Abstract
Neuropeptides are small proteinaceous molecules (3–100 amino acids) that are secreted by neurons and act on both neuronal and non-neuronal cells. Neuropeptide Y (NPY), a highly conserved and expressed neuropeptide in the central nervous system of mammals, plays a major role in stress response and resilience. Increasing evidence suggests that NPY and its receptors are altered in depression and suicide, pointing to their antidepressant-like nature. The objective of this study was to examine the role of NPY system in depression and suicidal behavior. Expression of NPY and its four receptors, NPY1R, NPY2R, NPY4R, and NPY5R was studied at the transcriptional and translational levels in the prefrontal cortex (PFC) and hippocampus regions of the postmortem brain of normal control (NC) (n=24) and depressed suicide (DS) (n=24) subjects. We observed a significant decrease in NPY mRNA and upregulation in NPY1R and NPY2R mRNA in both brain regions of DS subjects compared with NC subjects. We also observed a significant decrease in NPY protein expression in the PFC of subjects with DS. This study provides the first detailed evidence of alterations in the NPY system and the associated stress response in depression and suicidal behavior in humans. The outcomes of this study could be applied in the development of novel NPY system-targeted approaches for the treatment of depression.
Keywords: Suicide, Depression, Prefrontal Cortex, Hippocampus, Neuropeptide Y
1. Background
Maladaptive changes in the stress response system lead to the development of depression (Holsboer, 2000). Extensive alterations occur in neuroendocrine and hormonal cascades under chronic stress and depression, which further induce neuronal dendrite remodeling, impaired immune system, and memory deficits, leading to depression-like behaviors. Overactivation of the hypothalamic-pituitary-adrenal (HPA) axis has been observed in depression and other mood-related disorders. The HPA axis further regulates the expression of various amino acids and neuropeptide systems in depression (Morales-Medina et al., 2010; Morales-Medina et al., 2009). Neuropeptides are small proteinaceous molecules (3–100 amino acids) that are secreted by neurons and act on both neuronal and non-neuronal cells. More than 100 neuropeptides have been identified in the human brain, which can affect the physiology of neurons and modulate gene expression within seconds or for hours and days (Meriney and Fanselow, 2019; Salio et al., 2006). Corticotropin releasing factor (CRF), arginine vasopressin (AVP), oxytocin, neuropeptide Y (NPY), orexin, dynorphins, and cholecystokinin (CCK) are some of the neuropeptides studied by different research groups in association with depression and suicide (Serafini et al., 2013).
NPY, a 36 amino acid long peptide, is a widely distributed neuropeptide in the CNS and has been implicated in neurogenesis, neuroprotection, circadian rhythms, energy regulation, cognition, emotional processing, feeding behavior, and stress response (Serafini et al., 2013; Wu et al., 2011). Several reports have suggested alterations of NPY in depression and suicidal behavior and have implicated anxiolytic and antidepressant-like properties of NPY (Heilig et al., 1989; Kask et al., 2002; Olsson et al., 2004). A postmortem study of depressed suicide (DS) subjects reported reduced NPY-like immunoreactivity in the caudate nucleus and frontal cortex regions, whereas other studies indicated reduced NPY levels in the plasma and cerebrospinal fluid (CSF) of depressed subjects in contrast to normal subjects (Nilsson et al., 1996; Wahlestedt and Heilig, 1995; Widdowson et al., 1992). Studies based on the effects of antidepressants on NPY levels also provide strong evidence of NPY alterations in depression and suicide, such as treatment with standard antidepressants, such as desipramine and citalopram, which were reported to increase NPY levels in multiple brain regions of experimental animals (Heilig et al., 1988).
NPY signaling is mediated by class A or rhodopsin-like G-coupled receptors. There are five mammalian NPY receptors, NPY1R, NPY2R, NPY4R, NPY5R, and NPY6R; however, only four of them (NPY1R, NPY2R, NPY4R, and NPY5R) have been reported to be functional in humans (Morales-Medina et al., 2010; Pedragosa-Badia et al., 2013; Starback et al., 2000). In general, NPY receptors couple with [ISP CHK]Go or Gi receptors which result in the inhibition of adenylyl cyclase and thus suppress cAMP accumulation (Sah and Geracioti, 2013). However, NPY2R and NPY4R have also been reported to couple with Gq receptors, resulting in activation of PLCβ, thus enhancing IP3 production (Pedragosa-Badia et al., 2013). All four receptors are distributed in the brain, but there are variations in their region-specific expression.
In addition to NPY, there is ample evidence of altered expression of NPYRs in depression and suicide, as reported earlier. NPY receptor subtypes NPY1R, NPY2R, and NPY5R are reported to be functional in the limbic system and are implicated in chronic stress, anxiety, and depression-like behaviors (Caberlotto and Hurd, 2001). However, Y4R is mainly implicated in endocrine and feeding behavior regulation (Sajdyk, 2005). Central application of NPY1R and NPY5R agonists into the ventricles of animals via intracerebroventricular injection (i.c.v.) induced anxiolytic effects in acoustic startle and elevated plus maze experiments, but similar effects were not observed with NPY2R agonist administration (Broqua et al., 1995; Nakajima et al., 1998). These studies indicated the anxiolytic potential of NPY1R and NPY5R, whereas the NPY2R has anxiogenic activity (Nakajima et al., 1998; Sajdyk et al., 2002a; Sajdyk et al., 2002b). The NPY2R is also proposed to act synergistically with NPY1R in other aspects of stress, such as fear conditioning (Verma et al., 2012). NPY4R knockout (KO) studies suggest that receptor is implicated in emotional processes. There are limited clinical reports on the examination of NPY1R and NPY2R and their agonists in relation to depression and suicide. Further investigation of these receptors is needed to clearly understand their regulation in depression and suicide.
Major depressive disorder (MDD) is characterized by a state of depressed mood. It is a debilitating and heterogeneous form of mental illness that has a high socioeconomic effect (Webb et al., 2020). According to a report from the World Health Organization (WHO), 4.4 % of the world’s population is estimated to suffer from depression (WHO, 2017). Depression is also associated with other medical comorbidities and a high risk of suicide, which is the second leading cause of death among young adults worldwide. Although none of the elucidated mechanisms completely explains disease conditions in depression and its treatment requires a multicomponent approach, identification of different molecules as drug targets or targets for modification-based therapies will boost the pharmacological interventions in depression treatment.
The involvement of neuropeptides in the pathophysiology of depression and suicide has been investigated in the last decade, with increasing reports of their receptors being implicated in the stress response pathway (Millon et al., 2017). In the current study, we examined the role of NPY in depression and suicidal behavior. Alterations in NPY at transcriptional and translational levels were studied in specific regions (prefrontal cortex [PFC], hippocampus) of postmortem brains of subjects who died by suicide and normal controls who died by natural or other modes of death. To our knowledge, this is the first clinical study examining the expression of NPY4R and NPY5R in brain samples of DS human subjects. We performed all the experiments in the PFC and hippocampus regions (of postmortem brains), which are critically implicated in emotion and memory processing, stress response, and mood disorders (Alvina et al., 2021; Morales-Medina et al., 2010).
2. Methods
2.1. Subjects, diagnostic procedure, and criteria for inclusion/exclusion
The current study was performed on the PFC [Brodmann area (BA)9] and hippocampus (head region) from the left hemisphere of DS (n=24) and normal control (n=24) subjects. Postmortem brains were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center. The PFC and hippocampus tissue samples were dissected by a trained neuroanatomist at the Maryland Brain Collection. Tissues were collected only after a family member provided informed consent. All tissues from NC and DS subjects were grossly examined by experienced neuropathologists. Toxicology data were obtained by analyzing urine and blood samples. All procedures were approved by the University of Maryland Institutional Review Board (IRB) and the University of Illinois at the Chicago IRB.
At least one family member of the deceased provided a detailed interview. The interviews were conducted by a trained psychiatrist, and brain tissue from all subjects was thoroughly examined by experienced neuropathologists. Blood and urine samples were used to assess toxicology details.
Structured Clinical Interview for DSM-IV criteria were used to perform diagnoses (First et al., 1997). For the same, the detailed interviews of family members, toxicology data, medical records of the case, and other records from the office of medical examiner were analyzed by two psychiatrists, and their consensus report was considered as the final diagnosis. Individuals diagnosed with MDD who committed suicide were included in the DS group. A consensus diagnostic report ensured that no NC subjects were diagnosed with any mental illness. Subjects with a history of substance abuse, family history of psychiatric illness, accidental deaths, or death after prolonged hospitalization were excluded from the NC group. Additionally, none of the subjects with any other mental illness, major history of medical or neurological disorders, and HIV were included in any of the study groups. All subjects were considered to have no sexual or ethnic differentiation. Detailed characteristics of the subjects are shown in Supplementary Table 1.
