Abstract
Context:
Fine-needle aspiration cytology (FNAC) is a rapid and accurate first-line diagnostic modality in lymphadenopathy.
Aims:
To determine the utility of FNAC for the diagnosis of lymphoma and highlight the various pitfalls in morphological interpretation.
Settings and Design:
This was a retrospective study of 3 years duration in which cytology diagnosis was compared with the follow-up histopathology diagnosis wherever available.
Results:
A cytodiagnosis of lymphoma was made in a total of 868 cases (2.8%) out of nearly 33,000 FNAC performed during the study period; 556 (64.1%) cases were diagnosed as non-Hodgkin lymphoma (NHL), 198 (22.8%) as Hodgkin lymphoma (HL), and in 114 (13.1%) cases, a cytological diagnosis of lymphoma without further categorization was given. Histopathological reports were available in 348 cases, with an overall concordance rate of 93.1% (324), which was slightly higher in the HL cases (95.8%) as compared to NHL (91.7%). Twenty-four cases (6.9%) showed discordant cytological diagnosis with subsequent histopathology. The main reasons for the erroneous diagnosis were the over-interpretation of the germinal center cells as atypical lymphoid cells, over-interpretation of immunoblasts with prominent nucleoli as Hodgkin cells, and sheets of monomorphic lymphoid cells interpreted as low-grade lymphoma.
Conclusion:
Cytomorphology alone can make a correct basic diagnosis of lymphoma with a high degree of accuracy. The errors in interpretation can be further reduced by careful attention to the diagnostic pitfalls and common differential diagnoses.
Keywords: Cytomorphology, FNAC, lymph node, lymphoma, pitfalls
INTRODUCTION
Fine-needle aspiration cytology (FNAC) is a rapid, reliable, and accurate first-line diagnostic modality in lymphadenopathy, including cases suspicious of hematolymphoid neoplasia.[1,2,3,4,5] It is minimally invasive and provides easy sample acquisition for multiple ancillary techniques, including cytological smears, cell block preparation, flow cytometry, and molecular testing at a single time, and at the same time, provides us an early provisional diagnosis.[6,7,8,9] No doubt, excisional biopsy of the lymph node and histopathological examination is the gold standard for diagnosing lymphoma, but FNAC has its place and usefulness for obtaining an early diagnosis. However, the accuracy of FNAC in providing a provisional diagnosis of lymphoid neoplasia based on cytomorphology alone is not well-documented. Further, the choice of the most appropriate ancillary technique to be applied in a given case would depend on the initial morphological diagnosis. Hence, in this study, we compared the FNAC diagnosis of lymphoma to histopathology in 348 cases to determine its utility and highlight the various pitfalls in morphological interpretation.
MATERIALS AND METHODS
This study was a retrospective study conducted in the Departments of Cytology and Histopathology, PGIMER, Chandigarh. The study was conducted as per the ethical guidelines of the Institute Ethics Committee and according to the Helsinki Declaration. Since it is a retrospective study, no additional intervention or invasive procedure was performed on any patient, precluding any ethical violation. FNAC was done in all the cases as a routine procedure with implied informed consent. In the case of palpable node or swelling, FNAC was done without any guidance using a 22- or 23-gauge needle. In non-palpable cases or deep-seated lymph nodes in the abdomen or mediastinum, aspiration under ultrasound guidance using a 22-gauge, 6-inch-long Chiba needle was done. About 3–4 air-dried smears and another 2–3 alcohol-fixed smears were made in every case for May-Grunwald Giemsa and H and E smears, respectively. The sample was also collected for cell blocks in most cases. The study period was from January 2017 to December 2019 (36 months). The cytology diagnosis (without any ancillary technique) was obtained from the reports. This was compared to the follow-up histopathology of the core biopsy or excisional biopsy wherever available. Statistical analysis: Descriptive statistics of the comparison of cytodiagnosis on morphology with the histopathology diagnosis to derive the rate of concordance. A detailed analysis of discrepant cases for the type of error was done and the frequency was provided.
