Abstract
As the global coronavirus disease-19 (COVID-19) pandemic caused by severe acute respiratory distress syndrome coronavirus 2 continues to cause higher mortality and hospitalization rates among older adults, strategies such as frailty screening have been suggested for resource allocation and clinical management. Frailty is a physiologic condition characterized by a decreased reserve to stressors and is associated with disability, hospitalization, and death. Measuring frailty can be a useful tool to determine the risk and prognosis of COVID-19 patients in the acute setting, and to provide higher quality of care for vulnerable individuals in the outpatient setting. A literature review was conducted to examine current research regarding frailty and COVID-19. Frailty can inform holistic care of COVID-19 patients, and further investigation is needed to elucidate how measuring frailty should guide treatment and prevention of COVID-19.
Keywords: coronavirus, COVID-19, frailty, frailty screening
The coronavirus disease-19 (COVID-19) pandemic, caused by severe acute respiratory distress syndrome coronavirus 2, continues to particularly affect older adults worldwide. The novel coronavirus strain was first detected in December 2019 and the World Health Organization declared the severe acute respiratory distress syndrome coronavirus 2 outbreak a pandemic on March 11, 2020.1 As of February 26, 2021, COVID-19 has infected nearly 113 million people around the world, and has caused more than 2.4 million deaths.2 In the United States, there have been more than 28 million reported cases and more than 500,000 deaths.2
Since the beginning of the pandemic, older patients have been at increased risk from COVID-19. In fact, people over the age of 65 represent half of the hospital admissions, more than half of the intensive care unit (ICU) admissions, and 80% of deaths related to COVID-19.3 These findings are supported by reports from China, Italy, and the United Kingdom, which showed the higher risk in older adults, particularly in those with pre-existing diseases.4
This phenomenon has prompted research on the causes of increased vulnerability of older patients to severe clinical presentation; frailty is thought to be one such factor. Frailty is defined as a condition characterized by declining function across several homeostatic systems leading to increased vulnerability to stressors and risk of adverse health outcomes.4 Previous studies have shown that frailty is associated with worse outcomes in hospitalized older adults.5
The assessment of frailty has emerged as a potential tool for the allocation of care resources. The clinical management guidelines produced by the United Kingdom National Institute for Health and Care Excellence recommend the assessment of frailty as the initial step in the triage of suspected COVID-19 patients. These guidelines advise the use of the clinical frailty scale (CFS) in patients aged 65 years and older, and state that decisions about admission to the ICU should be made on the basis of potential for medical benefit.6 While the Centers for Disease Control and Prevention have released guidance regarding the increased risks for hospitalization and dying among older adults, there are no specific recommendations regarding frailty assessments in the United States.7
This article examines various studies to evaluate the value of frailty metrics in clinical decision-making for elderly patients with COVID-19.
IMPORTANCE OF FRAILTY ASSESSMENT
Age has been shown to be a strong risk factor for severe illness, complications, and death in COVID-19 patients.8 For example, among a cohort of more than 44,000 confirmed cases of COVID-19 in China, the case fatality ratio increased with age and was highest among the oldest cohort.8 Mortality among people 80 years and older was 14.8%; 70–79 years, 8.0%; 60–69 years, 3.6%; 50–59 years, 1.3%; 40–49 years, 0.4%; and for those younger than 40 years, 0.2%.8 Based on initial epidemiologic data from the United States through March 16, 2020, the case fatality ratio was highest in people aged 85 years or older (range 10–27%), followed by people aged 65–84 years (3–11%), aged 55–64 years (1–3%), and was lowest in people younger than 55 years (<1%).8 These data suggest that age plays an important role in the clinical outcomes of COVID-19. However, using age cutoffs is a myopic approach to critical care resource allocation that ultimately harms the elderly population. During the early stages of the pandemic, there was an increased prevalence of discussions regarding rationing and withholding life-saving ventilation based on age. While such care measures may be futile in some frail older people with multimorbidity, this is not true for all elderly adults.
