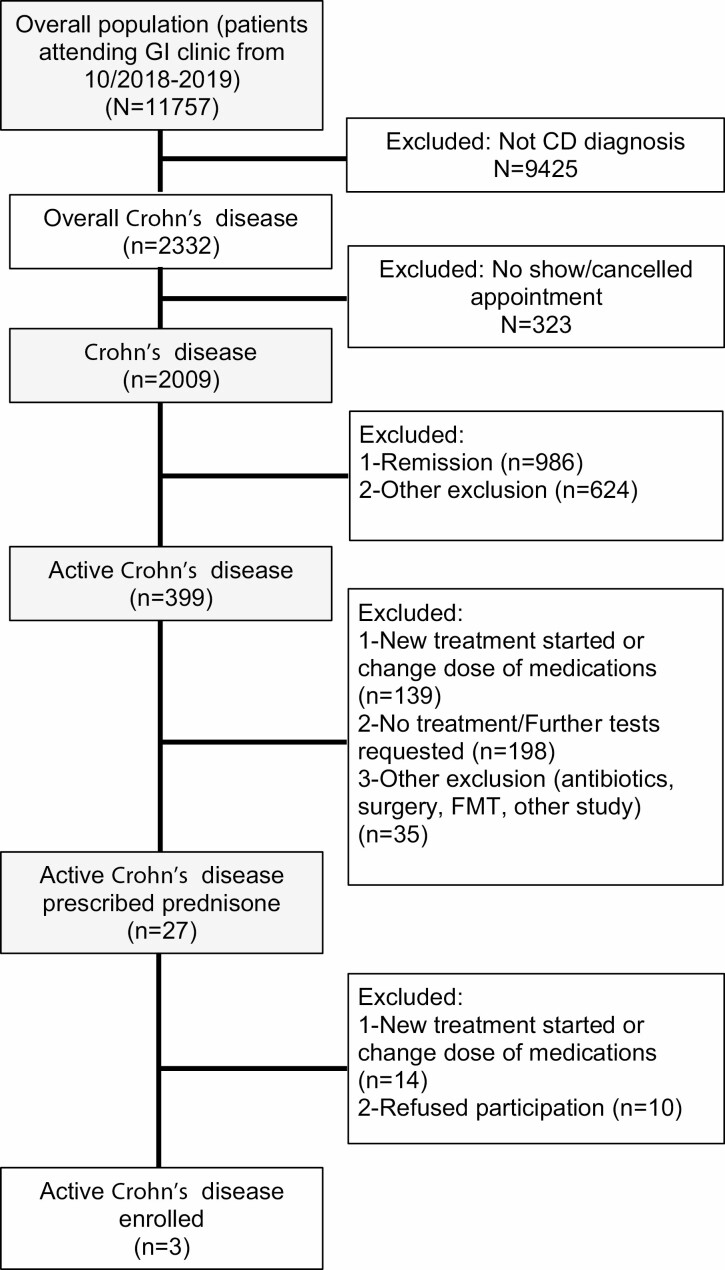
The screening process for potential candidates started in October 2018 at McMaster University Medical Centre and was subsequently rolled out to the other centres (Supplementary Table 3). In 1 year, we pre-screened 11,757 patient visits of which 2332 were for patients with CD. Of these, 323 did not attend their appointment and they were not, therefore, approached for the study. Of the remaining 2009 CD patients, 399 had active CD and, of these, 27 (6.7%) were prescribed prednisone in the clinic (Figure 2). Additional 67 patients were already on prednisone for more than 5 days before they were approached, and therefore, they were not offered participation in the study. These patients were prescribed prednisone at the emergency department (n = 34), at the ambulatory clinic by a gastroenterologist before screening started (n = 8), at the endoscopy unit immediately after colonoscopy (n = 5); or by the family doctor (n = 6). Seven patients were already steroid-dependent and additional seven patients were on prednisone due to other diagnoses, such as pyoderma gangrenosum, Addison’s disease, or other autoimmune conditions.