RESULTS
A cytodiagnosis of lymphoma/suspicious for lymphoma was made in a total of 868 cases (2.8%) out of nearly 33,000 FNAC performed during the study period. Out of these 868 cases, 556 (64.1%) cases were diagnosed as non-Hodgkin lymphoma (NHL), 198 (22.8%) as Hodgkin lymphoma (HL), and 114 (13.1%) cases, a cytological diagnosis of lymphoma, not otherwise specified, was given. Histopathological reports were available in 348 cases with an overall concordance rate for morphological diagnosis of lymphoma being 93.1% cases (324/348). The concordance was slightly higher in the cases diagnosed as HL (113/118, 95.8%) as compared to the cases diagnosed as NHL (211/230, 91.7%). Twenty-four cases (6.9%) showed cytohistopathology discordance and are detailed in Tables 1 and 2.
Table 1.
Discordant cases on comparison of cytodiagnosis with histopathology: Category 1: False-positive cases (n=14)
| Age | Sex | Site LN | FNA Dx | HPE | Error Type | Cause of Error |
|---|---|---|---|---|---|---|
| 70 | F | Cx | NHL | RLH | I | Low cellularity |
| 34 | F | Cx | NHL | RLH | I | Low cellularity |
| 48 | M | Cx | Lymphoma | RLH | I | |
| 38 | F | PA | s/o HL | RLH | I | Low cellularity |
| 6 | M | Cx | NHL | RLH | I | |
| 47 | M | Ax | s/o Lymphoma | RLH | I | |
| 63 | M | Ing | NHL | RLH | I | Low cellularity |
| 22 | M | Ax | NHL | RLH | I | Low cellularity, Crush artifact |
| 24 | F | Cx | NHL | RLH | I | Low cellularity |
| 60 | F | Cx | NHL, s/o FL | RLH | I | Low cellularity |
| 52 | M | Cx | s/o Lymphoma | RLH | I | Low cellularity |
| 17 | M | Cx | s/o HL | RLH | I | Low cellularity |
| 23 | F | Cx | Lymphoma s/o HL | PTGC | I | |
| 43 | M | Ax | NHL, s/o FL | RLH | I |
Ax, Axillary; Cx, cervical; F, Female; FL, Follicular lymphoma; HL, Hodgkin lymphoma; Ing, Inguinal; I, Interpretation; LN, Lymph node; M, Male; NHL, Non-Hodgkin lymphoma; PA, Para-aortic; PTGC, Progressive transformation of germinal center; RLH, Reactive lymphoid hyperplasia; S/O, Suggestive of
Table 2.
Discordant cases on comparison of cytodiagnosis with histopathology
| Category 2: Lymphoma vs. other malignancies (n=3) | ||||||
|---|---|---|---|---|---|---|
| Age | Sex | Site LN | FNA Dx | HPE | Error type | Cause of Error |
| 50 | M | Cx | NHL, HG | Small-cell carcinoma metastatic | I | Low cellularity, crush artifact |
| 62 | M | Cx | NHL | Carcinoma, Metastatic | I | Crush artifact |
| 37 | M | Cx | NHL, HG | Nasopharyngeal Carcinoma, Metastatic | I | |
| Category 3: Hodgkin vs. non-Hodgkin lymphoma (n=7) | ||||||
| 65 | M | Cx | HL | DLBCL | I | Low cellularity, few atypical cells |
| 2 | F | SM | HL | DLBCL | I | Low cellularity |
| 65 | F | SC | HL | ALCL | I | Low cellularity |
| 47 | M | RPN | NHL, s/o ALCL | HL, NOS | I | Segmental involvement |
| 42 | M | Cx | NHL, s/o ALCL | HL, NS | I,S | Low cellularity, segmental involvement |
| 32 | M | Ax | NHL, s/o ALCL | HL, NS | I,S | Low cellularity, segmental involvement |
| 43 | F | Cx | NHL, s/o ALCL | HL, NS | I,S | Low cellularity, segmental involvement |
ALCL, Anaplastic large-cell lymphoma; Ax, Axillary; Cx, cervical; DLBCL, Diffuse large B-cell lymphoma; Dx, Diagnosis; F, Female; FL, Follicular lymphoma; HG, High grade; HL, Hodgkin lymphoma; HPE, Histopathological examination; I, Interpretation; LN, Lymph node; M, Male; NHL, Non-Hodgkin lymphoma; NOS, Not otherwise specified; RPN, Retroperitoneal; S, sampling ; SM, Sub-mandibular; S/O, Suggestive of; SC, Supraclavicular
Category 1: False-positive cases
Category 1 represents false-positive cases [Table 1]. There were 14 cases (4.0%) wherein a diagnosis of lymphoma was rendered on cytomorphology but were reported as reactive lymphoid hyperplasia on subsequent histopathology. All except one case occurred in adults ranging in age from 22 to 70 years. The size of the lymph node in these cases was also around 2–3 cm, and they also had a clinical suspicion of lymphoma. In a single case with a cytodiagnosis of lymphoma suggestive of HL, subsequent histopathology showed the progressive transformation of the germinal center [Figure 1]. The re-evaluation of the smears revealed low cellularity of smears in 9/14 cases and crushing artifact in 1 case. However, the main reason for the inaccurate diagnosis was the (i) over-interpretation of the germinal center cells as atypical lymphoid cells (eight cases) [Figure 2a], (ii) over-interpretation of immunoblasts with prominent nucleoli as Hodgkin cell (three cases) [Figure 2b], and (iii) sheets of monomorphic lymphoid cells interpreted as low-grade lymphoma (three cases) [Figure 2c].