Instead, Le Couteur et al9 proposes a frailty-based approach that prioritizes individual functional abilities, multimorbidity, prognosis, and treatment preferences. Moreover, frailty is independent of age when predicting poorer outcomes.10 Measuring frailty allows for comparison of patients who are the same chronological age yet have different physical traits and comorbidities. It is important to recognize that frailty is not synonymous with older age because younger adults can also be frail. While the majority of frail individuals are older persons, a substantial segment of this group is middle-aged.11 Therefore, frailty may be a better metric than age for guiding clinical decisions and informing health policy in response to COVID-19.
Moreover, frailty can be reversible with early identification as a modifiable risk factor in the aging population. For example, frail patients with cardiovascular disease (CVD) typically have worse outcomes compared with nonfrail patients.12 Verma et al12 recommend that providers utilize therapeutic measures to target frailty in patients with CVD by referring patients to exercise rehabilitation programs and decreasing the risk of falls. Patients with CVD should also be stratified based on degree of frailty, and frail patients should be evaluated before procedural interventions due to an increased risk for complications.12 In the same vein, physicians can also potentially treat frailty in the outpatient setting as a preventive measure against severe COVID-19 illness.
MECHANISM OF FRAILTY IN COVID-19
The mechanism of increased illness severity in frail patients with COVID-19 is likely linked to a decreased immune response. Due to the failure of biological systems that regulate homeostasis, frail patients are vulnerable to environmental stressors and poor outcomes. The pathobiological changes in aging and frailty are due to immunosenescence and “inflamm-aging.”13 Immunosenescence is an age-associated shift in the immune system that causes a diminished ability to fight novel infections and contributes to a chronic state of inflammation called “inflamm-aging.”14 Proinflammatory cytokines such as interleukin-6, interleukin-1, tumor necrosis factors-alpha, C-reactive protein, and fibrinogen may promote muscle protein degradation and exacerbate frailty.15 This chronic inflammatory state downregulates the innate and adaptive immune responses, reducing the body’s ability to counter acute respiratory infections such as COVID-19.16 Inflamm-aging may also disrupt cell clearance mechanisms that help resolve inflammation after injury, leading to the development of acute respiratory distress syndrome in COVID-19.17
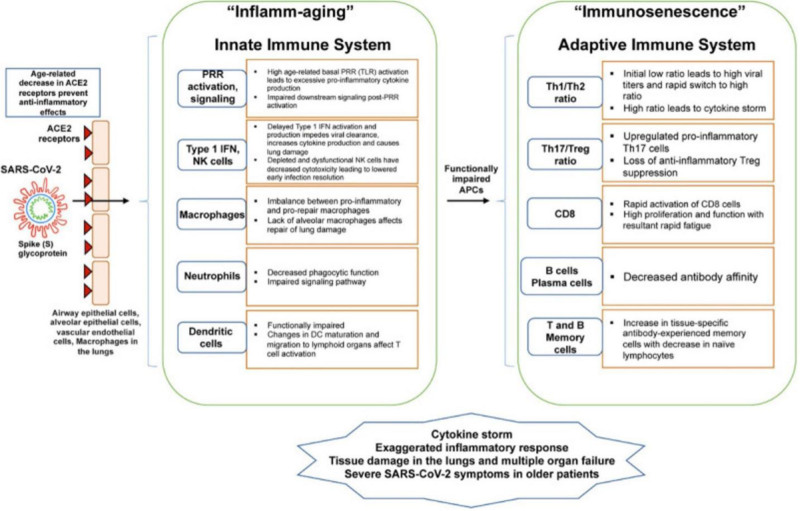
In a prospective cohort study of 114 older COVID-19 patients in Wuhan, China, Ma et al18 reported that CD8+ counts in frail COVID-19 patients were significantly lower than those in nonfrail COVID-19 patients. Because cell-mediated immune responses play an important role in virus clearance, frailty-related suppression of the immune system may explain the association between aging and the increased risk of adverse outcomes in COVID-19 patients (Fig. 1).18
FIGURE 1.
This diagram shows how age-related changes in the innate and adaptive immune system contribute to inflamm-aging and immunosenescence and how such changes relate to SARS-CoV2 infection. From ACE indicates angiotensin converting enzyme; APC, antigen presenting cells; DC, dendritic cells; IFN, interferon; PRR, pattern recognition receptor; SARS-CoV2, severe acute respiratory distress syndrome coronavirus 2; TLR, toll-like receptor. Republished from Bajaj et al.14 Copyright Bajaj, Gadi, Spihlman, Wu, Choi, and Moulton. Published by Frontiers Media. All permission requests for this image should be made to the copyright holder.