Figure 2.
Prisma flowchart patient recruitment.
Thirteen out of 27 patients prescribed with prednisone in the clinic were eligible for the study, and 10 of 13 patients refused participation as they were not willing to take the prednisone (n = 4), could not commit to completing the study procedures (n = 3) or were not willing to take EEN (n = 3). Five out of these 10 patients were started on EEN after refusing participation in the study.
Three patients out of the 13 (25%) were ultimately enrolled in the study (Table 1) and, of these, only one finished the study period. One patient dropped out due to the need for surgery and antibiotics for a complicated abscess, and the other required rescue therapy. The only patient who finished the study reported resolution of symptoms after 6 weeks of EEN and a standard course of prednisone (Supplementary Table 4 and Supplementary Figure 2).
Table 1.
Baseline characteristics of patients enrolled
| ECS | S-ECS | CS | |
|---|---|---|---|
| Number of participants (n) | 1 | 1 | 1 |
| Age, years | 57 | 25 | 23 |
| Gender | Male | Male | Female |
| Smoking | No | No | No |
| BMI | 19.8 | 20.8 | 17.1 |
| Subjective global assessment | B | A | B |
| Location of CD | Ileocolonic | Ileocolonic | Ileocolonic |
| Years of diagnosis | 7 | 12 | 1 |
| Previous surgery | Yes | No | No |
| Prior treatment failure | |||
| Immunosuppressants | Yes | Yes | No |
| Biologics | No | No | No |
| Hospitalization last 6 months | No | No | Yes |
| Hb | 85 | 126 | 90 |
| Albumin | 31 | 31 | 22 |
| CRP | 5.8 | 42 | 25 |
| Fecal calprotectin | 6932 | 693 | 707 |
| Vitamin A | 1.2 | 0.9 | 0.8 |
| Vitamin D | 18.3 | 14.5 | 51 |
| Vitamin B12 | 269 | 233 | 182 |
| Chromium | 2.1 | 2.7 | 7.7 |
| Copper | 18.1 | 2.7 | – |
| Zinc | 8.6 | 8.6 | 5.7 |
| SHAPE markers | 1 | 0 | 0 |
| Beck (depression) | 15 | 22 | 27 |
| CDAI | 447 | 261 | 440 |
| SIBDQ score | 3.6 | 3.2 | 2.1 |
| HAD score–anxiety | 10 | 11 | 6 |
| HAD score–depression | 8 | 7 | 11 |
| Volume Modulen (mL/day) | 1893 | 2578 | 0 |
| Study finished | Yes | No | No |
BMI, Body mass index; CD, Crohn’s disease; CDAI, Crohn's Disease Activity Index; CRP, C-reactive protein; CS, corticosteroids; ECS, exclusive enteral nutrition (EEN) + CS arm; HAD, Hospital Anxiety and Depression score; S-ECS, short-course-CS + EEN arm; SIBDQ, Short form of the Inflammatory Bowel Disease Questionnaire.
Tools to Improve Recruitment Rates
During the 1-year recruitment period, we implemented different techniques and tools to improve recruitment rates. The research coordinator was available on site for 90% of the gastroenterology and inflammatory bowel disease (IBD) clinic days. In order to increase our catchment area, we invited gastroenterologists from other centres to collaborate with the study. We sent e-mails as a reminder of the study recruitment to all co-investigators and attended rounds to approach gastroenterologists and residents. We distributed flyers, designed informative brochures for patients and developed a study webpage to promote the study. We identified that a proportion of patients were prescribed prednisone in the emergency department or during admission to hospital, and therefore, we amended the protocol to allow participation by those patients for whom prednisone had been prescribed for less than 3 days (Supplementary Table 3).
Despite all of these efforts, recruitment rates remained very low and therefore, we considered the study non-feasible after 1 year.