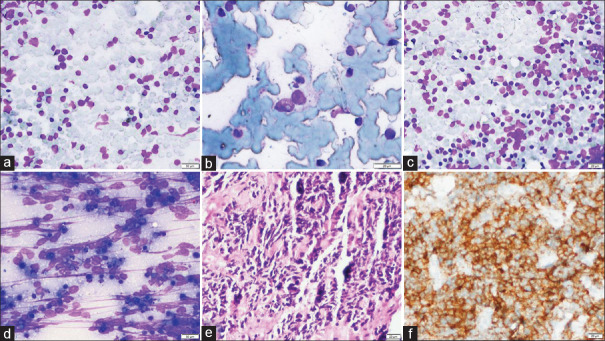
Figure 1.
Category 1: FNA cytology smears (case no. 13) showing scattered RS-like cells with prominent nucleoli (a and b; MGG, 100X), whereas on histology, excisional biopsy shows reactive lymphoid hyperplasia with prominent transformation germinal center (c; H and E, 40X) anti-BCL2 immunostain highlights invasion by BCL2-positive mantle zone cells (d; BCL2, 200X)
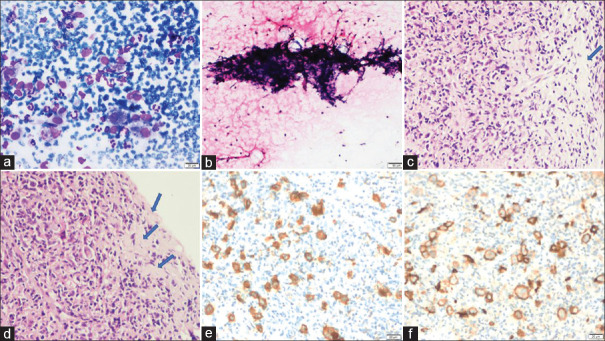
Figure 2.
Category 1 (a–c): FNA cytology smears (case no. 14) showing predominantly large germinal center cells mimicking atypical lymphoid cells (a; MGG, 40X), binucleated RS-like cell (case no. 4) (b; MGG, 200X), and sheets of large monomorphic cells mimicking low-grade lymphoma (case no. 11) (c; MGG, 40X); Category 2 (d–f): FNA cytology smears (case no. 1) shows singly scattered atypical cells with marked crushing artifact (a; MGG, 200X), histopathology shows sheets of tumor cells (b; H and E, 100X) which are positive for CD56 immunostain confirming a small cell carcinoma (c; CD56, 100X)
Category 2: Lymphoma versus other malignancies
Three cases (3/348,0.9%) were diagnosed as lymphoma on cytology but showed other malignancies on histology [Table 2]. All the patients presented with cervical lymphadenopathy and had a clinical suspicion of lymphoma. The cytological smears were paucicellular in two cases, which was the cause of the discrepancy. There was an associated marked crushing artifact in one case [Figure 2d]. Immunohistochemistry on the biopsy confirmed metastatic small-cell carcinoma and metastatic carcinoma, respectively [Figure 2e and f]. In the third case, the smears were cellular and showed large, singly scattered atypical cells with coarse chromatin admixed with lymphoid cells and many lymphoglandular bodies. Numerous mitotic figures and apoptotic bodies were also seen. Hence, the cytodiagnosis was high-grade lymphoma. Subsequent clinical correlation revealed a mass lesion in the nasopharynx, and core-needle biopsy from the lymph node and biopsy from the primary confirmed nasopharyngeal carcinoma.
