Abstract
The coronavirus 2019 (COVID-19) pandemic has presented many new challenges to the healthcare community with the sheer number of individuals affected and the range of symptoms at presentation. Early findings have shown that increased age is an independent risk factor for COVID-19 severity. Diabetes and hypertension were also found to be strong independent risk factors for severe COVID-19. It was later discovered that obesity is a strong risk factor for severe disease as well. Possible mechanisms for the increased risk associated with metabolic disease include the increased prevalence of acute respiratory syndrome, immune cell dysfunction, and chronic inflammatory states associated with obesity and diabetes. Acknowledging these risk factors has consequences for addressing vaccination strategies as well as healthcare disparities.
Keywords: coronavirus, coronavirus 2019, metabolic risk
The emergence of coronavirus 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has presented new challenges to the global healthcare community due to its highly contagious nature, as well as its high morbidity and mortality. One of those challenges is in its vast diversity of symptoms, with most who contract the virus remaining entirely asymptomatic, while others experience severe symptoms from respiratory failure to multiorgan failure and death. It is imperative to determine the risk factors most strongly associated with severe disease in COVID-19 to better allocate resources during a pandemic.
Preliminary findings have shown that the elderly are more susceptible to mortality from COVID-19. However, as the pandemic has progressed, it has been observed that hypertension and metabolic disease, including diabetes and obesity, are also risk factors for COVID-19 mortality. This article will review the literature and research available to assess the data on the mortality risk for COVID-19 among patients with diabetes, hypertension, and obesity.
REVIEW OF METABOLIC RISK FACTORS FOR SEVERE COVID-19
Multiple meta-analyses have been performed to assess the comorbidity of COVID-19 and cardiovascular metabolic diseases in diabetes and hypertension. The earliest studies showed diabetes and hypertension as common comorbidities of the disease.1 Li et al2 performed a meta-analysis of 6 studies with 1527 patients that found the incidences of hypertension, cardiac-cerebrovascular diseases, and diabetes were about twofold, threefold, and twofold higher, respectively, in severe cases of COVID-19 relative to nonsevere cases. Tascioglu et al3 provided the perspective that some studies, including Wang et al4 have shown hypertension and diabetes to not be independent risk factors for COVID-19 mortality, and age was the only independent prognostic risk factor. In that study,4 hypertension and diabetes were present in patients who died due to COVID-19 in equivalent proportion to the prevalence of hypertension and diabetes in the general Chinese population.
New York City was one of the earliest cities in the United States to be hit by the COVID-19 pandemic. With its uniquely diverse patient population and its existence as a hub for travel into and out of the United States, the data that have come out of New York are important for understanding COVID-19. The first major study was from Richardson et al5 analyzing the outcome of 5700 patients hospitalized with COVID-19 in the New York City area. The 3 most common comorbidities of hospitalized patients in that study were hypertension, obesity, and diabetes, at 56.6%, 41.7%, and 33.8% of hospitalized patients, respectively.
There is an ever-growing obesity epidemic in the United States. More than two-thirds of the US adults and one-third of the US children and adolescents are overweight or obese, and obesity has become the second leading cause of preventable disease or death in the United States.6 Multiple studies have shown that obesity increases morbidity and mortality.7 It is necessary to understand the relationship between obesity and COVID-19, in particular, to better care for those who may have an increased risk for morbidity and mortality due to the virus.
Gao et al8 performed a retrospective study of 150 patients from China with COVID-19, and found a higher proportion of obese patients had severe COVID-19 compared with nonobese patients (33.3% vs 14.7%), as well as a higher median hospital stay of 23 versus 18 days. In another study,9 the symptoms and severity of COVID-19 patients with nonalcoholic fatty liver disease and stratified results between nonobese and obese patients were assessed. These investigators found that obese patients had more severe disease (37.5% vs 9.5%, P = 0.021). One retrospective cohort study of 1158 patients from Kuwait showed obesity and diabetes to be independent risk factors for COVID-19 severity.10 Tartof et al11 found a J-shaped association between obesity and risk of death due to COVID-19 among 6919 patients in the Kaiser Permanente Southern California system. The relative risk of mortality among individuals with a body mass index (BMI) between 40 and 44, and above 45, was found to be 2.68 and 4.18, respectively, when compared to individuals with a normal BMI. Hamer et al12 found a linear increase in the rates of COVID-19 hospitalization with increasing BMI, based on a cohort from the United Kingdom.
