Abstract
Context:
Differentiating NSCLC as either adeno or squamous type and identification of Epidermal Growth Factor Receptor (EGFR) mutations is clinically relevant for lung cancer patients for selecting treatment. Thyroid transcription factor-1 (TTF-1) and p63 were demonstrated as useful markers for histologic typing of lung cancer. Mutation and overexpression of EGFR has been reported in a subset of non-small cell lung cancers. If these markers can be validated for the differential diagnosis of adenocarcinoma in a sputum sample itself, it will be highly beneficial for lung cancer patients.
Aims:
To evaluate whether immunocytochemical expression of TTF-1, p63, and EGFR proteins in sputum samples can be used for differential diagnosis of lung adenocarcinoma by comparing with that of the corresponding tissue samples.
Settings and Design:
Ninety sputum samples and matched tissue samples were used for the study.
Subjects and Methods:
Monolayered smears and cell blocks of sputum and the corresponding tissue samples were immunostained with the standard ABC method. The expression patterns of these markers were analyzed statistically and compared with clinic-pathological parameters.
Statistical Analysis Used:
Chi-square test and paired t-test.
Results:
The p63 protein had a positive expression in 73.9% of SCC whereas TTF1 had positive expression in 75.8% of ADC. The EGFR expression was positive in 27 cases of adenocarcinoma, 21 cases of SCC and 19 cases of NSCLC.
Conclusions:
Immunocytochemistry of the aforementioned antibodies in sputum samples can be used as supplementary evidence for the subtyping of NSCLC.
Keywords: Epidermal growth factor receptor, non-small cell lung carcinoma, p63, sputum cytology, TTF1
INTRODUCTION
Non-small cell lung carcinoma (NSCLC) accounts for approximately 75–85% of the lung cancers and is a highly heterogeneous group of disease with variable phenotypes.[1] Earlier non-small cell lung carcinoma (NSCLC) was lumped together without attention to more specific histologic typing (i.e., adenocarcinoma, squamous cell carcinoma) as there was no therapeutic implication on classifying histologic subtypes such as adenocarcinoma and squamous cell carcinoma. But now a days the differential diagnosis of non-small cell carcinoma as either adeno or squamous type and identification of molecular abnormalities such as Epidermal Growth Factor Receptor (EGFR) mutations and Echinoderm Microtubule Associated Protein like 4 –Anaplastic Lymphoma Kinase (EML4-ALK) rearrangements are clinically relevant. These mutations are found more frequently in females, never smokers, patients diagnosed with adenocarcinoma, and patients of East Asian ethnicity.[2,3] EGFR tyrosine kinase inhibitors are suggested as the first-line therapy in patients who have advanced lung adenocarcinoma with EGFR mutations.[4] EGFR targeted drugs, such as gefitinib and erlotinib, are found to be more efficacious against adenocarcinoma, and the use of antiangiogenic modalities are contraindicated in squamous cell carcinoma, as it can cause life-threatening pulmonary haemorrhage.[5] Moreover, another new antifolate drug pemetrexed is reported to be very effective in adenocarcinoma but is not effective in squamous cell carcinomas.[6]
With the introduction of modified processing technique, sputum cytology can have an important role in lung cancer diagnosis. The application of immunocytochemistry in sputum sample is also well documented.[7] If a panel of reliable markers can be validated for the differential diagnosis of adenocarcinoma in sputum sample itself, it will be highly advantageous to lung cancer patients in selecting specific treatments, without sophisticated investigations like bronchoscopy and biopsy.
TTF-1 has been identified as a 38 k Da homeodomain-containing transcription factor essential for the morphogenesis and differentiation of the thyroid, lung, and ventral forebrain.[8] In the lung, TTF-1 is responsible for transcriptional activation of surfactant proteins A, B, C, and Clara cell secretory proteins. Neoplasms of pulmonary origin have been found to retain variable TTF-1 expression according to histologic type.[9]
The p63 protein is a member of the p53 family, located on chromosome 3q27–29.[10] It encodes six splice variants and possessing a p53-like NH2- terminal transactivating domain known as TAp63 and has properties similar to p53.[10] In lung neoplasms, it is expressed consistently in SCC but is not detected in small cell carcinoma.
