Abstract
Objectives
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease associated with neuro-psychiatric (NP) manifestations. Frequency and patterns of neuro-psychiatric systemic lupus erythematosus (NPSLE) vary substantially between patients. We conducted a systematic review (SR) of the literature and examined prevalence and characteristics of NPSLE in the Swiss SLE cohort study (SSCS).
Methods
The SR search was performed between January 1999 and January 2020. We included prospective/cross-sectional studies focusing on NPSLE. We secured study characteristics, cohort compositions and frequencies of NP manifestations, assessed heterogeneity across reports and investigated sources of variation using meta-regression models. Regarding the SSCS, we reviewed all patients included and classified NP manifestations.
Results
The SR searches identified 530 studies. We included 22 studies in our meta-analysis, the mean frequency of NPSLE ranged from 10.6% to 96.4%. The frequency of NPSLE in the SSCS was 28.1%. Severe events including cerebrovascular insults, seizures and psychosis appeared in 7.1%, 5.3% and 6.5% respectively. There was a linear relationship between duration of SLE and cumulative incidence of NPSLE.
Conclusions
The spectrum of NPSLE is very broad. The diagnostic work-up and rates of reported manifestations varied substantially across studies. We call for concerted efforts and consensus regarding definitions of NPSLE that will facilitate accurate diagnosis and attribution to SLE, particularly with a view to timely intervention and patient outcomes.
Keywords: Systemic lupus erythematosus, neuropsychiatric, NPSLE, SSCS, meta-analysis, prevalence
Introduction
Systemic lupus erythematosus (SLE) is a chronic, systemic autoimmune disease, often presenting with neuro-psychiatric manifestations. The frequency and patterns of severe neurologic and psychiatric events in SLE are extremely heterogeneous and remain incompletely understood. In the literature, the occurrence of neuro-psychiatric systemic lupus erythematosus (NPSLE) varies from 21-95%.1–4 The wide range of NPSLE prevalence is mainly due to the difficulty of attributing NP events to SLE and classification of events, which leads to inconsistent reporting of its incidence.5–8 With regard to the course of NPSLE, as per current knowledge, at least half of NP events occur within the first 1-2 years after SLE diagnosis.2,5,9–11
According the American College of Rheumatology (ACR) nomenclature and case definitions from 1999, in SLE 19 neuro-psychiatric syndromes are described.12 These syndromes are differentiated in 12 central and seven peripheral, diffuse or focal manifestations.1,12 Syndromes range from subtle abnormalities like headache, cognitive dysfunction or mood disorder to severe presentations such as seizures, psychosis or strokes. In clinical practice, expert physician judgement based on clinical tests remains the most appropriate reference standard for NPSLE diagnosis.5,11 Uncertainty regarding atypical presentations may delay targeted and timely interventions. In this study, we evaluated the occurrence and distribution of neuro-psychiatric manifestations in a large prospective nationwide cohort of Swiss SLE patients13 and performed a systematic literature review to contextualize our findings.
Methods
Systematic review/meta-analysis
Our review of studies regarding NPSLE has been registered on Prospero. Included were studies published between January 1999 and January 2020 investigating the occurrence of NP events in SLE patients, provided they met the following criteria: 1) prospective or cross-sectional study design; 2) the use of the ACR criteria to establish a diagnosis for SLE; 3) definition of NP events by applying the ACR classification from 1999. Excluded were retrospective cohort studies or case reports and studies that presented duplicate data of the same cohort in a similar time period as another included study.
Searches were conducted in electronic databases, i.e. Pre-(Medline), PubMed, interface and the Science Citation Index database for papers citing relevant studies. We also checked the reference lists of all papers and reviews. Finally, we contacted authors of pertinent papers to clarify ambiguities and request the availability of additional data. Abstracts published in all languages of the identified articles were reviewed (A.M.). Retrospective studies, case reports and reviews were excluded, as well as papers not investigating the topic of NPSLE. The remaining studies were read in full text and checked for inclusion and exclusion criteria (A.M.). The process of data extraction was performed by two independently working investigators to assure uniformity of the data (A.M. and C.W.). The extraction files were subsequently compared, and discrepancies corrected. For each study included, we recorded the following data: study design, total number of SLE patients, number of NPSLE patients, number of NP events and patient characteristics including gender, ethnicity, mean age at SLE diagnosis, mean age at study assessment, duration from SLE diagnosis to NP event, cardiovascular risk factors, autoantibodies if available, as well as medication received. Furthermore, we conducted data on the mean ACR criteria for SLE, the mean SELENA-SLEDAI and the mean SDI score.
Prevalence estimates for NPSLE in each study and for each of the 19 NP manifestations individually were calculated with their 95% confidence intervals. Prevalence is shown as percentage of the entire study population (SLE patients). In order to minimize the risk of bias, a quality assessment was carried out for each study. We assessed how representative the study cohorts were, the accuracy of the outcome measurements, the identification of confounders and the measures taken to minimize their influence. We also examined the presentation of outcomes, whether patient enrolment was consecutive, the number and handling of dropouts, the duration of follow-up and finally, the generalizability of study results to patients seen in everyday clinical practice. Despite substantial unexplained heterogeneity of 22 high quality studies, we performed a random effects meta-analysis, calculating exact 95% confidence intervals using the Stata 16.1 routine “metaprop”. All statistical analyses were performed using the Stata 16.1 statistics software package (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.)
Swiss SLE cohort study
Study design
This cross-sectional and retrospective study encompasses data collected between April 2007 and August 2019 from 688 patients sequentially included in the Swiss SLE Cohort Study (SSCS) form eight tertiary centers located in Switzerland. Patient recruitment included both incident and prevalent cases of SLE. Patients were seen at inclusion and then annually by specialists from Clinical Immunology, Internal Medicine, Nephrology and Rheumatology. Clinical data as well as biological characteristics were collected as previously published.13 This study complies with the Declaration of Helsinki and was approved by the local ethics board (BASEC-Nr. 2019-01807). Patients gave their written consent for inclusion in the SSCS database.
