Abstract
Objectives. To evaluate the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) over 6 months in the Brazilian State of Rio Grande do Sul (population 11.3 million), based on 8 serological surveys.
Methods. In each survey, 4151 participants in round 1 and 4460 participants in round 2 were randomly sampled from all state regions. We assessed presence of antibodies against SARS-CoV-2 using a validated lateral flow point-of-care test; we adjusted figures for the time-dependent decay of antibodies.
Results. The SARS-CoV-2 antibody prevalence increased from 0.03% (95% confidence interval [CI] = 0.00%, 0.34%; 1 in every 3333 individuals) in mid-April to 1.89% (95% CI = 1.36%, 2.54%; 1 in every 53 individuals) in early September. Prevalence was similar across gender and skin color categories. Older adults were less likely to be infected than younger participants. The proportion of the population who reported leaving home daily increased from 21.4% (95% CI = 20.2%, 22.7%) to 33.2% (95% CI = 31.8%, 34.5%).
Conclusions. SARS-CoV-2 infection increased slowly during the first 6 months in the state, differently from what was observed in other Brazilian regions. Future survey rounds will continue to document the spread of the pandemic.
More than 118 million COVID-19 cases have been reported worldwide, and more than 2.6 million persons have died, as of March 11, 2021.1 Since May 2020, Brazil has been a hotspot for the pandemic; it is the third country in the world in absolute number of confirmed cases (11.2 million as of March 11, 2021) and the second in the number of deaths (270 900 as of March 11, 2021).1 However, there has been marked regional variability in progression of the pandemic in Brazil. A countrywide survey in mid-May 2020 showed that whereas the proportion of the population with antibodies was 6.3% in the North (Amazon) region, it was below 1% in the 4 remaining regions of the country. In early June 2020, figures remained below 1% in 3 regions, but increased to 9% in the North (Amazon) and 3.2% in the Northeast region.2
Brazil’s South region comprises 3 states with a combined population of 27.4 million people, of whom 11.3 million live in the southernmost state of Rio Grande do Sul (hereafter “the State”; Figure A, available as a supplement to the online version of this article at http://www.ajph.org), where the first COVID-19 death was reported on March 24, 2020. Eighteen days later, we started the first round of a series of statewide seroprevalence surveys. In August 2020, we published results from the first 3 survey rounds. Although prevalence increased by 4-fold between the first and third rounds, it remained far below 0.5%, suggesting that the epidemic was at an early stage in the State.3 Eight rounds have been completed between April and September 2020, allowing us to document the spread of the virus in the State over 6 months.
Social distancing measures were widely adopted early in the pandemic. On May 9, 2020, the State’s government launched the Controlled Distancing Model, a color-coded strategy aimed at defining how much each region of the State would be allowed to relax social distancing measures. The scheme was based on reported cases and deaths, prevalence (based upon our surveys), and hospital bed occupancy rates. Further information on the model and on the indicators used is available in Box A (available as a supplement to the online version of this article at http://www.ajph.org). Our objective was to report on the 6-month spread of COVID-19 infections in the State based on 8 sequential statewide population-based serological surveys.
METHODS
We present results from the 8 rounds that were completed in 2020. Of the 8 rounds,4 surveys 1 through 4 took place 2 weeks apart. Given the slow increase in prevalence, the interval was increased to 4 weeks until prevalence reached 1%, after which the interval was reduced to 3 weeks. The exact dates of each round were April 11–13, 2020 (round 1), April 25–27 (round 2), May 9–11 (round 3), May 23–25 (round 4), June 26–28 (round 5), July 24–26 (round 6), August 14–16 (round 7), and September 4–6 (round 8). Further details on the study protocol are available elsewhere.4
The Brazilian Institute of Geography and Statistics divides Rio Grande do Sul State into 8 intermediate regions and 497 municipalities (Figure A). A multistage sampling approach was adopted (Box B, available as a supplement to the online version of the article at http://www.ajph.org).