Discussion
We aimed to assess the feasibility of a study on the effects of adding EEN to CS to increase rates of remission and decrease adverse events compared with CS alone, and we demonstrated this study was non-feasible due to very low rates of recruitment.
In this pilot study, we aimed to mimic a future trial with the overall aim to assess the feasibility rather than the effectiveness of a study protocol (37–39). Although our primary aim was to assess the feasibility of recruitment, retention and intervention adherence, we also intended to advance scientific inquiry related to intervention safety, appropriate dose of CS to induce remission and potential treatment effect (39).
We have determined that a study involving CS and EEN is non-feasible due to poor recruitment. Patient recruitment is challenging for research studies. Indeed, an assessment of over 500 research protocols revealed that the most frequent setback in the completion of these studies was patient enrolment, with 44.3% of studies reporting this problem (40). A 2015 analysis of 2579 clinical trials in multiple diseases found that 19% of trials were either terminated for failed accrual or completed with less than 85% expected enrolment (41). Moreover, recruitment into IBD trials has been proven to be challenging, (42) especially in investigator-initiated trials, due to great competition of industry-sponsored trials, insufficient sites, study design with restrictive inclusion/exclusion criteria or patient preferences (43). A 2012 survey by Ravikoff et al. (43) found that 32% of IBD patients at a tertiary centre were not interested in participating in therapeutic trials. In our study, we encountered similar rates with 37% (10/27) of active CD patients who were prescribed prednisone declining to participate, and the majority refused to take prednisone. Another factor is the accessibility of multiple approved biologic therapies on the market, which is a change from a time when only infliximab was available for patients (44). We found the principal challenge for recruitment in our study was related to changes in prescription practices in recent years, with a major trend to avoiding corticosteroid therapy in clinical practice, especially in outpatient setting. Overall, CS were prescribed in less than 25% of our CD patients, and the majority of those prescriptions were provided in the emergency department. During the screening process, we have explored prescription practices in gastroenterology and IBD specialized clinics, and with the new era of biologic therapies, gastroenterologists from tertiary centres have considerably decreased the prescription of CS therapy. There is increased awareness and reluctance on the part of physicians to prescribe and patients to take CS due to the associated adverse events; therefore, studies requiring prescription of CS therapy may no longer be feasible. There was no alternative to CS in our study, and this may have contributed to low rates of acceptance for participation. Similar findings were recently published by Selinger et al. (45), reporting a dramatic reduction in steroid prescription in CD patients, especially in tertiary centres from 2015 to 2017. Thus, they propose low steroid use as a key performance indicator or quality improvement. In a similar direction, a recent work in pediatric patients in Switzerland (46) found a significant earlier initiation of anti-tumour necrosis factor alpha agents (anti-TNFα) in the past decade with concomitant steroid-sparing effect in CD patients.
We found a high proportion of patients in whom CS was prescribed were excluded due to other reasons; this suggests an additional limitation related to the restrictive exclusion criteria for enrolment in the study. However, exclusion criteria were carefully planned to avoid confounders, and therefore, omitting them would reduce the validity of the findings. Regardless, a retrospective analysis of our screened patients suggests that less restrictive criteria would not have been sufficient to recruit the intended population in the period of time proposed.
After participants were enrolled, we strategically planned to contact and motivate participants to improve retention (47); however, retention rates in our study were poor. Two out of three enrolled patients dropped out from the study due to adverse events; however, these were related to complications of the underlying disease rather than to the intervention. The participants enrolled in the study had severe disease activity based on CDAI, which may have contributed to the increased rates of complications. However, restricting the inclusion criteria to non-severe cases would have decreased recruitment rates even more and limited the generalizability of results.