2.2. mRNA expression studies
RNA extraction and quantification were performed as described by Pandey et al. (2019), and total RNA was extracted using TRIzol (Invitrogen, Life Technologies, MA) method followed by DNase treatment. After quantification and purity assessment using NanoDrop ND-1000 (NanoDrop Technologies, DE) and Agilent Bioanalyzer 2100 (Agilent Technologies, CA), 1 μg of RNA from each sample was reverse transcribed to obtain cDNA in a GeneAmp PCR system (Applied Biosystems, CA) using random hexamers (50 ng), RNase out (10 units), and M-MLV reverse transcriptase enzyme (200 units) (Invitrogen) according to the manufacturer’s instructions (described in Pandey et al., 2019). The cDNA was diluted 1:10 using DEPC-treated water (Ambion, Thermo Fisher Scientific, TX, USA) and stored at 4 °C for further analysis. All RNA samples considered for cDNA preparation had values of 1.8–2.0 for 260/280 nm and ≥5.5, respectively.
Further diluted cDNA was used for quantitative PCR (qPCR analysis) using the MX3005p sequence detection system from Agilent. Taqman primers for NPY and its receptors for gene expression assays (Applied Biosystems) are listed in Table 1. GAPDH and β-actin were used as housekeeping genes as suitable control genes for our current data set [described in (Pandey et al., 2019)] after extensive testing. To eliminate non-specific amplification events, no template and no MMLV (reverse transcriptase) enzyme controls were included in each plate. The mRNA expression of each isozyme was normalized to the geometric mean of housekeeping genes and analyzed using Livak’s method (2−ΔΔCt) with respect to control samples.
Table 1:
TaqMan primers/probes used for real-time polymerase chain reaction analysis
| Taqman accession | Probe Location (exon boundary) | Assay Function | |
|---|---|---|---|
| ACTB | Hs99999903_m1 | 1-1 | House Keeping |
| GAPDH | Hs99999905_m1 | 3-3 | House Keeping |
| NPY | Hs00173470_m1 | 3-4 | target gene |
| NPY1R | Hs00702150_s1 | 3-3 | target gene |
| NPY2R | Hs01921296_s1 | 2-2 | target gene |
| NPY4R | Hs00275980_s1 | 2-2 | target gene |
| NPY5R | Hs00178914_m1 | 4-4 | target gene |
2.3. Protein expression studies
2.3.1. Total protein
Cytoplasmic protein was isolated from 100 mg tissue of the PFC region, and changes in protein expression were studied using a previously described protocol (Pandey et al., 1999). Briefly, 30 μg of cytoplasmic protein from each sample was electrophoresed on a 7.5% (w/v) agarose gel, followed by electrophoretic transfer of separated protein bands on a nitrocellulose membrane (Amersham, IL). The membrane was blocked with TBST buffer containing 5% skimmed milk, followed by monoclonal anti-(NPY 1R and 2R) primary antibody (1:3000) overnight at 4 °C. The next day, the blots were washed with Tris-buffered saline with 0.1% Tween® 20 detergent (TBST) and incubated with HRP-conjugated secondary antibody (1:5000). Detection of proteins of interest was carried out by exposing the blot to enhanced chemiluminescence film. Each membrane was then incubated in a stripping solution (Chemicon International, CA) and re-probed for the detection of β-actin. The autoradiogram was captured, and the optical density of the bands was analyzed using the Loats Image Analysis System (Westminster, MD). Each protein was normalized to the housekeeping gene β-actin, and results were plotted as relative protein expression [in terms of percentage change (PC)] in comparison to the control.
2.3.2. ELISA
NPY levels were determined in aliquots of the cytosolic fraction by enzyme-linked immunosorbent assay (ELISA) using commercially available Quantakine® kits for human NPY purchased from R&D Systems, Minneapolis, MN.
2.4. Statistical analysis and effect of confounding variables
The data obtained in the current study were analyzed using IBM SPSS Statistics software version 25 (IBM Tech, NY, USA). This analysis was individually performed in the PFC regions of the brain for both mRNA and protein to determine the difference in the levels of NPY and its receptors between the DS and NC subjects. Means of demographic parameters—age, brain pH, and postmortem time interval (PMI)—were compared using one-way ANOVA with Bonferroni post-hoc test. To study the overall effect in multiple comparisons, both groups were analyzed jointly in the MANCOVA analysis, adjusting for the effects of confounding variables such as age, gender, PMI, antidepressants, and brain pH as covariates. Multiple and paired comparisons were performed using the Bonferroni post-hoc test. The p-values ≤0.05 were considered statistically significant. We also performed false discovery rate (FDR) and Pearson’s correlation, and the values are reported in Table 2A and Table 2B, respectively.
Table 2 A:
Uncorrected and False Discovery Rate corrected p Values for mRNA expression
| PFC | HIPPO | |||
|---|---|---|---|---|
| Raw | Corrected | Raw | Corrected | |
| NPY | 0.002 | 0.01 | 0.001 | 0.01 |
| NPY1R | 0.008 | 0.02 | 0.004 | 0.01 |
| NPY2R | 0.008 | 0.01 | 0.007 | 0.01 |
| NPY4R | 0.064 | 0.08 | 0.223 | 0.28 |
| NPY5R | 0.691 | 0.69 | 0.452 | 0.45 |
Table 2B:
Pearson’s correlation and ANOVA
| NPY Vs NPYRs (Pearson’s correlation) (mRNA) | ||||
| PFC | NPY/NPY1R | NPY/NPY2R | NPY/NPY4R | NPY/NPY5R |
| NC | −0.069 | 0.361 | 0.124 | −0.091 |
| DS | −0.359 | −0.384 | −0.283 | 0.072 |
| HIPPO | NPY/NPY1R | NPY/NPY2R | NPY/NPY4R | NPY/NPY5R |
| NC | −0.141 | −0.242 | −0.275 | 0.128 |
| DS | 0.005 | 0.25 | 0.073 | 0.084 |
| NPY Vs NPYRs (Pearson’s correlation) (Protein) | ||||
| PFC | NPY/NPY1R | NPY/NPY2R | NPY/NPY4R | NPY/NPY5R |
| NC | 0.026 | 0.037 | 0.259 | *0.506 |
| DS | 0.084 | −0.202 | 0.225 | −0.362 |
| mRNA Vs Protein (Pearson’s correlation) | |||||
| PFC | NPY/NPY | NPY1R/NPY1R | NPY2R/NPY2R | NPY4R/NPY4R | NPY5R/NPY5R |
| NC | −0.229 | 0.181 | 0.122 | 0.048 | −0.324 |
| DS | −0.14 | 0.155 | *−0.453 | −0.142 | −0.169 |
| ANOVA for antidepressants (DS group) (p values) | |||||
| mRNA | NPY | NPY1R | NPY2R | NPY4R | NPY5R |
| PFC | 0.960 | 0.576 | 0.775 | 0.415 | 0.509 |
| HIPPO | 0.427 | 0.136 | 0.792 | *0.005 | 0.714 |
| Protein | NPY | NPY1R | NPY2R | NPY4R | NPY5R |
| PFC | 0.453 | 0.354 | 0.074 | 0.374 | 0.075 |
3. Results
The study was conducted on postmortem brain samples of 24 NC and 24 DS subjects from the adult Maryland population irrespective of origin, race, and sex discrimination. The demographic characteristics of the subjects together with PMI (in hours), brain pH and RNA integrity number (RIN) are summarized in Table 3, and detailed characteristics of the subjects are shown in Supplementary Table 1. In addition to variables such as age, sex, race, PMI, and brain pH, Supplementary Table 1 also includes the suicide method and the presence of antidepressants or ethanol at the time of death for each DS subject.
Table 3.
Demographic characteristics of subjects
| Parameter | Normal Controls | Depressed Suicide |
|---|---|---|
| Subjects (N) | 24 | 24 |
| Age | 42.08 ± 15.35 | 38.95 ± 15.39 |
| Race | 7 B, 17W | 2 B, 22W |
| Gender | 20 M, 4F | 14 M, 10 F |
| PMI (hours) | 16.54 ± 6.4 | 18.91 ± 6.02 |
| Brain pH | 7.01 ± 0.15 | 6.96 ± 0.25 |
| RIN | 6.8 ± 1.2 | 7.6 ± 1.4 |
N, number, B, black, W, white; M, male, F, female, RIN, RNA integrity number
Values are presented as means ± SD
3.1. mRNA expression studies
3.1.1. mRNA levels of NPY and its receptors in PFC of DS and NC subjects
To study the possible alterations associated with depression and suicide in NPY and its receptors at the transcriptional level, we examined the mRNA levels in the PFC of DS subjects and compared them to those of the NC subjects (Figure 1). A generalized linear model using multivariate analysis showed that NPY [F(4,44)=2.889, p=0.035], NPY1R [F(4,44)=2.597, p=0.051], and NPY2R [F(4,44)=3.037, p=0.028] mRNA levels differed significantly between the groups; however, the mRNA levels of NPY4R and NPY5R were not significantly different between the DS and NC subjects.