We used the rapid point-of-care lateral-flow Wondfo SARS-CoV-2 Antibody Test (Wondfo Biotech Co, Guangzhou, China). The manufacturer’s own validation study reported a sensitivity of 86.4% and specificity of 99.6%, using samples collected from 361 confirmed cases and 235 negative controls in China. We conducted 2 separate validation studies on this test.5 , 6 In the first study, carried out in April,5 we estimated a sensitivity of 77.1% by administering the Wondfo test to 83 patients with positive reverse-transcriptase polymerase chain reaction (RT-PCR) tests within the past 60 days. However, emerging evidence on the decline of antibodies over time motivated us to conduct a second validation study, in which we administered the rapid test from mid-October to mid-November to 133 patients who had positive RT-PCR results from April to October. In the second study, test sensitivity ranged from about 80% (among participants with positive RT-PCR within 2 months) to as low as 42% after 5 months of the RT-PCR, with a mean value of 63.2%.5 In our publication using data from the first 3 rounds,3 we used a meta-analytical technique to summarize the results of 4 validation studies available until that time—with a combined sensitivity of 84.8% and specificity of 99.0%—to correct prevalence obtained in our study.3 In a later publication analyzing data from national serological surveys,4 we further refined the correction parameters using the same sensitivity of 84.8% and setting specificity at 99.95% based on the results of the first survey in Rio Grande do Sul, where only 2 positive results were obtained in 4500 participants. Details on this approach are provided elsewhere.4 In the present analyses, we present the unadjusted prevalence, the adjusted prevalence using the same strategy applied in the national study, and the prevalence adjusted for the decay in antibodies over time.
To account for the time-dependent decline in sensitivity of the Wondfo, we estimated how the pandemic behaved over time in each of the 9 cities. We used official statistics of COVID-19 death rates (which are more reliable than reported cases, which depend on intensity of testing), and we assumed that infected individuals who died had become positive in the test 2 weeks before dying.7 Therefore, for each survey round in each city, we corrected the unadjusted prevalence using a sensitivity value that represented a weighted average of the values obtained in our second validation study,6 with the COVID-19 all-ages mortality rate curve in each city providing the statistical weights. For example, if most deaths in a city were recent, sensitivity had a higher value than in another city where most deaths had occurred in the past, and, thus, sensitivity values had already declined by the time of the survey round. Uncertainty in the model that describes the relationship between sensitivity and time was incorporated by resampling the model coefficients in the parametric bootstrap procedure implemented in the previously developed correction method.4 Figure B (available as a supplement to the online version of this article at http://www.ajph.org) presents the fitted curves.
A questionnaire was applied to participants in all rounds, including information on gender, age, schooling, self-reported skin color, and compliance with social isolation measures. Schooling was recorded as the highest level completed with a pass grade. Given Brazil’s widespread multiethnic population, the classification of ethnicity recognizes 5 groups, based on the question: “How do you classify yourself in terms of color or race?” The 5 response options are “White,” “Brown” (“Pardo” in Portuguese), “Black,” “Yellow (Asian),” and “Indigenous.” We instructed interviewers to check the “Yellow” option when the respondent mentioned being of Asian descent, and “Indigenous” when any of the multiple First Nations were mentioned. The “Brown” category reflects mixed ancestry including European, African, or Indigenous backgrounds. In terms of social distancing measures, individuals were asked about their routine activities through the following question: “Which of the following options best describes your current routine?”:
-
a.
stays at home all the time;
-
b.
leaves the household only for essential activities, such as buying food;
-
c.
leaves the household sometimes, to buy things or to stretch the legs;
-
d.
leaves the household daily; or
-
e.
leaves the household daily, to work or other regular activity.
In the analyses, we combined categories “b” and “c,” and categories “d” and “e.”
We fitted LOWESS regression8 to the time-trend data on the number of deaths and cases per 100 000 inhabitants in Brazil made available by the State (https://covid.saude.gov.br). Our intent was 2-fold: adjust a smoothed curve to the data and generate interpolated values based on this curve.
All analyses took the clustering of the sample into account. For the analysis of the association of SARS-CoV-2 infection with sociodemographic and socioeconomic variables, we calculated the prevalence of infection (8 rounds combined) in each subgroup of the independent variables. The combined prevalence is simply the number of positive tests across all surveys divided by the number of tests performed across all surveys. We calculated P values with the χ2 test. We also compared the distribution of social distancing behavior between the 8 surveys using a χ2 test with Rao and Scott second-order correction,9 which accounts for the sampling design and yields a statistic that follows an F-distribution with 1 and 2 degrees of freedom (df). We performed all analyses with R version 3.6.1.10 We used the “survey” package11 , 12 to incorporate the sampling design and to compare the distribution of social distancing behavior across surveys.