Finally, it is unclear if the implications for study recruitment are limited to nutrition studies or may be relevant to other therapies. A recent study found that a CD exclusion diet, combined with supplemental enteral nutrition (SEN), was similar to EEN for inducing remission in children with mild/moderate CD (48). This raises the possibility that SEN may be as effective as EEN and more tolerable; however, this finding has not been reproduced in adults or in patients with moderate/severe disease requiring CS (48). We found 23% of eligible patients refused participation related to the EEN, which seems a lower rate compared to previous studies, which quote as high as 39% (8,49). Although this could suggest a trend to increased acceptance of the use in EEN in adult population, this may not necessarily apply to pediatric population and future studies may be helpful to determine the generalizability of our findings.
In conclusion, our study suggests that studies requiring CS prescription alone may no longer be feasible. The addition of EEN may be an additional barrier to recruitment; however, this was not a major obstacle in our study as a lower proportion of patients refused participation on the basis of EEN compared to prior studies. Due to current changes in clinical practice and rarity of CS prescription alone, a larger study with this design is discouraged. In the era of increasing early use of biologic, a future study looking at the incremental value of EEN or PEN to biologic agents could be considered. Future studies should focus on improving efficacy and decreasing adverse events of currently prescribed therapies; and studies evaluating the addition of EEN or other nutritional support to current therapies should be encouraged.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank NESTEC, Nestle Health Sciences, for providing in kind IBD Modulen formula for this pilot study.
Disclosures: MIP-S received in kind support for the Modulen formula from Nestle Canada. NN holds a McMaster University Department of Medicine Internal Career Award and honoraria from Janssen, Abbvie, Takeda, Pfizer, Merck and Ferring, unrelated to this manuscript. KJK is a honoraria speaker for Takea, Janssen and Abbvie and has consulting fees with Takeda and Abbvie, unrelated to this manuscript. JKM has Honoraria from AbbVie, Allergan, Amgen, Bristol-Meyer-Squibb, Celgene, Celltrion, Ferring, Hospira, Janssen, Lilly, Lupin, Merck, Novartis, Pfizer, Pharmascience, Roche, Shire, Takeda and Teva, unrelated to this manuscript. UC has received honoraria as consultant and speaker from Ferring, Takeda, Abbvie and Shire and is in the advisory board of Merck, unrelated to this manuscript. GB has received honoraria as consultant from Jansen, Takeda, Abbvie and Pfizer and is on the advisory board of Takeda, unrelated to this manuscript. EFV received grant support from Biocodex and Gilead, unrelated to this manuscript and holds a Canada Research Chair. MTB, LR, EC, JB, SH, FT, ANM, SMC, PM, PB, GIL and DA have nothing to disclose.
Funding: MIP-S received an Innovation Grant from Crohn’s and Colitis Canada, Medicine Internal Career Research Award and Farncombe Family Digestive Health Research Institute Award.
Author Contributions: MTB: Drafted the proposal, registered the protocol and drafted the manuscript equally with co-first author LR. NN, JB, KJK, SH, FT, JKM, SMC, PM, GIL and PB: Protocol design, recruitment of patients and reviewed the manuscript. GB, UC, ANM and EC: Recruitment of patients and reviewed the manuscript. EFV: Protocol design and reviewed the manuscript. DA and MIP-S: Designed the study, drafted the proposal and interpreted the results.