EGFR is a member of the HER/erbB family of tyrosine kinase receptor proteins and somatic mutation of the EGFR kinase domain (exon 18-21) is the main mechanism of receptor activation. Mutation and overexpression of EGFR has been reported in a subset of non-small cell lung cancers (NSCLC). The altered activation of EGFR activates the Ras- Raf- MEK-MAPK pathway, that induce the proliferation, differentiation, cell motility, adhesion, and angiogenesis.[11]
In most of the previous studies the immunoexpression of these markers were studied in tissue samples. But now a days liquid-based cytology (LBC) preparations are being used increasingly, especially bronchial washings and brushings and have been shown to be superior to the conventional method.[12] The introduction of LBC has improved the diagnostic accuracy, decreased screening time, and helped in the removal of obscuring background materials and blood.[13] Furthermore, cell fixation by LBC may be better than 95% ethanol fixation for immunocytochemistry. SurePath technique is a thin-layer, LBC technique that is based on a sedimentation process using CytoRich Red solution in non-gynaecologic cytology.[14] Sputum homogenization using the CytoRich Red solution provide the whole cell content of the sputum sample which can be used for morphological evaluation as well as molecular analysis including immunocytochemistry.[7] Even though some studies have examined the utility of immunostaining for Napsin A, TTF-1, and p63 using cytological or histologic samples, its application in sputum samples has not yet been reported.[15]
The aim of the current study was to evaluate the expression levels of TTF-1, p63, and EGFR proteins in sputum samples and to compare the expression pattern of these proteins in corresponding tissue samples by immunocytochemistry to see whether these markers can be employed for the differential diagnosis of NSCLC in sputum sample itself. The expression of these markers was correlated with various clinico-pathological features to see whether expression of any of these proteins is associated with tumor grade, stage, and metastasis or smoking habits.
SUBJECTS AND METHODS
The subjects for the study were selected from a cohort of 3185 patients referred from the Sanatorium for Chest Disease and Medical College Hospital, with a history of chronic obstructive pulmonary disease and/radiologic findings suspicious of malignancy. From this, sputum samples of 90 patients were selected based on the presence of desired number of cells and availability of corresponding tissue samples and clinical data. This includes 33 adenocarcinoma (ADC), 23 squamous cell carcinomas (SCC), 25 non-small cell carcinomas (NSCLC-NOS), and 9 small cell carcinomas (SCLC). The study was approved by the Institutional Review Board and Human Ethics Committee and informed consent was obtained from each subject. [IRB-9-2010 dated 5-12-2009, HEC-7-2010 dated 5-3-2010].
Sputum samples collected for five consecutive days were homogenized and processed using “Cytorich red solution” (Tripath Imaging Inc. Burlington NC, 27215, USA). From the sediment obtained, monolayer smears were prepared on pre-coated slides and the remaining samples were used for cellblock preparation.[7] Immunohisto/chemistry was performed in 4 micron sections obtained from cell blocks of sputum and corresponding tissue samples according to the standard ABC technique using DAB as chromogen. Immunocytochemistry was done on monolayer smears also whenever adequate sample was available for which cell permeability was enhanced by treating with Sodium deoxy cholate. Primary antibodies were procured from Santha Cruz Laboratories (EGFR, p63, Mouse monoclonal, dilution 1:100) and Novacastra (TTF, Mouse monoclonal antibody, dilution 1:50). Appropriate positive lung adenocarcinoma for TTF-1 and breast sclerosing adenosis for p63 and human breast carcinoma tissue for EGFR were used as positive control. The primary antibody was substituted by Phosphate Buffer Saline (PBS) in negative control.
Immunoscoring was done by two of the investigators independently and a repeat scoring was performed if 100% agreement was not obtained for sputum samples and corresponding tissue samples. H-scores were then calculated as the product of intensity (0-3) and distribution (0-100%) with H-score ranging from 0 to 300, by counting a minimum of 200 well-defined malignant cells. H-scores 30 and above was taken as positive expression. Tumors were considered to be immune positive for TTF-1 or p63, if the tumor cells demonstrated unequivocal nuclear staining. On the other hand, for EGFR, cell membrane and cytoplasmic staining were considered as specific criteria for immune positivity. Western blot analysis was performed for all the markers to assess the sensitivity of the antibodies.
Statistics
The expression pattern of different markers in different lung lesions and its association with various clinico-pathological features were assessed by Chi-square test. Paired t test was performed to compare the expression pattern of these proteins in cytology and histology samples. All statistical tests were two sided and P = < 0.05 was considered to be significant. Analysis was performed with SPSS software version 11.0.
RESULTS
Protein extract isolated from bronchial biopsy samples from tumor tissue showed prominent bands at molecular weight corresponding to 38 k Da and 62 k Da which are specific for TTF-1 and p63, respectively. Samples with metaplastic cells showed mild expression at 62 k Da and 170 k Da corresponding to p63 and EGFR [Figure 1].