Only interviewers with negative tests for SARS-CoV-2 and absence of any symptoms collected data. They used individual protection equipment that was discarded after visiting each household.
RESULTS
We were able to interview and test 4151 people in the first round, 4460 in the second, and 4500 participants in each of the remaining rounds of data collection. Nonresponse rates increased in the later rounds. In the first round, refusals accounted for 8.9%. The corresponding proportions were 8.8%, 7.1%, 7.9%, 10.0%, 12.0%, 13.3% and 14.0% in rounds 2 through 8, respectively.
Table 1 describes the sample in each round according to gender, age, skin color, and education levels. The proportion of males was stable at around 40% in all rounds. Around 10% of the participants were aged 0 to 19 years in each round, and the proportion of participants aged 80 years or older was stable at around 4%. Around three quarters of the sample reported White skin color. Taking the 8 rounds together, only 262 Asian and 173 Indigenous participants were included, and, therefore, these categories were grouped as “other” for the analyses. The samples of the 8 rounds were also stable in terms of schooling—around 35% of the participants had primary education or less. Despite the slight increase in refusal rates, the samples were similar across surveys in terms of gender, age, skin color, and education.
TABLE 1—
Description of the Sample in Terms of Gender, Age, Skin Color, and Schooling: The State of Rio Grande do Sul, Brazil, April 11 to September 6, 2020
| Variable | Round of Data Collection, % or No. | |||||||
| 1 (Apr 11–13) | 2 (Apr 25–27) | 3 (May 9–11) | 4 (May 23–25) | 5 (Jun 26–28) | 6 (Jul 24–26) | 7 (Aug 14–16) | 8 (Sep 4–6) | |
| Gender | ||||||||
| Male | 41.7 | 40.6 | 41.1 | 42.1 | 39.5 | 39.9 | 38.1 | 39.1 |
| Female | 58.3 | 59.4 | 58.9 | 57.9 | 60.5 | 60.1 | 61.9 | 60.9 |
| Age, y | ||||||||
| 0–4 | 1.7 | 1.1 | 1.1 | 0.8 | 1.1 | 0.8 | 0.8 | 0.8 |
| 5–9 | 2.0 | 1.5 | 1.5 | 1.3 | 1.8 | 1.7 | 1.3 | 1.3 |
| 10–19 | 5.4 | 5.1 | 5.9 | 5.4 | 5.2 | 5.6 | 5.7 | 5.6 |
| 20–39 | 27.5 | 28.3 | 28.0 | 29.2 | 26.0 | 27.5 | 27.4 | 27.4 |
| 40–59 | 33.3 | 32.5 | 32.4 | 33.3 | 33.4 | 32.8 | 33.6 | 33.3 |
| 60–79 | 25.7 | 28.0 | 26.6 | 26.4 | 28.4 | 27.4 | 27.2 | 27.6 |
| ≥ 80 | 4.4 | 3.5 | 4.5 | 3.6 | 4.1 | 4.2 | 4.0 | 4.0 |
| Skin colora | ||||||||
| White | 76.5 | 75.8 | 76.0 | 76.7 | 75.9 | 76.4 | 75.9 | 75.8 |
| Brown | 15.8 | 16.2 | 15.3 | 16.2 | 15.4 | 14.9 | 15.5 | 15.6 |
| Black | 6.6 | 6.7 | 7.4 | 6.1 | 7.4 | 7.2 | 7.2 | 7.5 |
| Schooling | ||||||||
| Primary or less | 40.9 | 34.2 | 36.1 | 34.1 | 36.4 | 35.0 | 35.5 | 35.4 |
| Secondary | 32.8 | 31.9 | 31.5 | 29.6 | 31.5 | 32.2 | 30.5 | 31.7 |
| University or higher | 26.3 | 33.9 | 32.4 | 36.3 | 32.1 | 32.8 | 34.0 | 32.9 |
| Sample size | 4151 | 4460 | 4500 | 4500 | 4500 | 4500 | 4500 | 4500 |
Because of small numbers, Indigenous and Asians were not included in the analyses.