FRAILTY METRICS
There are several frailty metrics that may be used to evaluate COVID-19 patients. Some recent studies have highlighted the CFS, a rapid frailty screening tool that is scored between 1 (very fit) and 7 (severely frail) based on self-report of comorbidities and the ability to perform activities of daily living.19
De Smet et al5 conducted a retrospective single-center observational study of 81 patients in Belgium to determine the association between frailty graded by CFS and short-term mortality in older adults hospitalized for COVID-19.5 The authors argue that frailty is significantly but weakly associated with a higher risk of mortality in COVID-19 patients; however, many severely frail patients survived (72%), and the CFS by itself had poor specificity and no useful cut-off for mortality prediction. It should be noted that this was a single-center study that cannot be readily extrapolated to other settings.
The CFS has also been studied in larger settings. The COVID-19 in Older PEople (COPE) study aimed to establish the prevalence of frailty in patients with COVID-19 who were admitted to the hospital, and investigated the influence of frailty on mortality and length of hospital stay.20 This multicenter European observational cohort study of 1564 COVID-19 patients (median age of 74 years) was conducted at 11 hospitals in the United Kingdom and Italy, showing that patients classified as frail by the CFS were more likely to die from COVID-19 and were discharged less quickly than nonfrail patients.20
However, the COPE study is not without limitations. Though outcomes were adjusted for baseline patient factors such as age and comorbidities, the study did not report data on treatment, illness severity, or process measures after admission.21 This raises concern regarding the degree to which frailty is intrinsically associated with increased mortality in COVID-19, or whether the reported mortality may be related to differences in illness severity or variation in treatment type offered to patients with advanced frailty.21
Although further study is required to determine the reproducibility and reliability of using the CFS in COVID-19 patients, there are still advantages to using this clinical tool. Compared with the frailty index, a tool that requires the physician to consider a list of 70 possible disorders, the CFS is easier to use and can be readily administered in the clinical setting.19 However, this does not mean that the frailty index has not been studied in COVID-19 patients. In fact, a recent study from Italy by Bellelli et al22 showed that in a sample of 105 COVID-19 patients, frailty, as assessed by the frailty index, was associated with in-hospital mortality and ICU admission, independent of age and sex.
Other researchers have examined different methods of frailty assessment. Woolford et al11 investigated whether frailty and multimorbidity were associated with risk of hospitalization with COVID-19 in the UK Biobank, a large prospective community cohort of over half a million UK residents. They calculated frailty using a modified version of 5 frailty phenotype indicators originally reported by Fried et al.23 The authors found no differences for frailty status or number of morbidities when comparing those who tested positive for COVID-19 and those who tested negative. They concluded that people living with frailty and multimorbidity are no more likely to require a hospital admission due to COVID-19 compared with other conditions resulting in similar disease severity.11 However, these specific findings do not inform the predictive value of frailty for subsequent outcomes of COVID-19, such as noninvasive ventilation, ICU admission, or length of hospital stay.
APPLICATIONS OF FRAILTY FOR COVID-19
While frailty does not aid in risk stratification in terms of positive versus negative results of COVID-19 testing, the assessment of frailty may have utility in predicting mortality or length of hospital stay in patients who do test positive for COVID-19. Previously, frailty had largely been investigated in regard to its association with overall mortality, hospital infection, ICU admission rates, and disease phenotypes.4
However, only a few observational studies have been carried out, resulting in limited clinical data. Specific interventions related to frailty or its impact on COVID-19 treatments have not been evaluated. Therefore, the question of how to use frailty scales in guiding interventions remains unanswered. Since CFS is an easy-to-use and reliable screening tool to identify frailty, it can provide valuable clinical information when triaging in the emergency department or escalating care on the wards. On the other hand, it appears that frailty alone does not provide sufficient information for clinical decision-making in COVID-19 patients; thus, clinicians should consider illness severity at presentation and likelihood of success of interventions together with the degree of frailty.4 It is likely that multiple factors, including frailty and comorbidities, contribute to the increased vulnerability of older people with COVID-19. Assessing frailty may help physicians to identify older people who require close monitoring and implement preventive interventions.