Patients
Chart reviews of all SSCS cohort patients were performed to obtain a complete reporting of NP manifestations. Patients without NP events served as a control group. All patients fulfilled at least 4 of the 11 revised ACR criteria.14,15 At time of database inclusion, ACR criteria were documented. The additionally assessed parameters included age, gender, ancestry, cardiovascular risk factors, smoking status, date of first SLE manifestation and time of SLE diagnosis. Preceding as well as newly occurring NP events and the time lag between NP event and SLE diagnosis were recorded. At the time of the patients’ visits a panel of autoantibodies (ANA and its specificities anti-dsDNA, anti-Sm, antiphospholipid (aPL) including lupus anticoagulant (LA), anticardiolipin (aCL), and beta 2 glycoprotein I (IgG and IgM), anti-SSA and anti-U1-RNP) were collected. Disease activity was assessed using the SELENA-SLEDAI score,16 while cumulative organ damage was assessed using the ACR/SLICC damage index (SDI).17
Cardiovascular risk factors
Cardiovascular risk factors included hyperlipidaemia, diabetes mellitus, arterial hypertension, and cigarette smoking and were defined by a clearly documented history.
NP events
NP events were defined according to the 19 ACR nomenclature and case definitions for NPSLE.12 For patients suffering from more than one NP event, each event was documented separately. Screening for NP events was performed primarily by clinical evaluation by the local interdisciplinary attending team. Diagnosis was supported with appropriate additional investigations if indicated, as per the ACR nomenclature of 1999. Specific investigations for NP disease such as brain imaging and cognitive testing were not performed routinely on all patients but only if indicated following clinical assessment. Patients were followed up annually, or at shorter intervals when an event occurred.
Statistical analyses
Values are documented as the mean ± standard deviation, unless otherwise indicated. The relationship between occurrence of a NP event and sex, ethnicity, cardiovascular risk factors, ACR criteria, SELENA-SLEDAI severity score SDI as well as autoantibodies was assessed using multivariate logistic regression analysis. Within the subset of the register, where the onset date of SLE (prior to register entry) and time point of first NPSLE was available (548/688; 79.7%) we performed a time-to-event analysis using a Cox regression model to assess the relationship between time point of the occurrence of different NPSLE manifestations in the disease course.
Results
Systematic review/meta-analysis
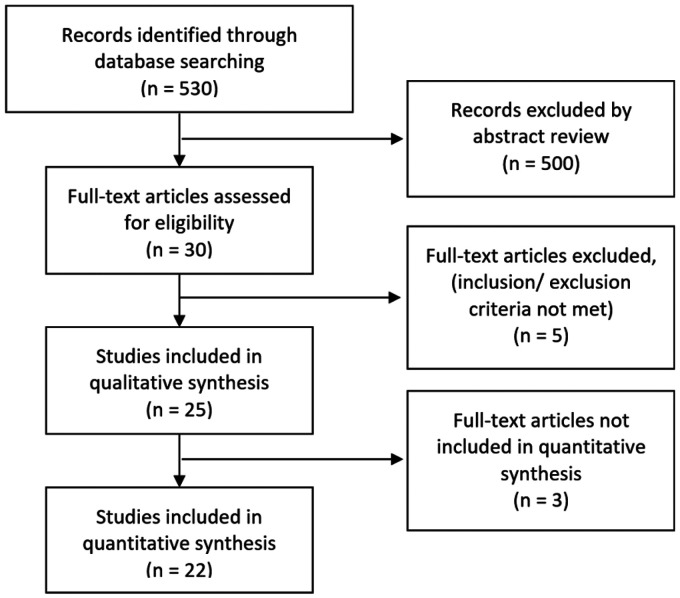
We identified 530 records through searching databases, of which 25 met the inclusion criteria. Twenty-two studies qualified for quantitative analysis (Figure 1).4,10,11,18–35 Nineteen of 25 studies were single-centered. The mean number of patients per study was 275.2 (SD 328.6). The total number of patients in all studies was 6’055, of which 2’569 (weighted mean, random effects model: 0.53 (95% CI: 0.39 to 0.67)), experienced at least one NP event with a mean of 1.9 events per NPSLE-patient (SD 0.6). The majority of patients were female (89.6%) with an average age at study inclusion of 36.5 years (SD 7.1) and an average disease duration of 7.3 years (SD 3.6). Data regarding ethnicity was available in 70% of the studies, with predominantly Caucasians observed. The mean value of SELENA-SLEDAI score was 10.6 (SD 7.4) and the mean SDI was 1.6 (SD 1.2). Study characteristics of the reviewed literature and flow chart of the inclusion process are shown in Table 1 and Figure 1.
Figure 1.
PRISMA flow chart showing inclusion process.
Table 1.
Study characteristics and patient population in the studies examining NSPLE identified by literature search.