Category 3: Hodgkin lymphoma versus Non-Hodgkin lymphoma
There were seven cases in this category (7/348, 2.0%) [Table 2], out of which three cases were initially diagnosed on cytology as HL and subsequent histopathology revealed NHL and four cases were converse. In all these cases, low cellularity and segmental nodal involvement were the reasons for the interpretational error. The cases of NHL misdiagnosed as HL on cytology revealed fewer atypical cells due to the sclerotic nodes (two cases) or a few atypical cells in a polymorphous background (one case) due to the segmental nodal involvement [Figure 3]. Histology revealed anaplastic large-cell lymphoma (ALCL) in one case and diffuse large-cell B-cell lymphoma (DLBCL) in the other two cases with partial effacement of the lymph nodal architecture. Conversely, out of the four cases of HL misdiagnosed as NHL, three were HL, nodular sclerosing type 2, and one was HL, mixed cellularity type. The smears showed numerous atypical cells in a background of the polymorphic population of the reactive lymphoid cells mimicking the ALCL type of NHL [Figure 4].
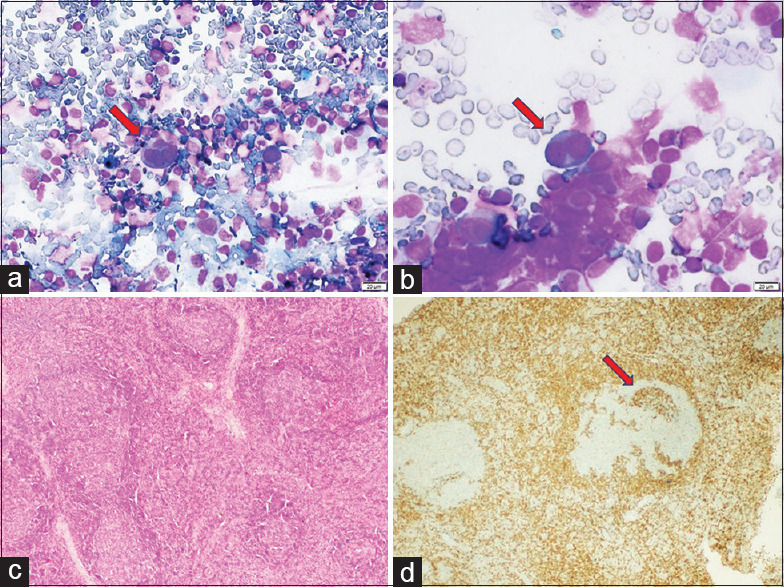
Figure 3.
Category 3: FNA cytology smears (case no. 1) showing scattered RS-like cells with prominent nucleoli (a and b; MGG, 200X), whereas on histology, excisional biopsy shows sheets of atypical lymphoid cells (c; H and E, 100X) which are diffusely positive for anti-CD20 antibody on immunostaining (d; CD20, 200X)
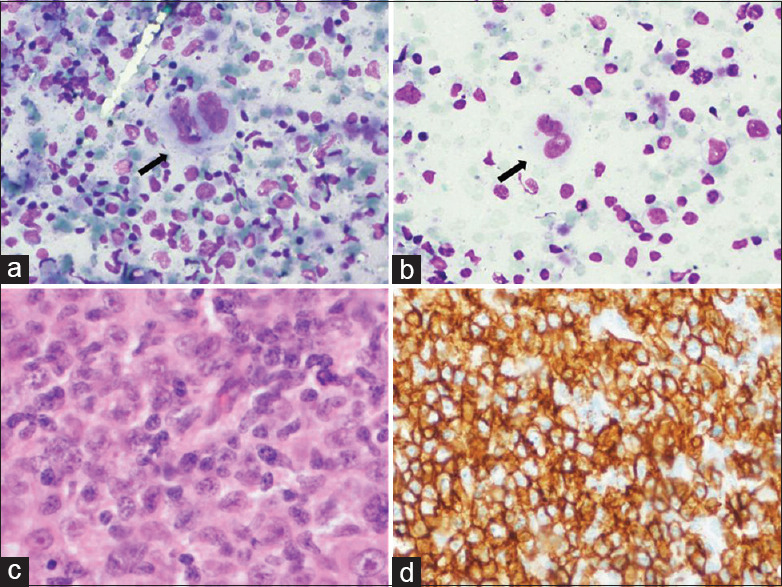
Figure 4.