Table 2 presents the unadjusted prevalence of participants with SARS-CoV-2, as well as the prevalence figures adjusted solely for the test’s initial sensitivity and specificity (as used in our previous publication),2 and the prevalence estimates further adjusted for the decay in antibodies over time identified in our validation study.6 The number of positive tests increased from 2 out of 4151 in the first round to 62 out of 4500 in the eighth round, with a total of 197 over the 8 rounds. The corrected prevalence increased from 0.03% (95% confidence interval [CI] = 0.00, 0.34) in the first round in April to 1.89% (95% CI = 1.36, 2.54) in the eighth round in September. Prevalence figures were below 0.5% from April to May, and above 1.0% from July onward.
TABLE 2—
Prevalence of SARS-CoV-2 Antibodies: The State of Rio Grande do Sul, Brazil, April 11 to September 6, 2020
| Round (Date) | No. of Positive Tests / Sample Size | Uncorrected Prevalence, % (95% CI) | Prevalence Adjusted for Test’s Validity, % (95% CI) | Prevalence Adjusted for the Decay in Antibodies, % (95% CI) |
| 1 (Apr 11–13) | 2 / 4151 | 0.05 (0.01, 0.17) | 0.03 (0.00, 0.34) | 0.03 (0.00, 0.34) |
| 2 (Apr 25–27) | 6 / 4460 | 0.13 (0.05, 0.29) | 0.10 (0.01, 0.38) | 0.10 (0.01, 0.40) |
| 3 (May 9–11) | 10 / 4500 | 0.22 (0.11, 0.41) | 0.21 (0.06, 0.49) | 0.21 (0.06, 0.51) |
| 4 (May 23–25) | 8 / 4500 | 0.18 (0.08, 0.35) | 0.15 (0.03, 0.44) | 0.16 (0.03, 0.46) |
| 5 (Jun 26–28) | 21 / 4500 | 0.47 (0.28, 0.72) | 0.49 (0.27, 0.83) | 0.55 (0.29, 0.94) |
| 6 (Jul 24–26) | 43 / 4500 | 0.96 (0.70, 1.27) | 1.07 (0.75, 1.48) | 1.18 (0.79, 1.68) |
| 7 (Aug 14–16) | 55 / 4500 | 1.22 (0.91, 1.60) | 1.39 (1.01, 1.85) | 1.56 (1.10, 2.14) |
| 8 (Sep 4–6) | 62 / 4500 | 1.38 (1.05, 1.77) | 1.57 (1.17, 2.06) | 1.89 (1.36, 2.54) |
Note. CI = confidence interval; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 3 presents the combined prevalence (number of positive tests across the 8 rounds divided by the number of tests performed across the 8 rounds) of antibodies against SARS-CoV-2 according to gender, age, skin color, and education. There were no differences according to gender (0.54% in male and 0.61% in female participants; P = .35) or skin color (prevalence ranged from 0.55% to 0.68%; P = .65). Older adults (0.41%) were significantly less likely (P = .028) to present antibodies compared with children and adolescents (0.63%), and young (0.61%) and middle-aged adults (0.70%). The intermediate education group presented significantly higher (P = .005) prevalence (0.76%), compared with those with lower (0.45%) or higher schooling duration (0.56%).
TABLE 3—
Combined Seroprevalence of SARS-CoV-2 According to Gender, Age, Skin Color, and Schooling: The State of Rio Grande do Sul, Brazil, April 11 to September 6, 2020
| Variables | Seroprevalence,a % (95% CI) | P |
| Gender | .35 | |
| Male | 0.54 (0.43, 0.67) | |
| Female | 0.61 (0.51, 0.73) | |
| Age, y | .028 | |
| 0–19 | 0.63 (0.38, 0.99) | |
| 20–39 | 0.61 (0.46, 0.79) | |
| 40–59 | 0.70 (0.56, 0.87) | |
| ≥ 60 | 0.41 (0.30, 0.55) | |
| Skin color | .65 | |
| White | 0.55 (0.46, 0.65) | |
| Brown | 0.68 (0.48, 0.93) | |
| Black | 0.65 (0.36, 1.08) | |
| Schooling | .005 | |
| Primary or less | 0.45 (0.34, 0.58) | |
| Secondary | 0.76 (0.60, 0.94) | |
| University or higher | 0.56 (0.43, 0.71) |
Note. CI = confidence interval; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Combined seroprevalence: number of positive tests across the 8 rounds divided by the number of tests performed across the 8 rounds.