Figure 1.
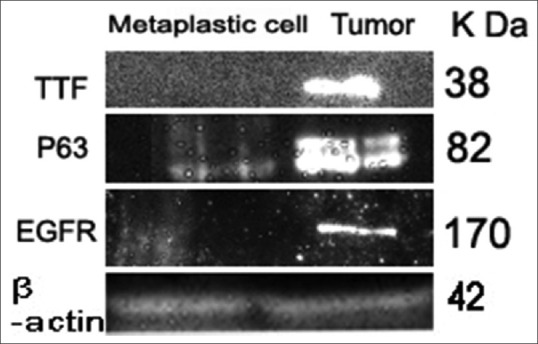
Western blot analysis of TTF1, p63 & EGFR. Lane. 1. Shows dense bands for tumor samples, diffuse expression for samples with metaplastic cells at 38 k Da corresponding to TTF1 protein. Lane. 2. Shows dense bands for tumor sample, mild bands for samples with metaplastic cells at 63 k Da corresponding to p63 protein. Lane. 3. Shows dense bands for tumor and metaplastic cells at 42 k Da corresponding to ß actin
Figure 2a shows mean H- score of p63 with respect to different histologic types of lung cancers. A higher mean H-score was observed for SCC (107.6) whereas it was significantly lower for ADC (33). A higher expression of TTF1 was found in adenocarcinoma with a mean H score of 116.2 whereas for SCC it was comparatively low. Figure 2b shows boxplot representing the mean H-score of TTF1 with respect to different lung lesions. Whereas EGFR had mean scores of 116.4 for NSCLC, 119.45 for ADC, and 124.78 for SCC. Figure 2c represents boxplot showing the mean H-score of EGFR with different lung lesions.
Figure 2.
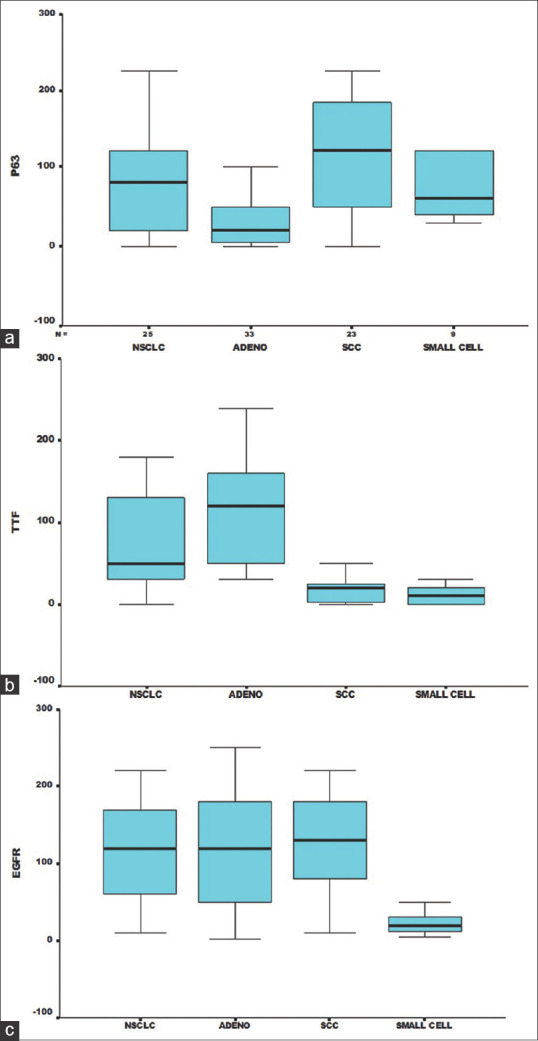
(a) Boxplot showing mean H score of p63 with respect to different histologic types of lung cancer. (b) Boxplot showing mean H score of TTF1 with respect to different histologic types of lung cancer. (c) Boxplot showing mean H score of EGFR with respect to different histologic types of lung cancer
The morphological features of squamous cell carcinoma are shown in Figure 3a. In squamous cell carcinoma, malignant cells show squamous differentiation with more or less centrally placed nucleus and squamoid cytoplasm, they often show heavy keratinization, and usually presented with marked pleomorphism (tadpoles, snakes, and pumpkin cells) and with hyper chromatic, dark, ink dot nucleus. Cytoplasm is usually dense, waxy, and varies from scant to abundant [Figure 3a]. Whereas in adenocarcinoma, the cells are found mainly in characteristic groups such as acini, tubules, cell balls, papillae depending on the degree of differentiation. Poorly differentiated adenocarcinoma is seen in syncytial aggregates. Adenocarcinoma cells are relatively large and vary from cuboidal to columnar with nucleocytoplasmic polarity. Cytoplasm is homogeneous to foamy or finely vacuolated and usually basophilic. Enlarged nuclei with high N/C ratio, finely granular chromatin with prominent nucleoli may also be noticed [Figure 3b]. NSCLC-NOS lack morphological features of glandular, squamous, and neuroendocrine tumors. Malignant cells are relatively large and undifferentiated, sometimes uniform, but pleomorphic, plenty of single cells and groups also present. Groups of cells appear as syncytia with irregularly arranged nuclei [Figure 3c].