Category 3: FNA cytology smears (case no. 5) showing many large atypical cells (a; MGG, 100X), along with fibrotic fragments (b; H and E, 40X), histology of excisional biopsy shows numerous atypical RS-like cells in a sclerotic background (blue arrow) (c; H and E, 100X) (d; H and E, 200X), these atypical cells were positive for CD30 and CD20 on immunostaining (e; CD30, 200X) (f; CD20, 200X)
Role of ancillary techniques
Although this article aimed to highlight the accuracy of the cytomorphological diagnosis, ancillary techniques of cell block immunocytochemistry (CB-ICC) and flow-cytometric immunophenotyping (FCI) were performed in 176 cases in this cohort of cases. After integrating the immunophenotyping data, accurate typing was done in 126 cases with a sign-out diagnosis of DLBCL (47), precursor T-lymphoblastic lymphoma (29), Burkitt lymphoma (17), Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (8), mantle cell lymphoma (8), ALCL (7), follicular lymphoma (7), and angioimmunoblastic T-cell lymphoma (AITCL) (1). In the remaining 50/176 cases, a diagnosis of NHL, a mature B-cell type, was given. Histopathology was available in 33 cases with perfect correlation in 31 cases and discordance in 2 cases only [Table 3]. Both these cases with cytodiagnosis of DLBCL (ABC sub-type) were categorized as Burkitt lymphoma based on C-Myc positivity, which had not been evaluated on the cell block immunocytochemistry.
Table 3.
Sub-categorization of NHL cases by using CB-ICC and FCI
| Cytomorphology diagnosis | Cytology diagnosis after CB-ICC and FCI | Histopathological diagnosis | No. of cases |
|---|---|---|---|
| NHL, large-cell type, high grade | DLBCL | DLBCL | 16 |
| Burkitt lymphoma | 2 | ||
| NHL small-cell type, low grade | Follicular lymphoma | Follicular lymphoma | 3 |
| NHL small-cell type, low grade | Mantle Cell lymphoma | Mantle Cell lymphoma | 4 |
| NHL, large-cell type, high grade | Lymphoblastic lymphoma | Lymphoblastic lymphoma | 5 |
| Lymphoma | ALCL | ALCL | 2 |
| NHL, large-cell type, high grade | Burkitt lymphoma | Burkitt lymphoma | 1 |
| Total | 33 |
ALCL, Anaplastic large-cell lymphoma; CB-ICC, Cell block immunocytochemistry; DLBCL, Diffuse large B-cell lymphoma; FCI, Flow cytometric immunophenotyping
DISCUSSION
The previous literature suggests a comparative analysis of cytopathology and histopathological evaluation of lymphomatous pathology with acceptable specificity and sensitivity. Whether this holds even in today's era with a recognition of a multitude of lymphomas was the central theme of this paper. In low- and middle-income countries with inadequate resources, morphology still retains its importance. The overall diagnostic accuracy was nearly 93%, and this was more in HL as compared to NHL. This is slightly more than the previous reports of 81[7] and 86.6% by Zhang et al.[6] It is important to make as accurate a diagnosis as possible on morphology for many reasons. One is that the confirmation of a clinical diagnosis of lymphoma can aid the clinician in quickly initiating further investigations that are relevant to the case, such as a bone marrow examination and Positron Emission Tomography-Computed Tomography (PET-CT) scan for staging apart from biopsy and histopathology. If immunophenotyping facilities are available, cytomorphology can help choose the most appropriate panel for flow cytometry or immunohistochemistry.
The significant challenge in a cytomorphological diagnosis of HL was in its distinction from a progressive transformation of germinal center (PTGC) and ALCL. Hartmann et al.[10] describe that PTGC can show different patterns on histology with variations in infiltration by the mantle zone cells, and that the subsequent cytomorphology will comprise of variable numbers of germinal center cells depending upon the extent of infiltration by the mantle zone. Like in our case, histology shows a scalloped mantle zone pattern with minimal infiltration by the mantle zone cells resulting in sheets of germinal center cells like centrocytes, centroblasts, and immunoblasts. Because of minimal mantle zone involvement, mature lymphocyte proportion is much less. All these findings, along with a favorable clinical setting, the case was reported as lymphoma with suspicion of HL.
Analysis of discrepancies
Twenty-four cases were under the cytohistopathology discorrelation category and were further sub-classified under three different categories. The erroneous diagnosis of a benign condition as malignant is always dangerous for the patient. Several factors come out as a cause for the misdiagnoses like interpretational error, low cellularity, crushing artifact, segmental involvement of the lymph node, and sampling error.