Figure 1: mRNA expression of neuropeptide Y (NPY) and its receptors NPY1R, NPY2R, NPY4R and NPY5R in the PFC.
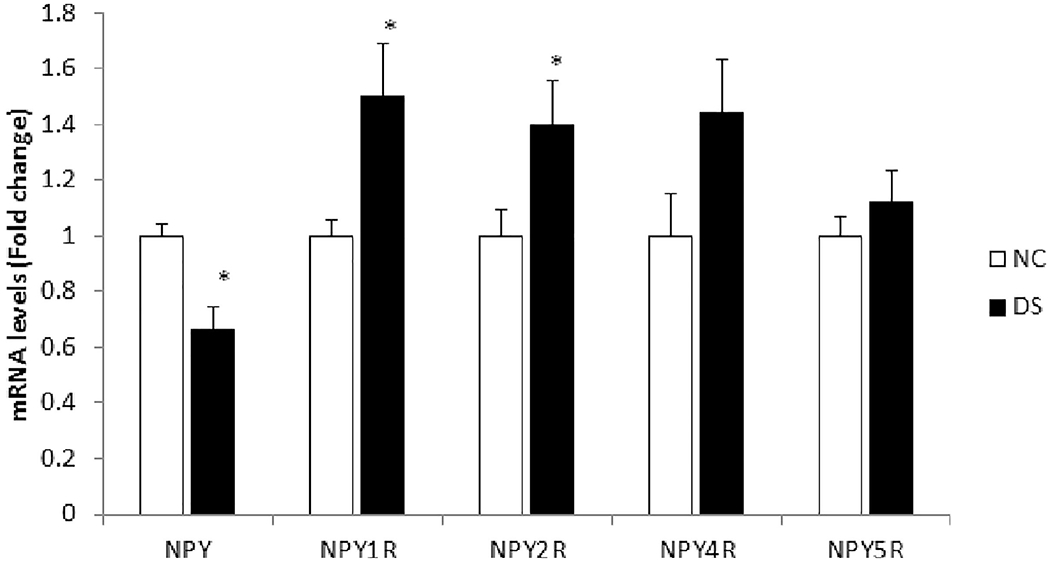
The histogram represents fold change in mRNA expression in the prefrontal cortex (PFC) of normal control (NC) and depressed suicide (DS) subjects. Values are represented as mean fold change ± SEM.
(*) represents a statistically significant difference (P≤0.05).
Univariate analysis using Bonferroni post-hoc test revealed that NPY mRNA was significantly decreased (p=0.002) in the PFC of DS subjects as compared to NC subjects. Univariate comparisons for NPYRs between both groups showed significant increases in the mRNA levels of NPY1R (p=0.008) and NPY2R (p=0.008) in DS subjects. NPY4R and NPY5R also showed a trend of increased expression in the DS subjects as compared to NC subjects, but the changes observed were not significant. Changes in NPY (p=0.01), NPY1R (p=0.02), and NPY2R (p=0.01) mRNA also passed the level of significance after FDR analysis (Table 2A).
3.1.2. mRNA levels of NPY and its receptors in the hippocampus of DS and NC subjects
We further studied the mRNA levels of NPY and its receptors in the hippocampus of DS and NC subjects and found similar results to those observed in the PFC (Figure 2). In the generalized linear model, multivariate analysis showed that NPY [F(4,42)=3.736, p=0.012], NPY1R [F(4,42)=2.895, p=0.035], and NPY2R [F(4,42)=3.037, p=0.017] mRNA levels were significantly different in both groups; however, the mRNA levels of NPY4R and NPY5R were not significantly different between the DS and NC groups.
Figure 2: mRNA expression of NPY and its receptors NPY1R, NPY2R, NPY4R, and NPY5R in the hippocampus.
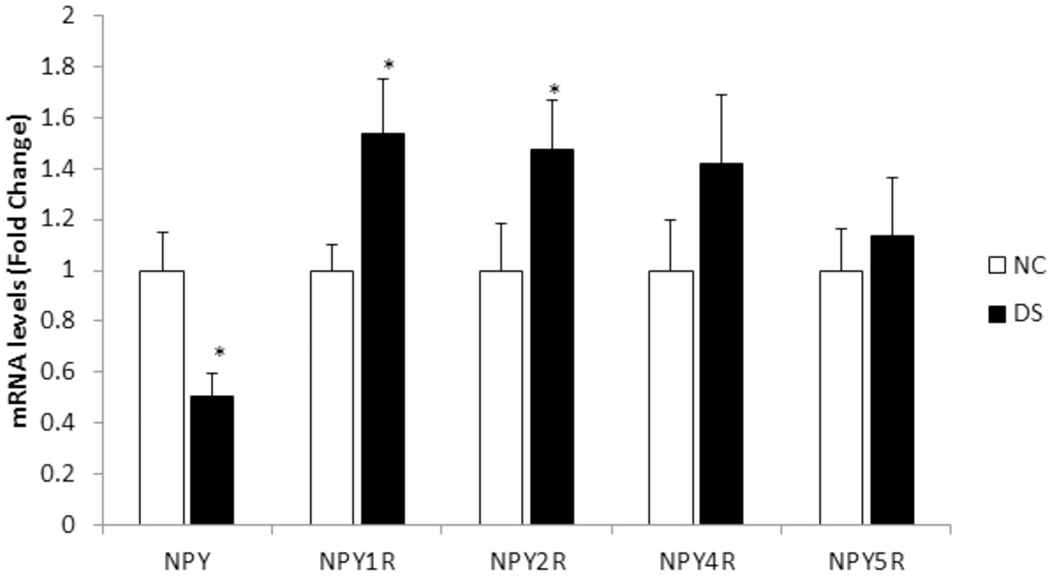
The histogram represents fold change in the mRNA expression in the hippocampus of NC and DS subjects. Values are represented as mean fold change ± SEM.
(*) represents a statistically significant difference (P≤0.05).
Univariate analysis using Bonferroni post-hoc test showed that NPY mRNA was significantly decreased (p=0.001) in the hippocampus region of DS subjects as compared to NC subjects. Univariate comparisons for NPYRs between both groups showed significant increases in the mRNA levels of NPY1R (p=0.004) and NPY2R (p=0.007) in DS subjects. As we observed in the PFC, NPY4R and NPY5R in the hippocampus also showed a trend of increased expression in DS subjects as compared to NC subjects, but the changes observed were not significant. Changes in NPY (p=0.01), NPY1R (p=0.01), and NPY2R (p=0.01) mRNA also passed the level of significance after FDR analysis (Table 2A).
3.2. Protein expression studies
To study the depression- and suicide-associated changes at the translational level, we studied the protein expression of NPY in the PFC of DS and NC subjects (Figure 3a). Since we observed changes in NPY1R and NPY2R at the mRNA level, we also examined the protein levels of these two receptors in the PFC region of DS and NC subjects. Representative blots of NPY1R and NPY2R from two NC and two DS subjects are shown in Figure 3b. Please note that the blots for NPY could not be shown because NPY was analyzed by ELISA. The mean protein levels of NPY1R and NPY2R in the DS and NC groups are shown in Figure 3c. Statistical analysis using post-hoc t-test showed significant differences in protein expression of NPY (p=0.0004) among PFC of DS and NC subjects; however, no significant changes were observed in protein levels of either NPY1R or NPY2R.
Figure 3: Protein expression of NPY and two of its receptors, NPY1R and NPY2R, in the PFC.