Frailty scales can also be used outside of the hospital. For example, residents of long-term care facilities face increased vulnerability to COVID-19. Not only do they have chronic conditions that contribute to a high level of frailty, but they also experience isolation from advocates, close proximity to other residents and staff, and less resources than in acute care settings.24 In this challenging situation, clinical assessments of frailty can serve as a surveillance tool by predicting illness severity and recovery. In fact, primary care may be the most effective setting to prevent adverse outcomes in frail adults, by directing patients to the appropriate setting and curbing excess hospitalizations.
Limitations of Frailty Screening Tools
It is important to recognize the strengths and weaknesses of any clinical tool. Hubbard et al3 both affirm the value of the CFS in allocation of limited resources and address its limitations, suggesting that frailty is a continuum rather than a dichotomous variable. Moreover, frailty does not suggest futility. Therefore, frailty should not be used as the sole factor for decision-making; rather, frailty should identify risk and inform discussions regarding escalation of treatment, mortality, patient wishes, and code status.25
One concern with recommendations like the National Institute for Health and Care Excellence guidelines is the use of a defined score as a cutoff when there is uncertainty surrounding each patient case. Chong et al26 published data on acutely hospitalized older adults (mean age 89.4 ± 4.6 years; n = 210), where they compared patients with a CFS score of 1–5 (nonfrail to mildly frail) against those with a score of 6–8 (moderate to severely frail). Data revealed that being in the more frail group was independently predictive of mortality and institutionalization following hospitalization. The mortality rate during hospitalization was found to be higher in CFS 6 (4.7%) and CFS 7–8 (8.6%) and patients with no mortality were recorded among CFS 1–3, CFS 4, and CFS 5 patients.26
At 12-month follow-up, the mortality rate in CFS 5 patients was low (8.3%) in comparison with CFS 6 (29.2%) and CFS 7–8 (60.0%) (P < 0.001); this was similarly observed for institutionalization.26 These data indirectly demonstrate that mildly frail older adults are able to endure the stressors of hospitalization and recover well, raising concerns about the use of CFS score of 5 as a hard cut-off to define a “more frail” state. Frailty should not automatically disqualify less frail individuals from the potential benefits of ICU care, especially when resources are available. Frailty should not be used solely as a patient label for rationing care; rather, frailty metrics should inform clinical management in a context-appropriate manner.
FUTURE STUDIES
There is a growing body of work describing frailty and its effect on COVID-19 outcomes. It is clear that clinicians can use early assessments of frailty as part of their strategy to identify vulnerable patients in the community, inform decision-making during acute hospitalizations, and guide critical care resource allocation. However, looking to the future, there are further implications for the use of frailty tools in the COVID-19 pandemic. Post–COVID-19 patients may exhibit extrapulmonary manifestations, including neurological, cardiovascular, and musculoskeletal disorders that restrict functional status.4 Frailty assessment will be necessary in the rehabilitation setting. Furthermore, the use of frailty tools may become more prevalent as providers use telemedicine to remotely monitor isolated patients with chronic conditions.
Finally, it will also be necessary to assess the relation between frailty, vaccine effectiveness, and vaccine priority. The Advisory Committee on Immunization Practices has recommended that long-term care facility residents who are frail, or older adults unable to reside independently in the community, be prioritized in the earliest phase of COVID-19 vaccination.27 The concept of immunosenescence is important for understanding vaccine responses because immunosenescence contributes to the variability in susceptibility that is seen with frailty and an increasing burden of health conditions. Frailty is also understood to affect older adults’ responses to vaccines for infections such as influenza, shingles, and pneumococcus.28 Frailty may ultimately affect vaccine safety, reactogenicity, immunogenicity, and efficacy in older adults.28 As more vaccines are approved for emergency use, the role of frailty in COVID-19 vaccine efficacy will become clearer with time.