In the present study, the erroneous interpretation of germinal follicular center cells as atypical cells was one of the common causes for false positivity seen in 14/24 (58.3%) cases. Out of these 14 cases, a few cases showed large cells like the immunoblasts and centroblasts with prominent nucleoli and frequent mitotic activity. Hehn et al.[11] supported a similar finding and described it in a large reactive lymph node. The needle usually hits a large germinal center and the aspirated material can show a mixed population of lymphoid cells, including all stages of maturity. Mendon[12] suggests that the presence of large centroblasts and immunoblast-like cells can be commonly seen in cases of viral etiology. Landgren et al.[13] suggest that these large atypical-looking cells should be considered significant only when their proportion will be >50% among all the cells.
A few cases showed sheets of monomorphic cells, which were 1.5–2.0 times the size of mature lymphocytes with slightly opened-up chromatin. Nasuti et al.[14] also observed a similar monomorphic population of lymphoid cells in cases of reactive lymphoid hyperplasia when the needle strikes in the widened inter-follicular area. One should also take care of the background cell population; thoroughly examining all the smears for polymorphous background population and the presence of numerous body macrophages could lower the chances of low-grade lymphoma. It is always better to confirm these cases with ancillary techniques like flow cytometry.[3,4,10] In cases of follicular lymphoma where the atypical cell population comprises a mixed population of centrocytes, centroblasts, and immunoblasts, it becomes challenging to rule out or confirm the diagnosis based on cytology smears. In our study, two cases on cytology were suggested as follicular lymphoma turned out reactive on histopathology. So, the concurrent use of flow cytometry is suggested in such cases to confirm this possibility.[15,16]
Three cases in our study were diagnosed as HL but turned out to be non-malignant after histopathology. Two cases showed reactive pathology on histopathology, while the remaining one was diagnosed as a progressive transformation of the germinal center. On cytology, there were a few scattered large cells with large prominent nucleoli, so again, erroneous interpretation is the cause of misdiagnosis. Zhang et al.[6] described that the presence of atypical mononuclear cells with Reed-Sternberg cell (RS cell) cell-like morphology in a background of the polymorphous lymphoid population is enough to label a case as suspicious for HL. However, Malakar et al.[17] suggest to always look for classical RS cells for making a diagnosis of Hodgkin lymphoma. If the patient is a known case of HL, then mononuclear cells can also be considered significant. The progressive transformation of the germinal center is always a close differential for HL. Hartmann et al.[10] described that lymphocyte-predominant HL is commonly associated with PTGC; the patient may present with concurrent or later transformation into HL. In PTGC, there is a marked proliferation of the germinal center; on cytology, one can misinterpret large immunoblasts as RS-like cell with a polymorphous background, if the needle hits the germinal center. Again, in cases with cytological suspicion of HL and lacking classical RS, histopathological examination or CB-ICC is advisable.
The other important pitfall in diagnosis is low cellularity seen as a secondary cause in 16/24 (66.6%) cases while it was the primary cause of misdiagnosis in 8/24 (33.3%) cases. The common reasons behind low cellular smears were either sclerosed nodes or deep location of aspirated mass/node. Sclerosis in cases of nodular sclerosing Hodgkin lymphoma or metastatic lymph node is the most common cause for limited cellularity. Some authors include limited cellularity as a sampling error being one of the most common reasons for a false-positive or false-negative diagnosis.[18,19] Jimenez-Heffernan et al.[20] and Perrone et al.[21] in their studies found low cellularity as one of the leading causes of misdiagnosis primarily in sclerosed masses. They have suggested histopathological examination in such cases. Besides, significant background cell populations such as the presence of eosinophils, plasma cells in HL, and scarcity of lymphoglandular bodies in the metastatic lesions could be considered as a soft pointer for better diagnosis.