Figure 3.
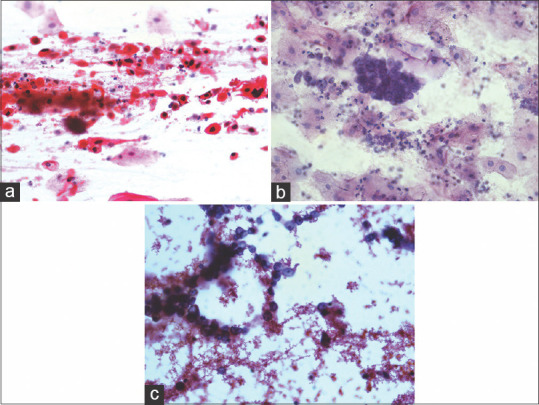
Malignant cells in sputum samples showing characteristic features. (a) Squamous cell carcinoma (sputum 40x, Pap stain). (b) Adenocarcinoma cells (Sputum 40x, Pap stain). (c) Non-small cell carcinoma (Sputum 40x, Pap stain)
The expression of TTF1 and p63 was concentrated in the nucleus and the pattern of expression was either diffuse, dense, or focal [Figure 4a–d]. But EGFR protein expression was concentrated mainly in the cell membrane and cytoplasm of tumor cells [Figure 5a–d] but the intensity was found to vary in different lesions. Immuno expression was considered positive when H-score was 30 and above.
Figure 4.
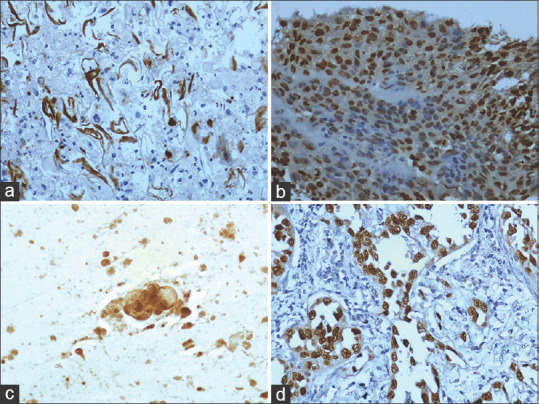
Immunoexpression of p63 and TTF1. (a) Intense nuclear expression of p63 squamous cell carcinoma (Sputum 40x). (b) Dense nuclear expression of p63 in squamous cell carcinoma (Tissue 40x). (c) Intense nuclear expression of TTF1 in adenocarcinoma (Sputum 40x). (d) Intense nuclear expression of TTF1 in adenocarcinoma (Tissue 40x)
Figure 5.
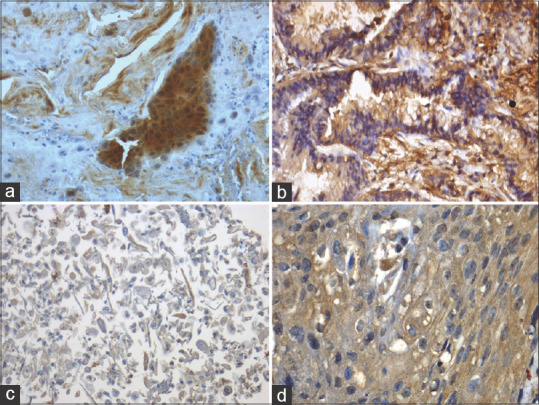
Immunoexpression of EGFR. (a) Intense expression of EGFR in cell membrane and cytoplasm of adenocarcinoma (Sputum cellblock 40x). (b) Intense expression of EGFR in cell membrane and cytoplasm of adenocarcinoma (Tissue 40x). (c) Diffuse EGFR expression in cell membrane and cytoplasm of squamous cell carcinoma (Sputum cell block 40x). (d) EGFR cell membrane and cytoplasmic expression in squamous cell carcinoma (Tissue 40x)
Immunoexpression of different lesions of the lung revealed that p63 had a positive expression in 73.9% of SCC whereas TTF1 had positive expression in 75.8% of ADC. Among the morphologically diagnosed cases, 4 (12.1%) adenocarcinoma, 2 (8.7%) squamous cell carcinoma and 5 (20%) samples of non-small cell carcinoma had both p63 and TTF1 expression [Table 1]. After IHC study, 10 NSCLC cases with positive TTF1 expression and negative p63 expression were included in NSCLC favors adenocacinoma, 9 cases with positive p63 expression and negative TTF1 expression were included in NSCLC favors squamous cell carcinoma, remaining 5 (20%) cases with positive expression for p63 and TTF1 were included in NSCLC-NOS [Table 1].