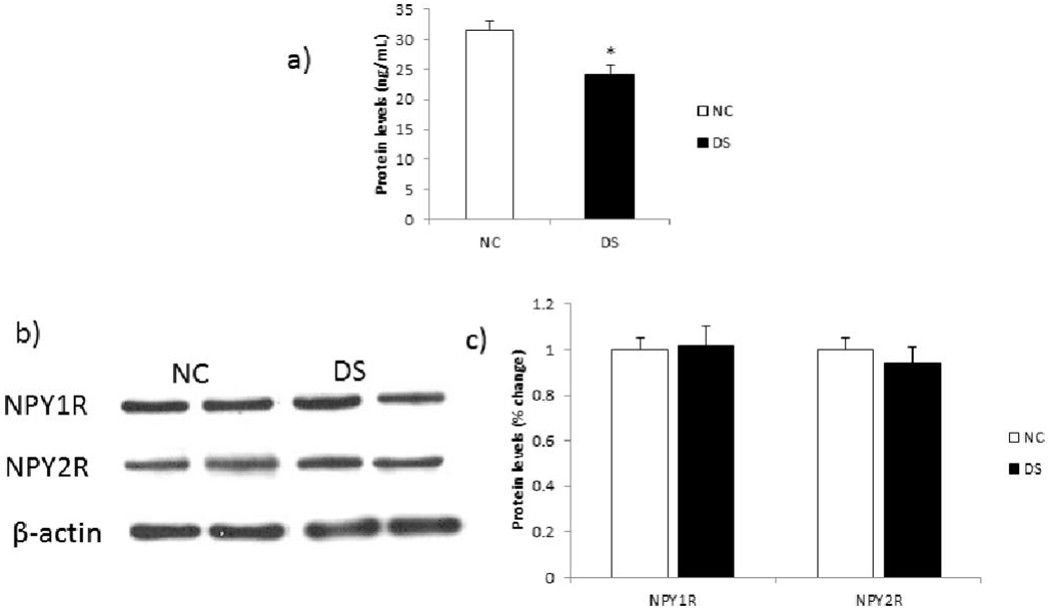
(a) Histogram showing protein expression of NPY (measured by ELISA) in the PFC of DS and NC subjects. Values represent mean percent change ± SEM. (*) represents a statistically significant difference (P≤0.05).
(b) Representative blots of two NPY receptors (NPY1R and NPY2R) and β-actin in the PFC of two NC and two DS subjects.
(c) Histogram showing corresponding percentage change in protein expression of these NPY receptors in the PFC of NC and DS subjects. Values represent mean percent change ± SEM.
3.3. Effect of confounding variables and correlations
To study the effect of confounding variables such as age, brain pH, gender, antidepressants, and PMI, we included those as covariates in the generalized linear model, and multivariate analysis showed that these confounding variables had no significant effect on the mRNA expression either in the PFC or hippocampus of DS and NC subjects, except for the effect of antidepressants on NPY4R in hippocampus. Similarly, these variables had no significant effect on the protein expression of NPY and its receptors in the PFC region of either group, except for the effect of gender on NPY5R.
Since some of the subjects (n=12) in the DS group were on antidepressants, we performed one-way ANOVA to analyze the difference in mRNA expression in DS subjects with and without antidepressants. We observed no significant difference (p>0.05) between these two groups in the PFC or hippocampus regions for NPY and its receptors, except for the expression of NPY4R in the hippocampus (p<0.05), suggesting that most of the measures we studied are not influenced by the presence of antidepressants in the blood of some of the DS subjects at the time of their death.
Further, we determined the Pearson’s correlation between NPY and NPY receptors (for both mRNA and protein expression), as well as mRNA vs. protein expression (NPY and its receptors). The results of the Pearson’s correlation are given in Table 2B. No correlations of NPY vs NPYRs were significant in case of mRNA in PFC and hippocampus. Similarly, for protein no correlations were significant except NPY vs NPY5R. In the correlations of mRNA and protein, only NPY2R vs NPY2R was significant.
4. Discussion
In the current study, we examined whether depression and suicidal behavior are associated with alterations in the expression of NPY and its four receptors NPY1R, NPY2R, NPY4R, and NPY5R in the PFC and hippocampal regions in postmortem brains of adult NC and DS subjects. We observed a significant decrease in NPY protein and mRNA expression in both PFC and hippocampus of DS subjects compared with NC subjects, suggesting that this decrease is associated with depression and suicide. Significant increases were also observed for NPY receptors NPY1R and NPY2R at the mRNA level, whereas other receptors were unchanged. At the protein level, NPY receptors showed no significant changes in the DS group subjects compared to the NC group subjects.
Our observation of reduced NPY levels is consistent with other reports associated with stress, depression, and suicide. Disrupted levels of NPY in various brain regions have been reported in different studies based on animal models of depression and clinical studies; for example, reduced NPY expression has been observed in rat brains of different animal models (Caberlotto et al., 1999; Jimenez-Vasquez et al., 2001; Miragaia et al., 2018). Reduced NPY levels have also been associated with anxiety, which is one of the major characteristics of depression-like behavior indirectly due to loss of NPY+ cells and reduced NPY signaling in GAD65 KO mice (Qi et al., 2018). Furthermore, reduced levels of NPY were found in different studies of plasma and CSF in patients with major depression and suicide attempts than in healthy subjects (Hashimoto et al., 1996; Heilig et al., 2004; Hou et al., 2006). Lower NPY expression was also observed in the frontal cortex and CSF of patients with bipolar disorder (Kuromitsu et al., 2001; Sandberg et al., 2014). Our observation of significantly reduced NPY mRNA as well as protein expression in the PFC and hippocampus regions of DS subjects is consistent with these reported studies.
Some studies have also reported no significant alterations or contrasting observations of NPY levels in depression-related behavior. Soleimani et al. (2014) found increased NPY levels in the CSF of MDD patients and suggested it as a compensatory mechanism, a functional but insufficient NPY stress response system to inhibit the development of depression in such individuals. In a recent study, increased plasma levels of NPY were observed in suicide subjects with comorbid psychiatric illness or stressful life events compared to matched healthy control subjects.
The inconsistency between our findings of decreased NPY in the postmortem brain (PFC and hippocampus) of DS subjects and those who find increased levels of NPY in plasma and CSF of depressed or suicidal patients may be attributed to several factors. Some possible factors that may explain these differences are: (1) the CSF studies were conducted in living patients as opposed to the postmortem brain from deceased suicide subjects as in our study; (2) May be related to the diagnosis, such as depression and suicide; (3) or a more severe form of depression; (4) or to the presence and prior treatment with antidepressants, as in the case of DS subjects. Some of the DS subjects were on antidepressant treatment at the time of death, or severity of depression.
Thus, besides NPY, CRF is another neuropeptide involved in stress response. Both NPY and CRF are co-localized in different areas like the paraventricular nucleus (PVN) in the hypothalamus or different amygdala nuclei (Sajdyk et al., 2004), but no clear molecular mechanism/pathway has been described showing the NPY-CRF interaction.
Anti-stress, anxiolytic, and other effects of NPY are mediated by its receptors. Pre-clinical evidence suggests that Y1R is widely expressed in areas associated with memory processing and emotional regulation (Dumont et al., 2000). Y1R exhibits the highest affinity for NPY and its agonists, such as [Leu31, Pro34] PYY and [Leu31, Pro34] NPY and mediates the anti-stress, anxiolytic, and antidepressant effects of NPY (Dumont et al., 2000). The involvement of the NPY1R receptor in the anti-stress activity of NPY has been suggested by various genetic and pharmacological approaches based on pre-clinical studies in naive animals with no previous exposure to stress (Nwokafor et al., 2020). The anxiolytic effect of NPY has also been shown to be mediated by Y1R in various studies (Heilig et al., 2004; Nwokafor et al., 2020; Sajdyk et al., 2002a); however, Sorensen et al. (2004) suggested the involvement of two other receptors, NPY2R and NPY5R. In a study by Caberlotto et al. (1999), reduced NPY mRNA levels were followed by increased NPY1R receptor binding sites in the hippocampus of Flinders Sensitive Line (FSL) rats, which could be a compensatory mechanism to altered NPY levels in stress (Caberlotto et al., 1999). Gene KO studies of NPY1R receptor showed different outcomes, such that NPY1R KO female mice exhibiting normal activity in the forced swim test (FST) but reduced freezing time in tail suspension test as compared to wild type mice (Painsipp et al., 2010). In contrast, Karlsson et al. (2008) showed that NPY1R KO results in depression-like behavior in the FST in both male and female mice. As the anti-stress and antidepressant-like activity of NPY is associated with its upregulated expression, deletion or suppression of NPY2R receptors has been proposed as an indirect method to increase NPY levels and enhance anxiolytic and antidepressant-like effects (Morales-Medina et al., 2010; Redrobe et al., 2003).
NPY expression was found to be increased after standard antidepressant treatments such as citalopram, quetiapine, electroconvulsive therapy etc. in CSF of preclinical samples and increased NPY expression in transgenic rodents was linked to reduced depression and anxiety like behavior (Mathe et al., 2020; Mathe et al., 1995; Nikisch et al., 2012; Thorsell et al., 2000). These studies suggest upregulation of NPY and modulation of receptors as the common mechanism for all the anti-depressant drugs (Mathe et al., 2020; Wu et al., 2011).