Another important pitfall is the crushing artifact; in our study, 3/24 (12.5%) cases show crushing artifact; two cases are of category two, lymphoma versus other malignancy. These two cases which show marked crushing artifact with singly scattered atypical cells showed metastatic small blue round cell tumors on histopathology. In these cases, again, the primary clinical suspicion of lymphoma with atypical cells on cytology smears lead to a diagnosis of lymphoma. Lymphoma is always a close differential diagnosis for small blue round cell tumors on morphology; besides, metastasis and lymphoma follow the same age group pattern.[22] The crushing artifact is more commonly associated with small blue round cell tumour. Layfield et al.[23] in their study conclude that several morphological features can help us to avoid mistakes in giving a final diagnosis in a tumor with small round cell morphology. They suggest that lymphoglandular bodies can also be seen in cases with Ewing's tumor or rhabdomyosarcoma; however, it is their frequency which matters. Likewise, careful observation for any rosette, molding, or clustering can favour a diagnosis of small blue round cell tumour over lymphoma. Further studies suggest proceeding for CB-ICC or histopathological examination for verification.[24,25]
Segmental involvement of lymph node is another common cause of misdiagnosis seen in 4/24 (16.7%) cases leading to compromised material and low cellularity. Segmental involvement of the lymph node is commonly seen in cases of metastatic disease and sometimes in low-grade lymphoma. Most of the time, metastatic disease leads to the partial involvement of the lymph node, and the rest of the lymph node shows reactive changes.
There are many aspects to consider before making a diagnosis; a cytopathologist can diagnose a case accurately with the help of clinical history, radiological findings, and careful interpretation of cytology smears, along with the use of ancillary techniques. Ancillary techniques may not be available in many centers or may not be affordable by the patient, and hence, an excellent morphological cytodiagnosis of lymphoma is important.
To conclude, FNAC is the first-line modality for screening lymphadenopathy with a sensitivity of more than 90% with a high degree of accuracy for a primary diagnosis of lymphoma based on cytomorphology alone. The errors in interpretation can be further reduced by careful attention to the diagnostic pitfalls and common differential diagnoses. Accurate morphological interpretation can direct appropriate and economical use of the ancillary techniques of immunophenotyping to enable accurate typing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sandhaus LM. Fine-needle aspiration cytology in the diagnosis of lymphoma. The next step. Am J Clin Pathol. 2000;113:623–7. doi: 10.1309/BEBH-BLA2-J5AY-YE2L. [DOI] [PubMed] [Google Scholar]
- 2.Zeppa P, Vigliar E, Cozzolino I, Troncone G, Picardi M, De Renzo A, et al. Fine needle aspiration cytology and flow cytometry immunophenotyping of non-Hodgkin lymphoma: Can we do better? Cytopathology. 2010;21:300–10. doi: 10.1111/j.1365-2303.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeppa P, Marino G, Troncone G, Fulciniti F, De Renzo A, Picardi M, et al. Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-Hodgkin lymphoma: A critical review of 307 cases with technical suggestions. Cancer. 2004;102:55–65. doi: 10.1002/cncr.11903. [DOI] [PubMed] [Google Scholar]
- 4.Schwock J, Geddie WR. Diagnosis of B-cell non-hodgkin lymphomas with small-/intermediate-sized cells in cytopathology. Patholog Res Int. 2012;2012:164934. doi: 10.1155/2012/164934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CJ, Duncan JA, Farquharson M, Richmond J. Fine needle aspiration cytology diagnosis of malignant lymphoma and reactive lymphoid hyperplasia. J Clin Pathol. 1998;51:197–203. doi: 10.1136/jcp.51.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Yu X, Zheng Y, Yang Y, Xie J, Zhou X. Value of fine needle aspiration cell blocks in the diagnosis and classification of lymphoma. Int J Clin Exp Pathol. 2014;7:7717–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Ingersoll KF, Zhao Y, Harrison GP, Li Y, Yang LH, Wang E. Limited tissue biopsies and hematolymphoid neoplasms. Am J Clin Pathol. 2019;152:782–98. doi: 10.1093/ajcp/aqz107. [DOI] [PubMed] [Google Scholar]