Figure C (available as a supplement to the online version of this article at http://www.ajph.org) presents the number of daily deaths per million inhabitants in the 5 Brazilian regions (see Figure A for the Brazilian map), as well as the dates on which the 8 statewide surveys took place in Rio Grande do Sul, which is 1 of 3 states in the South region. The 3 states in the South Region are Rio Grande do Sul (population 11.3 million), Santa Catarina (population 7.2 million), and Paraná (population 11.1 million). This region was consistently below all other regions in terms of deaths from the beginning of the pandemic until around early September 2020, when our eighth survey took place. At that point, first-wave epidemic curves were descendent in all regions, except the South, where numbers were still increasing. From mid-November 2020 onward, the South showed the highest mortality rates among all regions. Table A (available as a supplement to the online version of this article at http://www.ajph.org) confirms these trends by comparing official statistics on cases and deaths per 100 000 inhabitants in Brazil and the State of Rio Grande do Sul.
Figure 1 shows reported social distancing practices in our samples. The proportion of participants who reported going out daily increased from 21.4% in April (round 1) to 30.4% in May (round 3), after which the proportion stabilized at around one third until the eighth round. The proportion of participants who reported staying at home all the time was reduced from 22.0% in April (round 1) to around 12% to 13% from June onward.
FIGURE 1—
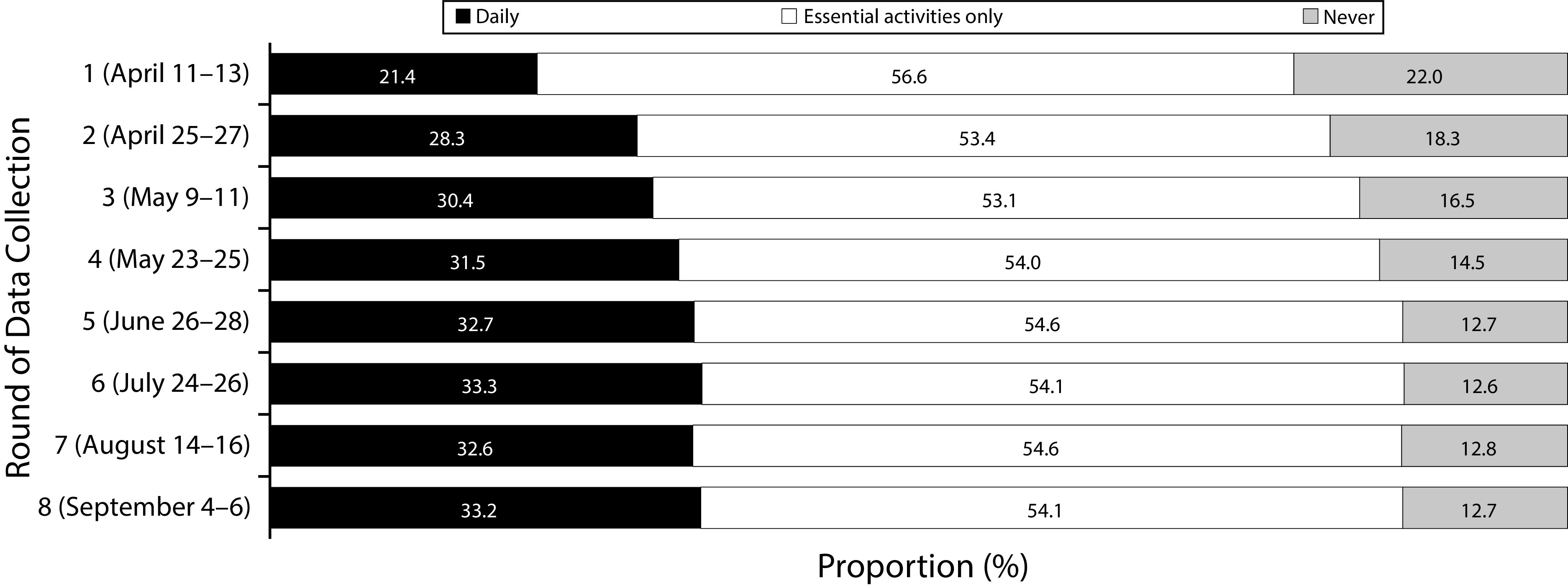
Social Distancing Practices Reported by the Population Between April and September 2020, in the State of Rio Grande do Sul, Brazil
Note. Values are the proportion (%) of the population that go out daily (black), for essential activities only (white), or stay at home all the time (gray).