Age is often considered the most significant risk factor for COVID-19 morbidity and mortality, while younger people are largely protected from severe disease.13 Of note, obesity also increases the risk of severe COVID-19 for young adults. Hendren et al14 found that obesity is overrepresented in patients hospitalized for COVID-19 and those with severe disease, including adults under 50 years of age. They found that obese adults hospitalized for COVID-19 are generally younger than those with normal weight. The protections against severe COVID-19 afforded by young age do not apply to Class III obese individuals (BMI > 40). Cunningham et al15 reports an increased risk for in-hospital mortality or mechanical ventilation in adults under 35 years old.
Pranata et al16 performed a systematic literature review on the association between COVID-19 mortality and severity of disease in patients with hypertension. In an analysis of 6560 patients from 30 studies, hypertension was associated with an increased risk of mortality, severe COVID-19, acute respiratory distress syndrome (ARDS), intensive care unit care, and disease progression, with a composite poor outcome (relative risk: 2.11, P < 0.001). It is significant that both obesity and hypertension are 2 of the strongest negative prognostic risk factors linked with COVID-19, as they are both rising issues in the United States. Not only is there an obesity epidemic in the United States, but one serial cross-sectional study has shown a recent decrease in the proportion of the US adults with hypertension whose hypertension is controlled.17
PATHOPHYSIOLOGY OF COVID-19 IN METABOLIC DISEASE
Based on clinical studies, it appears that diabetes, hypertension, and obesity are strong risk factors for Covid-19 mortality. It is important to explore the pathophysiologic basis for the increased risk of mortality and severe disease in this patient population. Understanding the underlying reasons for the increased risk would better prepare us to treat this high-risk population.
It is first necessary to review the pathophysiology of COVID-19. Coronaviruses in general are large, single-stranded, enveloped RNA viruses which cause respiratory, neurologic, and gastrointestinal disease.18 Coronaviruses most commonly cause common cold symptoms. SARS-CoV-2 causes more severe disease and is the third known coronavirus to cause severe SARS. SARS-CoV-2 targets nasal and bronchial epithelial cells and pneumocytes through the structural spike (S) protein which binds to the angiotensin-converting enzyme 2 (ACE2) receptor. The host cell’s type 2 transmembrane serine protease (TMPRSS2) cleaves ACE2 to promote viral uptake, and activates the SARS-CoV-2 S protein, allowing entry into the cell. ACE2 and TMPRSS2 are primarily expressed in alveolar epithelial type II cells.
SARS-CoV-2 may cause profound lymphopenia when it infects and kills T lymphocytes. The innate and adaptive inflammatory responses further impair lymphopoiesis and increase lymphocyte apoptosis. As viral replication accelerates, the epithelial–endothelial barrier integrity breaks down. Infection of the pulmonary capillary endothelium exacerbates the inflammatory response and triggers an influx of monocytes and neutrophils. Computed tomographic imaging shows ground-glass opacities in the lungs due to interstitial mononuclear inflammatory infiltrates and pulmonary edema filling the alveolar spaces with hyaline membrane formation. This marks early-phase ARDS. In summation, endothelial barrier disruption, impaired oxygen diffusion, and dysfunctional alveolar-capillary oxygen transmission are characteristics of COVID-19.
Severe COVID-19 is characterized by fulminant activation of coagulation and consumption of clotting factors in diffuse intravascular coagulation. Inflamed lung tissues and pulmonary endothelium may result in the formation of microthrombi which results in a high incidence of thrombotic complications such as deep venous thrombosis, pulmonary embolism, and arterial complications such as limb ischemia, stroke, and myocardial infarction in critical patients. Multiorgan failure may also occur due to the development of a dysregulated host response to infection in viral sepsis.