- 8.Jelloul FZ, Navarro M, Navale P, Hagan T, Cocker RS, Das K, et al. Diagnosis of lymphoma using fine-needle aspiration biopsy and core-needle biopsy: A single-institution experience. Acta Cytol. 2019;63:198–205. doi: 10.1159/000497252. [DOI] [PubMed] [Google Scholar]
- 9.Liu K, Mann KP, Vitellas KM, Paulson EK, Nelson RC, Gockerman JP, et al. Fine-needle aspiration with flow cytometric immunophenotyping for primary diagnosis of intra-abdominal lymphomas. Diagn Cytopathol. 1999;21:98–104. doi: 10.1002/(sici)1097-0339(199908)21:2<98::aid-dc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann S, Winkelmann R, Metcalf RA, Treetipsatit J, Warnke RA, Natkunam Y, et al. Immunoarchitectural patterns of progressive transformation of germinal centers with and without nodular lymphocyte-predominant Hodgkin lymphoma. Hum Pathol. 2015;46:1655–61. doi: 10.1016/j.humpath.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–52. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 12.Mendon ME. Fine needle aspiration cytology of lymph nodes. Prog Diagn Cytol. 1999;32:453–6. [Google Scholar]
- 13.Landgren O, Porwit MacDonald A, Tani E, Czader M, Grimfors G, Skoog L, et al. A prospective comparison of fine-needle aspiration cytology and histopathology in the diagnosis and classification of lymphomas. Hematol J. 2004;5:69–76. doi: 10.1038/sj.thj.6200316. [DOI] [PubMed] [Google Scholar]
- 14.Nasuti JF, Yu G, Boudousquie A, Gupta P. Diagnostic value of lymph node fine needle aspiration cytology: An institutional experience of 387 cases observed over a 5-year period. Cytopathology. 2000;11:18–31. doi: 10.1046/j.1365-2303.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 15.Brandao GD, Rose R, McKenzie S, Maslak P, Lin O. Grading follicular lymphomas in fine-needle aspiration biopsies: The role of ThinPrep slides and flow cytometry. Cancer. 2006;108:319–23. doi: 10.1002/cncr.22173. [DOI] [PubMed] [Google Scholar]
- 16.Dong HY, Harris NL, Preffer FI, Pitman MB. Fine-needle aspiration biopsy in the diagnosis and classification of primary and recurrent lymphoma: A retrospective analysis of the utility of cytomorphology and flow cytometry. Mod Pathol. 2001;14:472–81. doi: 10.1038/modpathol.3880336. [DOI] [PubMed] [Google Scholar]
- 17.Malakar D, Swarup K. A clinical evaluation of fine needle aspiration of cytology in the diagnosis of lymphadenopathy. Ind J Tuberc. 1991;38:17–8. [Google Scholar]
- 18.Jogai S, Dey P, Al Jassar A, Amanguno HG, Adesina AO. Role of fine needle aspiration cytology in nodular sclerosis variant of Hodgkin's lymphoma. Acta Cytol. 2006;50:507–12. doi: 10.1159/000326004. [DOI] [PubMed] [Google Scholar]
- 19.Rashmi Kumari T, Rajalakshmi T. Fine needle aspiration cytology in the diagnosis of Hodgkin lymphoma: Hits and misses. J Cytol. 2008;25:10–2. [Google Scholar]
- 20.Jimenez-Heffernan JA, Vicandi B, Lopez-Ferrer P, Hardisson D, Viguer JM. Value of fine needle aspiration cytology in the initial diagnosis of Hodgkin's disease. Analysis of 188 cases with an emphasis on diagnostic pitfalls. Acta Cytol. 2001;45:300–6. doi: 10.1159/000327622. [DOI] [PubMed] [Google Scholar]
- 21.Perrone T, Frizzera G, Rosai J. Mediastinal diffuse large-cell lymphoma with sclerosis. A clinicopathologic study of 60 cases. Am J Surg Pathol. 1986;10:176–91. doi: 10.1097/00000478-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Rajwanshi A, Srinivas R, Upasana G. Malignant small round cell tumors. J Cytol. 2009;26:1–10. doi: 10.4103/0970-9371.54861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layfield LJ, Liu K, Dodge RK. Logistic regression analysis of small round cell neoplasms: A cytologic study. Diagn Cytopathol. 1999;20:271–7. doi: 10.1002/(sici)1097-0339(199905)20:5<271::aid-dc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Llombart-Bosch A, Navarro S. Immunohistochemical detection of EWS and FLI-1 proteinss in Ewing sarcoma and primitive neuroectodermal tumors: Comparative analysis with CD99 (MIC-2) expression. Appl Immunohistochem Mol Morphol. 2001;9:255–60. doi: 10.1097/00129039-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Silverman JF, Dabbs DJ, Ganick DJ, Holbrook CT, Geisinger KR. Fine needle aspiration cytology of neuroblastoma, including peripheral neuroectodermal tumor, with immunocytochemical and ultrastructural confirmation. Acta Cytol. 1988;32:367–76. [PubMed] [Google Scholar]