DISCUSSION
At the early stages of the SARS-CoV-2 pandemic, most countries relied solely on official statistics on cases and deaths.13–15 It took a few months for population-based seroprevalence studies to start providing a more accurate picture of the burden of SARS-CoV-2 infection, including asymptomatic patients and those with mild symptoms. Spain and Brazil, 2 countries that were hit hard by COVID-19, published nationwide survey-based estimates in August and September 2020. The ENE-COVID study estimated that 5.0% of the Spanish population had been infected at that stage.16 The EPICOVID-19 study in Brazil estimated that 3.1% of the Brazilian population had been infected with the virus at that time.4 These results are not strictly comparable as different antibody tests were used. Both the Spanish and Brazilian surveys continued to take place, and decays in antibodies over time were reported after the first wave, as in many other studies.17–20
In Rio Grande do Sul, a multisectoral effort including universities, the state government, and the private sector allowed for the launch of a seroprevalence household survey only 18 days after the first death was reported. In addition to this early start, we were able to complete 8 survey rounds between April and September 2020. We are unaware of any other seroprevalence study that included 8 sequential rounds anywhere in the world. In August 2020, we published the findings of the first 3 rounds of this statewide survey; prevalence figures increased by 4-fold between the first and third rounds but remained well below 0.5%.3 We now present updated information including data from 8 rounds of data collection, confirming the slow spread of the epidemic that had been reported earlier.
A critical methodological challenge was to estimate the prevalence given the decay in antibodies over time, which was confirmed by most of the literature, and which we managed to quantify in our second validation study.6 We applied a statistical model to correct for the decline in sensitivity over time, which used official statistics to model the progression of the epidemic in each of the cities included in the analyses. This strategy yields estimates that are more closely interpretable as the cumulative prevalence of infection when compared with the unadjusted estimates. However, both unadjusted and adjusted estimates presented similar trends, likely because of the slow spread of the virus in the State.
Limitations
Our study had some limitations. First, the proportion of refusals increased over time. As the pandemic hit other Brazilian states extremely hard, people became less willing to admit researchers to their homes. Nevertheless, the distributions of the samples in terms of sociodemographic and socioeconomic variables were similar across the 8 rounds of the study. Second, in the first rounds of the survey, we did not collect detailed information on socioeconomic status, so that analyses here use schooling as a proxy. Third, the sensitivity of the test declined over time,6 a finding that we were unaware of when the survey started. At that time (April 2020), this test, which had been donated to the Ministry of Health by a private company, was the only test suitable for large-scale epidemiological studies in the country. By conducting 2 validation studies and correcting our estimates, we attempted to correct the test limitations. Fourth, because of low prevalence, the associations between SARS-CoV-2 infection and the sociodemographic and socioeconomic variables should be interpreted with caution.
The lack of association between prevalence of SARS-CoV-2 antibodies and gender was also observed in many seroprevalence studies.2 , 16 , 21 , 22 Variations by age are less consistent in the literature. The Brazilian national study2 reported higher antibody prevalence among middle-aged participants compared with younger or older participants. The Spanish national survey found children to be at a lower risk of SARS-CoV-2 infection,16 but a survey in 10 US sites found no differences according to age.21 Similarly to our findings, a Swiss survey reported older adults to be at lower risk of SARS-CoV-2 infection.22 It is important to highlight that age patterns in antibody prevalence do not reflect disease severity. Case-fatality rates increase with age,23 , 24 a finding that is confirmed by the fact that 81.2% of COVID-19 deaths in Rio Grande do Sul during 2020 occurred among individuals aged 60 years or older.25
Although early COVID-19 infections in most countries were observed among the rich, who are more likely to travel abroad, after community transmission starts, the scenario can change rapidly. The EPICOVID-19 study in Brazil found those in the bottom quintile of household assets to have a 2-fold increase in SARS-CoV-2 infection prevalence.2 In August 2020, Khalatbari-Soltani et al.26 published a study on the importance of collecting data on socioeconomic determinants of COVID-19, because “disadvantaged socioeconomic position is widely associated with disease and mortality, and there is no reason to think this will not be the case for the newly emerged coronavirus disease.”26(p620) In Spain, immigrant populations had a higher risk of SARS-CoV-2 infections compared with Spanish participants.16 In our study, those with secondary education were more likely to present antibodies against SARS-CoV-2 than those with higher or lower education, a finding that was also observed in a study among blood donors in Saudi Arabia.27 Unfortunately, a detailed questionnaire on household assets was not administered in the first rounds of the statewide surveys, impeding in-depth analyses of inequalities in SARS-CoV-2 infection. In addition, because of the low prevalence figures detected, statistical power was reduced for this specific analysis.