CONCLUSIONS
Assessing frailty represents a valuable opportunity to provide higher quality care for older adults with COVID-19. Early detection and careful monitoring of frailty can help providers care for vulnerable older adults in this challenging time when social isolation is very common. In the acute setting, tools such as the CFS may provide clinicians with useful information to guide resource allocation and escalation of care. Many recent studies have shown that frailty is linked to adverse outcomes in COVID-19, but frailty screening tools cannot be used as the sole component for clinical decision-making.29 While frailty gives a more holistic understanding than age or comorbidities alone of susceptibility to adverse outcomes, it is best used to highlight risk and guide a patient-centered approach to treating COVID-19 patients.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
REFERENCES
- 1.Ducharme J. World Health Organization declares COVID-19 a “Pandemic.” Here’s what that means. Time. 2020. Available at: https://time.com/5791661/who-coronavirus-pandemic-declaration. Accessed January 18, 2021. [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. 2020. WHO. Available at: https://covid19.who.int/. Accessed February 26, 2021. [Google Scholar]
- 3.Hubbard RE, Maier AB, Hilmer SN, et al. Frailty in the face of COVID-19. Age Ageing. 2020;49:499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maltese G, Corsonello A, Di Rosa M, et al. Frailty and COVID-19: a systematic scoping review. J Clin Med. 2020;9:E2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Smet R, Mellaerts B, Vandewinckele H, et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J Am Med Dir Assoc. 2020;21:928–932.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 rapid guideline: critical care in adults. 2020. NICE. Available at: https://www.nice.org.uk/guidance/ng159/resources/covid19-rapid-guideline-critical-care-in-adults-pdf-66141848681413. Accessed January 18, 2021. [PubMed] [Google Scholar]
- 7.Older Adults At greater risk of requiring hospitalization or dying if diagnosed with COVID-19. 2020. CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html. Accessed January 18, 2021. [Google Scholar]
- 8.Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). 2020. CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed January 18, 2021. [Google Scholar]
- 9.Le Couteur DG, Anderson RM, Newman AB. COVID-19 through the lens of gerontology. J Gerontol A Biol Sci Med Sci. 2020;75:e119–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt J, Carter B, McCarthy K, et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. 2019;48:388–394. [DOI] [PubMed] [Google Scholar]
- 11.Woolford SJ, D’Angelo S, Curtis EM, et al. COVID-19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants. Aging Clin Exp Res. 2020;32:1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma G, O’Laughlin JP, Bunker L, et al. Trial of time: review of frailty and cardiovascular disease. Cardiol Rev. 2017;25:236–240. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajaj V, Gadi N, Spihlman AP, et al. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol. 2020;11:571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Salminen A, Kaarniranta K, Kauppinen A. Immunosenescence: the potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell Mol Life Sci. 2019;76:1901–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sendama W. The effect of ageing on the resolution of inflammation. Ageing Res Rev. 2020;57:101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Hou L, Yang X, et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: a prospective cohort study. BMC Med. 2020;18:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt J, Carter B, Vilches-Moraga A, et al. ; COPE Study Collaborators. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darvall JN, Bellomo R, Young PJ, et al. Frailty and mortality in patients with COVID-19. Lancet Public Health. 2020;5:e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellelli G, Rebora P, Valsecchi MG, et al. ; COVID-19 Monza Team members. Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020;46:1634–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 24.Andrew M, Searle SD, McElhaney JE, et al. COVID-19, frailty and long-term care: implications for policy and practice. J Infect Dev Ctries. 2020;14:428–432. [DOI] [PubMed] [Google Scholar]
- 25.Moug S, Carter B, Myint PK, et al. Decision-making in COVID-19 and frailty. Geriatrics (Basel). 2020;5:E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong E, Chan M, Tan HN, et al. COVID-19: use of the clinical frailty scale for critical care decisions. J Am Geriatr Soc. 2020;68:E30–E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Interim Considerations for COVID-19 Vaccination of Healthcare Personnel and Long-Term Care Facility Residents. 2020. CDC. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19/clinical-considerations.html. Accessed January 18, 2021. [Google Scholar]
- 28.Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet. 2021;396:1942–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma G, O'Laughlin JP, Bunker L, et al. Trial of time: review of frailty and cardiovascular disease. Cardiol Rev. 2017;25:236–240. [DOI] [PubMed] [Google Scholar]