References
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380(9853):1590–605. [DOI] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011;474(7351):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocchi A, Benchimol EI, Bernstein CN, et al. . Inflammatory bowel disease: A Canadian burden of illness review. Can J Gastroenterol 2012;26(11):811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruemmele FM. Role of diet in inflammatory bowel disease. Ann Nutr Metab 2016;68(Suppl. 1):33–41. [DOI] [PubMed] [Google Scholar]
- 5.Mack DR, Benchimol EI, Critch J, et al. . Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn’s Disease. Gastroenterology 2019;157(2):320–48. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Montiel MP, Casis-Herce B, Gómez-Gómez GJ, et al. . Pharmacologic therapy for inflammatory bowel disease refractory to steroids. Clin Exp Gastroenterol 2015;8:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan KJ, Ullman TA, Ford AC, et al. . Antibiotic therapy in inflammatory bowel disease: A systematic review and meta-analysis. Am J Gastroenterol 2011;106(4):661–73. [DOI] [PubMed] [Google Scholar]
- 8.Narula N, Dhillon A, Zhang D, et al. . Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2018;4:CD000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day AS, Lopez RN. Exclusive enteral nutrition in children with Crohn’s disease. World J Gastroenterol 2015;21(22):6809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahidi L, Day AS, Lemberg DA, et al. . Paediatric inflammatory bowel disease: A mechanistic approach to investigate exclusive enteral nutrition treatment. Scientifica (Cairo) 2014;2014:423817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto T, Nakahigashi M, Saniabadi A.R. Review article: Diet and inflammatory bowel disease—epidemiology and treatment. Aliment Pharmacol Therapeut 2009;30:99–112. [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Milo T, Buller H, et al. . Consensus and controversy in the management of pediatric Crohn disease: An international survey. J Pediatr Gastroenterol Nutr 2003;36(4):464–9. [DOI] [PubMed] [Google Scholar]
- 13.Di Caro S, Fragkos KC, Keetarut K, et al. . Enteral nutrition in adult Crohn’s disease: Toward a paradigm shift. Nutrients 2019;11(9):2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Moráin C, Segal AW, Levi AJ. Elemental diet as primary treatment of acute Crohn’s disease: A controlled trial. Br Med J (Clin Res Ed) 1984;288(6434):1859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Critch J, Day AS, Otley A, et al. ; NASPGHAN IBD Committee . Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2012;54(2):298–305. [DOI] [PubMed] [Google Scholar]
- 16.Grover Z, Burgess C, Muir R, et al. . Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J Crohns Colitis 2016;10(10):1159–64. [DOI] [PubMed] [Google Scholar]
- 17.Gatti S, Galeazzi T, Franceschini E, et al. . Effects of the exclusive enteral nutrition on the microbiota profile of patients with Crohn’s disease: A systematic review. Nutrients 2017;9(8):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes A. Review article: Crohn’s disease—the role of nutritional therapy. Aliment Pharmacol Ther 2002;16(Suppl. 4):48–52. [DOI] [PubMed] [Google Scholar]
- 19.Pinto-Sanchez MI, Nardelli, A., Borojevic, R., et al. . Antigliadin antibodies predict the symptomatic response to gluten-free diet and improvement in gastrointestinal motility in IBS patients. Gastroenterology 2017;152(5):S45. [Google Scholar]
- 20.Guo Z, Wu R, Zhu W, et al. . Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn’s disease. Nutr Clin Pract 2013;28(4):499–505. [DOI] [PubMed] [Google Scholar]
- 21.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology 1979;77(4 Pt 2):843–6. [PubMed] [Google Scholar]
- 22.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980;1(8167):514. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Feagan BG, Hanauer SB, et al. . A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 2002;122(2):512–30. [DOI] [PubMed] [Google Scholar]
- 24.Kim ER, Rhee PL. How to interpret a functional or motility tes—colon transit study. J Neurogastroenterol Motil 2012;18(1):94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oz HS, Ray M, Chen TS, et al. . Efficacy of a transforming growth factor beta 2 containing nutritional support formula in a murine model of inflammatory bowel disease. J Am Coll Nutr 2004;23(3):220–6. [DOI] [PubMed] [Google Scholar]