| Author, year (Cohort-name) | Patient recruitmentStart–End | Number centers | Number of patients | Mean age | Female proportion | SLE duration in months | Mean SELENA SLEDAI | Included in meta-analysis |
|---|---|---|---|---|---|---|---|---|
| Abdel-Nasser, 2008 | 01/2003–09/2003 | 1 | 32 | 25 | 0.875 | 46.8 | 22.43 | + |
| Abdul-Sattar, 2013 | 07/2011–12/2012 | 1 | 84 | a) 31b) 29 | a) 0.944b) 0.9375 | a) 80.4b) 67.2 | a) 15.6b) 5 | + |
| Ahn, 2018(Hanyang BAE lupus cohort) | 02/1998–12/2015 | 1 | 1121 | a) 26.6*b) 27.7* | a) 0.949b) 0.916 | a) 150b) 141.6 | a) 5.1b) 5 | + |
| Ainiala, 2001 | 01/1980–12/1997 | 5 | 46 | 45 | 0.848 | 168 | n.r. | + |
| Alfetra, 2003 | n.r. | 61 | 40 | 0.869 | 120 | n.r. | + | |
| Appenzeller, 2005 | n.r. | 1 | 115 (+44 healthy controls) | 33.5 | 0.948 | 66.5 | n.r. | + |
| Brey, 2002 (SALUD) | n.r. | 128 | 43 | 0.9375 | 96 | 4.8 | + | |
| Briani, 2009 | 01/1986–10/2004 | 1 | 219 | 28 | 0.845 | n.r. | n.r. | + |
| Hanly, 2009 | 06/2000–12/2007 | 1 | 209 | a) 40.9b) 46.3 | a) 0.89b) 0.862 | a) 103.2b) 97.2 | a) 4.4b) 3.4 | + |
| Hanly, 2011(SLICC) | 10/1999–02/2008 | 24 | 1206 | 34.5 | 0.896 | 5.4 | 5.4 | + |
| Kakati, 2017 | 08/2009–07/2010 | 1 | 52 | 25.59 | 0.923 | n.r. | 22.44 | + |
| Kampylafka, 2013 | 07/2008–07/2011 | 1 | 370 | a) 30b) 32.1 | a) 0.94b) 0.88 | a) 68.4b) 109.8 | a) 18.1b) 2.95 | −a |
| Katsumata, 2010 | 08/1994–10/2003 | 1 | 191 | a) 28b) 34 | a) 0.930b) 0.918 | a) 12b) 12 | a) 15b) 9 | −a |
| Magro-Checa, 2017(Leiden NPSLE Cohort) | 09/2007–03/2016 | 1 | 304 | 42.5 | 0.897 | 55.2 | n.r. | + |
| Mok, 2006 | 01/1990–10/2004 | 1 | 282 | 31.8 | 0.915 | n.r. | 11.67 | + |
| Morrison, 2014 | 11/1983–01/2010 | 1 | 108 | a) 40.8b) n.r. | a) 0.85b) n.r. | a) 120b) n.r. | a) 8.66b) n.r. | + |
| Muhammed, 2018 | 01/2015–07/2016 | 1 | 101 | a) 31.9b) 32.5 | a) 0.97b) 0.88 | a) 54b) 56.4 | a) 31.1b) 20.9 | + |
| Pradhan, 2015 | 01/2008–12/2012 | 1 | 120 | a) 23.07*b) 26 | a) 0.867b) n.r. | a) 41.3b) n.r. | a) 28.7b) n.r. | −b |
| Sanna, 2003 | 09/1999–04/2000 | 1 | 323 | a) 43.1b) 40.3 | a) 0.951b) 0.957 | a) 142.8b) 115.1 | n.r. | + |
| Schenatto, 2006 | 01/2000–12/2002 | 1 | 87 (+25 healthy controls) | a) 31.2b) 41.0 | a) 0.957b) 0.875 | a) 71.48b) 96 | a) 9.93b) 2.89 | + |
| Sibbitt, 2002(University of New Mexico Lupus Cohort) | n.r. | 1 | 75 | 21.6c | 0.9 | 93.6 | 15.6 | + |
| vdMeulen, 2017(Leiden NPSLE Cohort) | n.r. | 3 | 272 (+51 healthy controls) | a) 42b) 40 | a) 0.87b) 0.897 | n.r. | n.r. | + |
| Zavada, 2013 | 01/2002–01/2009 | 1 | 471 | a) 43 | a) 0.9 | n.r. | n.r. | + |
| Zirkzee, 2012(Leiden NPSLE Cohort) | 09/2007–12/2009 | 1 | 71 | a) 42.2b) 42 | a) 0.873b) 0.92 | a) 102.3b) 100.8 | a) 7.88b) 4.1 | + |
| Meier, 2020 (SSCS) | 04/2007–08/2019 | 7 | 688 | a) 46.1 b) 43.7 | a) 0.824 b) 0.857 | a) 140.4 b) 99.6 | a) 10.05 b) 5.37 | + |
Subgroups: (a) NPSLE-patients, (b) Non-NPSLE-Patients; Mean age refers to mean age at inclusion (*mean age at diagnosis).
aFocused on central nervous manifestations only.
bNo prevalence due recruitment of 60 cases/controls.
cPediatric patients with inclusion criteria: diagnosis of SLE at 17 years of age or younger.
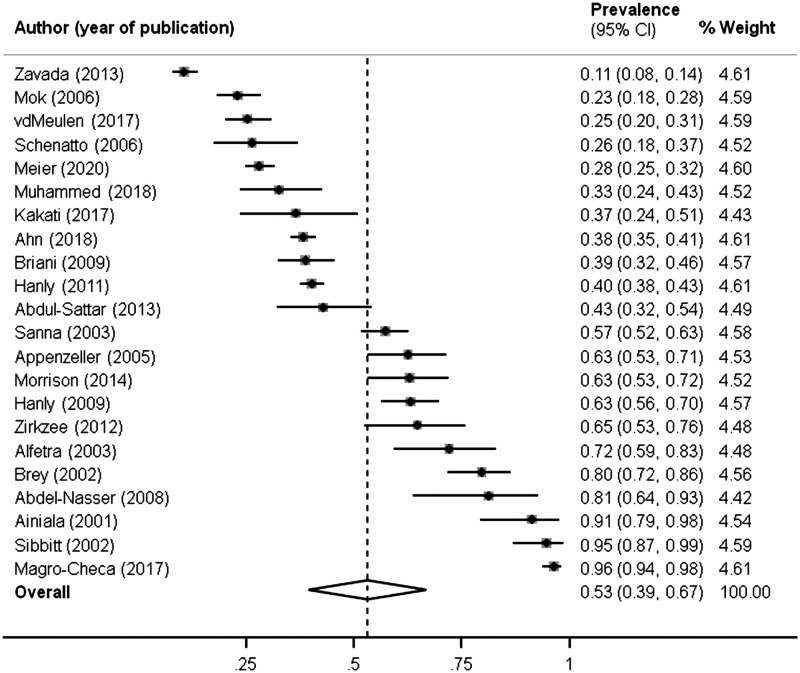
The overall NPSLE prevalence when all studies were pooled together was 52.2% (Figure 2). A large proportion of events (4407, 93.7%) was related to the CNS. The most common NP syndromes were headache (1394/4702), mood disorder (770/4702) and cognitive dysfunction (554/4702). Demyelinating syndrome, Guillain-Barré syndrome, autonomic disorder, myasthenia gravis and plexopathy each showed a prevalence rate of less than 0.5%. The cumulative prevalence of each NP syndrome of the different studies is shown in Table 2.
Figure 2.
Forest plot of NPSLE prevalence in studies identified by literature search.
Table 2.