The increased risk of severe COVID-19 in obese patients could be related to ARDS. BMI is associated with the development of ARDS. Gong et al19 found a relationship between higher BMI with longer hospital stays and ICU admission due to ARDS, although not with ARDS mortality. There are multiple proposed mechanisms for this increased association. They found that ventilator settings vary by weight and that high peak inspiratory pressure, increased positive end-expiratory pressure, and larger tidal volumes were associated with ARDS development. The ARDSnet trials found that obese patients had ventilator settings with significantly higher tidal volumes and peak airway pressures than normal weight patients.20 Clinical studies have shown an association between ARDS development and 2 inflammatory adipokines that are elevated in obesity, pre-B cell enhancing factor and leptin.
Popkin et al21 suggest several mechanisms for increased severity of COVID-19 in obese patients. One suggestion is that obesity’s strong association with hyperglycemia and type 2 diabetes is responsible. Uncontrolled serum glucose has been shown to be strongly correlated to COVID-19 mortality due to impairment of immune cell function either directly or via oxidant and glycation product generation.22 Furthermore, immune cell metabolism is impaired by dysfunction in insulin and leptin as in hyperglycemic states. Individuals with both obesity and diabetes have increased Th17 inflammation due to impaired immune cell oxidation of fatty acid metabolites.
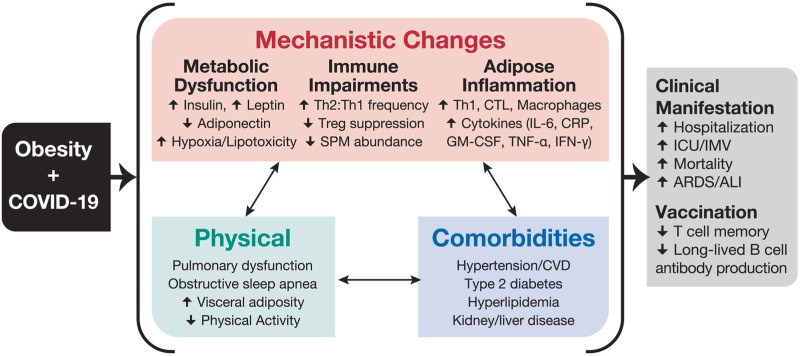
Increased BMI is also associated with increased anti-inflammatory CD4 Th2 and T regulatory cells which may inhibit the ability to control viral spread. Studies on hyperinsulinemic mice with obesity have shown regulatory T cells have reduced interleukin-10 (IL-10) production and are 40% less suppressive despite being in higher abundance in the lungs during influenza infections.23,24 RNA virus immune responses rely on type 1 inflammatory responses by Th1 cells for protection balanced with Tregs anti-inflammatory responses and so the dysregulation seen in metabolic disease would likely impair the immune response to SARS-CoV-2. Furthermore, there is an accumulation of proinflammatory macrophages, dendritic cells, cytotoxic T cells, and Th1 cells in the adipose tissue of obese individuals which increase serum inflammatory cytokines such as IL-6, type I and III interferons, and C-reactive protein (Fig. 1).21
FIGURE 1.
Clinical manifestations and mechanisms for COVID-19 risk in individuals with obesity. Reprinted with permission from John Wiley & Sons from Popkin et al.21 Copyright Popkin et al. All permission requests for this image should be made to the copyright holder.
Another suggestion of Popkin et al21 is that dietary consumption of fatty acids increases the inflammatory response due to the formation of various inflammatory mediators. Prostaglandins are derivatives of long chain fatty acids which initiate the local inflammatory response during infection. In the United States, omega-6 fatty acids, which mediate the proinflammatory cyclooxygenase (COX) activity via prostaglandin production, are consumed in a 10:1 ratio with omega-3 fatty acids, which mediate the anti-inflammatory response of COX. Cholesterol and other fatty acids are essential for spread of enveloped RNA viruses. SARS-CoV uses cholesterol to facilitate viral budding after binding to ACE2 receptors with viral S protein, while low cholesterol levels in ACE2-producing cells reduces viral S protein binding. The changes to the immune system and the chronic inflammatory state that come with obesity all contribute to ARDS, acute lung injury, and respiratory failure, which are characteristics of severe COVID-19.