Table 1.
Number and Percentage of Samples positive for p63, TTF1 and EGFR in different lesions
| Marker protein | Different Lesions of lung |
|||||||
|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma (n-33) |
Squamous cell carcinoma (n-23) |
Non small cell carcinoma (n-25) |
Small cell carcinoma (n-9) |
|||||
| Positive # (%) | Negative # (%) | Positive # (%) | Negative # (%) | Positive # (%) | Negative # (%) | Positive # (%) | Negative # (%) | |
| P63 | 5 (15.2) | 24 (72.7) | 17 (73.9) | 4 (17.4) | 9 (36) * | 11 (44) | 9 (100) | 0 |
| TTF1 | 25 (75.8) | 4 (12.1) | 5 (21.7) | 16 (69.6) | 10 (40) † | 10 (40) | 0 | 9 (100) |
| Both P63 & TTF1 | 4 (12.1) | 0 | 2 (8.7) | 0 | 5 (20)‡ | 0 | 0 | 0 |
| EGFR | 10 (30.3) | 1 (3.03) | 5 (21.73) | 0 | 4 (16) | 1 (4) | 1 (11.1) | 1 (11.1) |
| EGFR & p63 | 1 (3.03) | 1 (3.03) | 12 (52.17) | 1 (4.35) | 5 (20) | 1 (4) | 0 | 5 (55.6) |
| EGFR & TTF1 | 15 (45.46) | 2 (6.06) | 3 (13.04) | 0 | 8 (32) | 2 (8) | 0 | 0 |
| EGFR, P63 & TTF1 | 1 (3.03) | 2 (6.06) | 1 (4.35) | 1 (4.35) | 2 (8) | 2 (8) | 1 (11.1) | 1 (11.1) |
*NSCLC favors SCC, †NSCLC favors ADC, ‡NSCLC -NOS
The EGFR expression was positive in 27 cases of adenocarcinoma, 21 cases of SCC and 19 cases of NSCLC. Among them 3.03% of adenocarcinoma, 4.35% SCC and 8% NSCLC showed positivity of EGFR, p63, TTF1. [Table 1]. Among the 27 EGFR positive cases of adenocarcinoma, 15 (45.46%) cases showed TTF1 positivity also. On the other hand, 10 cases had only EGFR positivity. In SCC 12 (52.17%) cases of EGFR positive cases had p63 positivity. Among NSCLC 32% showed both EGFR and TTF1 expression [Table 1].
The correlation analysis of various clinic-pathological features with the expression pattern of p63, TTF1 and EGFR revealed that p63 expression has significant association with smoking status (p = 0.006), histologic type of tumor (p = 0.000), tumor stage (p = 0.04), and tumor grade (p = 0.05). TTF1 expression was also found to have significant association with smoking status (p = 0.01), histologic type (p = 0.0001). Whereas EGFR expression had significant association with histologic type of tumor (p = 0.000) and metastasis (p = 0.028).
A slight variation has been noticed in the expression pattern of markers in monolayered smears and cellblocks with respect to tissue samples in the intensity of staining and number of cells having positive expression. Cell block and biopsy samples had slight difference in the staining intensity, which was not statistically significant. Nevertheless, all samples with positive expression in histology samples expressed positivity in cytology samples also.