Results: prevalence of neuro-psychiatric manifestations of SLE in relevant studies identified by literature search.
| Author, year (Cohort-name) | Number of patients | Prevalence NPSLE patients | NPSLE patients | NPSLE events | CNS events | PNS events |
CNS |
PNS |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aseptic meningitis | Cerebrovascular disease | Demyelinating syndrome | Headache | Movement disorder | Myelopathy | Seizure disorders | Acute confusional state | Anxiety disorder | Cognitive disfunction | Mood disorder | Psychosis | Guillain-Barré-Syndrome | Autonomic disorder | Mononeuropathy | Myasthenia gravis | Neuropathy cranial | Plexopathy | Polyneuropathy | Included in meta-analysis | |||||||
| Abdel-Nasser, 2008 | 32 | 0.813 | 26 | 57 | 57 | 0 | 0 | 1 | 0 | 15 | 0 | 0 | 4 | 2 | 3 | 12 | 19 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + |
| Abdul-Sattar, 2013 | 84 | 0.429 | 36 | 53 | 47 | 6 | 0 | 6 | 0 | 10 | 0 | 0 | 3 | 0 | 7 | 1 | 16 | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | + |
| Ahn, 2018 | 1121 | 0.383 | 429 | 652 | 586 | 66 | 16 | 47 | 2 | 258 | 7 | 3 | 71 | 19 | 36 | 12 | 89 | 26 | 1 | 1 | 22 | 0 | 14 | 0 | 28 | + |
| Ainiala, 2001 | 46 | 0.913 | 42 | 122 | 105 | 17 | 1 | 7 | 1 | 25 | 1 | 0 | 4 | 3 | 6 | 37 | 20 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 13 | + |
| Alfetra, 2003 | 61 | 0.721 | 44 | 103 | 90 | 13 | 0 | 15 | 0 | 13 | 0 | 2 | 7 | 0 | 4 | 32 | 17 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 8 | + |
| Appenzeller, 2005 | 115 | 0.626 | 72 | 133 | 126 | 7 | 1 | 0 | 0 | 40 | 1 | 3 | 15 | 10 | 0 | 36 | 14 | 6 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | + |
| Brey, 2002 | 128 | 0.797 | 102 | 290 | 259 | 31 | 0 | 2 | 0 | 73 | 1 | 0 | 21 | 0 | 27 | 67 | 62 | 6 | 0 | 0 | 9 | 0 | 2 | 0 | 20 | + |
| Briani, 2009 | 219 | 0.388 | 85 | 96 | 64 | 32 | 0 | 11 | 0 | 35 | 0 | 2 | 10 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 15 | 0 | 0 | 0 | 17 | + |
| Hanly, 2009 | 209 | 0.632 | 132 | 299 | 277 | 22 | 1 | 17 | 6 | 96 | 1 | 1 | 20 | 11 | 24 | 25 | 70 | 5 | 0 | 0 | 3 | 2 | 10 | 1 | 6 | + |
| Hanly, 2011 | 1206 | 0.403 | 486 | 843 | 785 | 58 | 6 | 40 | 3 | 397 | 6 | 10 | 63 | 22 | 42 | 43 | 139 | 14 | 2 | 2 | 18 | 0 | 15 | 1 | 20 | + |
| Kakati, 2017 | 52 | 0.365 | 19 | 53 | 43 | 10 | 1 | 0 | 0 | 5 | 1 | 0 | 8 | 6 | 2 | 11 | 5 | 4 | 0 | 2 | 1 | 0 | 3 | 0 | 4 | + |
| Kampylafka, 2013 | 370 | n.a. | n.a. | 23 | 21 | 2 | 1 | 6 | 0 | 0 | 0 | 5 | 8 | 0 | 0 | 0 | 0 | 1 | n.a. | n.a. | n.a. | n.a. | 2a | n.a. | n.a. | − |
| Katsumata, 2010 | 191 | n.a. | n.a. | 72 | 72 | n.a. | 5 | 1 | 5 | 5 | 0 | 2 | 18 | 24 | 1 | 0 | 9 | 2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | − |
| Magro-Checa, 2017 | 304 | 0.964 | 293 | 437 | 410 | 27 | 2 | 65 | 1 | 79 | 8 | 11 | 27 | 9 | 22 | 72 | 88 | 26 | 0 | 1 | 1 | 1 | 6 | 2 | 16 | + |
| Mok, 2006 | 282 | 0.23 | 65 | 115 | 105 | 10 | 1 | 23 | 0 | 8 | 2 | 6 | 17 | 10 | 3 | 10 | 10 | 15 | 0 | 0 | 1 | 2 | 7 | 0 | 0 | + |
| Morrison, 2014 | 108 | 0.63 | 68 | 126b | 107b | 18b | 1 | 10 | 2 | 23 | 1 | 2 | 7 | 7 | 6 | 12 | 29 | 7 | 0 | 0 | 4 | 0 | 10 | 1 | 3 | + |
| Muhammed, 2018 | 101 | 0.327 | 33 | 42 | 30 | 12 | 0 | 0 | 0 | 10 | 1 | 0 | 4 | 2 | 5 | 4 | 4 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 9 | + |
| Pradhan, 2015 | 120 | n.a. | 60 | 170 | 168 | 2 | 0 | 12 | 0 | 24 | 0 | 1 | 35 | 0 | 11 | 22 | 18 | 45 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | − |
| Sanna, 2003 | 323 | 0.573 | 185 | 351 | 324 | 27 | 0 | 57 | 3 | 78 | 4 | 4 | 27 | 13 | 24 | 35 | 54 | 25 | 2 | 0 | 6 | 5 | 5 | 0 | 9 | + |
| Schenatto, 2006 | 87 | 0.264 | 23 | 23 | 17 | 6 | 0 | 3 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 0 | 9 | 0 | 0 | 1 | 0 | 4 | 0 | 1 | + |
| Sibbitt, 2002 | 75 | 0.947 | 71 | 258 | 246 | 12 | 1 | 9 | 3 | 54 | 5 | 1 | 38 | 26 | 16 | 41 | 43 | 9 | 0 | 0 | 0 | 0 | 1 | 0 | 11 | + |
| vdMeulen, 2017 | 272 | 0.254 | 69 | 134 | 122 | 12 | 0 | 41 | 1 | 10 | 2 | 2 | 10 | 3 | 4 | 30 | 13 | 6 | 0 | 1 | 1 | 0 | 1 | 0 | 9 | + |
| Zavada, 2013 | 471 | 0.106 | 50 | 102 | 93 | 9 | 2 | 13 | 0 | 13 | 1 | 1 | 11 | 2 | 3 | 25 | 14 | 8 | 0 | 0 | 2 | 0 | 3 | 0 | 4 | + |
| Zirkzee, 2012 | 71 | 0.648 | 46 | 79 | 77 | 2 | 0 | 14 | 0 | 15 | 1 | 1 | 6 | 0 | 1 | 22 | 13 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | + |
| Meier, 2020 | 688 | 0.281 | 193 | 295 | 235 | 60 | 4 | 49 | 0 | 51 | 2 | 7 | 37 | 11 | 0 | 10 | 19 | 45 | 3 | 0 | 5 | 2 | 18 | 7 | 25 | + |
PNS: peripheral nervous system; CNS: central nervous system; n.a.: not applicable.
aAccording to ACR definition cranial neuropathy is a peripheral manifestation.
bOne event was not specified.