Although we did not find an association between seroprevalence and skin color, the EPICOVID-19 study in Brazil reported a 5-fold higher risk among Indigenous compared with White individuals, with intermediate levels of risk for Black and Brown participants.2 Systematic reviews have confirmed the association between ethnicity and COVID-19 infections, hospital admissions, and mortality.28 In Brazil, Black and Brown participants were more likely to present severe episodes.29 The lack of association reported in our study is likely attributable to the fact that there were very few Indigenous participants in the sample and there was low prevalence of infection in the State up to September 2020, resulting in lack of statistical power to find differences.
One of the explanations for the slow progression of the COVID-19 pandemic during the first 6 months in Rio Grande do Sul might be the adoption of the Controlled Distancing Model, under the assumption that economic activities in each region of the state should be maintained at a level determined by the burden of infection and by health system preparedness to deal with incoming patients. Whenever prevalence rose or the occupancy of hospitals beds—particularly for intensive care—increased, economic activities were restricted up to complete lockdowns. Figure D (available as a supplement to the online version of this article at http://www.ajph.org) shows that, despite the slow spread of the virus reported in our article, the color-coded system tended to become more severe over time, with a clear increase in the number of regions being coded toward more severe colors. This finding is in accordance with data presented in Table A, showing that although the State started much better than the entire country in terms of daily new cases and deaths, the difference tended to be reduced until September 2020. By January 2021, numbers in Rio Grande do Sul were far above those for the entire country.
Determining cause and effect in the association between SARS-CoV-2 and the Controlled Distancing Model is challenging. Although the model may have had an impact on dissemination of the virus across the State, one should bear in mind that the algorithm relies on statistics about the burden of COVID-19 and, therefore, the model is also influenced by the spread of the virus. Another issue to keep in mind is that trends in Rio Grande do Sul are not that different from those observed in the neighbor states of Santa Catarina and Paraná, which adopted their own nonpharmaceutical strategies to respond to the pandemic.
With the benefit of hindsight and the availability of 8 seroprevalence surveys, it is possible to summarize the progression of the pandemic in Rio Grande do Sul. Compliance with social distancing recommendations was high during the first months of the pandemic, as confirmed by individual data collected in our surveys (Figure B) and publicly available mobility information (Figure E, available as a supplement to the online version of this article at http://www.ajph.org). Only 2 months after the pandemic had started in the State, the Controlled Distancing Model was launched, the state government was strongly engaged in our surveys, and donor funding was easy to obtain.
After a few months, however, the scenario started changing. First, the slow spread of the virus in the State in the first months generated a natural feeling of safety, despite warnings from scientists. Second, the pressure from businessmen and from the federal government to reopen the economy became stronger. Third, donor funding for new rounds of the statewide survey became difficult to obtain. Fourth, the government started allowing regions to appeal against the color-coded results of the algorithm—therefore, the model became reactive, instead of its original goal of being proactive. Particularly close to the 2020 elections, many city mayors started appealing against the results of the algorithm, always requesting the city to be reallocated to a less severe color so that they could adopt a more flexible model. Fifth, elections taking place in November were problematic, as many candidates did not follow public health guidelines. Because of a combination of these factors, most of the positive results found in our 8 surveys in terms of the slow spread of the virus in the State had been lost by the eighth round of data collection (Figure 1).
Conclusions
We observed a slow spread of SARS-CoV-2 between April and September 2020 in Rio Grande do Sul, differently from what was observed in other Brazilian states. Our research group was recently awarded funding to conduct rounds 9 and 10 of the statewide survey. Round 9 will take place in February 2021, and round 10 will be conducted in April 2021. As the vaccination of the Brazilian population has recently started, the April 2021 survey will likely find participants with antibodies generated by the vaccine, as well as individuals with antibodies from infection. Therefore, the 10th round of the study may serve as a population-based evaluation of the effectiveness of the vaccination campaign in the State.