Only a few clinical studies have examined the role of NPY receptors NPY1R and NPY2R in postmortem brains in association with depression and stress. In a study by Caberlotto and Hurd (2001), individuals who died by committing suicide were found to have higher NPY1R expression in young and adult individuals; however, a negative correlation of NPY1R expression was observed with increasing age. This study also found an increase in NPY2R mRNA in the PFC of suicide victims (Caberlotto and Hurd, 2001).
NPY also plays an important role in mood stabilization and depression treatment, as suggested by an electrochemical study explaining the modulation of neurotransmitter release by NPY and NPY1R agonists in a manner similar to standard antidepressants (Crespi, 2011; Heilig et al., 1988; Husum and Mathe, 2002). NPY, a NPY1R receptor agonist, and antidepressants block the reuptake of noradrenaline and serotonin, making it available for release and receptor stimulation, thus alleviating depression (Crespi, 2011). Therefore, increased expression of the NPY1R in the current study can be explained as a compensatory mechanism against reduced NPY levels and the body’s attempt to cope with accumulating depressive conditions. An increase in NPY2R mRNA expression indicates increased anxiety and stress, as supported by previous reports; however, its correlation with the body’s compensatory mechanism is not clear.
Augmentation of the anxiolytic effect was observed in a double KO study (NPY2R and NPY4R KO) conducted by Tasan et al. (2009) suggesting a positive correlation between NPY2R and NPY4R and indicating the anxiogenic property of this receptor. Since pancreatic polypeptides (PPs) are produced in the peripheral system, either PPs breaching the blood-brain barrier or 100 times less potent NPY, which is present in the brain, are responsible for the anxiogenic properties of this receptor; however, the unavailability of any non-peptide antagonist of the NPY4R receptor hampers the elucidation of its clear implication in stress and depression.
The NPY5R is also known as a feeding receptor that regulates appetite; however, the role of NPY5R in stress and anxiety is controversial (Morales-Medina et al., 2010; Reichmann and Holzer, 2016). Kask et al. (2001) observed no changes in anxiety or stress levels exhibited by rodents treated with NPY5R antagonist in elevated plus maze (EPM) or social interaction tests; however, other studies suggested NPY5R to be implicated in anxiety- and mood-related behaviors in preclinical models; for example, Sorenson et al. (2004) and Sajdyk et al. (2002a) observed anxiolytic effects of different NPY5R receptor agonist after i.c.v. administration to experimental animals.
The HPA axis is the major stress response pathway, which is implicated in the pathophysiology of depression and suicide. HPA axis deregulation in mood disorders has been well established by various studies based on measuring altered levels of CRF, ACTH, cortisol, and corticosterone. Enhanced levels of cortisol in the CSF and blood plasma of depressed patients indicate hyperactivation of the HPA axis. Similarly, a recent study by our group, which examined the expression of CRF, its receptors, and binding factors in specific regions of postmortem brains of teenage suicide subjects, also suggested hyperactivation of the HPA axis by depression and suicidality (Pandey et al., 2013). NPY also plays a pivotal role in stress response and adaptation mechanisms. It interacts with the HPA axis, counteracting the effects of increased CRF, resulting in the neutralization of stress (Yang et al., 2018). Thus, the lower NPY levels, as observed in the current study and previous reports, are obviously associated with persistent stress and maladaptive changes. Administration of a NPY1R receptor antagonist to Sprague-Dawley rats in a chronic restraint stress model abolished the stress adaptation mechanism of NPY and NPY1R, accompanied by increased CRF and corticosterone levels in the serum (Yang et al., 2018). Based on a thorough literature review, Ehlers et al. (1997) suggested that a balanced expression of anxiolytic-NPY and anxiogenic-CRF neuropeptides exerts a major influence on stress response and related behavioral alterations. We have provided a summary of pre-clinical and clinical studies (Table 4).
Table 4:
Summary of pre-clinical and clinical studies
| Pre-clinical studies | Clinical studies |
|---|---|
| Early life stress has long term effect on NPY levels and vulnerability to depression and stress (Husum and Mathe, 2002) | Insuflated NPY as antidepressant, a randomized controlled trial in MDD patients (Mathe et al., 2020) |
| Anxiolytic effect of intracerebroventircularly administered NPY in brain of rats of anxiety models (Heilig et al., 1989) | Intranasal administration of NPY as a novel treatment approach for the patients of PTSD (Sayed et al., 2018) |
| Anxiolytic effect of NPY is mediated through NPY1R (Broqua et al., 1995; Nakajima et al., 1998; Nwokafor et al., 2020; Sajdyk et al., 2002a) | Lower NPY levels in depressed patients which increased upon treatment with anti-depressants (Ozsoy et al., 2016) |
| NPY1R receptor antagonist treatment to Sprague-Dawley rats of chronic restraint stress model abolished the adaptation to stress (Yang et al., 2018) | Increased expression of NPY1 & 2R in postmortem brains of suicide victims (Caberlotto and Hurd, 2001) |
| NPY1R knockout induced anxiety and depression like behavior in FST rats (Karlsson et al., 2008) | Increased NPY levels in CSF and plasma of patients having MDD and other psychiatric illness (Lu et al., 2019; Soleimani et al., 2014) |
| Deletion/suppression of NPY2R is correlated with increased NPY levels and enhanced anxiolytic /anti-depressant like effect (Morales-Medina et al., 2010; Redrobe et al., 2003) | Reduced NPY levels in different brain regions of bipolar disorder patients (Kuromitsu et al., 2001; Sandberg et al., 2014) |
| Anxiolytic function of NPY1R and NPY5R, anxiogenic effect of NPY5R (Sajdyk et al., 2002a; Sajdyk et al., 2002b) | Higher CSF NPY levels in baseline depressed patients (Martinez et al., 2012) |
| Altered NPY levels in brain of different animal models of depression such as Flinders sensitive rats, maternal separation as genetic model of depression (Caberlotto et al., 1999; Jimenez-Vasquez et al., 2001; Miragaia et al., 2018) | Region specific NPY reduction in brain and reduced NPY levels in plasma and CSF of patients with history of depression and suicide attempts (Hashimoto et al., 1996; Heilig et al., 2004; Hou et al., 2006; Nilsson et al., 1996; Wahlestedt and Heilig, 1995; Widdowson et al., 1992) |
| NPY levels play important role in stress and depression treatment (Crespi, 2011) | CSF NPY reduced with anti-depressant treatment (Olsson et al., 2004) |
| Antidepressants desipramine and citalopram induced increase in NPY levels in brain of experimental animals (Heilig et al., 1988) | Decreased NPY levels correlated with suicide attempts (Westrin et al., 1999; Westrin et al., 1998; Widdowson et al., 1992) |
| No changes in anxiety or stress levels upon treatment with NPY5R antagonist (Kask et al., 2001) |
References for the Table 4:
Broqua, P., Wettstein, J.G., Rocher, M.N., Gauthier-Martin, B., Junien, J.L., 1995. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol 6(3), 215-222.
Caberlotto, L., Hurd, Y.L., 2001. Neuropeptide Y Y(1) and Y(2) receptor mRNA expression in the prefrontal cortex of psychiatric subjects. Relationship of Y(2) subtype to suicidal behavior. Neuropsychopharmacology 25(1), 91-97.
Caberlotto, L., Jimenez, P., Overstreet, D.H., Hurd, Y.L., Mathe, A.A., Fuxe, K., 1999. Alterations in neuropeptide Y levels and Y1 binding sites in the Flinders Sensitive Line rats, a genetic animal model of depression. Neurosci Lett 265(3), 191-194.
Crespi, F., 2011. Influence of Neuropeptide Y and antidepressants upon cerebral monoamines involved in depression: an in vivo electrochemical study. Brain Res 1407, 27-37.
Hashimoto, H., Onishi, H., Koide, S., Kai, T., Yamagami, S., 1996. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci Lett 216(1), 57-60.
Heilig, M., Soderpalm, B., Engel, J.A., Widerlov, E., 1989. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 98(4), 524-529.
Heilig, M., Wahlestedt, C., Ekman, R., Widerlov, E., 1988. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur J Pharmacol 147(3), 465-467.
Heilig, M., Zachrisson, O., Thorsell, A., Ehnvall, A., Mottagui-Tabar, S., Sjogren, M., Asberg, M., Ekman, R., Wahlestedt, C., Agren, H., 2004. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res 38(2), 113-121.