DISCUSSION
Recently, tumor subtyping has been emerged as a critical point in selecting treatment strategies for NSCLC patients.[16] So differentiating adeno and squamous cell carcinomas is of prime importance in lung cancer management. As most of the lung cancer patients present in an advanced unresectable stage, cytology material, or small biopsy samples are the only available specimen for diagnosis.[17]
The key morphologic features often followed to differentiate ADC and SCC is glandular pattern with vacuolated cytoplasm and isolated cells with eccentric nucleus and prominent nucleoli for the former and keratinized or non-keratinized cells with marked pleomorphism and ink dot nucleus for SCC. The distinction of the ADC and SCC can be made in majority of cases by assessing morphologic features alone. But poorly differentiated malignancies are difficult to classify on the basis of morphologic criteria. Other factors that mislead the differential diagnosis of NSCLC include sampling errors and poor specimen preparations.
Cytology samples, especially sputum samples have advantages over histology specimens, a cellblock also can be made from sputum samples for immunocytochemistry and other prospective studies. LBC of sputum samples using Cytorich® red preservative can provide adequate samples with good preservation of morphology.[7] Nicholson et al.,[18] have demonstrated the advantages of LBC and have reported that this method has been found to improve the diagnostic rate; however, a few artifactual sampling errors also have been reported.
In the current study sputum monolayered smears/cell blocks prepared by LBC were used for immunocytochemistry. It is well demonstrated that cytology samples and corresponding tissue samples have shown concordance in most of the cases studied, even though slight variation in staining pattern was noticed. So, it can be employed as an alternate method of sample preparation for immunocytochemistry.
The p63 protein has been well demonstrated as a marker of squamous differentiation and overexpression of this gene has been identified in lung SCCs by global gene expression profiling as well as by IHC.[19] In the current study among 23 cases of SCCs, 82.6% cases were positive for p63 and this was in concordance with other studies.[20] Kargi et al.[21] had reported p63 positivity by IHC in 80% of SCC samples, but it was also demonstrated that the better differentiated areas and even well differentiated tumors may be negative for p63. Since the samples with well differentiated SCC can easily be diagnosed by morphology, p63 IHC is not necessary. In samples which were diagnosed as NSCLC, 36% cases were positive for p63, and negative for TTF1 and 40% cases were positive for TTF1 only. The original tissue sections of these samples were subjected to review and found that these tissues could have been sub typed if we could do immunocytochemistry in these samples at the time of reporting.
In our study, 15.2% of the ADCs were p63 positive, among these 4 cases had TTF1 positivity also. A similar observation has been found in previous studies also, where 0 to 33% of lung ADCs was found to express p63.[22] These differences have been reported to be either due to the non-specificity of the antibody used to detect p63 or due to the faulty interpretation of the staining. The chances of first possibility are rare as most of the p63 antibodies used in IHC studies is polyclonal, which detects all p63 isoforms (TAp63a, TAp63b, TAp63c, DNp63a, DNp63b, and DNp63c).[23] In the current study, we have used monoclonal antibodies and the sensitivity of the antibody was confirmed in western blot. The current study has considered H-score above 30 as positive expression. But while analyzing the interpretation of staining, faint or focal immunostaining for p63 should be considered as non-specific unless there is proof suggesting that it was not positive. The positivity for p63 immunoexpression should be considered when high intensity staining present in at least 50% of tumor cells which will increase the specificity of p63.[24] Our study also supports this observation. So p63 can be employed as a marker for SCC.
In the lungs, the major role of TTF-1 is transcriptional activation of surfactant proteins A, B, and C and Clara cell secretory proteins and different histologic types of lung neoplasms have been found to retain variable TTF-1 expression.[25] The antibodies against TTF-1 antigen are well established for the distinguishing between primary lung adenocarcinomas and metastatic adenocarcinomas from other sites. In this study 75.8% adenocarcinomas and 40% of NSCLCs expressed immunopositivity for TTF-1, but 5 cases of adenocarcinoma had p63 positivity. It is reported that 7–10% of primary SCC also expresses positivity for TTF-1.[26] In the current study also 7 cases of SCC also showed positivity for TTF-1. TTF-1 positivity is not universally specific to the lung and is seen in up to 100% of primary thyroid carcinomas and in up to 90% of small cell carcinomas of any origin.[27] So TTF-1 can be considered as a reliable marker for ADC, only if we have staining pattern of p63 also. A TTF 1 positive and p63 negative tumor can reliably be diagnosed as ADC. Hence, a panel of p63 and TTF1 is suggested as reliable markers rather than using a single marker. Hence, the final diagnosis should be based on a combined approach to morphologic parameters as well as marker expression.