SSCS
Patient characteristics
A total of 688 patients were registered prospectively in the SSCS database of which 583 (84.7%) were female. Patients were predominantly Caucasian (556; 80.8%), which reflects the ethnic distribution in Switzerland.36 Mean age at SLE diagnosis was 37.1 years (SD 15.9), mean age at SSCS inclusion was 44.3 years (SD 15.6) with a mean SLE disease duration of 9.3 years (SD 10.1) (Table 3).
Table 3.
Patient characteristics of the SSCS.
| Total | NPSLE patients | Non-NPSLE patients | P value | |
|---|---|---|---|---|
| Patients (%) | 688 (100) | 193 (28.1) | 495 (71.9) | |
| Gender, female (%) | 583 (84.7) | 159 (82.4) | 424 (85.7) | |
| Ethnicity (%) | ||||
| Caucasian | 556 (80.8) | 156 (80.8) | 400 (80.8) | |
| African | 46 (6.7) | 11 (5.8) | 35 (7.1) | |
| Asian | 59 (8.6) | 16 (8.4) | 43 (8.7) | |
| Native American | 26 (3.8) | 0 (0) | 26 (5.3) | |
| Other | 1 (0.1) | 0 (0) | 1 (0.1) | |
| Age at first diagnosis, years (mean ± SD) | 37.1 ± 15.9 | 36.5 ± 16.1 | 37.3 ± 15.9 | |
| Age at study inclusion, years (mean ± SD) | 44.3 ± 15.6 | 46.1 ± 15.3 | 43.7 ± 15.7 | |
| Disease duration, years (mean ± SD) | 9.3 ± 10.1 | 11.7 ± 11.6 | 8.3 ± 9.3 | |
| Cardiovascular risk factors (%) | ||||
| Smoking (incl. former smoking) | 251 (36.5) | 115 (59.6) | 136 (27.5) | 0.735 |
| Arterial hypertension | 158 (22.9) | 60 (31.1) | 98 (19.8) | 0.002 |
| Diabetes mellitus | 31 (4.5) | 13 (6.7) | 18 (3.6) | 0.078 |
| Hyperlipidemia | 66 (9.4) | 28 (14.5) | 39 (7.9) | 0.014 |
| SELENA-SLEDAI Score (mean ± SD) | 6.7 ± 7.7 | 10.0 ± 10.5 | 5.4 ± 5.8 | <0.001 |
| SDI Score (mean ± SD) | 1.5 ± 2.3 | 2.2 ± 3.0 | 1.1 ± 1.9 | <0.001 |
| ACR criteria (mean ± SD) | 5.2 ± 1.6 | 5.5 ± 1.9 | 5.0 ± 1.4 | <0.001 |
| Cumulative ACR manifestations (%) | ||||
| Malar rash | 263 (38.2) | 73 (37.8) | 190 (38.4) | |
| Discoid rash | 136 (19.8) | 41 (21.2) | 95 (19.2) | |
| Photosensitivity | 324 (47.1) | 97 (50.3) | 227 (45.9) | |
| Oral ulcers | 194 (28.2) | 54 (28.0) | 140 (28.3) | |
| Arthritis | 489 (71.1) | 141 (73.1) | 348 (70.3) | |
| Pleuritis | 147 (21.4) | 42 (21.8) | 105 (21.2) | |
| Pericarditis | 124 (18.0) | 42 (21.8) | 83 (16.8) | |
| Renal disorder | 261 (37.9) | 91 (47.2) | 170 (34.3) | |
| Seizures | 32 (4.7) | 32 (16.6) | 0 (0) | |
| Psychosis | 40 (5.8) | 40 (20.7) | 0 (0) | |
| Haematological disorder | 412 (59.9) | 107 (55.4) | 305 (61.6) | |
| Auto antibodies (%) | ||||
| ANA | 669 (97.2) | 184 (95.3) | 485 (98.0) | |
| Anti-dsDNA-Ab | 447 (65.0) | 133 (68.9) | 314 (63.4) | |
| Anti-Sm-Ab | 139 (20.2) | 41 (21.2) | 98 (19.8) | |
| Anti-phospholipid-Ab | 287 (41.7) | 85 (44.0) | 202 (40.8) | |
| Anti-SSA-Ab | 242 (44.7) | 68 (35.2) | 174 (35.2) | |
| Anti-U1-RNP-Ab | 121 (29.3) | 35 (18.1) | 86 (17.4) |
Cardiovascular risk factors
The prevalence of smoking was 36.5% (251/688), of arterial hypertension 22.9% (158/688), diabetes mellitus 4.5% (31/688) and hyperlipidemia 9.4% (66/688).
SLE specific parameters
The mean number of ACR criteria was 5.2 (SD 1.6). The mean SDI was 1.5 (SD 2.3). The SLEDAI for the entire cohort was 6.7 (SD 7.7). We observed the following frequencies of autoantibodies (ab) in the SSCS cohort: ANA 97.2% (669/688), anti-dsDNA-ab 65% (447/688), anti-Sm-ab 20.2% (139/688), aPL-ab 41.7% (287/688), anti-SSA-ab 44.7%) (242/688) and anti-U1-RNP-ab 29.3% (121/688).
NPSLE
The nervous system (NS) was involved in 193 of 688 patients (28.1%). Isolated involvement of the central nervous system (CNS) was present in 136 patients (70.5%) and isolated involvement of the peripheral nervous system (PNS) was present in 34 patients (17.6%), in 23 patients (11.9%) both systems were involved. The 193 patients had 295 events, which results in 1.5 events per patient suffering from NPSLE with a range from 1-6 events, encompassing 16/19 NP manifestations. The most common CNS manifestations were headache (n = 51) followed by cerebrovascular disease (n = 49), psychosis (n = 45) and seizures (n = 37). Among the PNS manifestations, the most common was peripheral polyneuropathy (n = 25). We did not observe demyelinating syndrome, autonomic disorder and anxiety disorder. The prevalence of NPSLE of the SSCS contextualized with the relevant literature is outlined in Table 2.
Comparison of patients with and without NPSLE in the SSCS
SLE specific parameters
The number of ACR criteria in patients with NPSLE was higher compared to non-NPSLE subjects (5.5; SD 1.9 vs 5.0; SD 1.4; p < 0.001). NPSLE patients also had an increased SDI (2.2; SD 3.0 vs. 1.1; SD 1.9; p < 0.001).