DISCUSSION
COVID-19 severity and mortality are strongly correlated to metabolic disease. In this review, various studies were discussed that show the extent to which metabolic disease is an independent risk factor for the development of severe COVID-19. The pathophysiology of COVID-19 and possible mechanisms of its severity in patients with metabolic disease were discussed as well. In light of this information, there are several important discussion points that require further investigation.
At the time of this review, several COVID-19 vaccines have been approved for use across the world, and vaccinations are in progress. One of the critical policy issues that are being discussed is that of determining the proper distribution of vaccines, given their limited availability. One common suggestion is that the most vulnerable populations should be prioritized, and so it could be suggested that individuals with metabolic disease should be prioritized for receiving the vaccine. However, it is also important to note that obesity has been shown to impair immunologic memory for the influenza vaccine,25 as well as others. Furthermore, it has been suggested that T cells are critical in lasting immunity to COVID-19 and so memory T cells are critical in vaccination,26 yet individuals with obesity have impaired T cell responses. Considering this information, it is critical that more research be done with regards to vaccinations and the correlation between lasting immunity and BMI, in order to best care for this population.
The COVID-19 pandemic should further inspire a serious effort at tackling healthcare disparities and structural racism. The United States, Black, Hispanic, and Native American individuals have increased COVID-19 infection rates and worse outcomes than non-Hispanic White individuals.27 Additionally, race and lower socioeconomic status are associated with increased rates of metabolic and cardiovascular disease.28,29 Metzl et al30 discuss the various ways the healthcare disparities due to structural inequality in the United States have had a serious impact on the severity of the COVID-19 pandemic for vulnerable populations. Tackling issues of systemic inequality is a necessity, and would simultaneously address healthcare disparities and disparities in the prevalence of metabolic and cardiovascular diseases, which both greatly contributed to the severity of this pandemic.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tascioglu D, Yalta K, Yetkin E. Hypertension and diabetes mellitus in patients with COVID 19: a viewpoint on mortality. Cardiovasc Endocrinol Metab. 2020;9:108–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression Epidemiol Rev. 2007;29:6–28. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. World Health Organization. (Technical report series no. 894); 2000. [PubMed] [Google Scholar]
- 8.Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e74. [DOI] [PubMed] [Google Scholar]
- 9.Zheng KI, Gao F, Wang XB, et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Sabah S, Al-Haddad M, Al-Youha S, et al. COVID-19: impact of obesity and diabetes on disease severity. Clin Obes. 2020; 10:e12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamer M, Gale CR, Kivimäki M, et al. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A. 2020;117:21011–21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. COVIDView: a weekly surveillance summary of U.S. COVID-19 activity. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. Accessed October 16, 2020.
- 14.Hendren NS, deLemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized With COVID-19. Circulation 2020;143:135–144. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JW, Vaduganathan M, Claggett BL, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020;181:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pranata R, Lim MA, Huang I, et al. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21:1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. [DOI] [PubMed] [Google Scholar]
- 19.Gong MN, Bajwa EK, Thompson BT, et al. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien JM, Jr, Welsh CH, Fish RH, et al. National heart, lung, and blood institute acute respiratory distress syndrome network. Ann Intern Med. 2004;140:338–345. [DOI] [PubMed] [Google Scholar]
- 21.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obesity Rev 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han JM, Patterson SJ, Speck M, et al. Insulin inhibits IL-10-mediated regulatory T cell function: implications for obesity. J Immunol. 2014;192:623–629. [DOI] [PubMed] [Google Scholar]
- 24.Milner JJ, Sheridan PA, Karlsson EA, et al. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J Immunol. 2013;191:2474–2485.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond). 2012;36:1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altmann DM, Boyton RJ. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol 2020;5:eabd6160. [DOI] [PubMed] [Google Scholar]
- 27.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hales CM, Fryar CD, Carroll MD, et al. Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. 2018;319:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzl JM, Maybank A, De Maio F. Responding to the COVID-19 pandemic: the need for a structurally competent health care system. JAMA. 2020;324:231–232. [DOI] [PubMed] [Google Scholar]