Hou, C., Jia, F., Liu, Y., Li, L., 2006. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res 1095(1), 154-158.
Husum, H., Mathe, A.A., 2002. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology 27(5), 756-764.
Jimenez-Vasquez, P.A., Mathe, A.A., Thomas, J.D., Riley, E.P., Ehlers, C.L., 2001. Early maternal separation alters neuropeptide Y concentrations in selected brain regions in adult rats. Brain Res Dev Brain Res 131(1-2), 149-152.
Karlsson, R.M., Choe, J.S., Cameron, H.A., Thorsell, A., Crawley, J.N., Holmes, A., Heilig, M., 2008. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 195(4), 547-557.
Kask, A., Vasar, E., Heidmets, L.T., Allikmets, L., Wikberg, J.E., 2001. Neuropeptide Y Y(5) receptor antagonist CGP71683A: the effects on food intake and anxiety-related behavior in the rat. Eur J Pharmacol 414(2-3), 215-224.
Kuromitsu, J., Yokoi, A., Kawai, T., Nagasu, T., Aizawa, T., Haga, S., Ikeda, K., 2001. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns 1(1), 17-21.
Lu, J., Li, S., Li, H., Mou, T., Zhou, L., Huang, B., Huang, M., Xu, Y., 2019. Changes In Plasma NPY, IL-1beta And Hypocretin In People Who Died By Suicide. Neuropsychiatr Dis Treat 15, 2893-2900.
Martinez, J.M., Garakani, A., Yehuda, R., Gorman, J.M., 2012. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety 29(1), 32-38.
Mathe, A.A., Michaneck, M., Berg, E., Charney, D.S., Murrough, J.W., 2020. A Randomized Controlled Trial of Intranasal Neuropeptide Y in Patients With Major Depressive Disorder. Int J Neuropsychopharmacol 23(12), 783-790.
Miragaia, A.S., de Oliveira Wertheimer, G.S., Consoli, A.C., Cabbia, R., Longo, B.M., Girardi, C.E.N., Suchecki, D., 2018. Maternal Deprivation Increases Anxiety- and Depressive-Like Behaviors in an Age-Dependent Fashion and Reduces Neuropeptide Y Expression in the Amygdala and Hippocampus of Male and Female Young Adult Rats. Front Behav Neurosci 12, 159.
Morales-Medina, J.C., Dumont, Y., Quirion, R., 2010. A possible role of neuropeptide Y in depression and stress. Brain Res 1314, 194-205.
Nakajima, M., Inui, A., Asakawa, A., Momose, K., Ueno, N., Teranishi, A., Baba, S., Kasuga, M., 1998. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides 19(2), 359-363.
Nilsson, C., Karlsson, G., Blennow, K., Heilig, M., Ekman, R., 1996. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides 17(3), 359-362.
Nwokafor, C., Serova, L.I., Nahvi, R.J., McCloskey, J., Sabban, E.L., 2020. Activation of NPY receptor subtype 1 by [D-His(26)]NPY is sufficient to prevent development of anxiety and depressive like effects in the single prolonged stress rodent model of PTSD. Neuropeptides 80, 102001.
Olsson, A., Regnell, G., Traskman-Bendz, L., Ekman, R., Westrin, A., 2004. Cerebrospinal neuropeptide Y and substance P in suicide attempters during long-term antidepressant treatment. Eur Neuropsychopharmacol 14(6), 479-485.
Ozsoy, S., Olguner Eker, O., Abdulrezzak, U., 2016. The Effects of Antidepressants on Neuropeptide Y in Patients with Depression and Anxiety. Pharmacopsychiatry 49(1), 26-31.
Redrobe, J.P., Dumont, Y., Herzog, H., Quirion, R., 2003. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res 141(2), 251-255.
Sajdyk, T.J., Schober, D.A., Gehlert, D.R., 2002a. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology 43(7), 1165-1172.
Sajdyk, T.J., Schober, D.A., Smiley, D.L., Gehlert, D.R., 2002b. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav 71(3), 419-423.
Sandberg, J.V., Jakobsson, J., Palsson, E., Landen, M., Mathe, A.A., 2014. Low neuropeptide Y in cerebrospinal fluid in bipolar patients is associated with previous and prospective suicide attempts. Eur Neuropsychopharmacol 24(12), 1907-1915.
Sayed, S., Van Dam, N.T., Horn, S.R., Kautz, M.M., Parides, M., Costi, S., Collins, K.A., Iacoviello, B., Iosifescu, D.V., Mathe, A.A., Southwick, S.M., Feder, A., Charney, D.S., Murrough, J.W., 2018. A Randomized Dose-Ranging Study of Neuropeptide Y in Patients with Posttraumatic Stress Disorder. Int J Neuropsychopharmacol 21(1), 3-11.
Soleimani, L., Oquendo, M.A., Sullivan, G.M., Mathe, A.A., Mann, J.J., 2014. Cerebrospinal fluid neuropeptide Y levels in major depression and reported childhood trauma. Int J Neuropsychopharmacol 18(1).
Wahlestedt, C., Heilig, M., 1995. Neuropeptide Y and related peptides, in: Bloom, F.E., Kupfer, D.J. (Eds.), Psychopharmacology: A fourth Generation of Progress. Raven Press, New York, pp. 543-552.
Westrin, A., Ekman, R., Traskman-Bendz, L., 1999. Alterations of corticotropin releasing hormone (CRH) and neuropeptide Y (NPY) plasma levels in mood disorder patients with a recent suicide attempt. Eur Neuropsychopharmacol 9(3), 205-211.
Westrin, A., Engstom, G., Ekman, R., Traskman-Bendz, L., 1998. Correlations between plasma-neuropeptides and temperament dimensions differ between suicidal patients and healthy controls. J Affect Disord 49(1), 45-54.
Widdowson, P.S., Ordway, G.A., Halaris, A.E., 1992. Reduced neuropeptide Y concentrations in suicide brain. J Neurochem 59(1), 73-80.
Yang, Z., Han, S., Keller, M., Kaiser, A., Bender, B.J., Bosse, M., Burkert, K., Kogler, L.M., Wifling, D., Bernhardt, G., Plank, N., Littmann, T., Schmidt, P., Yi, C., Li, B., Ye, S., Zhang, R., Xu, B., Larhammar, D., Stevens, R.C., Huster, D., Meiler, J., Zhao, Q., Beck-Sickinger, A.G., Buschauer, A., Wu, B., 2018. Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature 556(7702), 520-524.
Stress causes a rapid increase in CRF release (leading to increased anxiety) and a delayed increase in NPY release (leading to reduced anxiety and adaptation to stress). NPY-containing neurons project from the arcuate nucleus (ARC) to the PVN in the hypothalamus, and this may be the site of NPY and CRF interaction (Heilig et al., 1994; Schmidt et al., 2008). Yang et al. (2018) have provided experimental evidence showing that NPY-CRF interaction leads to stress adaptation. A schematic diagram linking CRF and NPY is given in Figure 4.
Figure 4: Interaction of CRF and NPY in stress.