- 26.Fell JM, Paintin M, Arnaud-Battandier F, et al. . Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther 2000;14(3):281–9. [DOI] [PubMed] [Google Scholar]
- 27.Harris JA, Benedict FG. A Biometric study of human basal metabolism. Proc Natl Acad Sci USA 1918;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanderson IR, Udeen S, Davies PS, et al. . Remission induced by an elemental diet in small bowel Crohn’s disease. Arch Dis Child 1987;62(2):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faiman A, Mutalib M, Moylan A, et al. . Standard versus rapid food reintroduction after exclusive enteral nutritional therapy in paediatric Crohn’s disease. Eur J Gastroenterol Hepatol 2014;26(3):276–81. [DOI] [PubMed] [Google Scholar]
- 30.Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol 2015;50(1):74–80. [DOI] [PubMed] [Google Scholar]
- 31.Bjelland I, Dahl AA, Haug TT, et al. . The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- 33.Sökülmez P, Demirbağ AE, Arslan P, et al. . Effects of enteral nutritional support on malnourished patients with inflammatory bowel disease by subjective global assessment. Turk J Gastroenterol 2014;25(5):493–507. [DOI] [PubMed] [Google Scholar]
- 34.Evans RC, Kamm MA, Hinton JM, et al. . The normal range and a simple diagram for recording whole gut transit time. Int J Colorectal Dis 1992;7(1):15–7. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead AL, Julious SA, Cooper CL, et al. . Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016;25(3):1057–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31(2):180–91. [DOI] [PubMed] [Google Scholar]
- 37.Eldridge SM, Chan CL, Campbell MJ, et al. ; PAFS Consensus Group . CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eldridge SM, Lancaster GA, Campbell MJ, et al. . Defining feasibility and pilot studies in preparation for randomised controlled trials: Development of a conceptual framework. PLoS ONE 2016;11(3):e0150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud 2018;4:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cullati S, Courvoisier DS, Gayet-Ageron A, et al. . Patient enrollment and logistical problems top the list of difficulties in clinical research: A cross-sectional survey. BMC Med Res Methodol 2016;16:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahon E, Roberts, J., Furlon, P., Uhlenbrauck, G., Bull, J.. Barriers to Clinical Trial Recruitment and Possible Solutions: A Stakeholder Survey 2015. https://www.appliedclinicaltrialsonline.com/view/barriers-clinical-trial-recruitment-and-possible-solutions-stakeholder-survey. [Google Scholar]
- 42.Herfarth HH, Jackson S, Schliebe BG, et al. . Investigator-initiated IBD trials in the United States: Facts, obstacles, and answers. Inflamm Bowel Dis 2017;23(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravikoff JE, Cole EB, Korzenik JR. Barriers to enrollment in inflammatory bowel disease randomized controlled trials: An investigation of patient perspectives. Inflamm Bowel Dis 2012;18(11):2092–8. [DOI] [PubMed] [Google Scholar]
- 44.Harris MS, Wichary J, Zadnik M, et al. . Competition for clinical trials in inflammatory bowel diseases. Gastroenterology 2019;157(6):1457–1461.e2. [DOI] [PubMed] [Google Scholar]
- 45.Selinger CP, Parkes GC, Bassi A, et al. . Assessment of steroid use as a key performance indicator in inflammatory bowel disease—analysis of data from 2385 UK patients. Aliment Pharmacol Ther 2019;50(9):1009–18. [DOI] [PubMed] [Google Scholar]
- 46.Guilcher K, Fournier N, Schoepfer A, et al. ; Swiss IBD Cohort Study . Change of treatment modalities over the last 10 years in pediatric patients with inflammatory bowel disease in Switzerland. Eur J Gastroenterol Hepatol 2018;30(10):1159–67. [DOI] [PubMed] [Google Scholar]
- 47.Bower P, Brueton V, Gamble C, et al. . Interventions to improve recruitment and retention in clinical trials: A survey and workshop to assess current practice and future priorities. Trials 2014;15:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine A, Wine E, Assa A, et al. . Crohn’s disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019;157(2):440–450.e8. [DOI] [PubMed] [Google Scholar]
- 49.Malchow H, Steinhardt HJ, Lorenz-Meyer H, et al. . Feasibility and effectiveness of a defined-formula diet regimen in treating active Crohn’s disease. European Cooperative Crohn’s Disease Study III. Scand J Gastroenterol 1990;25(3):235–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.