In lung cancer, it was well documented that most of the EGFR mutations occur in ADC and also seen in adenosquamous carcinoma.[3] But in some of the studies no correlation could be found between EGFR overexpression and histology and no difference was seen between adenocarcinoma and squamous cell carcinoma. On the contrary, in certain other studies, the highest expression of EGFR was observed in squamous cell carcinomas.[28] Overexpression of EGFR was noticed in the present study also in most of the tumors even though significant difference (p = 0.0001) was observed between different histologic types. This is in concordance with other studies that observed overexpression of EGFR in NSCLC ranging from 43% to 89%.[29] The variations in the EGFR expression pattern from one study to another may be due to differences in the assessment techniques, definition of the level of overexpression, and differences in the study populations. There is increasing evidence for an especially high EGFR expression in bronchioalveolar carcinoma and one preliminary study has demonstrated a significant correlation between nonmucinous bronchioalveolar carcinoma (BAC) histology and EGFR expression.[29] However, the current study is not in concordance with this observation as EGFR expression was noticed in 27.5% adenocarcinoma, 39.1% of SCC, 30.4% of NSCLC and 2.9% of SCLC.
In conclusion the differential diagnosis of ADC and SCC can be achieved by selecting an optimal combination of antibodies along with morphologic evaluation. Immunocytochemistry of the aforementioned antibodies in monolayered smear/cell block preparations can be used as supplementary evidence for the subtyping of NSCLC. Samples positive for TTF1 and negative for p63 could be diagnosed as adenocarcinoma. Similarly, p63 positive TTF1 negative samples can safely be diagnosed as squamous cell carcinoma. However recent reports suggest p40 as the best marker to differentiate squamous cell carcinoma.[30] Even though EGFR status cannot be employed in differential diagnosis, its analysis is important for targeted therapies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Department of Biotechnology, Govt. of India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Authors acknowledge the Department of Biotechnology, Govt. of India, for funding this project.
REFERENCES
- 1.Junker K. Prognostic factors in stage I/II non-small cell lung cancer. Lung Cancer. 2001;33:17–24. doi: 10.1016/s0169-5002(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 2.Kawahara A, Yamamoto C, Nakashima K, Azuma K, Hattori S, Kashihara M, et al. Molecular diagnosis of activating EGFR mutations in non-small cell lung cancer using mutation-specific antibodies for immunohistochemical analysis. Clin Cancer Res. 2010;16:3163–70. doi: 10.1158/1078-0432.CCR-09-3239. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–23. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 4.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, Maione P, Bareschino MA, Schettino C, Sacco PC, Ferrara ML, et al. The emerging role of histology in the choice of first-line treatment of advanced non–small cell lung cancer: Implication in the clinical decision-making. Curr Med Chem. 2010;17:1030–8. doi: 10.2174/092986710790820589. [DOI] [PubMed] [Google Scholar]
- 7.Veena VS, George PS, Jayasree K, Sujathan K. Comparative analysis of cell morphology in sputum samples homogenized with dithiothreitol, N-acetyl-L cysteine, cytorich red preservative and in cellblock preparations to enhance the sensitivity of sputum cytology for the diagnosis of lung cancer. Diagn Cytopathol. 2015;43:551–8. doi: 10.1002/dc.23266. [DOI] [PubMed] [Google Scholar]
- 8.Wu M, Szporn AH, Zhang D, Wasserman P, Gan L, Miller L, et al. Cytology applications of p63 and TTF-1 immunostaining in differential diagnosis of lung. Diagn Cytopathol. 2005;33:223–7. doi: 10.1002/dc.20337. [DOI] [PubMed] [Google Scholar]
- 9.Reis-Filho JS, Schmitt FC. Taking advantage of basic research: p63 is a Reliable myoepithelial and stem cell marker. Adv Anat Pathol. 2002;9:280–9. doi: 10.1097/00125480-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 11.Ladanyi M, Pao W. Lung adenocarcinoma: Guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21:16–22. doi: 10.1038/modpathol.3801018. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Uehara T, Ota H. Liquid-based thin-layer cytology can be routinely used in samples obtained via fiberoptic bronchoscope. Acta Cytol. 2011;55:69–78. doi: 10.1159/000320872. [DOI] [PubMed] [Google Scholar]