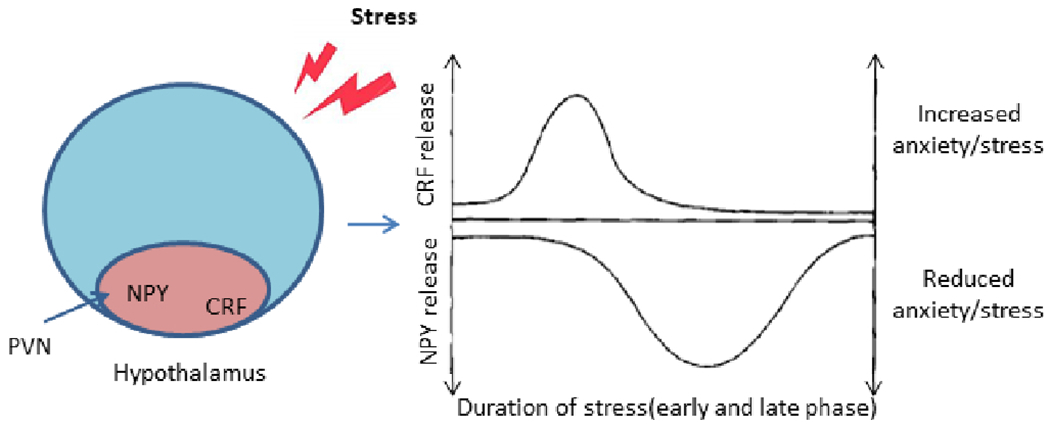
Proposed representation of counter-regulation of stress and anxiety by CRF and NPY. After stress induction, there is a rapid increase in CRF release and a slower increase in NPY release. The delayed increase in NPY release counteracts the effect of CRF by reducing anxiety leading to stress adaptation. NPY neurons projecting from the arcuate nucleus (ARC) to the paraventricular nucleus (PVN) in hypothalamus may be a common region where NPY and CRF interact [adapted from (Heilig et al., 1994; Schmidt et al., 2008)]
In summary, in the present study, we observed reduced NPY expression at both the protein and mRNA levels, which suggests a dysfunctional NPY stress response and adaptation system in depression and suicide. The compromised NPY system is further linked to the hyperactive HPA system, as we have observed in the HPA axis hyperactivation in previous studies by our group in relation to depression and suicide. The elevated level of NPY1R observed in the current study may be due to a compensatory mechanism against a reduced NPY expression. Being anxiogenic, the enhanced expression of the NPY2R is suggestive of aggravated anxiety in DS subjects. There are few preclinical studies on the roles of NPY4R and NPY5R in stress and depression, but we did not observe any significant changes; thus, they cannot comment on their implication in depression and suicide. Some preclinical studies related to depression also implicate an interaction of the NPY system with the dopaminergic system and NLRP3 inflammasome pathway (Serafini et al., 2013; Wang et al., 2019); however, for their detailed elucidation in human systems, further investigation is required. There are a few recent clinical studies which have also demonstrated the role of NPY as a novel treatment approach, such as NPY insufflation to MDD subjects and intranasal administration of NPY to post-traumatic stress disorder (PTSD) subjects (Mathe et al., 2020; Sayed et al., 2018).
Although some of the DS subjects were on chronic antidepressant treatment, no significant difference in mRNA and protein expression among subgroups (DS-with antidepressants and DS-without antidepressants) was observed except the expression of NPY4R (in hippocampus), which suggested that there is no significant influence of antidepressants on expression of NPY and its receptors.
5. Limitation
Due to limited amounts of hippocampus tissue, we were not able to perform NPY receptor protein studies. Further, the number of subjects in the two groups was reasonable (n=24 in each group); however, some of the subjects of the DS group were on different antidepressants for different time periods before committing suicide, thus the number of subjects in sub-groups to study the effect of antidepressants was small (n=12).
6. Conclusion
Preclinical and a few clinical studies suggest the involvement of the NPY system in stress response and pathophysiology of depression; however, there is no sufficient and detailed evidence describing the effect of depression and suicide on NPY and its four functional receptors specifically in the postmortem brain. The outcomes of this study suggest that depression and suicidal behavior modulate the NPY system and could thus help in the development of novel NPY-system-based treatment approaches. Specific alterations in this system may also be established as markers or predictors of suicide.
Supplementary Material
Highlights.
Neuropeptide Y (NPY) plays a major role in stress response and may have antidepressant-like effect
Abnormalities of NPY and its receptors may be present in depression and suicide
NPY mRNA and protein expression was decreased in postmortem brain of depressed suicide subjects
mRNA expression of NPY1R and NPY2R was increased in postmortem brain of depressed suicide subjects
Our results show NPY abnormalities in postmortem brain of depressed suicide subjects
Funding
This research was supported by grants RO1MH098554 and RO1MH106565 (Dr. Pandey) from the National Institute of Mental Health, Rockville, MD. The funding source had no role in the study design, collection, analysis, and interpretation of data, or writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Approval and consent to participate
All procedures were approved by the University of Maryland Institutional Review Board (IRB) and University of Illinois at Chicago IRB. Postmortem brain tissue was collected only after a family member gave informed consent.
Consent for publication
Not applicable
Declaration of competing interests
All authors declare that they have no competing interests, directly or indirectly related to this work.
Availability of data and material
The data sets of the current study are available from the corresponding author upon reasonable request.
References:
- Alvina K, Jodeiri Farshbaf M, Mondal AK, 2021. Long term effects of stress on hippocampal function: Emphasis on early life stress paradigms and potential involvement of neuropeptide Y. J Neurosci Res 99(1), 57–66. [DOI] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL, 1995. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol 6(3), 215–222. [PubMed] [Google Scholar]
- Caberlotto L, Hurd YL, 2001. Neuropeptide Y Y(1) and Y(2) receptor mRNA expression in the prefrontal cortex of psychiatric subjects. Relationship of Y(2) subtype to suicidal behavior. Neuropsychopharmacology 25(1), 91–97. [DOI] [PubMed] [Google Scholar]
- Caberlotto L, Jimenez P, Overstreet DH, Hurd YL, Mathe AA, Fuxe K, 1999. Alterations in neuropeptide Y levels and Y1 binding sites in the Flinders Sensitive Line rats, a genetic animal model of depression. Neurosci Lett 265(3), 191–194. [DOI] [PubMed] [Google Scholar]
- Crespi F, 2011. Influence of Neuropeptide Y and antidepressants upon cerebral monoamines involved in depression: an in vivo electrochemical study. Brain Res 1407, 27–37. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, St-Pierre J-A, Tong Y, Parker R, Herzog H, Quirion R, 2000. Neuropeptide Y, peptide YY and pancreatic polypeptide receptor proteins and mRNAs in mammalian brains, in: Quirion R, Björklund A, Hökfelt T (Eds.), Handbook of Chemical Neuroanatomy. Elsevier, pp. 375–475. [Google Scholar]
- Ehlers CL, Somes C, Seifritz E, Rivier JE, 1997. CRF/NPY interactions: a potential role in sleep dysregulation in depression and anxiety. Depress Anxiety 6(1), 1–9. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 1997. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). American Psychiatric Publishing, Inc., Arlington, Virginia, USA. [Google Scholar]
- Hashimoto H, Onishi H, Koide S, Kai T, Yamagami S, 1996. Plasma neuropeptide Y in patients with major depressive disorder. Neurosci Lett 216(1), 57–60. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT, 1994. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci 17(2), 80–85. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E, 1989. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 98(4), 524–529. [DOI] [PubMed] [Google Scholar]
- Heilig M, Wahlestedt C, Ekman R, Widerlov E, 1988. Antidepressant drugs increase the concentration of neuropeptide Y (NPY)-like immunoreactivity in the rat brain. Eur J Pharmacol 147(3), 465–467. [DOI] [PubMed] [Google Scholar]
- Heilig M, Zachrisson O, Thorsell A, Ehnvall A, Mottagui-Tabar S, Sjogren M, Asberg M, Ekman R, Wahlestedt C, Agren H, 2004. Decreased cerebrospinal fluid neuropeptide Y (NPY) in patients with treatment refractory unipolar major depression: preliminary evidence for association with preproNPY gene polymorphism. J Psychiatr Res 38(2), 113–121. [DOI] [PubMed] [Google Scholar]
- Holsboer F, 2000. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23(5), 477–501. [DOI] [PubMed] [Google Scholar]
- Hou C, Jia F, Liu Y, Li L, 2006. CSF serotonin, 5-hydroxyindolacetic acid and neuropeptide Y levels in severe major depressive disorder. Brain Res 1095(1), 154–158. [DOI] [PubMed] [Google Scholar]
- Husum H, Mathe AA, 2002. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology 27(5), 756–764. [DOI] [PubMed] [Google Scholar]
- Jimenez-Vasquez PA, Mathe AA, Thomas JD, Riley EP, Ehlers CL, 2001. Early maternal separation alters neuropeptide Y concentrations in selected brain regions in adult rats. Brain Res Dev Brain Res 131(1-2), 149–152. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M, 2008. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 195(4), 547–557. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R, 2002. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev 26(3), 259–283. [DOI] [PubMed] [Google Scholar]
- Kask A, Vasar E, Heidmets LT, Allikmets L, Wikberg JE, 2001. Neuropeptide Y Y(5) receptor antagonist CGP71683A: the effects on food intake and anxiety-related behavior in the rat. Eur J Pharmacol 414(2-3), 215–224. [DOI] [PubMed] [Google Scholar]
- Kuromitsu J, Yokoi A, Kawai T, Nagasu T, Aizawa T, Haga S, Ikeda K, 2001. Reduced neuropeptide Y mRNA levels in the frontal cortex of people with schizophrenia and bipolar disorder. Brain Res Gene Expr Patterns 1(1), 17–21. [DOI] [PubMed] [Google Scholar]