The frequency of autoantibodies did not differ significantly between patients with and without NPSLE. We exclusively investigated the occurrence of aPL-antibodies in patients with cerebrovascular disease. They are clearly associated with the presence of cerebrovascular disease in patients with NPSLE (33/49 vs. 254/639; p < 0.001).
Cardiovascular risk factors
Arterial hypertension (60/193 vs. 98/495; p = 0.002) and hyperlipidemia (28/193 vs. 39/495; p = 0.014) were more common among patients with NPSLE. With regard to smoking (115/193 vs. 136/495; p = 0.735) and diabetes mellitus (13/193 vs. 18/495; p = 0.078) differences were not significant.
Course of NPSLE in the SSCS
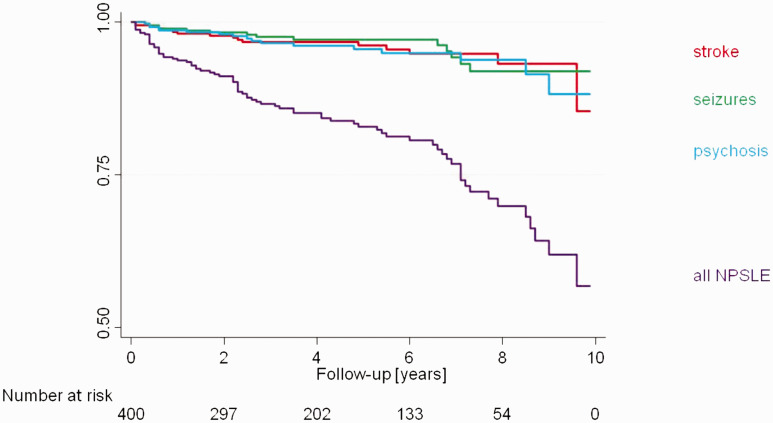
In the analysis regarding the temporal occurrence of the first NP event the mean time lag between the diagnosis of SLE and NP presence was 4.5 years (range 0.1 to 16.1 years). A comparison of the different NP manifestations did not reveal any major differences in the temporal course of the individual events (Figure 3).
Figure 3.
Kaplan-Meier curve of NP occurrence over 10 years of follow-up, overall and stratified for stroke, seizures and psychosis.
Discussion
Our study has two findings: over two dozen studies addressed the prevalence of neuro-psychiatric manifestations in SLE and we observed a striking range and diversity of clinical patterns in reporting NPSLE across studies, including our analysis of the SSCS cohort that could not be explained by divergent study characteristics.
SSCS patients suffering from NPSLE documented higher cumulative organ damage (SDI) and higher than average number of fulfilled ACR criteria compared to patients without NP events. The association of NPSLE with greater morbidity and mortality has already been described in numerous studies.4,11,18–24,37–40 The higher scoring of cumulative organ damage and also ACR criteria in patients with NPSLE suggest that NP events in SLE are a marker for a severe disease course with increased organ damage.
In the analysis of the SSCS patients, arterial hypertension (31.1% vs. 19.8%) and hyperlipidemia (14.5% vs.7.9) are positively associated with NP events. Patients with SLE have an increased cardiovascular risk and blood pressure and serum cholesterol levels are positively associated with cardiovascular events.41–45 Accelerated atherosclerosis and its long-term sequelae are most probably also responsible for NP events among patients with SLE. Therefore, cardiovascular risk factors in SLE patients should be treated aggressively to prevent the occurrence of cardiovascular and NPSLE events. However, traditional cardiovascular risk factors do not fully explain the high incidence of vascular events.46 Additional inflammatory processes involving antibodies and cytokines are likely to contribute to further neuropsychiatric damage. In a recent study, it was shown that anti-nervous system (NS) antibodies, most prevalently anti-MOG antibodies, are significantly associated with NPSLE. Anti-neuronal antibodies have to cross the blood-brain barrier to cause damage in the NS, it is conceivable that a disruption of blood-brain/blood-nerve barrier integrity may be mediated by vasculopathy that enables and drives autoantibody-mediated inflammation in the NS tissue.47–49 In accordance with the current literature we demonstrated that elevated values for aPL-ab are associated with the presence of cerebrovascular disease (33/49 vs. 254/639; p < 0.001).21,25–27,50 We did not find a higher prevalence of autoantibodies against dsDNA, Sm, SSA or U1-RNP in patients with NPSLE in general. Although anti-dsDNA- as well as anti-ribosomal-P-antibodies may be related with NPSLE, there is little agreement on positive or negative correlations.2,18,49–52
In the SSCS, NP events occurred even ten years after SLE diagnosis. This contradicts previously published studies in which NP events are described at disease onset or within the first 1-2 years after SLE diagnosis.1,2,5,9–11,18,20 Rather, we found a linear relationship between duration of follow-up and the probability of occurrence of NP events, both overall and for specific manifestations, such as stroke, seizures and psychosis (Figure 3).
The prevalence of NPSLE differs largely in the various studies and ranges from 10.6% to 96.4%.4,10,11,18,35,50 The prevalence of 28% within the SSCS cohort seems to be underreported when compared to the current literature (Figure 2). However minor NP events including mild cognitive dysfunction, headache, mild depression, anxiety and ENMG-negative polyneuropathy are common and nonspecific features and differ hardly between SLE patients and the normal population.30 These subtle NP manifestations cause the large prevalence differences between studies about NPSLE.2 Standard clinical practice applied by physicians does not routinely include assessments for cognitive dysfunction and mood disorders with test batteries as proposed by the ACR committee. Psychiatric diagnoses of mood disorder, anxiety and cognitive dysfunction, should be defined according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria usually applied in psychiatry.53 But neuropsychological test batteries to diagnose cognitive dysfunction are time consuming and not regularly performed during follow up visits or within studies.10,19,22,26 Due to the lack of the above-mentioned testing, none of the studies included in our review distinguished between e.g. mild or severe depression, cognitive dysfunction, and so forth. Therefore, a prevalence analysis of NPSLE only considering major events in our meta-analysis by omitting the five syndrome-categories, that are considered as minor and mild forms, would not be correct.
Prevalence of major NP events with clear diagnostic criteria including movement disorder, seizure and stroke, did not differ much between studies (Table 2). The most frequent major NP events in the SSCS were cerebrovascular disease, psychosis and seizures. This is in line with the current literature, also shown in Table 2.4,10,11,18–35 Five of the 19 ACR case definitions including demyelinating syndrome, Guillain-Barré syndrome, autonomic disorder, myasthenia gravis and plexopathy, were underrepresented or not represented at all in the SSCS. This is despite the fact that our cohort is sufficiently large to be able to make general statements. However, certain NP syndromes are also scarcely present in other large cohorts (Table 2).