ACKNOWLEDGMENTS
Funding for data collection was provided by Unimed Porto Alegre, Instituto Cultural Floresta, Instituto Serrapilheira, Banrisus, and Todos pela Saúde.
This work was started through the Data Committee created by the State of Rio Grande do Sul government to fight the COVID-19 pandemic. The tests used in the study have been provided by the Brazilian Ministry of Health.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest.
HUMAN PARTICIPANT PROTECTION
Ethical approval was obtained from the Brazilian National Ethics Committee (30415520.2.0000.5313), and written informed consent was obtained from all participants. A separate informed consent form was used to obtain permission from parents or legally authorized representatives for minors. Positive cases were reported to the statewide SARS-CoV-2 surveillance system.
Footnotes
See also Tereshchenko, p. 1387.
REFERENCES
- 1.Worldometer. 2020. https://www.worldometers.info/coronavirus
- 2.Hallal PC, Hartwig FP, Horta BL, et al. SARS-CoV-2 antibody prevalence in Brazil: results from two successive nationwide serological household surveys. Lancet Glob Health. 2020;8(11):e1390–e1398. doi: 10.1016/S2214-109X(20)30387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveira MF, Barros AJD, Horta BL, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26(8):1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 4.Hallal PC, Horta BL, Barros AJD, et al. Trends in the prevalence of COVID-19 infection in Rio Grande do Sul, Brazil: repeated serological surveys. Cien Saude Coletiva. 2020;25:2395–2401. doi: 10.1590/1413-81232020256.1.09632020. [DOI] [PubMed] [Google Scholar]
- 5.Pellanda LC, da Ros Wendland EM, McBride AJA, et al. 2020. . medRxiv. 10.1101/2020.05.06.20093476 [DOI]
- 6.Silveira MF, Mesenburg M, Dellagostin OA, et al. 2021. . SSRN. 10.2139/ssrn.3757411 [DOI]
- 7.Faes C, Abrams S, Van Beckhoven D, et al. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. Int J Environ Res Public Health. 2020;17(20):7560. doi: 10.3390/ijerph17207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. doi: 10.1080/01621459.1979.10481038. [DOI] [Google Scholar]
- 9.Diggle PJ. Estimating prevalence using an imperfect test. Epidemiol Res Int. 2011;2011:1–5. doi: 10.1155/2011/608719. [DOI] [Google Scholar]
- 10.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.R-project.org [Google Scholar]
- 11.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(8):1–19. doi: 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- 12.Lumley T. 2019. https://CRAN.R-project.org/package=survey
- 13.COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: epidemiology report 5 (reporting week ending 19:00 AEDT 29 February 2020) Commun Dis Intell. 2020;44:1–14. doi: 10.33321/cdi.2020.44.20. [DOI] [PubMed] [Google Scholar]
- 14.Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):1–6. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korean Society of Infectious Diseases, Korean Society of Pediatric Infectious Diseases, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-Associated Infection Control and Prevention, and Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seow J, Graham C, Merrick B, et al. 2020. . medRxiv. 10.1101/2020.07.09.20148429 [DOI]
- 19.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 20.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23‒May 12, 2020. JAMA Intern Med. 2020;180(12):1576–1586. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- 22.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 24.Russell TW, Hellewell J, Jarvis CI, et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill. 2020;25(12):2000256. doi: 10.2807/1560-7917.ES.2020.25.12.2000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Governo do Estado do Rio Grande do Sul. 2021. https://ti.saude.rs.gov.br/covid19
- 26.Khalatbari-Soltani S, Cumming RC, Delpierre C, et al. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J Epidemiol Community Health. 2020;74:620–623. doi: 10.1136/jech-2020-214297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banjar A, Al-Tawfiq JA, Alruwaily A, et al. Seroprevalence of antibodies to SARS-CoV-2 among blood donors in the early month of the pandemic in Saudi Arabia. Int J Infect Dis. 2021;104:452–457. doi: 10.1016/j.ijid.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan D, Sze S, Minhas JS, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health. 2020;8(8):e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