- Mathe AA, Michaneck M, Berg E, Charney DS, Murrough JW, 2020. A Randomized Controlled Trial of Intranasal Neuropeptide Y in Patients With Major Depressive Disorder. Int J Neuropsychopharmacol 23(12), 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathe AA, Rudorfer MV, Stenfors C, Manji HK, Potter WZ, Theodorsson E, 1995. Effect of electroconvulsive treatmetn on somatostatin, neuropeptide Y, endothelin, and neurokinin A concentrations in cerebrospinal fluis of depressed patients: a pilot study. Depression 3(5), 250–256. [Google Scholar]
- Meriney SD, Fanselow EE, 2019. Neuropeptide Transmitters, in: Meriney SD, Fanselow EE (Eds.), Synaptic Transmission. Academic Press, pp. 421–434. [Google Scholar]
- Millon C, Flores-Burgess A, Narvaez M, Borroto-Escuela DO, Gago B, Santin L, Castilla-Ortega E, Narvaez JA, Fuxe K, Diaz-Cabiale Z, 2017. The neuropeptides Galanin and Galanin(1-15) in depression-like behaviours. Neuropeptides 64, 39–45. [DOI] [PubMed] [Google Scholar]
- Miragaia AS, de Oliveira Wertheimer GS, Consoli AC, Cabbia R, Longo BM, Girardi CEN, Suchecki D, 2018. Maternal Deprivation Increases Anxiety- and Depressive-Like Behaviors in an Age-Dependent Fashion and Reduces Neuropeptide Y Expression in the Amygdala and Hippocampus of Male and Female Young Adult Rats. Front Behav Neurosci 12, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Medina JC, Dumont Y, Quirion R, 2010. A possible role of neuropeptide Y in depression and stress. Brain Res 1314, 194–205. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R, 2009. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat 38(4), 266–272. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, Baba S, Kasuga M, 1998. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides 19(2), 359–363. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Baumann P, Liu T, Mathe AA, 2012. Quetiapine affects neuropeptide Y and corticotropin-releasing hormone in cerebrospinal fluid from schizophrenia patients: relationship to depression and anxiety symptoms and to treatment response. Int J Neuropsychopharmacol 15(8), 1051–1061. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Karlsson G, Blennow K, Heilig M, Ekman R, 1996. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides 17(3), 359–362. [DOI] [PubMed] [Google Scholar]
- Nwokafor C, Serova LI, Nahvi RJ, McCloskey J, Sabban EL, 2020. Activation of NPY receptor subtype 1 by [D-His(26)]NPY is sufficient to prevent development of anxiety and depressive like effects in the single prolonged stress rodent model of PTSD. Neuropeptides 80, 102001. [DOI] [PubMed] [Google Scholar]
- Olsson A, Regnell G, Traskman-Bendz L, Ekman R, Westrin A, 2004. Cerebrospinal neuropeptide Y and substance P in suicide attempters during long-term antidepressant treatment. Eur Neuropsychopharmacol 14(6), 479–485. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Sperk G, Herzog H, Holzer P, 2010. Delayed stress-induced differences in locomotor and depression-related behaviour in female neuropeptide-Y Y1 receptor knockout mice. J Psychopharmacol 24(10), 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Pandey SC, Teas SS, Conley RR, Roberts RC, Tamminga CA, 1999. Low phosphoinositide-specific phospholipase C activity and expression of phospholipase C beta1 protein in the prefrontal cortex of teenage suicide subjects. Am J Psychiatry 156(12), 1895–1901. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Bhaumik R, Ren X, 2019. Innate immunity in the postmortem brain of depressed and suicide subjects: Role of Toll-like receptors. Brain Behav Immun 75, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M, 2013. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology 38(11), 2628–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedragosa-Badia X, Stichel J, Beck-Sickinger AG, 2013. Neuropeptide Y receptors: how to get subtype selectivity. Front Endocrinol (Lausanne) 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Kim M, Sanchez R, Ziaee SM, Kohtz JD, Koh S, 2018. Enhanced susceptibility to stress and seizures in GAD65 deficient mice. PLoS One 13(1), e0191794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R, 2003. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res 141(2), 251–255. [DOI] [PubMed] [Google Scholar]
- Reichmann F, Holzer P, 2016. Neuropeptide Y: A stressful review. Neuropeptides 55, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R, Geracioti TD, 2013. Neuropeptide Y and posttraumatic stress disorder. Mol Psychiatry 18(6), 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, 2005. Neuropeptide Y receptors as therapeutic targets in anxiety and depression. Drug Development Research 65(4), 301–308. [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, 2002a. Neuropeptide Y receptor subtypes in the basolateral nucleus of the amygdala modulate anxiogenic responses in rats. Neuropharmacology 43(7), 1165–1172. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR, 2002b. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav 71 (3), 419–423. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, Gehlert DR, 2004. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides 38(4), 225–234. [DOI] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A, 2006. Neuropeptides as synaptic transmitters. Cell Tissue Res 326(2), 583–598. [DOI] [PubMed] [Google Scholar]
- Sandberg JV, Jakobsson J, Palsson E, Landen M, Mathe AA, 2014. Low neuropeptide Y in cerebrospinal fluid in bipolar patients is associated with previous and prospective suicide attempts. Eur Neuropsychopharmacol 24(12), 1907–1915. [DOI] [PubMed] [Google Scholar]
- Sayed S, Van Dam NT, Horn SR, Kautz MM, Parides M, Costi S, Collins KA, Iacoviello B, Iosifescu DV, Mathe AA, Southwick SM, Feder A, Charney DS, Murrough JW, 2018. A Randomized Dose-Ranging Study of Neuropeptide Y in Patients with Posttraumatic Stress Disorder. Int J Neuropsychopharmacol 21(1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Liebl C, Sterlemann V, Ganea K, Hartmann J, Harbich D, Alam S, Muller MB, 2008. Neuropeptide Y mediates the initial hypothalamic-pituitary-adrenal response to maternal separation in the neonatal mouse. J Endocrinol 197(2), 421–427. [DOI] [PubMed] [Google Scholar]
- Serafini G, Pompili M, Lindqvist D, Dwivedi Y, Girardi P, 2013. The role of neuropeptides in suicidal behavior: a systematic review. Biomed Res Int 2013, 687575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani L, Oquendo MA, Sullivan GM, Mathe AA, Mann JJ, 2014. Cerebrospinal fluid neuropeptide Y levels in major depression and reported childhood trauma. Int J Neuropsychopharmacol 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G, Lindberg C, Wortwein G, Bolwig TG, Woldbye DP, 2004. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J Neurosci Res 77(5), 723–729. [DOI] [PubMed] [Google Scholar]
- Starback P, Wraith A, Eriksson H, Larhammar D, 2000. Neuropeptide Y receptor gene y6: multiple deaths or resurrections? Biochem Biophys Res Commun 277(1), 264–269. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G, 2009. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience 158(4), 1717–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M, 2000. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A 97(23), 12852–12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Tasan RO, Herzog H, Sperk G, 2012. NPY controls fear conditioning and fear extinction by combined action on Y(1) and Y(2) receptors. Br J Pharmacol 166(4), 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlestedt C, Heilig M, 1995. Neuropeptide Y and related peptides, in: Bloom FE, Kupfer DJ (Eds.), Psychopharmacology: A fourth Generation of Progress. Raven Press, New York, pp. 543–552. [Google Scholar]
- Wang W, Xu T, Chen X, Dong K, Du C, Sun J, Shi C, Li X, Yang Y, Li H, Xu ZD, 2019. NPY Receptor 2 Mediates NPY Antidepressant Effect in the mPFC of LPS Rat by Suppressing NLRP3 Signaling Pathway. Mediators Inflamm, 7898095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CA, Cohen ZD, Beard C, Forgeard M, Peckham AD, Bjorgvinsson T, 2020. Personalized prognostic prediction of treatment outcome for depressed patients in a naturalistic psychiatric hospital setting: A comparison of machine learning approaches. J Consult Clin Psychol 88(1), 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2017. Depression and other common mental disorders. [Google Scholar]
- Widdowson PS, Ordway GA, Halaris AE, 1992. Reduced neuropeptide Y concentrations in suicide brain. J Neurochem 59(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Wegener G, Bailey C, Saxena S, Charney D, Mathe AA, 2011. Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opin Ther Targets 15(11), 1317–1331. [DOI] [PubMed] [Google Scholar]
- Yang Z, Han S, Keller M, Kaiser A, Bender BJ, Bosse M, Burkert K, Kogler LM, Wifling D, Bernhardt G, Plank N, Littmann T, Schmidt P, Yi C, Li B, Ye S, Zhang R, Xu B, Larhammar D, Stevens RC, Huster D, Meiler J, Zhao Q, Beck-Sickinger AG, Buschauer A, Wu B, 2018. Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature 556(7702), 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets of the current study are available from the corresponding author upon reasonable request.