Limitations
There are a number of potential limitations to our cohort study. As a cross-sectional study, the validity of disease history depends on the documentation of past disease episodes. However, since the SSCS data were collected prospectively, a certain reliability of the documentation can be assumed. Therefore, we think that this is unlikely to have had a substantial effect on the prevalence rates of the NP syndromes. In addition, SSCS is more likely to include patients with a more severe course of the disease since they were recruited in tertiary centers. Another downside of the study is the lack of use of the test batteries defined by ACR for the diagnosis of cognitive impairment and mood disorder. However, these tests have not been used by most of the reviewed literature,10,19,22,26 since they are very time consuming for daily practice. Furthermore, we did not differ between primary and secondary NP events, as there is no clinically reliable method to determine the attribution of NP events to SLE with certainty.
The studies included in the meta-analysis showed a high heterogeneity in the frequency of neuro-psychiatric syndromes. We tried to reduce this variability by including only prospective studies from the beginning. A potential bias is the various ethnicities represented in different studies. Several studies examined only a single ethnic group, while others looked at ethnically diverse groups. We also included studies with both adults and pediatric patients, which may have increased the heterogeneity of the results. However, the occurrence of a publication bias is relatively unlikely, as there is no “wrong” or “right” in the investigation of frequencies.
Conclusion
In summary, there are large differences in the frequency of NPSLE in comparison to individual studies. This is mainly due to the common symptoms headache, anxiety, mood disorders, depression and psychosis being categorized under the criteria of NPSLE. It should be discussed whether all SLE patients should receive routine neuropsychological testing and imaging at regular intervals. On one hand, this would provide more clarity regarding the prevalence of so-called “minor” events in SLE patients; on the other hand, it would enable prompt intervention in case of their occurrence.
Furthermore, the temporal occurrence of symptoms can present at different stages of the disease and as clinicians, it is important to be aware that neuro-psychiatric events can often present many years after an initial SLE diagnosis.
We call for concerted interdisciplinary teamwork within cohort studies including the expertise of neurologists and psychiatrists to provide a better understanding of NPSLE. Moreover, mechanisms of psychiatric lupus manifestations require a better understanding in order to establish objective biomarkers. Due to these minor symptoms, there is the need for broad consensus regarding stringent definitions of neuro-psychiatric SLE manifestations that facilitate accurate diagnosis, particularly with a view to timely intervention and improving patient outcomes.
Acknowledgements
We thank Dr. med. John Hanly and Dr. med. David D’Cruz for their valuable comments and advice. We would also like to thank Dr. med. Alessandra Bortoluzzi, who provided us with an additional data set.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aline L Meier https://orcid.org/0000-0003-0698-711X
References
- 1.Hanly JG.Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol 2014; 10: 338–347. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz N, Stock AD, Putterman C.Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 2019; 15: 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001; 45: 419–423. [DOI] [PubMed] [Google Scholar]
- 4.Hanly JG, Urowitz MB, Su L, et al.; Systemic Lupus International Collaborating Clinics (SLICC). Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2010; 69: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tay SH, Mak A.Diagnosing and attributing neuropsychiatric events to systemic lupus erythematosus: time to untie the gordian knot? Rheumatology (Oxford) 2017; 56: i14–i23. [DOI] [PubMed] [Google Scholar]
- 6.Borowoy AM, Pope JE, Silverman E, et al. Neuropsychiatric lupus: the prevalence and autoantibody associations depend on the definition: results from the 1000 faces of lupus cohort. Semin Arthritis Rheum 2012; 42: 179–185. [DOI] [PubMed] [Google Scholar]
- 7.Hanly JG.Attribution in the assessment of nervous system disease in SLE. Rheumatology (Oxford) 2015; 54: 755–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanouriakis A, Pamfil C, Rednic S, et al. Is it primary neuropsychiatric systemic lupus erythematosus? Performance of existing attribution models using physician judgment as the gold standard. Clin Exp Rheumatol 2016; 34: 910–917. [PubMed] [Google Scholar]
- 9.Bertsias GK, Ioannidis JP, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010; 69: 2074–2082. [DOI] [PubMed] [Google Scholar]
- 10.Hanly JG, Su L, Farewell V, et al. Prospective study of neuropsychiatric events in systemic lupus erythematosus. J Rheumatol 2009; 36: 1449–1459. [DOI] [PubMed] [Google Scholar]
- 11.Magro-Checa C, Zirkzee EJ, Beaart-van de Voorde LJJ, et al. Value of multidisciplinary reassessment in attribution of neuropsychiatric events to systemic lupus erythematosus: prospective data from the Leiden NPSLE cohort. Rheumatology (Oxford) 2017; 56: 1676–1683. [DOI] [PubMed] [Google Scholar]
- 12.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42: 599–608. [DOI] [PubMed] [Google Scholar]
- 13.Ribi C, Trendelenburg M, Gayet-Ageron A, et al.; Swiss Systemic Lupus Erythematosus Cohort Study Group. The Swiss systemic lupus erythematosus cohort study (SSCS) – cross-sectional analysis of clinical characteristics and treatments across different medical disciplines in Switzerland. Swiss Med Wkly 2014; 144: w13990. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC.Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40: 1725. [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Kim MY, Kalunian KC, et al.; OC-SELENA Trial. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005; 353: 2550–2558. [DOI] [PubMed] [Google Scholar]
- 17.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39: 363–369. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Sattar AB, Goda T, Negm MG.Neuropsychiatric manifestations in a consecutive cohort of systemic lupus erythematosus; a single center study. Int J Rheum Dis 2013; 16: 715–723. [DOI] [PubMed] [Google Scholar]
- 19.Ahn GY, Kim D, Won S, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus 2018; 27: 1338–1347. [DOI] [PubMed] [Google Scholar]