- 13.Hansen T, Pedersen H, Brauner V, Hariri J. Control specimens for immunocytochemistry in liquid-based cytology. Cytopathology. 2011;4:243–6. doi: 10.1111/j.1365-2303.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu DY, Nassar A, Siddiqui MT. High-grade urothelial carcinoma: Comparison of sure path liquid-based processing with cytospin processing. Diagn Cytopathol. 2009;37:16–20. doi: 10.1002/dc.20957. [DOI] [PubMed] [Google Scholar]
- 15.Aikawa E, Kawahara A, Hattori S, Yamaguchi T, Abe H, Taira T, et al. Comparison of the expression levels of napsin a, thyroid transcription factor-1 and p63 in non small cell lung cancer using cytocentrifuged bronchial brushings. Cancer Cytopathol. 2011;119:335–45. doi: 10.1002/cncy.20162. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch FR, Spreafico A, Novello S, Wood MD, Simms L, Papotti M. The prognostic and predictive role of histology in advanced non small cell lung cancer: A literature review. J Thorac Oncol. 2008;3:1468–81. doi: 10.1097/JTO.0b013e318189f551. [DOI] [PubMed] [Google Scholar]
- 17.Nizzoli R, Tiseo M, Gelsomino F, Bartolotti M, Majori M, Ferrari L, et al. Accuracy of fine needle aspiration cytology in the pathological typing of non-small cell lung cancer. J Thorac Oncol. 2011;6:489–93. doi: 10.1097/JTO.0b013e31820b86cb. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson AG, Gonzalez D, Shah P, Pynegar MJ, Deshmukh M, Rice A, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol. 2010;5:436–41. doi: 10.1097/JTO.0b013e3181c6ed9b. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98:13790–5. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au NH, Gown AM, Cheang M, Huntsman D, Yorida E, Elliott WM, et al. P63 expression in lung carcinoma: A tissue microarray study of 408 cases. Appl Immunohistochem Mol Morphol. 2004;12:240–7. doi: 10.1097/00129039-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Kargi A, Gurel D, Tuna B. The diagnostic value of TTF-1, CK 5/6 and p63 immunostaining in classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15:415–20. doi: 10.1097/PAI.0b013e31802fab75. [DOI] [PubMed] [Google Scholar]
- 22.Au NH, Cheang M, Huntsman DG, Yorida E, Coldman A, Elliott WM, et al. Evaluation of immunohistochemical markers in non-small cell lung cancer by unsupervised hierarchical clustering analysis: A tissue microarray study of 284 cases and 18 markers. J Pathol. 2004;204:101–9. doi: 10.1002/path.1612. [DOI] [PubMed] [Google Scholar]
- 23.Wang BY, Gil J, Kaufman D, Gan L, Kohtz DS, Burstein D. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol. 2002;33:921–6. doi: 10.1053/hupa.2002.126878. [DOI] [PubMed] [Google Scholar]
- 24.Conde E, Angulo B, Redondo P, Toldos O, García-García E, Suárez-Gauthier A, et al. The use of P63 immunohistochemistry for the identification of squamous cell carcinoma of the lung. PLoS One. 2010;5:e12209. doi: 10.1371/journal.pone.0012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loo PS, Thomas SC, Nicolson MC, Fyfe MN, Kerr KM. Subtyping of undifferentiated non–small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol. 2010;5:442–7. doi: 10.1097/JTO.0b013e3181d40fac. [DOI] [PubMed] [Google Scholar]
- 26.DiLoreto C, Puglisi F, DiLauro V, Damante G, Fabbro D, Beltrami CA. Immunocytochemical expression of tissue specific transcription factor-1 in lung carcinoma. J Clin Pathol. 1997;50:30–2. doi: 10.1136/jcp.50.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner BM, Cagle PT, Sainz IM, Fukuoka J, Shen SS, Jagirdar J. Napsin A, a new marker for lung adenocarcinoma, is complementary and more sensitive and specific than thyroid transcription factor 1 in the differential diagnosis of primary pulmonary carcinoma. Evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med. 2012;136:163–71. doi: 10.5858/arpa.2011-0320-OA. [DOI] [PubMed] [Google Scholar]
- 28.Hendler FJ, Ozanne BW. Human squamous cell lung cancers express Increased epidermal growth factor receptors. J Clin Investig. 1984;74:647–51. doi: 10.1172/JCI111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: Perspectives for targeted therapies. Lung Cancer. 2003;41:29–41. doi: 10.1016/s0169-5002(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 30.Tatsumori T, Tsuta K, Masai K, Kinno T, Taniyama T, Yoshida A, et al. P40 is the best marker for diagnosing pulmonary squamous cell carcinoma: Comparison with p63, cytokeratin 5/6, desmocollin-3, and Sox2. Appl Immunohistochem Mol Morphol. 2014;22:377–82. doi: 10.1097/PAI.0b013e3182980544. [DOI] [PubMed] [Google Scholar]