- 20.Kakati S, Barman B, Ahmed SU, et al. Neurological manifestations in systemic lupus erythematosus: a single centre study from North East India. J Clin Diagn Res 2017; 11: OC05–OC9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok CC, To CH, Mak A.Neuropsychiatric damage in Southern Chinese patients with systemic lupus erythematosus. Medicine (Baltimore) 2006; 85: 221–228. [DOI] [PubMed] [Google Scholar]
- 22.Morrison E, Carpentier S, Shaw E, et al. Neuropsychiatric systemic lupus erythematosus: association with global disease activity. Lupus 2014; 23: 370–377. [DOI] [PubMed] [Google Scholar]
- 23.Muhammed H, Goyal M, Lal V, et al. Neuropsychiatric manifestations are not uncommon in Indian lupus patients and negatively affect quality of life. Lupus 2018; 27: 688–693. [DOI] [PubMed] [Google Scholar]
- 24.Zirkzee EJ, Steup-Beekman GM, van der Mast RC, et al. Prospective study of clinical phenotypes in neuropsychiatric systemic lupus erythematosus; multidisciplinary approach to diagnosis and therapy. J Rheumatol 2012; 39: 2118–2126. [DOI] [PubMed] [Google Scholar]
- 25.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002; 58: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 26.Briani C, Lucchetta M, Ghirardello A, et al. Neurolupus is associated with anti-ribosomal P protein antibodies: an inception cohort study. J Autoimmun 2009; 32: 79–84. [DOI] [PubMed] [Google Scholar]
- 27.Sanna G, Bertolaccini ML, Cuadrado MJ, et al. Neuropsychiatric manifestations in systemic lupus erythematosus: prevalence and association with antiphospholipid antibodies. J Rheumatol 2003; 30: 985–992. [PubMed] [Google Scholar]
- 28.Abdel-Nasser AM, Ghaleb RM, Mahmoud JA, et al. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clin Rheumatol 2008; 27: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 29.Afeltra A, Garzia P, Mitterhofer AP, et al. Neuropsychiatric lupus syndromes: relationship with antiphospholipid antibodies. Neurology 2003; 61: 108–110. [DOI] [PubMed] [Google Scholar]
- 30.Ainiala H, Loukkola J, Peltola J, et al. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 2001; 57: 496–500. [DOI] [PubMed] [Google Scholar]
- 31.Appenzeller S, Rondina JM, Li LM, et al. Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum 2005; 52: 2783–2789. [DOI] [PubMed] [Google Scholar]
- 32.Schenatto CB, Xavier RM, Bredemeier M, et al. Raised serum S100B protein levels in neuropsychiatric lupus. Ann Rheum Dis 2006; 65: 829–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sibbitt WL, Jr, Brandt JR, Johnson CR, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol 2002; 29: 1536–1542. [PubMed] [Google Scholar]
- 34.van der Meulen PM, Barendregt AM, Cuadrado E, et al. Protein array autoantibody profiles to determine diagnostic markers for neuropsychiatric systemic lupus erythematosus. Rheumatology (Oxford) 2017; 56: 1407–1416. [DOI] [PubMed] [Google Scholar]
- 35.Zavada J, Nytrova P, Wandinger KP, et al. Seroprevalence and specificity of NMO-IgG (anti-aquaporin 4 antibodies) in patients with neuropsychiatric systemic lupus erythematosus. Rheumatol Int 2013; 33: 259–263. [DOI] [PubMed] [Google Scholar]
- 36.Bundedamt für Statistik Schweiz. Bevölkerung nach Migrationsstatus, https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/migration-integration/nach-migrationsstatuts.html (2020, accessed 15 April 2021).
- 37.Hanly JG, Urowitz MB, Su L, et al.; Systemic Lupus International Collaborating Clinics. Short-term outcome of neuropsychiatric events in systemic lupus erythematosus upon enrollment into an international inception cohort study. Arthritis Rheum 2008; 59: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kampylafka EI, Alexopoulos H, Kosmidis ML, et al. Incidence and prevalence of major central nervous system involvement in systemic lupus erythematosus: a 3-year prospective study of 370 patients. PLoS One 2013; 8: e55843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanly JG, Li Q, Su L, et al. Peripheral nervous system disease in systemic lupus erythematosus: results from an international, inception cohort study. Arthritis Rheumatol 2020; 72: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al.; Systemic Lupus International Collaborating Clinics. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum 2007; 56: 265–273. [DOI] [PubMed] [Google Scholar]
- 41.Fischer LM, Schlienger RG, Matter C, et al. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol 2004; 93: 198–200. [DOI] [PubMed] [Google Scholar]
- 42.Magder LS, Petri M.Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol 2012; 176: 708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta JL, Saldeen TG, Rand K.Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol 1998; 31: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 44.Stojan G, Petri M.Atherosclerosis in systemic lupus erythematosus. J Cardiovasc Pharmacol 2013; 62: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenig KF, Ribi C, Radosavac M, et al.; Swiss SLE cohort study (SSCS). Swiss SLEcs. Prevalence of vascular disease in systemic lupus erythematosus compared with type-1 diabetes mellitus: a cross-sectional study of two cohorts. Lupus 2015; 24: 58–65. [DOI] [PubMed] [Google Scholar]
- 46.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001; 44: 2331–2337. [DOI] [PubMed] [Google Scholar]
- 47.Govoni M, Bombardieri S, Bortoluzzi A, Caniatti L, et al. Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology (Oxford) 2012; 51: 157–168. [DOI] [PubMed] [Google Scholar]
- 48.Hanly JG, Kozora E, Beyea SD, et al. Review: nervous system disease in systemic lupus erythematosus: Current status and future directions. Arthritis Rheumatol 2019; 71: 33–42. [DOI] [PubMed] [Google Scholar]
- 49.Jafri K, Patterson SL, Lanata C.Central nervous system manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 2017; 43: 531–545. [DOI] [PubMed] [Google Scholar]
- 50.Hanly JG, Urowitz MB, Siannis F, et al.; Systemic Lupus International Collaborating Clinics. Autoantibodies and neuropsychiatric events at the time of systemic lupus erythematosus diagnosis: results from an international inception cohort study. Arthritis Rheum 2008; 58: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Probstel AK, Thanei M, Erni B, et al.; Swiss Systemic Lupus Erythematosus Cohort Study Group. Association of antibodies against myelin and neuronal antigens with neuroinflammation in systemic lupus erythematosus. Rheumatology (Oxford) 2019; 58: 908–913. [DOI] [PubMed] [Google Scholar]
- 52.Mok MY, Chan EY, Fong DY, et al. Antiphospholipid antibody profiles and their clinical associations in Chinese patients with systemic lupus erythematosus. J Rheumatol 2005; 32: 622–628. [PubMed] [Google Scholar]
- 53.Battle DE.Diagnostic and statistical manual of mental disorders (DSM). Codas 2013; 25: 191–192. [DOI] [PubMed] [Google Scholar]